Abstract
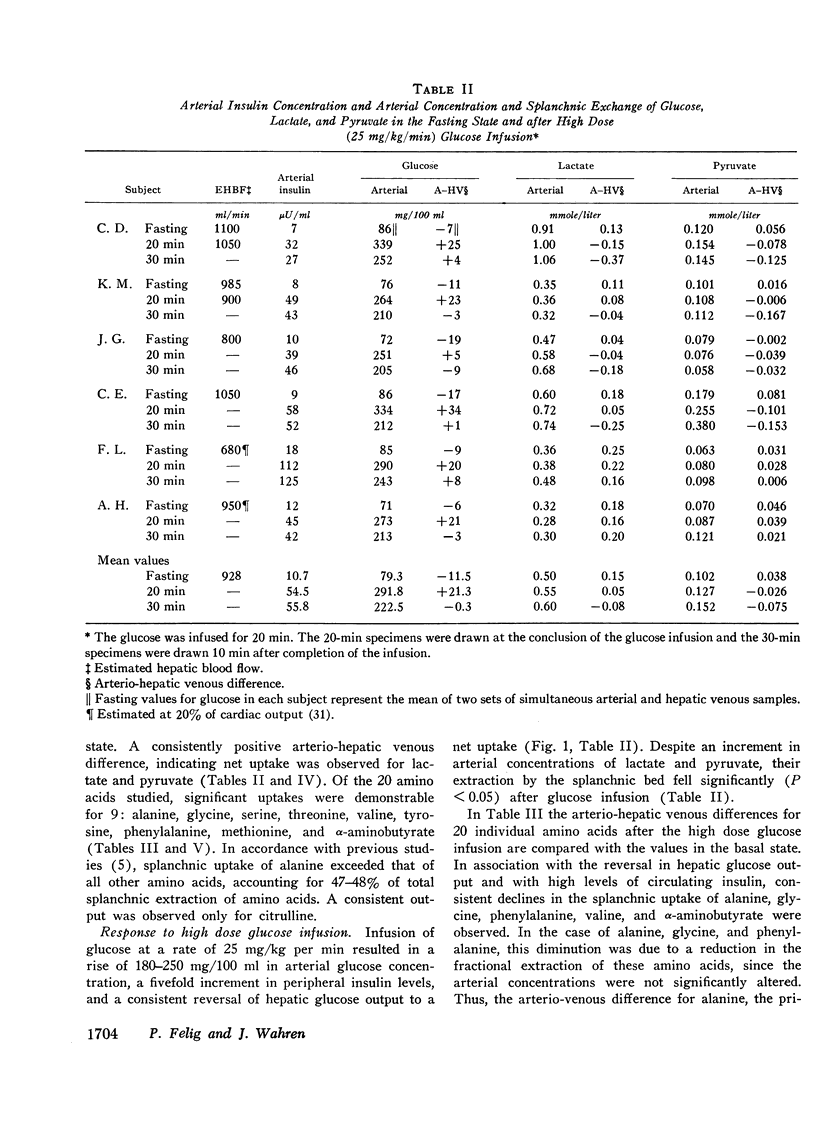
Splanchnic exchange of glucose, 20 individual amino acids, lactate, and pyruvate was studied in normal subjects in the postabsorptive state and after stimulation of endogenous insulin secretion by infusion of glucose at two dose levels. In the basal state, mean splanchnic glucose production was 3.4 mg/kg per min. A net uptake of lactate, pyruvate, and nine amino acids was observed, with alanine accounting for half of the total splanchnic-amino acid extraction.
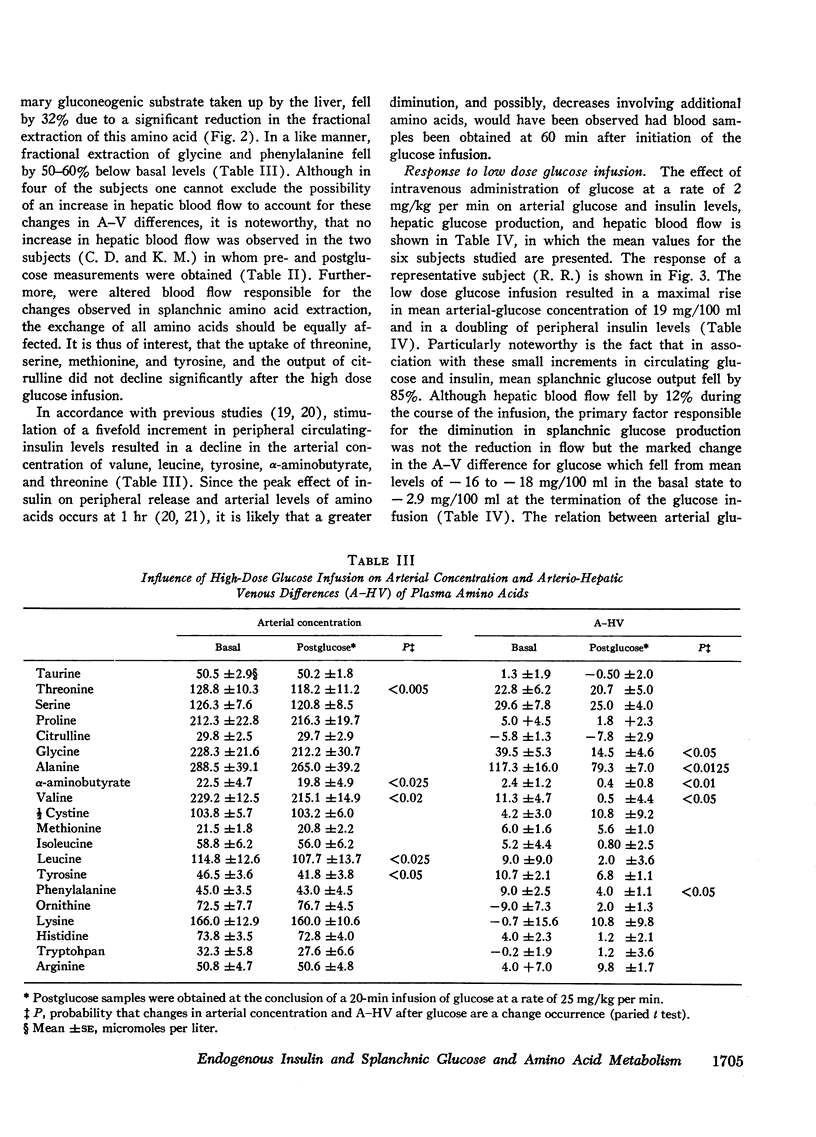
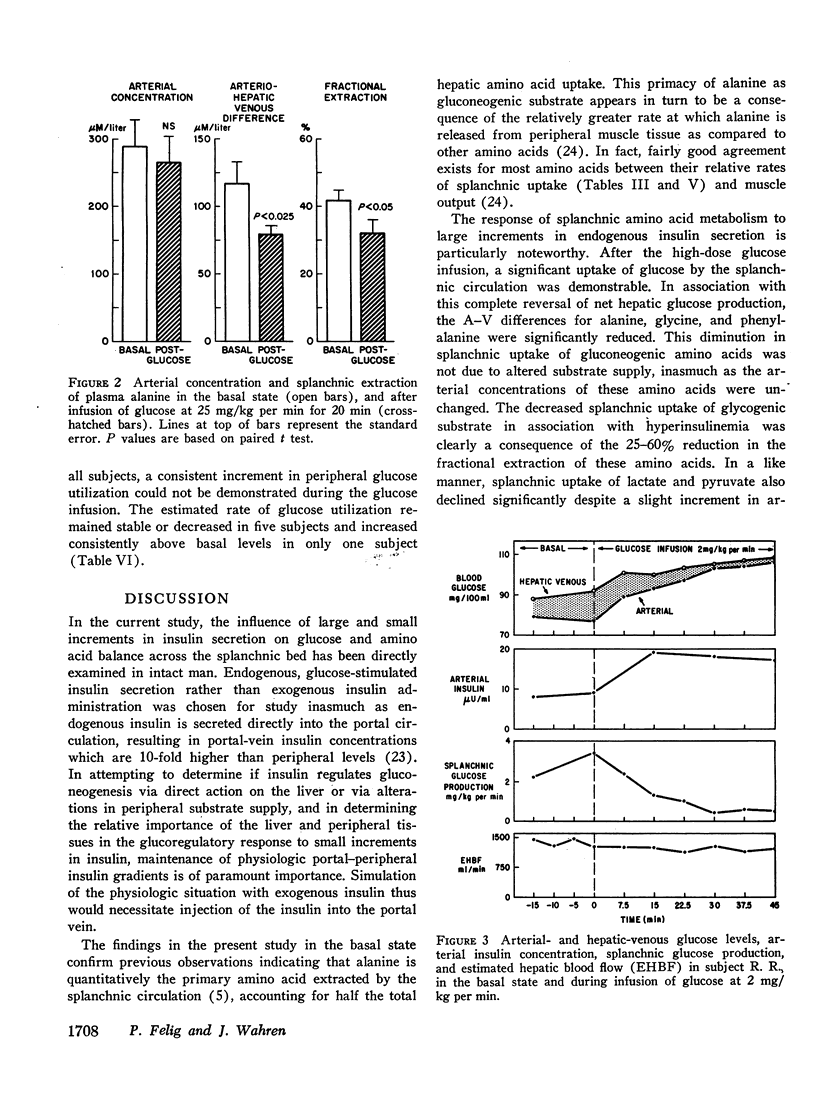
Infusion of glucose at 25 mg/kg per min for 20 min resulted in a fivefold increase in arterial insulin levels and in reversal of splanchnic glucose balance to a net uptake. Splanchnic uptake of alanine, glycine, phenylalanine, lactate, and pyruvate fell by 30-60% due to a reduction in fractional extraction of these substrates, inasmuch as their arterial concentrations did not decline.
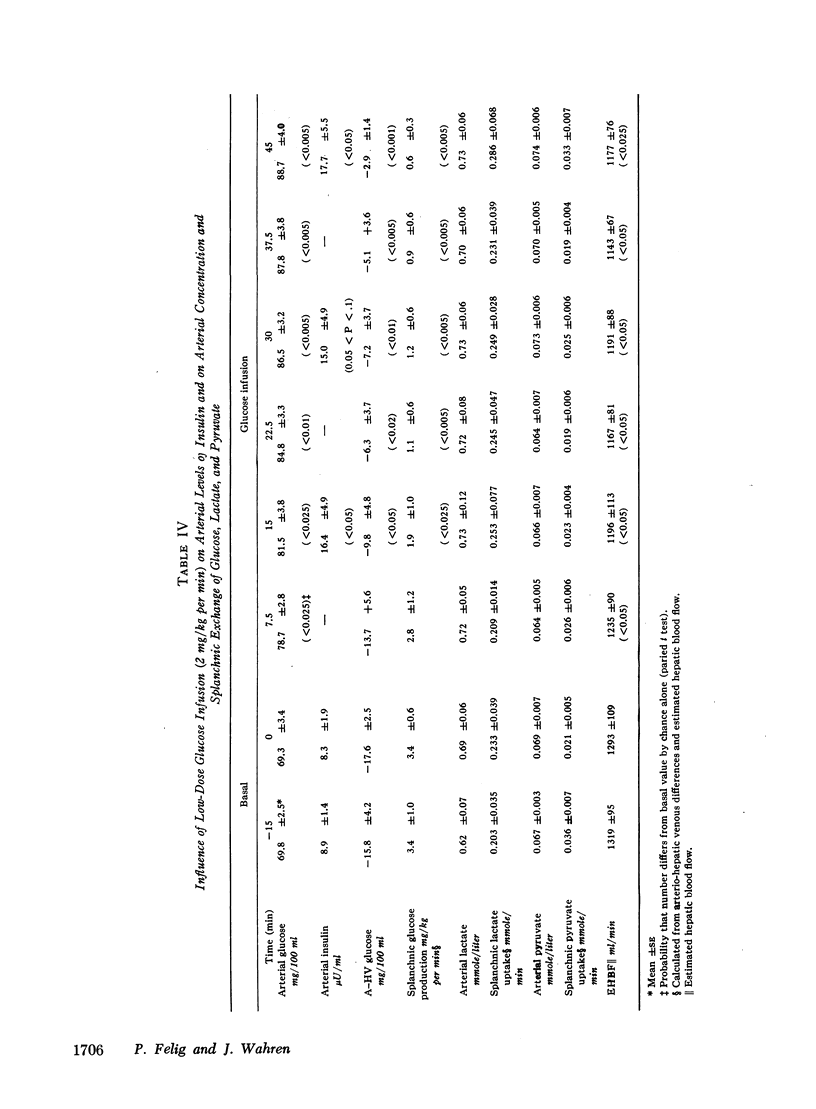
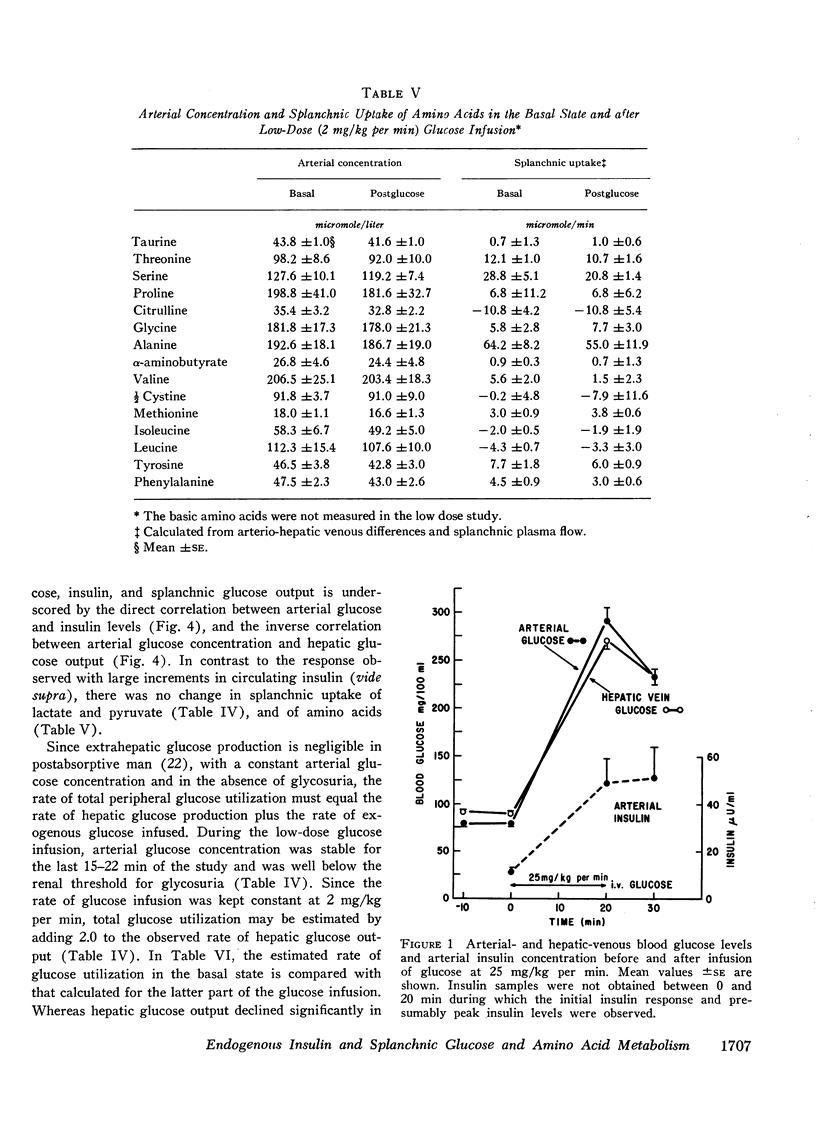
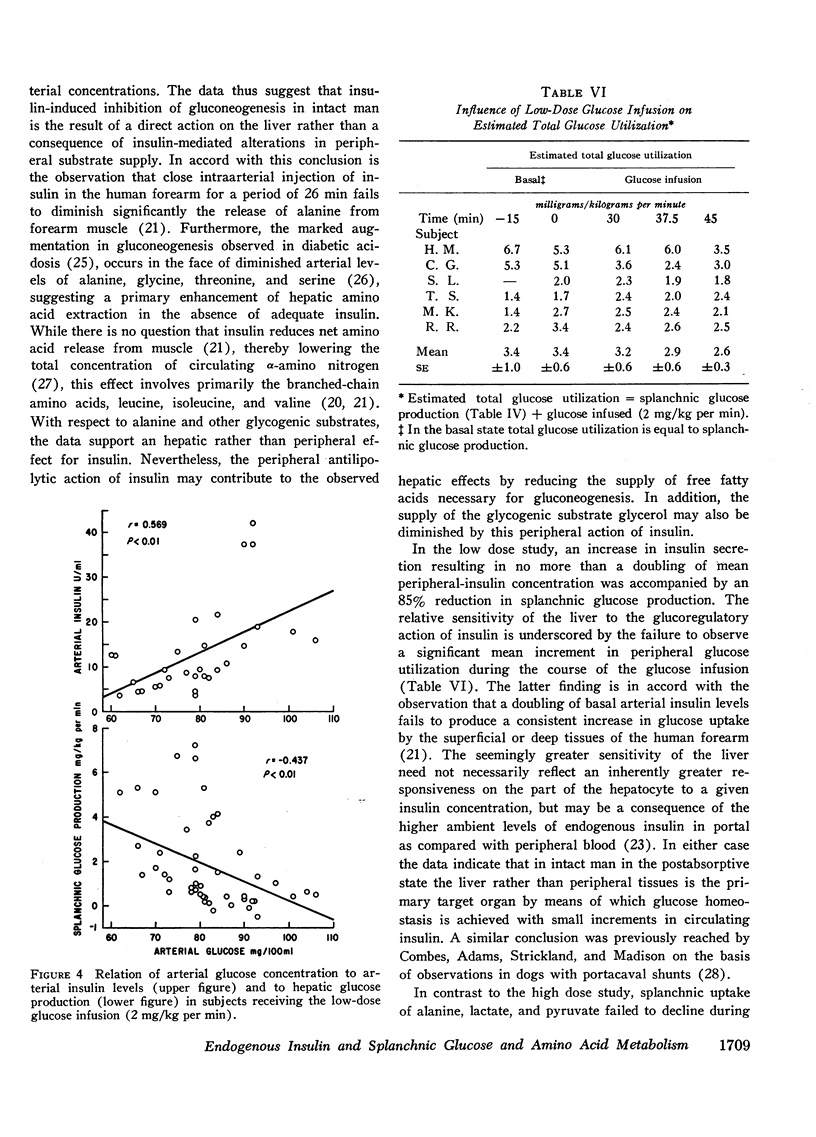
Administration of glucose at 2 mg/kg per min for 45 min resulted in a 19 mg/100 ml increase in arterial glucose concentration and a doubling of arterial insulin levels. Despite the small increment in insulin, hepatic glucose production fell by 85%. Splanchnic exchange of amino acids, lactate, and pyruvate was unaltered. Estimated total glucose utilization during the infusion was no greater than in the basal state, indicating lack of stimulation of peripheral glucose uptake.
It is concluded that: (a) inhibition of hepatic glucose production associated with glucose infusion and large increments in insulin levels occurs in the absence of a decrease in the concentration of circulating gluconeogenic substrate, suggesting an hepatic rather than peripheral effect; (b) the liver is the primary target organ whereby glucose homeostasis is achieved with small increments in insulin; (c) the relatively greater sensitivity of the liver's response to insulin as compared with an effect of insulin on the peripheral tissues, may be a consequence of the higher levels of endogenous insulin in portal as compared with peripheral blood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEARN A. G., BILLING B. H., SHERLOCK S. Hepatic glucose output and hepatic insulin sensitivity in diabetes mellitus. Lancet. 1951 Oct 20;2(6686):698–701. doi: 10.1016/s0140-6736(51)91476-6. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Block W. D., Markovs M. E., Steele B. F. Comparison between free amino acid levels in plasma deproteinated with picric acid and with sulfosalicylic acid. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1089–1091. doi: 10.3181/00379727-122-31333. [DOI] [PubMed] [Google Scholar]
- Bondy P. K., Bloom W. L., Whitner V. S., Farrar B. W. STUDIES OF THE ROLE OF THE LIVER IN HUMAN CARBOHYDRATE METABOLISM BY THE VENOUS CATHETER TECHNIC. II. PATIENTS WITH DIABETIC KETOSIS, BEFORE AND AFTER THE ADMINISTRATION OF INSULIN. J Clin Invest. 1949 Sep;28(5 Pt 2):1126–1133. doi: 10.1172/JCI102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy P. K., James D. F., Farrar B. W. STUDIES OF THE ROLE OF THE LIVER IN HUMAN CARBOHYDRATE METABOLISM BY THE VENOUS CATHETER TECHNIC. I. NORMAL SUBJECTS UNDER FASTING CONDITIONS AND FOLLOWING THE INJECTION OF GLUCOSE. J Clin Invest. 1949 Mar;28(2):238–244. doi: 10.1172/JCI102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMBES B., ADAMS R. H., STRICKLAND W., MADISON L. L. The physiological significance of the secretion of endogenous insulin into the portal circulation. IV. Hepatic uptake of glucose during glucose infusion in non-diabetic dogs. J Clin Invest. 1961 Sep;40:1706–1718. doi: 10.1172/JCI104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG J. W., DRUCKER W. R., MILLER M., WOODWARD H., Jr A prompt effect of exogenous insulin on net hepatic glucose output in man. Metabolism. 1961 Mar;10:212–220. [PubMed] [Google Scholar]
- CROFFORD O. B., FELTS P. W., LACY W. W. EFFECT OF GLUCOSE INFUSION ON THE INDIVIDUAL PLASMA FREE AMINO ACIDS IN MAN. Proc Soc Exp Biol Med. 1964 Oct;117:11–14. doi: 10.3181/00379727-117-29483. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Cahill G. F., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969 Oct 9;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970 Oct;19(10):727–728. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Ishiwata K., Hetenyi G., Jr, Vranic M. Effect of D-glucose or D-ribose on the turnover of glucose in pancreatectomized dogs maintained on a matched intraportal infusion of insulin. Diabetes. 1969 Dec;18(12):820–827. doi: 10.2337/diab.18.12.820. [DOI] [PubMed] [Google Scholar]
- MYERS J. D. Net splanchnic glucose production in normal man and in various disease states. J Clin Invest. 1950 Nov;29(11):1421–1429. doi: 10.1172/JCI102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Soeldner J. S., Cahill G. F., Jr Insulin blockade of amino acid release by human forearm tissues. Trans Assoc Am Physicians. 1968;81:258–265. [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- SANDERS C. A., LEVINSON G. E., ABELMANN W. H., FREINKEL N. EFFECT OF EXERCISE ON THE PERIPHERAL UTILIZATION OF GLUCOSE IN MAN. N Engl J Med. 1964 Jul 30;271:220–225. doi: 10.1056/NEJM196407302710502. [DOI] [PubMed] [Google Scholar]
- SEGAL S., BLAIR A. E., WYNGAARDEN J. B. An enzymatic spectrophotometric method for the determination of pyruvic acid in blood. J Lab Clin Med. 1956 Jul;48(1):137–143. [PubMed] [Google Scholar]
- Wahren J. Quantitative aspects of blood flow and oxygen uptake in the human forearm during rhythmic exercise. Acta Physiol Scand Suppl. 1966;269:1–93. [PubMed] [Google Scholar]



