Abstract
Objective
Tongue strength, timing, and coordination deficits may underlie age-related swallowing function. Retrusive tongue actions are likely important in retrograde bolus transport. However, age-related changes in retrusive tongue muscle contractile properties have not been identified in animal studies. Because previous studies employed whole hypoglossal nerve stimulation that activated both protrusive and retrusive tongue muscles, co-contraction may have masked retrusive muscle force decrements. The hypotheses of this study were: (1) retrusive tongue muscle contraction forces would be diminished and temporal characteristics prolonged in old rats when lateral nerves were selectively activated, and (2) greater muscle contractile forces with selective lateral branch stimulation would be found relative to whole hypoglossal nerve stimulation.
Design
Nineteen Fischer 344/Brown Norway rats (9 Old, 10 Young Adult) underwent tongue muscle contractile property recording elicited by: (1) bilateral whole hypoglossal nerve stimulation, and (2) selective lateral branch stimulation. Twitch contraction time (CT), half-decay time, maximal twitch and tetanic forces, and a fatigue index were measured.
Results
For whole nerve stimulation, CT was significantly longer in the old group. No significant age group differences were found with selective lateral nerve stimulation. Significantly reduced twitch forces (old group only), increased tetanic forces and significantly less fatigue were found with selective lateral nerve stimulation than with whole hypoglossal stimulation.
Conclusions
Retrusive tongue forces are not impaired in old rats. Deficits observed in swallowing with aging may be due to other factors such as inadequate bolus propulsive forces, mediated by protrusive tongue muscles, or timing/coordination of muscle actions.
Keywords: deglutition, deglutition disorders, aging, tongue, hypoglossal nerve
Introduction
Timing and coordination of swallowing behaviors changes with aging such that oral transit times may be increased, multiple swallows per intake may be observed, wet voice may be evidenced, time to relaxation of the upper esophageal segment may increase, and throat-clearing and coughing may be associated with the swallow, even in people not symptomatic of dysphagia [1–6]. Combinations of subtle strength, timing, and coordination deficits may result in longer-duration swallows that expose the airway to the bolus for a greater time, potentially increasing the probability of aspiration. The underlying causes of presbyphagia are not known, but have been linked with changes in cranial muscle structure and function. In particular, evidence supports a reduced maximum tongue isometric pressures in older adults [7–10]. While reduced tongue forces during the swallow have not been reported in prior studies of healthy elders, the amount of tongue force used represented a greater proportion of maximal isometric pressure than in younger adults [7–9]. However, reductions in tongue strength have been associated with swallowing deficits [11–13] and tongue exercise-based treatments appear to improve tongue strength [14] and swallowing [15, 16]. Accordingly, age-related changes in swallowing may be characterized by both temporal and strength changes, perhaps due to structural and functional decline within the muscles of the tongue.
The extrinsic muscles of the tongue are active during oral preparation of the bolus prior to transport into the pharynx [17], bolus propulsion [17], and throughout the entire oropharyngeal swallow [18]. Of the extrinsic muscles, the genioglossus acts to elevate the tongue tip and protrude the tongue, with a primary role in generating tongue-to-palate pressure during the swallow [19]. Motor innervation of the protrusive and retrusive muscles of the tongue is provided by the hypoglossal nerve. Stimulation of the medial branch of the hypoglossal nerve will elicit a tongue protrusion, [20, 21], while stimulation of the lateral branch elicits tongue retrusion, via the hyoglossus and styloglossus muscles [21]. The hyoglossus, styloglossus, and the genioglossus are active during oral preparation and retrograde movement of the bolus to the hypopharynx [18,22, 23]. Thus, both protrusive and retrusive tongue muscle actions are relevant to the swallow.
Because the hypoglossal nerve provides motor innervation to both retrusive and protrusive muscles of the tongue, stimulation of the whole hypoglossal nerve (proximal to its bifurcation into the medial and lateral branches) will produce a co-contraction of retrusive and protrusive muscles and a net retrusive tongue action [20, 21]. Stimulation of the lateral branch of the hypoglossal alone results in tongue retrusion of greater force than that reported whole nerve stimulation [20, 21]. Accordingly, co-contraction of protrusive and retrusive tongue muscles serves to reduce force output for evoked retrusive actions. With regard to temporal parameters, contraction times following isolated medial nerve stimulation are faster than both isolated lateral nerve stimulation and whole nerve stimulation [20]. How aging may affect tongue muscle responses to whole nerve stimulation versus isolated lateral nerve stimulation has not been examined.
Decreased swallow function may occur due to a decline in both retrusive and protrusive tongue functions. However, in rats, age-related changes in tongue muscle contractile properties have been found for evoked protrusive [24, 25] and not retrusive actions elicited with whole nerve stimulation [26, 27]. This is surprising given the retrusive nature of bolus transport during the swallow and previous findings of slower bolus transport speeds during videofluorography in old rats [28]. However, prior data examining retrusive tongue actions were derived by stimulating the whole hypoglossal nerve, and may have been confounded by antagonistic protrusive muscle activity. To examine this possibility, it is necessary to examine retrusive tongue actions following section of the medial branch of the hypoglossal nerve to remove the effects of the genioglossus and to allow precise targeting of the lateral branch and associated retrusive muscles.
The purpose of this study was to determine if age-related changes are manifested in muscle contractile properties of elicited tongue retrusion by whole hypoglossal nerve stimulation and lateral branch hypoglossal stimulation in a rat model. Our hypotheses were that: (1) retrusive tongue muscle contraction forces would be diminished and temporal characteristics prolonged in old rats relative to young adults when the lateral nerve branches are selectively activated, and (2) greater muscle contractile forces with selective lateral branch stimulation would be found relative to whole hypoglossal nerve stimulation in both young adult and old rats. That is, we hypothesized senescence would not alter the previously reported observation of greater tetanic forces with selective lateral nerve stimulation versus whole hypoglossal nerve stimulation [20, 21].
Materials and Methods
This research project was performed according to principles put forth in the NIH Guide for the Care and Use of Laboratory Animals, Eighth Edition, and was approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee.
Animal Subjects
Ten young adult (9 month, 433.8g [SD±35.75]) and 10 old (32 month, 531.2g [SD±67.84]) male Fischer 344/Brown Norway rats were used in this research. The median life expectancy of the Fischer 344/Brown Norway rat is approximately 36 months [29]. One old rat expired during anesthesia; thus data are reported for 10 young adult and 9 old rats.
Surgical Method
Following anesthesia with an intraperitoneal injection of sodium pentobarbital (70 mg/kg), the hypoglossal nerves were exposed bilaterally using a ventral approach under an operating microscope. Bipolar electrodes in a silicon nerve cuff were placed distal to the bifurcation of the whole hypoglossal nerve. The bifurcation of hypoglossal nerve was identified during placement of the cuff. In addition, identification of the bifurcation was further substantiated during the dissection of the medial branch of the hypoglossal nerve. Each nerve was tested for appropriate reaction to stimulation prior to data acquisition.
Two experimental conditions followed: (1) Whole nerve stimulation and muscle contractile property recordings, and (2) Isolated lateral branch recordings. That is, following whole nerve stimulation recordings as described below, the medial branch of the hypoglossal nerve was isolated and transected bilaterally using microscissors, leaving an intact lateral branch. Following a 45-minute waiting period, the hypoglossal nerves were stimulated again, resulting in the isolated stimulation of the lateral branch of the hypoglossal nerve.
Muscle Contraction Recordings
Tongue force was measured by attaching a suture to the tip of the tongue and then connecting the suture to an isometric force transducer. The rat was placed in a dorsal recumbent position onto a fixed stage. The transducer was slightly elevated to avoid interference of the suture by the incisors. This adjustment in suture angle was typically around 10 degrees. The rat’s jaw was retracted to enable access to the tongue. The upper jaw was secured to the fixed stage by wire to prevent movement of the head from interfering with data collection. The tongue was extended to a preload force of approximately of 20 mN to allow recording of tongue forces in response to stimulation.
Tongue contractile properties were measured in response to bilateral stimulation of the hypoglossal nerves, as described previously in Surgical Method.
Stimulation waveforms were 0.1-ms rectangular pulses at a supramaximally applied current, defined as 1.5 times the maximum current level required to obtain maximal twitch force (A-M Systems Differential AC amplifier, model 177; A-M Systems Isolated Pulse Stimulator, model 2100; A-M Systems Analog Stimulus Amplification Unit, model 2200, Carlsborg, WA). For muscle twitch, stimuli were delivered at 1 Hz. Three 10-s trials with a 1-min rest period between trials were recorded. Following twitch contraction data collection, tetanic forces were recorded using 200 ms trains with stimulus rates at 60, 80, and 100 Hz. Fatiguability was measured using a 2-minute fusion stimulation paradigm consisting of an 80 Hz train for 500 ms. Data were electronically acquired directly into a dedicated laboratory computer using software developed for this purpose (Acquire 1.5; Madison, WI). Maximal twitch contraction time (CT), twitch contraction half-decay time (HDT), maximal twitch force, maximal tetanic force, and a fatigue index were measured for each rat. The CT was measured as the duration between the onset of stimulation and the point of 50% maximal force. The HDT was measured as the duration between the onset of stimulation and the point of 50% decay from the maximal force. Tetanic force was the maximal force of each stimulated fused wave. The fatigue index was calculated as the ratio of the force following two minutes of stimulation divided by the initial recorded force [28]. A fatigue index closer to 1.0 reflects a resistance to fatigue.
Because of the exploratory nature of this work, pairwise comparisons were used to interrogate all possible pairs. That is, 2-sided, two-sample equal variance t-tests were used for comparison of age groups for temporal variables and fatigue index on the whole nerve and isolated lateral branch of the hypoglossal. Due to significant differences in weight between age groups (t (17) = 4.53; p < .001; Old mean = 540.6 [SD=67.8] g; Young adult mean = 433.8 [SD = 35.75]) that can affect muscle size and force measures [31,32], analysis of covariance (ANCOVA) was performed with a weight covariate for twitch and tetanic force variables. Paired t-tests were used to compare outcomes within each age group for both the whole nerve and the lateral branch. SAS statistical software was used for all analyses (SAS Institute, Inc., Cary, North Carolina). The critical value for obtaining statistical significance was set at α = 0.05.
Results
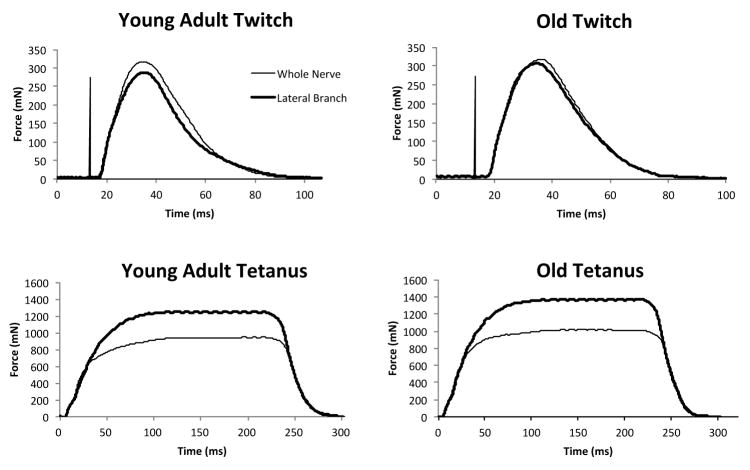
Representative twitch and tetanic contraction waveforms for a young adult and old rat in each of the two conditions, whole nerve and lateral branch stimulation, are shown in Figure 1.
Figure 1.
Representative twitch and tetanic tension signals during whole nerve and lateral branch stimulation of the hypoglossal nerve (HN) from young adult and old animals. There was a szignificant increase in tetanic forces for lateral branch HN stimulation when compared with stimulation of the whole nerve (p<.001). There was no age affect on either whole nerve or lateral branch stimulation values.
Whole Hypoglossal Nerve Stimulation
As shown in Figure 1 and Table 1, contraction time was significantly longer in the old group than in the young adult group (t (17) = 2.21; p = .049). There were no significant differences in decay time, twitch force, tetanic force, or fatigue ratio with whole hypoglossal nerve stimulation.
Table 1.
Muscle contractile property data expressed as mean (standard deviation) within each age group during retrusive actions of the tongue elicited by bilateral stimulation of the whole hypoglossal nerve or selective stimulation of the lateral branch of the hypoglossal nerve (after section of the medial branch).
| Whole and Lateral Branch Hypoglossal Nerve Stimulation | |||||
|---|---|---|---|---|---|
|
| |||||
| Age | Maximum twitch tension (mN) | Contraction time (ms) | Half-decay time (ms) | Maximum tetanic tension (mN) | Fatigue Index (%) |
| Young Adult | |||||
| Whole Nerve | 301.9 (21.2) | 9.4 (.5) | 37.0 (2.1) | 959.1 (104.5) | 83 (8) |
| Lateral Branch | 292.3 (15.1) | 9.7 (.8) | 35.4 (3.3) | 1299.9 (92.3)* | 93 (4)* |
| Old | |||||
| Whole Nerve | 329.8 (32.8) | 10.1 (.9) | 38.8 (3.7) | 959.8 (74.4) | 81 (4) |
| Lateral Branch | 311.9 (35.8)* | 10.3 (1.5) | 36.7 (6.1) | 1308.3 (108.1)* | 91 (7)* |
p < .05 for whole nerve vs. lateral branch comparisons.
Lateral Branch Stimulation
Following section of the medial branch of the hypoglossal nerve, isolated lateral branch stimulation revealed no significant differences (p >.05) between young adult and old groups on any force or temporal variables (Table 1).
Differences between Whole Nerve and Lateral Branch Stimulation
Force amplitudes and temporal measures were compared across the two hypoglossal nerve stimulation conditions in the same rats. With selective lateral nerve stimulation (Table 1), we found significantly reduced twitch forces only in the old group (old: t (8) = 2.43; p = .04; young adult: t (9) = 1.22; p = .25), significantly greater tetanic forces (at 100 Hz stimulation) in both groups (old: t (8) = 9.19; p <.0001; young adult: t (9) = 8.61; p<.0001), and significantly less fatigue (old: t (8) = 3.47; p =.009; young adult: t (9) = 3.5; p =.007).
There was not a significant difference between young adult and old groups in tetanic force evoked by whole nerve stimulation when expressed as a function of forces evoked by selective lateral nerve stimulation t (17) = .39; p =.70. That is, the proportional difference between tetanic force levels evoked by the two stimulation conditions did not change significantly with aging.
Discussion
The goal of this study was to investigate the degree to which retrusive tongue actions may be compromised by the aging process. It is intuitive that retrusive actions of the tongue may be important for bolus transport or propulsion into the pharynx, and have been implicated as a potential source of disruption in the aging swallow in human and animal studies [28, 33]. Yet, direct recordings of retrusive tongue actions elicited by stimulation of whole hypoglossal nerves bilaterally have not shown deficits in force in prior animal studies [26, 27]. To investigate this disparity, we examined selective stimulation of the lateral branches of the hypoglossal nerves to allow measurement of retrusive muscle contractile properties without co-contraction of protrusive muscles, which is known to diminish force amplitudes [21]. That is, we considered that whole hypoglossal nerve stimulation may: (1) compromise accurate measurement of retrusive muscle contractile properties due to coactivation of protrusive and retrusive tongue muscles [20], and (2) may mask potential age group differences, and thus account for previous negative findings. Our hypotheses were that selective lateral nerve stimulation would result in: (1) significantly lower retrusive tongue forces in old versus young adult rats and, (2) tetanic force amplitudes that were greater overall versus whole hypoglossal nerve stimulation. Our findings supported the latter, but not the former, hypothesis.
With whole hypoglossal nerve stimulation and with selective lateral branch stimulation, no significant group differences in twitch or tetanic force were detected. Accordingly, previous reports in the rat were confirmed by these findings [26, 27]. It did not appear that antagonistic co-contraction by muscles of tongue protrusion was masking underlying force decrements in retrusive muscles within the old or young adult groups. Because the aging rat model has been shown to have analogous deficits in the oropharyngeal swallow to elderly people [28], these findings were unexpected. Tongue force decrements have been observed in elderly people [9, 34], particularly those with swallowing impairments [11, 33]. An interpretation of these findings is that impaired swallowing in elderly people is not due to reductions in tongue force generated by retrusive muscles. If alterations in muscle function are underlying age-related dysphagia, it appears that other muscles, such as extrinsic tongue muscles with protrusive actions or intrinsic tongue muscles, may have a much larger role.
In fact, decrements in force have been found previously for evoked protrusive actions of the tongue in the rat model [24, 25]. The genioglossus is the major contributor to tongue tip elevation and protrusion and is active throughout the oropharyngeal swallow to the point of laryngeal closure [18]. It may follow, therefore, that age-related decrements in genioglossus force generation contribute to dysphagia due to impaired generation of bolus driving pressure. This possibility is supported by the results of tongue exercise studies where tongue elevation and press (e.g, protrusive actions) are used, resulting in increased tongue forces following training [14–16]. Swallowing function has been shown to improve in some, but not all of studies testing tongue exercise interventions [14–16, 35, 36]. Therefore, protrusive tongue muscles may be contributing to the oropharyngeal swallow in a substantive fashion and disruptions in protrusive muscle function may be an underlying factor in presbyphagia to a greater degree than retrusive muscle weakness or contraction timing.
However, other interpretations of these findings must be considered in light of results from human studies in which maximum isometric tongue pressures were impaired in elderly people, but tongue pressures during the swallow were not different from young controls [9]. One interpretation is that strength or functional reserve is reduced in elderly people, which may facilitate development of dysphagia in the event of illness or other health challenge [9]. However, deficits in functional reserve in elderly people have not been found when studied directly [37]. Although submaximal tongue forces are employed in swallowing actions [9], it appears that tongue force may have a role in presbyphagia. Recent studies of elderly people with dysphagia have reported reduced tongue propulsion forces versus healthy volunteers, and those patients with reduced tongue strength were more likely to aspirate than those with greater tongue strength [11, 33]. Therefore, reduced tongue strength may be part of a larger constellation of factors characterizing presbyphagia.
As in previous studies [20, 21], we found that selective lateral branch stimulation resulted in greater tetanic force output than whole hypoglossal nerve stimulation in young adult and old animals, while twitch forces were marginally, but significantly, greater in only the old group. Differences in force and temporal values reported in this work versus prior studies from our laboratory [27] may be due to differences in stimulation electrode configuration and methodology.
These results and the other findings reported here suggest that overall strength of retrusive tongue muscles is not impaired with aging. However, altered protrusive muscle strength, timing [24, 25], or coordination with retrusive muscle actions may contribute to the overall swallow impairment profile. In this work, we found that twitch contraction time was longer in duration in the old group with whole nerve stimulation, but not with selective lateral branch stimulation. That is, timing deficits were apparent only when protrusive muscles were co-activated. This finding suggests that coordination of co-contraction of all tongue muscles innervated by the hypoglossal nerve may be a factor in the prolonged contraction time. Previous studies have shown that retrusive and protrusive muscle co-contraction does not interfere with respiration and may serve to stabilize the tongue base and upper airway during breathing [21]. Perhaps these same co-contraction mechanisms apply during the swallow, with the goal of maintaining pharyngeal patency and airway safety during bolus transfer. If so, altered timing profiles in aged tongue muscles with retrusive/protrusive co-contraction may interfere with a normal swallow.
Taken with prior findings in the rat model, results suggest that strength in retrusive tongue muscles does not appear to be an explanation for the swallowing deficits manifested with aging. However, deficits in timing and protrusive muscle strength may be factors to consider, especially given improvements observed in swallowing in some human studies using tongue exercise maneuvers employing protrusive gestures.
Highlights.
Tongue muscle contractile properties were compared in young adult and old rats.
With whole hypoglossal nerve stimulation, contraction time was longer in old rats.
No significant age differences were found with selective lateral branch stimulation (SLBS).
With SLBS, increased tetanic forces were found vs. whole nerve stimulation.
With SLBS, less fatigue was found vs. whole nerve stimulation.
Acknowledgments
The authors gratefully acknowledge the assistance of Allison J. Schaser, Heidi Kletzien, Glen Leverson, and Chee Paul Lin for their contributions to this work. This work was funded in part by NIH grants R01DC005935 and R01DC008149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.González-Fernández M, Humbert I, Winegrad H, Cappola AR, Fried LP. Dysphagia in old-old women: prevalence as determined according to self-report and the 3-ounce water swallowing test. J Am Geriatr Soc. 2014;62(4):716–20. doi: 10.1111/jgs.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins JA, Hamilton JW, Lot GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–9. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 3.Shaw DW, Cook IJ, Gabb M, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268:G389–96. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 4.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156:1181–4. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180–4. doi: 10.1007/BF00366381. [DOI] [PubMed] [Google Scholar]
- 6.McKee GJ, Johnston BT, McBride GB, Primrose WJ. Does age or sex affect pharyngeal swallowing? Clin Otolaryngol. 1998;23:100–6. doi: 10.1046/j.1365-2273.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 7.Todd JT, Lintzenich CR, Butler SG. Isometric and swallowing tongue strength in healthy adults. Laryngoscope. 2013;123(10):2469–73. doi: 10.1002/lary.23852. [DOI] [PubMed] [Google Scholar]
- 8.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–62. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 9.Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55:M634–40. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 10.Clark HM, Solomon NP. Age and Sex Differences in Orofacial Strength. Dysphagia. 2012;27:2–9. doi: 10.1007/s00455-011-9328-2. [DOI] [PubMed] [Google Scholar]
- 11.Butler SG, Stuart A, Leng X, et al. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci. 2011;66(4):452–8. doi: 10.1093/gerona/glq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- 13.Lazarus CL, Logemann JA, Pauloski BR, et al. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res. 2000;43:1011–23. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 15.Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–8. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3(4):735–47. doi: 10.2147/cia.s3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahrilas PJ, Lin S, Logemann JA, Ergun GA, Facchini F. Deglutitive tongue action - Volume accomodation and bolus propulusion- Gastroenterology. 1993;104:152–62. doi: 10.1016/0016-5085(93)90847-6. [DOI] [PubMed] [Google Scholar]
- 18.van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: A combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114:478–87. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 19.Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51:828–35. doi: 10.1044/1092-4388(2008/060). [DOI] [PubMed] [Google Scholar]
- 20.Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol. 1995;74:547–55. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- 21.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–13. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol. 2012;112:1512–1519. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Research. 2001;915:185–194. doi: 10.1016/s0006-8993(01)02848-7. [DOI] [PubMed] [Google Scholar]
- 24.Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. 2008;23:116–21. doi: 10.1007/s00455-007-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential Effects of Targeted Tongue Exercise and Treadmill Running on Aging Tongue Muscle Structure and Contractile Properties. J Appl Physiol. 2013;114:472–81. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota F, Connor NP, Konopacki RA. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol. 2005;114:799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- 27.Connor NP, Ota F, Nagai H, Russell JA, Leverson GE. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51:818–27. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28(1):95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol [A] Biol Sci Med Sci [A] Biol S. 1999;54A:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 30.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507:265–76. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32:615–31. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hurd WJ, Morrey BF, Kaufman KR. The effects of anthropometric scaling parameters on normalized muscle strength in uninjured baseball pitchers. J Sport Rehabil. 2011;20(3):311–20. doi: 10.1123/jsr.20.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rofes L, Arreola V, Romea M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. 2010;22(8):851–8. E230. doi: 10.1111/j.1365-2982.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanderwegen J, Guns C, Van Nuffelen G, Elen R, De Bodt M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia. 2013;28(2):159–66. doi: 10.1007/s00455-012-9425-x. [DOI] [PubMed] [Google Scholar]
- 35.Robbins JA, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind J. The Effects of Lingual Exercise on Swallowing in Older Adults. J Am Geriatr Soc. 2005;53:1493–89. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 36.Lazarus CL, Husaini H, Falciglia D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg. 2014;43(5):523–30. doi: 10.1016/j.ijom.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele CM. Optimal approaches for measuring tongue-pressure functional reserve. J Aging Res. 2013;2013:542909. doi: 10.1155/2013/542909. [DOI] [PMC free article] [PubMed] [Google Scholar]