Abstract
The reported incidence of post allogeneic hematopoietic stem cell transplant (HSCT) auto-immune hemolytic anemia (AIHA) was between 4.4% and 6% following a single transplant. Cord blood transplantation, T-cell depletion and chronic GvHD are significantly associated with post-transplant AIHA. During an 11 year period, data for 500 pediatric HSCT recipients were eligible for evaluation of the incidence of AIHA post first and second transplants. Demographic, transplant, and post-transplant related variables were analyzed. Twelve/500 (2.4%) recipients at a median of 273 days and 7/72 (9.7%) recipients at a median of 157 days developed AIHA post first and second HSCT respectively. Post first HSCT, none of the matched related donor recipients developed AIHA (0/175 MRD vs. 12/325 other donors, p=0.04). Four/12 required a second HSCT to control the AIHA. Post the second HSCT, matched unrelated donor was significantly associated with the development of AIHA. No other variables were associated with the post-second transplant AIHA.
The incidence of AIHA post first and second HSCT was less than reported. The increased incidence of AIHA among recipients of second HSCT is most likely due to the profound immune dysregulation. A much larger, prospective study would be needed to evaluate the incidence, complications and management of post-transplant AIHA.
Keywords: bone marrow transplantation, pediatrics, Coombs-positive hemolytic anemia, anemia, Hematopoietic Stem Cell Transplantation
Introduction
Immune-mediated hemolytic anemia(IHA) is a recognized post hematopoietic stem cell transplant (HSCT) complication(1). Allo-immune hemolytic anemia is attributed to donor-recipient ABO blood groups incompatibility and is usually short-lived in the immediate post allogeneic HSCT setting(2). Autoimmune hemolytic anemia (AIHA) is less common and is reported either as an isolated event or coincident with other immune-mediated cytopenias(3-5), and is usually attributed to immune dysregulation(6). Cord blood transplantation, Tcell-depleted HSCT, and chronic graft versus host disease (GvHD) are significantly associated with AIHA and other autoimmune cytopenias(5, 7-11). AIHA is caused by donor antibodies directed against donor red blood cells (RBC)(12, 13). The incidence of AIHA is rare in the general population with prevalence of 1:80,000 in pediatric age group(14). On the other hand, Sanz et al. and O'Brien et al. reported cumulative incidence of post-HCST AIHA of 4% (12/272) and 6% (19/303), respectively (12, 15).
Aim
The objectives of this study were to analyze the incidence of autoimmune hemolytic anemia among pediatric recipients of allogeneic HSCT and to determine whether a second HSCT increases the risk for autoimmune hemolytic anemia.
Methods
Study Subjects
We performed a retrospective chart review of HSCT transplant patients' records at Texas Children's Hospital – Baylor College of Medicine after obtaining the required IRB approval. Over a period of 11 years, 515 pediatric patients underwent allogeneic HSCT for treatment of a variety of neoplastic and non-neoplastic hematologic diseases, bone marrow failure syndromes, immunodeficiency and metabolic disorders. Direct anti-globulin test (DAT) was performed for clinical indications at the direction of the treating physician. Recipients' case records, blood bank data, transfusion history, and HSCT program research database were reviewed. AIHA post-HSCT was defined by the presence of positive DAT and non specific antibody elution when detected, along with other lab and clinical evidence of hemolytic anemia including increased reticulocyte count, elevated indirect bilirubin, increased lactate dehydrogenase, decreased haptoglobin, and increased transfusion requirement(12, 15). The patient transplant characteristics are shown in Table 1.
Table 1. Transplant Recipient Characteristic Post First and Second Hematopoietic Stem Cell Transplant.
| Transplant Recipient Characteristics | First HSCT N=500 |
Second HSCT N=72 |
||||
|---|---|---|---|---|---|---|
| No AIHA N=488 |
AIHA N=12 |
p | No AIHA N = 65 |
AIHA N = 7 |
p | |
| Age (years) at 1st transplant (mean±std) | 9±5.9 | 7.4±7.2 | NS | 7.5± 5.8 | 10 ± 5.9 | |
| Age (years) | ||||||
| <10 | 285 | 7 | NS | 46 | 5 | NS |
| ≥10 | 203 | 5 | 19 | 2 | ||
| Gender | ||||||
| Female | 209 | 4 | NS | 22 | 3 | NS |
| Male | 279 | 8 | 43 | 4 | ||
| Race | ||||||
| Caucasian | 188 | 3 | NS | 24 | 4 | NS |
| Hispanic | 203 | 6 | 31 | 2 | ||
| African American | 64 | 2 | 7 | 0 | ||
| Other | 33 | 1 | 3 | 1 | ||
| Diagnosis | ||||||
| Malignant (Total) | 326 | 7 | NS | 45 | 3 | NS |
| Acute Leukemia | 245 | 3 | 35 | 1 | ||
| Lymphomas (HL & NHL) | 28 | 1 | 2 | 0 | ||
| MDS | 32 | 1 | 6 | 1 | ||
| CML | 18 | 2 | 1 | 1 | ||
| Other | 3 | 0 | 1 | 0 | ||
| Non-malignant (Total) | 162 | 5 | 20 | 4 | ||
| Hemoglobinopathy | 87 | 1 | 7 | 3 | ||
| Immune deficiency disorders | 38 | 2 | 7 | 1 | ||
| Metabolic disorders | 10 | 1 | 3 | 0 | ||
| Histiocytic disorders | 24 | 0 | 3 | 0 | ||
| Other | 3 | 1 | 0 | 0 | ||
| Conditioning | ||||||
| Bu/Cy +/- ATG or Campath +/- ARA-c/VP16 or MEL | 87 | 4 | NS | 11 | 2 | NS |
| Bu/Flu/Campath | 5 | 0 | 3 | 1 | ||
| Cy/ ATG or Campath | 29 | 0 | 1 | 0 | ||
| Cy +/- Flu +/- ATG or Campath | 3 | 0 | 1 | 0 | ||
| Cy/VP16 | 5 | 0 | 0 | 0 | ||
| Flu +/- ATG or Campath +/- MEL | 23 | 1 | 18 | 2 | ||
| No Conditioning | 5 | 1 | 9 | 0 | ||
| TBI/Cy +/- ARA-c +/- ATG or Campath | 299 | 6 | 6 | 0 | ||
| TBI/Flu +/- ATG or Campath | 30 | 0 | 13 | 2 | ||
| TBI/VP16 | 2 | 0 | 0 | 0 | ||
| TBI | 0 | 0 | 1 | 0 | ||
| Campath | 0 | 0 | 1 | 0 | ||
| Missing | 0 | 0 | 1 | 0 | ||
| Donor Type | ||||||
| HLA-matched | 341 | 5 | 0.054 | 25 | 5 | NS |
| HLA-mismatched | 147 | 7 | 40 | 2 | ||
| Matched related donor | 175 | 0 | 0.04* | 9 | 0 | NS |
| Mismatched related donor | 82 | 5 | 0.09* | 27 | 2 | NS |
| Mismatched unrelated donor | 65 | 2 | NS | 13 | 0 | NS |
| Matched Unrelated donor | 166 | 5 | NS | 16 | 5 | 0.04* |
| Stem cell source | ||||||
| Bone marrow a | 375 | 7 | NS | 27 | 2 | NS |
| Peripheral blood a | 103 | 4 | 37 | 5 | ||
| Cord Blood | 10 | 1 | 0 | 0 | ||
| ABO match | ||||||
| Match | 256 | 8 | NS | 33 | 3 | NS |
| Minor mismatch | 95 | 2 | 14 | 1 | ||
| Major mismatch | 134 | 2 | 18 | 3 | ||
p value adjusted for multiple comparisons
Comparison between Bone Marrow and Peripheral blood as stem cell source
Any HSCT recipients with positive DAT and antibody elution of specific antibody were excluded from the AIHA group. Fifteen patients were excluded from the analysis: one patient with pre-existing diagnosis of SCID and AIHA prior to the first-HSCT, two patients with allo-immune; anti-A specific antibody; hemolytic anemia, and twelve patients with post-HSCT positive DAT, for whom the relevant clinical information were missing. Five hundred patients were eligible for the final analysis. Seventy-two of these 500 patients received a second HSCT and were available for analysis.
Allogeneic HSCT Procedures
For the initial transplant, the conditioning regimens and selection of stem cell source were determined by diagnosis, presence of comorbidities, and donor availability. 437/500 recipients received myeloablative-conditioning therapy, 57 patients received reduced intensity-conditioning therapy and 6 did not receive conditioning therapy for the first HSCT (Table 1). Among patients undergoing second HSCT, 38/72 recipients received reduced intensity conditioning therapy (Table 1).
Recipients of un-manipulated HSCT received either cyclosporine (CSA) or tacrolimus in combination with methotrexate for prevention of acute GvHD. Patients, for whom a suitable matched related or unrelated donor was unavailable, were eligible for haploidentical donor transplant from CD34 selection of mobilized blood. GvHD prophylaxis therapy was not administered if the infused CD3+ number was less than 5×104/kg. Blood transfusion support for allogeneic HSCT recipients with ABO incompatible donors was administered according to the standard operating procedure considering recipient and donor ABO-incompatibility, and the time period from the transplant(16). CMV positive HSCT recipients received ganciclovir prophylaxis following the engraftment, until day 100 post-HSCT. Intravenous immunoglobulin (IVIG) was administered every four weeks until day 120 post-transplant.
Statistical Method
Descriptive statistics were calculated to describe the patient characteristics of the study sample. To evaluate the potential risk factors for the development of AIHA, Fisher exact tests and two-sample tests of proportions were used to compare patient characteristics and outcomes between patients who developed AIHA and those who did not. A conservative Bonferroni correction was applied to p-values resultant from proportion tests where multiple comparisons were made. Log rank tests were performed to determine whether survival times differed between patients who developed AIHA and those who did not. All analysis were performed in SAS 9.3 (SAS Institute Inc., Cary, NC,USA) and Stata SE/12.1 (StataCorp LP, College Station, TX, USA).
Results
Patients Characteristics
Five hundred evaluable patients underwent allogeneic HSCT during the study period. There were 287 males (57%) and 213 females (43%) with a median age of 8 years (range < 1-23 years). Of the 500 HSCT recipients, 428 (86%) patients received single HSCT, and 72 (14%) patients required a second HSCT for various indications. The median follow-up after the first and the second allo-HSCT was 30.5 months and 14.5 months, respectively. The characteristics of the recipients with and without AIHA post-first and second HSCT are shown in Table 1. Following the first HSCT, 12 (2.4%) out of 500 patients developed AIHA at a median of 273 days (range of 119-4505 days).
Seven out of 72 (9.7%) recipients of second HSCT developed AIHA. For patients undergoing a second HSCT, the same donor was used for 27/72 recipients (37.5%). Excluding patient #2 who had persistent AIHA post 2nd HSCT, AIHA was diagnosed at a median of 157 days (range of 70-256 days) following the second HSCT. Four out of the 12 patients with AIHA following first HSCT were refractory to medical treatment, patients number 2, 5, 7, and 9 in tables 2&3, underwent a second HSCT for refractory hemolysis. Two of those four patients; patients number 2 and 5 received the second HSCT from the same donor. Patient number 2 had persistent AIHA through the second HSCT that ultimately responded to steroid therapy. Patient number 5 re-developed the AIHA 95 days post the second HSCT; which was controlled after a third Allogeneic HSCT from a different donor, and the patient is alive without hemolysis. Patient number 9 received IVIG and steroids post the second transplant to control the AIHA, but the patient is deceased secondary to chronic GvHD complications. Also, patient number 7 is deceased from veno-occlusive disease and multi-organ failure following the second HSCT (Table 3). The 488 and 65 recipients without AIHA post first and second HSCT, respectively, served as the comparison group.
Table 2. Characteristics of Recipients' with Auto-immune Hemolytic Anemia Post First Hematopoietic Stem Cell Transplant.
| Case # | Age/ Diagnosis |
AGVHD | Time of onset of AIHA post- transplant (Days)/ Type of Antibody |
ABO Incompatibility |
HLA Match |
Subsequent Transplant/ Reason |
Stem Cell Source/ reason for HSCT |
Chronic GvHD | Conditioning / (GvHD Prophylaxis) |
Status/days alive from last HSCT/Cause of Death |
Donor/ Recipient Gender |
Donor Chimerism @ AIHA onset/ follow-up |
Treatment for AIHA/Responses C = controlled R = refractory |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22.15/2ry MDS | No | 402/ No AB Elute |
No | MMRD | No | PBSCT | Yes (Limited) | Flu/Mel/ATG (TAC) |
Deceased (508 days post HSCT/Other | M/F | Missing | Not Available |
| 2 | .07/SCID | No | 601/ WAAB |
No | MMRD | Yes/AIHA | BMT | No | None (CSA) |
Alive, post subsequent HSCT | F/M | Missing | Not Available |
| 3 | 2.95/CML | No | 263/ No AB Elute |
Major | MUD | No | BMT | No | TBI/Cy/Camp/Ara-C (TAC/MTX) |
Alive | M/M | 100% | Steroids/C |
| 4 | 14.33/NHL | No | 203/ No AB Elute |
No | MMUD | No | BMT | No | TBI/Cy/Camp/Ara-C (TAC/MTX) |
Alive | F/M | 100% | Steroids/C |
| 5 | 7/B-Thal | Grade 1 | 336/ WAAB |
No | MUD | Yes/AIHA | PBSCT | No | Bu/Cy/Flu/Campath (TAC/MTX) |
Alive | F/M | 96% / 100% (Hypocellular) | IVIG/R Rituximab/R 2nd HSCT/R |
| 6 | 2.89/ALL | Grade 1 | 284/ Cold antibodies |
Minor | MUD | No | BMT | No | TBI/Cy/Camp/Ara-C (TAC/MTX) |
Alive | M/M | 100% | IVIG/R Steroids/R Rituximab/C danazol/C |
| 7 | 2.11/AML | No | 130/ WAAB |
No | MMRD | Yes/AIHA | PBSCT | No | TBI/Cy/Camp/Ara-C (TAC) |
Deceased/235 days/VOD-multi. Organ failure with 2nd HSCT | F/F | 100% (Hypocellular) | Steroids/R Rituximab/R CTX&VCR/R 2nd HSCT/R |
| 8 | 11.59/Metabolic disorder | No | 512/ WAAB |
Major | MUD | No | BMT | No | Bu/Cy/Flu/Camp (TAC/MTX) |
Alive | F/M | Missing | |
| 9 | 1.95/CML | No | 4505/ WAAB |
No | MMRD | Yes/AIHA | BMT | No | Bu/Cy Missing |
Deceased/6980 days/Chronic GvHD post 2nd HSCT | M/F | Missing | IVIG/R Steroids/R Rituximab/R danazol/R 2nd HSCT/ |
| 10 | 0.31/SCID | No | 215/ WAAB |
Minor | MUD | No | Cord SCT | No | Bu/Cy/Ara-C (CSA) |
Alive | M/F | 100% | IVIG/C Prednisone/C |
| 11 | 12.18/SCAEBV | No | 230/ WAAB |
No | MMUD | No | BMT | No | TBI/Cy/Ara-C (TAC/MTX) |
Alive | F/M | 95% | IVIG/C Darbepoetin/C danazol/C |
| 12 | 15.39/ALL | No | 119/ No AB Elute |
No | MMRD | No | PBSCT | Yes (Extensive) | TBI/Cy/Camp/Ara-C None |
Deceased/565 days/Chronic GvHD-IFI | M/M | 100% | Rituximab/C |
AB= Antibodies; AIHA=autoimmune hemolytic anemia; ALL= acute lymphoblastic leukemia; AML= acute myeloid leukemia; Ara-C= Cytarabine; ATG= antithymocytic globulin; BFD= Blackfan Diamond Syndrome, BM= bone marrow; BMT= bone marrow transplant; B-Thal=beta thalassemia major; Bu= busulfan; C=controlled; Camp = Campath; CML= chronic myeloid leukemia; CSA=cyclosporine; Cy= cyclophosphamide; F= female; Flu= fludarabin; GvHD= graft versus host disease; HLA=human leukocyte antigens; HSCT= hematopoietic stem cell transplant; IFI=invasive fungal infection; M= male; MDS= myelodysplastic syndrome; MMRD=mismatched related donor; MMUD= mismatched unrelated donor; MRD=matched related donor; MUD= matched unrelated donor; MTX=methotrexate; NHL: Non-Hodgkin Lymphoma; PBSCT= peripheral blood stem cell transplant; R=refractory; SAA= severe aplastic anemia; SCAEBV= severe chronic active EBV infection syndrome; SCID= severe combined immunodeficiency; TAC=tacrolimus; WAAB=warm agglutinins antibodies
Table 3. Characteristics of Recipients' with Auto-immune Hemolytic Anemia Post Second Hematopoietic Stem Cell Transplant.
| Case # |
Age at 2nd Transplant/ Diagnosis |
Same donor (SD) vs. different donor (DD) from 1st HSCT |
Acute GvHD | Time of onset of AIHA post transplant (Days)/ Type of Antibody |
ABO Incompatibility |
HLA Match |
Stem Cell Source/ reason for HSCT |
Chronic GvHD |
Conditioning/ (GvHD Prophylaxis) |
Status/days alive from last HSCT/Cause of Death |
Donor/ Recipient Gender |
Donor Chimerism @ AIHA onset |
Treatment for AIHA/Responses C = controlled R = refractory |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2.92/SCID | SD (mother) | No | Persistent/ WAAB |
No | MMRD | PBSCT/AIHA | No | Bu/Cy/Ara-C (CSA) |
Alive | F/M | Missing | IVIG/R Steroids/C |
| 13 | 6.84/MDS | SD(mother) | No | 171/ No AB Elute |
Minor | MMRD | PBSCT / relapse | No | Flu/Camp (None) |
Deceased/ 868 days /Relapse | F/F | 62% | Steroids/C |
| 14 | 9.85/BFD | DD | No | 70/ WAAB |
Major | MUD | PBSCT /Failed engraftment | Yes (Limited) | Flu/Camp (TAC/MTX) |
Alive | M/M | 100% | Prednisone/R Splenectomy/C |
| 5 | 9.14/B-Thal | SD | No | 94/ WAAB |
No | MUD | PBSCT/AIHA | No | Flu/Camp (None) |
Alive | F/M | 75% Monocytes 98% Granulocytes | 3rd HSCT/C |
| 15 | 15.85/SAA | SD | No | 256/ No AB Elute |
Major | MUD | PBSCT / Failed engraftment | No | Flu/Camp (None) |
Alive | M/M | 100% (Hypocellular) | IVIG/C Steroids/C |
| 16 | 6.51/ALL | DD | No | 235/ WAAB |
Major | MUD | BMT/Relapse | No | Bu/Cy/Camp (TAC/MTX) |
Deceased/344 days /Relapsed | F/F | 100% | IVIG/R Steroids/R Rituximab/R |
| 9 | 20.12/AIHA | DD | No | 143/ WAAB |
No | MUD | BMT / AIHA | Yes/ Extensive | Bu/Flu/Camp (TAC/MTX) |
Deceased/346 days/CGvHD | F/F | 84% Monocytes 100% Granulocytes | IVIG/C Steroids/C |
AB= Antibodies; AIHA=autoimmune hemolytic anemia; ALL= acute lymphoblastic leukemia; AML= acute myeloid leukemia; Ara-C= Cytarabine; ATG= antithymocytic globulin; BFD= Blackfan Diamond Syndrome, BM= bone marrow; BMT= bone marrow transplant; B-Thal= beta thalassemia major; Bu= busulfan; C=controlled; Camp = Campath; CML= chronic myeloid leukemia; CSA=cyclosporine; Cy= cyclophosphamide; F= female; Flu= fludarabin; GvHD= graft versus host disease; HLA= human leukocyte antigens; HSCT= hematopoietic stem cell transplant; IFI=invasive fungal infection; M= male; MDS= myelodysplastic syndrome; MMRD= mismatched related donor; MMUD= mismatched unrelated donor; MRD= matched related donor; MTX=methotrexate; MUD= matched unrelated donor; NHL: Non-Hodgkin Lymphoma; PBSCT= peripheral blood stem cell transplant; R=refractory; SAA= severe aplastic anemia; SCAEBV= severe chronic active EBV infection syndrome; SCID= severe combined immunodeficiency; TAC=tacrolimus; WAAB=warm agglutinins antibodies
Of the 500 first HSCT recipients; comparing the donor type; the matched related donor (MRD) status was significantly associated with no incidence of AIHA when compared with other donor status (mismatched related, matched unrelated and mismatched unrelated) post first HSCT, (0/175; 0% MRD vs. 12/325; 3.7% other donors, p=0.04, adjusted for multiple comparisons). Furthermore, there was a trend for higher incidence of AIHA among HLA-mismatched vs. -matched HSCT recipients regardless of the donor related/unrelated status, (7/154; 4.5% HLA mismatched vs. 5/346; 1.4% HLA matched HSCT recipients, p=0.054), and no statistical significant between HLA-mismatched related or unrelated donors, (5/87 vs. 2/67recipients respectively, after adjustment for multiple comparisons). For the second HSCT recipients; there was a higher incidence of AIHA among matched unrelated donors (5/21; 23% MUD vs. 2/51; 3.9% of other donors, that included 9 MRD, 29 mismatched related (T-cell depleted) and 13 mismatched unrelated donors). Most patients with AIHA required medical intervention, ranging from IVIG, steroid therapy and frequent blood transfusions. Refractory cases received multimodal medical treatment, which included rituximab, Cytoxan/vincristine and/or danazol. Patient number 14 responded to splenectomy. Four patients received second HSCT to control the AIHA; three of the four were refractory to the second HSCT.
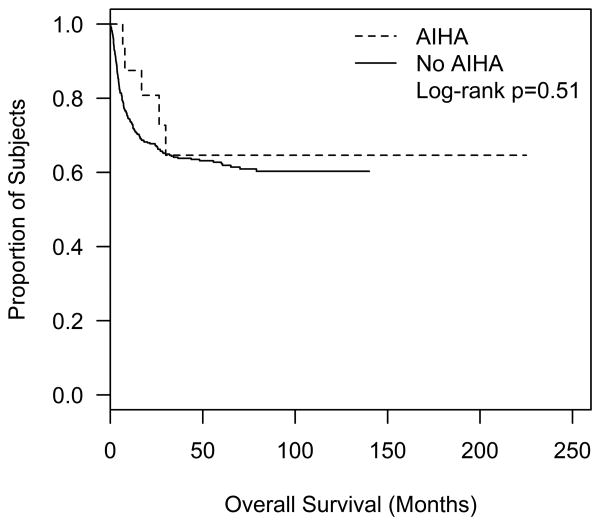
Of the twelve patients with AIHA post first HSCT, one patient deceased due to unspecified cause (patient number 1); the second patient deceased due to multiple organ failure post the second transplant (patient number 7); and the third patient deceased due to chronic GvHD (patient number 12). Of the patients with AIHA post second HSCT, two patients died due to relapse (patients number 13 and 16) and one patient died due to chronic GvHD (Patient number 9) (Tables 2&3). The overall survival did not differ significantly among recipients of single HSCT with and without AIHA (Figure I). There was no statistical significance observed comparing CSA vs. tacrolimus based GvHD prophylaxis. Age, gender, ethnicity, stem cell source (bone marrow versus peripheral blood), donor/recipient ABO/Rh blood group matching, development of GvHD (acute vs. chronic), alemtuzumab, and conditioning therapies were not identified as risk factors for development of AIHA post HSCT.
Figure I.
Discussion
AIHA is a delayed complication of HSCT the etiology of which is poorly understood. Only a few large series have described the incidence and the outcome of AIHA in HSCT recipients. The present study reports the incidence and outcome of AIHA in a large pediatric HSCT recipients group. Autoimmune hemolytic anemia was retrospectively identified upon review all pediatric patients who had positive DAT, with or without non-specific antibodies and clinical evidence of hemolysis post-allogeneic HSCT. Subclinical cases of AIHA may not have been identified in the study, as DAT testing was not routinely performed, or recipients had positive DAT, with absence clinical symptoms and signs of AIHA.
The reported incidence of AIHA post HSCT ranged between3 to 4.4% in allogeneic HSCT recipients; the current study reports a lower incidence of 2.4 % (12/500) following the first HSCT (7, 12, 15, 17). Although most patients (8/12) developed AIHA during the first year post the first HSCT, three patients developed AIHA between the first and second year and one was diagnosed more than 12 years after the first HSCT, with cumulative incidence of 1.8% within the first year, 2.2% within the second year of first HSCT.
The median time of onset of post HSCT AIHA varied between different reports, being 8 months (range: 6-63 months) for patients with severe combined immune deficiency after T-cell depleted HSCT(9); 147 days (range: 41-170 days) among adult recipients of HSCT, 4 months (range: 2-32 months) among pediatric HSCT recipients (12, 15), and 10 months (range: 7-25 months) among T-cell depleted HSCT recipients (7). In the current study, the median time to diagnosis of AIHA after first HSCT was 273 days (range: 119-4505 days), and 157 days (range: 70-256 days) post the second transplant.
The current study showed a trend toward increased incidence of AIHA among HLA mismatched HSCT recipients (5/341 HLA-matched vs. 7/147 HLA-mismatched HSCT, p= 0.054). This difference did not reach statistical significance in contrast to Sanz et al (HLA-matched vs. HLA mismatched is 6/196 vs. 6/76 respectively, p=0.04) (12).
O'Brien et al did not find a significant difference of the incidence of AIHA between HLA-matched vs. HLA mismatched HSCT (10/139 vs. 9/164 respectively, p=0.61(15).
In the current study, a significant difference was observed between MRD HSCT recipients' vs. others, which supports both O'Brian et al and Sanz et al. observation. The former reported no cases of AIHA in over 100 recipients of related donor transplantation (15); and Sanz et al, who reported lower incidence of AIHA among recipients of matched related donor (unrelated donor vs. family related donor was 9% vs. 2.6%, p=0.02) (12). In contrast, Chen et al reported six out-of the 9 patients with post-transplant AIHA received MRD-HSCT (17).
Only two out of 12 patients with AIHA post-first transplant received cyclosporine, 8/12 received tacrolimus and 2/12 received neither.
In the current study we found no significant association between the incidence of AIHA and the incidence of acute or chronic GvHD. The lack of association between the AIHA and GvHD is supported by Chen et al, O'Brien et al (15, 17), and disputed by Sanz et al and others (8, 11, 12).
We did not find an association between the source of stem cell; bone marrow (BM) vs. peripheral blood (PB) HSCT; and the incidence of AIHA. Sanz et al supported the current observation, comparing the source of HSCT (BM vs. PB, was 3/54 (5.6%) vs. 5/162 (2.6%), p= 0.46)(12). Unlike Horn et al who reported increased risk of development of AIHA utilizing PB as stem cell source (BM vs. PB, was 4/30 (13%) vs. 4/4 (100%), p= 0.02)(9). Multiple reports suggest an association between umbilical cord blood (UCB) as source for the HSCT and the incidence of AIHA (5, 11, 19, 20). This association was not present in a larger series reported by O'Brien et al(15). In the current study there were insufficient patients undergoing cord blood transplant to analyze.
Other than a few reports of pediatric patients receiving allogeneic HSCT(5, 15, 19), this is the first to report the incidence of AIHA for pediatric patients receiving a second allogeneic HSCT.
In the current study, the incidence of AIHA post-second HSCT was higher, at 9.7% (7 out of 72 HSCT recipients); furthermore, after adjustment for multiple comparisons of the donor type, a statistically significant association of increased incidence of AIHA is observed in recipients of matched unrelated donor for the second HSCT. We hypothesize that second transplantation is accompanied by more profound immune dysregulation, related to exposure to more intense therapy. The profound immune dysregulation may be explained by Daikeler and Tyndall hypothesis of either the imbalance between auto-reactive and regulatory lymphocyte population and the loss of self-tolerance mechanisms; the presence of genetic difference in the major or minor HLA genes between the donor and the recipient; the transfer of auto-immunity from the donor to the recipient; and as a direct effect to the conditioning regimen for the HSCT(4).
Cutting et al (22) reported the association between AIHA and graft failure following reduced intensity matched unrelated donor HSCT for severe aplastic anemia. They speculated that the higher incidence of AIHA for patients undergoing transplant for non-malignant disorders might be due to a relatively competent immune system prior to HSCT. These patients had not been exposed to cytotoxic or immunosuppressive therapy, which can lead to immune suppression (15). In the current study, higher incidence of AIHA was observed among recipients of second HSCT with nonmalignant disease, [4/24, (16.6%) recipients with nonmalignant disease vs.3/48, (6.2%) recipients with neoplastic disorders], but it did not reach a statistical significance, possibly due to the low number of the sampled population. We found no association between the type of conditioning therapy and the occurrence of immune hemolytic anemia.
AIHA Treatment
The majority of patients received multi-agent therapy for control of post-HSCT AIHA. Prednisolone and intravenous immunoglobulin were the initial therapeutic options for the twelve patients with AIHA post first HSCT. Refractory cases required alternative therapies, two of five patients receiving rituximab responded; one of three patients responded to danazole. Otherwise four patients failed to respond to the multi-agent therapy and received a second HSCT to control the AIHA (Table 2). Despite the second transplant for AIHA, 3/4 patients required multidrug intervention and third HSCT to control the AIHA developing after the 2nd transplant (Table 3). Multiple reports agreed on the difficulty in treating post-transplant AIHA, and alternative approaches are needed(7, 15, 20-25).
Survival and AIHA
Increased incidence of AIHA has been associated with patients receiving T-cell depleted HSCT, relapse, viral infection or GvHD (8-10). In the current study, no association was found with any of the reported risk factors. The individual patients' outcomes are listed in Tables 2 and 3, where the cause of death varied between relapse (2/16), veno-occlusive disease associated multiple organ failure (1/16), GvHD (1/16) and invasive fungal infection (1/16).
In summary, AIHA is a significant complication that occurs with increased incidence post-HSCT. We observed increased incidence of AIHA after second HSCT. We have observed a strong association of the onset of AIHA and HLA mismatched status, specifically mismatched related HSCT with second HSCT that might be a contributing element to the described relationship between immune dysregulation the source for the development of AIHA post-HSCT(8, 26, 27). Furthermore, we postulate that more immune dysregulation post-second allogeneic HSCT leads to increased incidence of autoimmune hemolytic anemia. Because of the retrospective nature the study has its limitations. It is difficult to defend the true incidence of AIHA post second HSCT, given that AIHA persisted in three out of 4 recipients of second transplant. A larger cohort of patients is needed to describe the true incidence of post-transplant(s) AIHA and to define its impact on the HSCT and patient survivor status.
Acknowledgments
Meng-Fen Wu, M.S for her support reviewing the data collection and initial analysis. Department of Biostatistics, Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX 77030, USA.
Source of Support: This project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center.
References
- 1.Petz LD. Immune hemolysis associated with transplantation. Semin Hematol. 2005;42:145–155. doi: 10.1053/j.seminhematol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Rowley SD. Hematopoietic stem cell transplantation between red cell incompatible donor-recipient pairs. Bone Marrow Transplant. 2001;28:315–321. doi: 10.1038/sj.bmt.1703135. [DOI] [PubMed] [Google Scholar]
- 3.Sherer Y, Shoenfeld Y. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant. 1998;22:873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]
- 4.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Page KM, Mendizabal AM, Prasad VK, et al. Posttransplant autoimmune hemolytic anemia and other autoimmune cytopenias are increased in very young infants undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2008;14:1108–1117. doi: 10.1016/j.bbmt.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagiolo E. Immunological tolerance loss vs. erythrocyte self antigens and cytokine network disregulation in autoimmune hemolytic anaemia. Autoimmun Rev. 2004;3:53–59. doi: 10.1016/S1568-9972(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 7.Drobyski WR, Potluri J, Sauer D, Gottschall JL. Autoimmune hemolytic anemia following T cell-depleted allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;17:1093–1099. [PubMed] [Google Scholar]
- 8.Godder K, Pati AR, Abhyankar SH, Lamb LS, Armstrong W, Henslee-Downey PJ. De novo chronic graft-versus-host disease presenting as hemolytic anemia following partially mismatched related donor bone marrow transplant. Bone Marrow Transplant. 1997;19:813–817. doi: 10.1038/sj.bmt.1700746. [DOI] [PubMed] [Google Scholar]
- 9.Horn B, Viele M, Mentzer W, Mogck N, DeSantes K, Cowan M. Autoimmune hemolytic anemia in patients with SCID after T cell-depleted BM and PBSC transplantation. Bone Marrow Transplant. 1999;24:1009–1013. doi: 10.1038/sj.bmt.1702011. [DOI] [PubMed] [Google Scholar]
- 10.Cwynarski K, Goulding R, Pocock C, et al. Immune haemolytic anaemia following T cell-depleted allogeneic bone marrow transplantation for chronic myeloid leukaemia: association with leukaemic relapse and treatment with donor lymphocyte infusions. Bone Marrow Transplant. 2001;28:581–586. doi: 10.1038/sj.bmt.1703206. [DOI] [PubMed] [Google Scholar]
- 11.Sevilla J, Gonzalez-Vicent M, Madero L, Diaz MA. Acute autoimmune hemolytic anemia following unrelated cord blood transplantation as an early manifestation of chronic graft-versus-host disease. Bone Marrow Transplant. 2001;28:89–92. doi: 10.1038/sj.bmt.1703087. [DOI] [PubMed] [Google Scholar]
- 12.Sanz J, Arriaga F, Montesinos P, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39:555–561. doi: 10.1038/sj.bmt.1705641. [DOI] [PubMed] [Google Scholar]
- 13.Rovira J, Cid J, Gutierrez-Garcia G, et al. Fatal Immune Hemolytic Anemia Following Allogeneic Stem Cell Transplantation: Report of 2 Cases and Review of Literature. Transfus Med Rev. 2013 doi: 10.1016/j.tmrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V, Shukla J, Bhatia BD. Autoimmune hemolytic anemia. Indian J Pediatr. 2008;75:451–454. doi: 10.1007/s12098-008-0071-0. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien TA, Eastlund T, Peters C, et al. Autoimmune haemolytic anaemia complicating haematopoietic cell transplantation in paediatric patients: high incidence and significant mortality in unrelated donor transplants for non-malignant diseases. Br J Haematol. 2004;127:67–75. doi: 10.1111/j.1365-2141.2004.05138.x. [DOI] [PubMed] [Google Scholar]
- 16.Warkentin PI. Transfusion of patients undergoing bone marrow transplantation. Hum Pathol. 1983;14:261–266. doi: 10.1016/s0046-8177(83)80028-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen FE, Owen I, Savage D, et al. Late onset haemolysis and red cell autoimmunisation after allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:491–495. doi: 10.1038/sj.bmt.1700677. [DOI] [PubMed] [Google Scholar]
- 18.Kako S, Kanda Y, Oshima K, et al. Late onset of autoimmune hemolytic anemia and pure red cell aplasia after allogeneic hematopoietic stem cell transplantation using in vivo alemtuzumab. Am J Hematol. 2008;83:247–249. doi: 10.1002/ajh.21086. [DOI] [PubMed] [Google Scholar]
- 19.Hows J, Beddow K, Gordon-Smith E, et al. Donor-derived red blood cell antibodies and immune hemolysis after allogeneic bone marrow transplantation. Blood. 1986;67:177–181. [PubMed] [Google Scholar]
- 20.Hall JG, Martin PL, Wood S, Kurtzberg J. Unrelated umbilical cord blood transplantation for an infant with beta-thalassemia major. J Pediatr Hematol Oncol. 2004;26:382–385. doi: 10.1097/00043426-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Mullen CA, Thompson JN, Richard LA, Chan KW. Unrelated umbilical cord blood transplantation in infancy for mucopolysaccharidosis type IIB (Hunter syndrome) complicated by autoimmune hemolytic anemia. Bone Marrow Transplant. 2000;25:1093–1097. doi: 10.1038/sj.bmt.1702397. [DOI] [PubMed] [Google Scholar]
- 22.Cutting R, Ezaydi Y, Edbrooke D, El-Ghariani K, Stamps R, Snowden JA. Graft failure and severe autoimmune haemolysis following fludarabine-based reduced-intensity matched unrelated donor bone marrow transplantation for severe aplastic anaemia: salvage by second transplant with conventional dose conditioning. Bone Marrow Transplant. 2006;38:317–318. doi: 10.1038/sj.bmt.1705433. [DOI] [PubMed] [Google Scholar]
- 23.Daikeler T, Hugle T, Farge D, et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 2009;44:27–33. doi: 10.1038/bmt.2008.424. [DOI] [PubMed] [Google Scholar]
- 24.Chao MM, Levine JE, Ferrara JL, et al. Successful treatment of refractory immune hemolysis following unrelated cord blood transplant with Campath-1H. Pediatr Blood Cancer. 2008;50:917–919. doi: 10.1002/pbc.21187. [DOI] [PubMed] [Google Scholar]
- 25.Robak T. Monoclonal antibodies in the treatment of autoimmune cytopenias. Eur J Haematol. 2004;72:79–88. doi: 10.1046/j.0902-4441.2003.00196.x. [DOI] [PubMed] [Google Scholar]
- 26.Raj K, Narayanan S, Augustson B, et al. Rituximab is effective in the management of refractory autoimmune cytopenias occurring after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35:299–301. doi: 10.1038/sj.bmt.1704705. [DOI] [PubMed] [Google Scholar]
- 27.Hilgendorf I, Wolff D, Wilhelm S, et al. T-cell-depleted stem cell boost for the treatment of autoimmune haemolytic anaemia after T-cell-depleted allogeneic bone marrow transplantation complicated by adenovirus infection. Bone Marrow Transplant. 2006;37:977–978. doi: 10.1038/sj.bmt.1705356. [DOI] [PubMed] [Google Scholar]