Abstract
Ipilimumab, a novel immune checkpoint inhibitor, is associated with long-term survival in ~20% of advanced melanoma patients and is also being evaluated in the adjuvant setting. With this growing cohort of survivors, long-term health outcomes, chronic toxicities, and functional outcomes among survivors treated with ipilimumab need to be defined. Using retrospective medical record abstraction, we evaluated disease status, chronic immune- and non-immune-related health events, pharmacologic management of symptoms, and functional status in melanoma patients with overall survival ≥2 years following ipilimumab treatment at Vanderbilt University. Ninety patients received ipilimumab for metastatic disease or as adjuvant therapy between January 2006 and September 2012, and 33 patients survived ≥2 years with a median overall survival of 60.1 months. Of these, 24 patients were alive at last follow-up (73%) with 14 patients free of disease (42%). Gastrointestinal and dermatologic adverse events were frequent but largely transient. By contrast, patients with hypophysitis universally required ongoing corticosteroids although largely remained asymptomatic with appropriate hormone replacement. Surviving patients generally had excellent performance status (ECOG 0-1 in 23/24). Chronic neurologic toxicities caused substantial morbidity and mortality in two patients who received whole brain radiotherapy >5 years prior to analysis, and in one patient with chronic, painful peripheral neuropathy. No previously undescribed cardiac, pulmonary, gastrointestinal, hematologic, or neoplastic safety signals were identified. In conclusion, ipilimumab was associated with largely excellent functional outcomes among long-term survivors. Chronic endocrine dysfunction and occasional neurologic toxicity (primarily associated with whole brain radiation) were observed in a small number of patients.
Keywords: Ipilimumab, CTLA-4, immune-related adverse events, colitis, hypophysitis, hepatitis, dermatitis, symptoms, melanoma, immune therapy, survivorship
Introduction
Historically, long-term survival from metastatic melanoma was a rarity. Durable remissions, however, were occasionally observed in patients treated with high-dose interleukin-2 (1, 2). Another immune modulator has more recently increased the proportion of patients with protracted survival. Ipilimumab, a monoclonal antibody to CTLA-4 (cytotoxic T lymphocyte-associated protein 4), activates antitumor immune responses. This agent has been shown to induce long-term disease control (3–5 years) in approximately 20% of patients in large prospective and retrospective analyses (3–6). Aberrant autoimmune T-cell activation occurs in a substantial minority of patients, resulting in acute immune-related toxicities such as colitis, hepatitis, endocrinopathies, dermatitis, and other rare but serious events (3, 7–11). These adverse events, which most commonly arise during the first three months of therapy, are of variable duration and intensity (5). Colitis, for example, often resolves rapidly with corticosteroid administration, whereas endocrinopathies may persist indefinitely (12, 13). Additional immune checkpoint inhibitors, particularly antibodies to programmed death-1 receptor and its ligand (PD-1/PD-L1), are also being developed for melanoma and in several other malignancies with promising results (14–16).
As an increasing proportion of patients derive sustained benefits from immune therapies, and these agents move into the adjuvant setting, survivorship concerns will become increasingly important. Despite the well-characterized acute toxicity profile, the long-term and chronic effects of immune therapies, including ipilimumab, have not been thoroughly explored. Thus, there is a critical need to characterize the impact of ipilimumab on intermediate and long-term health outcomes, including immune-related sequelae, pharmacologic management, and health-related quality of life.
To investigate the chronic effects of this agent, we identified 33 patients with melanoma treated with ipilimumab at our institution between 2006 and 2012 who survived for at least two years with evaluable follow-up. We assessed their current disease status, ongoing symptoms, interval health events, medication utilization, and functional status via retrospective chart review.
Materials and Methods
Patients
After obtaining IRB approval for the proposed studies, we extracted clinical data from the electronic medical record for patients at Vanderbilt University Medical Center treated with ipilimumab between January 2006 and September 2012; follow-up occurred through October 2014. Patients were included if they had ≥2 years of follow up data beginning at the first ipilimumab dose until death, loss to follow up, or the end of the study period, September 2014. Patients were eligible for inclusion if they had received at least one dose of ipilimumab, irrespective of dosage (3mg/kg or 10mg/kg), indication (metastatic disease or as adjuvant therapy for resected melanoma at high risk of recurrence), or setting (clinical trial or commercially available). Patients on the following clinical trials were included: NCT01274338 (ECOG 1609), NCT00094653, NCT00623766, and NCT00495066. Patients who received prior or subsequent anticancer therapies were included.
Study Design
We assessed ipilimumab efficacy in terms of overall survival (OS), defined as the time of ipilimumab initiation to time of death from any cause, and progression-free survival (PFS) as defined by RECIST 1.1 criteria. We recorded immune-related toxicities (including colitis, endocrinopathies, hepatitis, dermatitis, and neuropathy), health-related events (e.g. diagnosis of new illnesses, hospitalizations, etc.), medication doses, and Eastern Cooperative Oncology Group (ECOG) performance status as documented in provider notes. Side effects characteristic of and attributed to subsequent therapies or cancer-related symptoms were identified but not attributed to ipilimumab. We also interrogated the medical records for tumor mutation status. Our institution performs reflex testing on melanoma samples using the SNaPshot platform as described previously (17). This test assesses 49 point mutations in BRAF, NRAS, CKIT, GNAQ, GNA11, and CTNNB1.
Statistics
Categorical and continuous variables were summarized by percentages and means; no formal statistical hypothesis testing was performed with these variables. OS and PFS were estimated using the Kaplan-Meier method; patients were censored at last available follow up. OS for patients who received corticosteroids for immune-related adverse events was compared with those who did not by the logrank test. All analyses were performed using R version 3.1.1.
Results
Patients
We identified 90 patients treated with ipilimumab between 2006 and 2012; 33 survived for at least 24 months with evaluable follow-up (3 were lost to follow-up and 54 died prior to 24 months). Among the 33 long-term survivors, the median age upon initiation of ipilimumab was 60 years; the majority had stage IVc melanoma (52%) and 22% had brain metastases (Table 1). Fourteen patients (42%) had received prior systemic therapy including chemotherapy (36%), high-dose interleukin-2 (9%), and BRAF inhibitors or other targeted therapy (12%). Most patients (61%) received the now-FDA approved dose of 3mg/kg, the remainder received 10mg/kg or were treated at outside facilities with an unknown dose with subsequent treatment and/or follow-up at our center. Eight patients (24%) received ipilimumab as an adjuvant therapy as part of clinical protocols for resected disease at high risk of relapse.
Table 1.
Demographics and ipilimumab dose
| Number | Percent | |
|---|---|---|
| Age | 60 (median) | 21–75 (range) |
| Gender | ||
| Male | 20 | 61 |
| Female | 13 | 39 |
| Stage | ||
| III | 8 | 24 |
| IVa | 4 | 12 |
| IVb | 4 | 12 |
| IVc | 17 | 52 |
| Mutational status | ||
| BRAF | 8 | 24 |
| NRAS | 8 | 24 |
| CKIT | 1 | 3 |
| WT | 10 | 30 |
| Unknown | 6 | 18 |
| Prior therapy | ||
| Chemotherapy | 12 | 36 |
| IL-2 | 3 | 9 |
| Targeted therapy | 4 | 12 |
| Number of lines | 0–4 (range) | |
| Dose of ipilimumab | ||
| 3mg/kg | 20 | 61 |
| 10mg/kg | 8 | 24 |
| unknown | 5 | 15 |
| Indication | ||
| Metastatic, no brain mets | 18 | 55 |
| Metastatic, brain mets | 7 | 21 |
| Adjuvant | 8 | 24 |
Response to therapy and survival
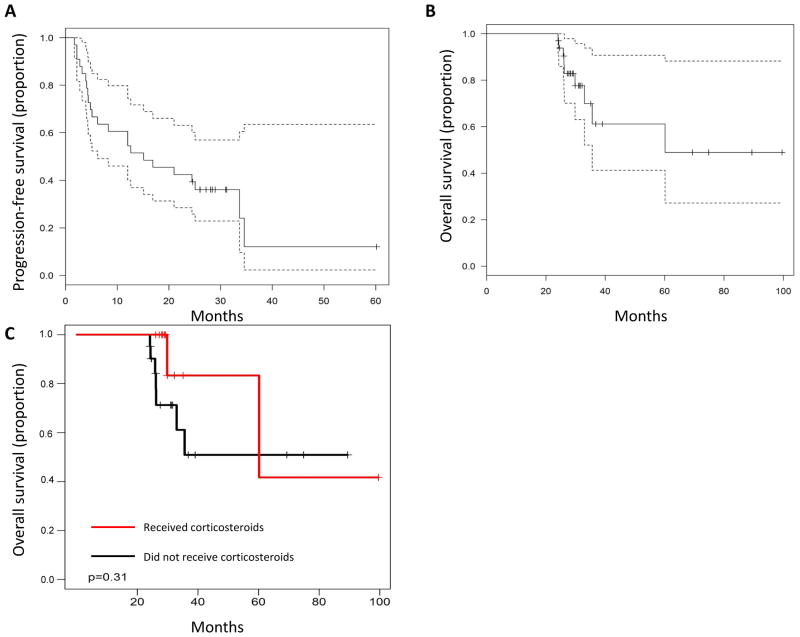
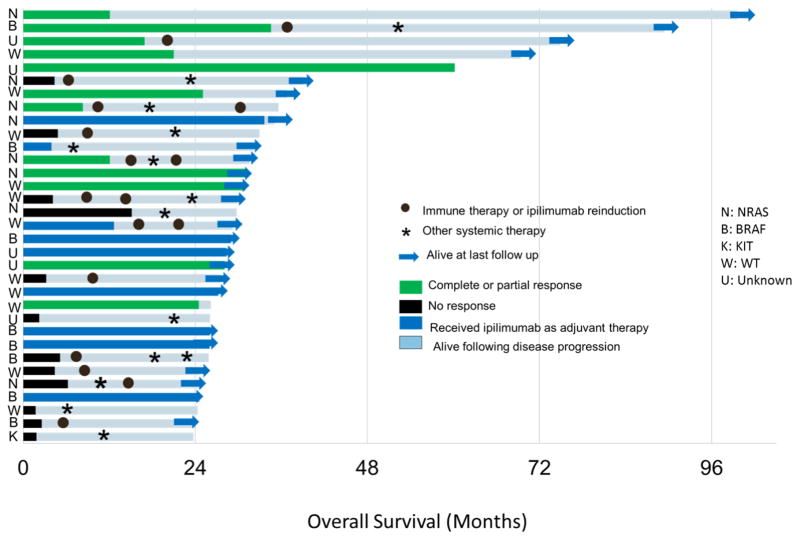
Although only patients who experienced an overall survival (OS) of ≥24 months were included, patient outcomes even within this select group were heterogeneous. The median progression-free survival (PFS) period for this group of patients was 15.1 months (Fig. 1A). Seventeen patients (52%) required additional systemic therapy and 4 received a second course of ipilimumab. The median OS was 60.1 months although many patients were censored prior to the median survival (Fig. 1B). Nine patients (27%) had died at the last evaluable follow up including 2 patients who died of causes unrelated to melanoma (see “Toxicities”, below). Given the concern that corticosteroids may blunt the effectiveness of ipilimumab, we assessed survival for patients who received corticosteroids for immune-related adverse events. Survival in the 10 patients who had received corticosteroids for greater than 1 month was not inferior to those that did not receive corticosteroids (logrank P=0.31, Fig 1C). Figure 2 summarizes the PFS, OS, and subsequent treatments for the study cohort.
Figure 1.
Figure 1A: Progression-free survival for all patients with 95% confidence intervals
Figure 1B: Overall survival for all patients with 95% confidence intervals
Figure 1C: Patients who received steroids (n=12) vs. no steroids (n=21)
Figure 2.
Duration of response and survival, subsequent anti-cancer therapy, and mutation status.
Acute and chronic immune-related toxicities
Next, we evaluated the treatment-related toxicities in this population. Given the extensive literature regarding acute immune-related adverse events (irAE) complicating ipilimumab, we focused on chronic toxicities.
Gastrointestinal
Seven patients experienced colitis (21%), all but one during the initial four doses of ipilimumab. One patient developed severe diarrhea with onset >2 years after starting ipilimumab although this resolved rapidly with corticosteroids. No cases of colitis were associated with protracted gastrointestinal symptoms.
Endocrine
Hypophysitis/hypopituitarism was diagnosed in 8/33 patients (24%). All patients initially received high-dose corticosteroids with subsequent taper except for one patient who received prednisone 5mg daily with clinical improvement. In contrast to other transient adverse events, all cases of hypophysitis required prolonged hormone replacement. Notably, although each patient required ongoing low-dose corticosteroid administration (prednisone 5mg daily or hydrocortisone 10mg/5mg daily), several patients were weaned off thyroid hormone replacement with subsequent normalization of thyroid functions. Testosterone replacement was also instituted in 5 patients (15%) and was continued during evaluable follow up in 4 of these patients. New onset, isolated hypothyroidism and adrenal insufficiency occurred in one patient each and required protracted hormone replacement for the duration of follow up.
Dermatologic
Pruritis and skin rashes were common (>50%) in the acute setting as previously described and were largely manageable with topical corticosteroids and antihistamines. One patient had a pruritic maculopapular skin eruption arising after his third dose that improved with a brief course of methylprednisolone but required ongoing topical hydrocortisone and oral antihistamines for over one year while receiving maintenance ipilimumab. Two patients experienced delayed skin conditions that may or may not be related to ipilimumab. One patient developed ulcerative lesions on the upper extremities 3 years after completing ipilimumab that resolved with topical antibiotics; another experienced scaly, pruritic plaque-like lesions consistent with eczema approximately 7 years after completing therapy. No other extended morbidity or mortality from cutaneous conditions was noted in this series.
Neurologic
One patient developed neuropathic pain in the lower extremities within one month following her fourth dose of ipilimumab. She has subsequently required chronic narcotic therapy during the next three years. She experienced a complete response of her melanoma although developed an isolated small bowel recurrence 12 months prior to this analysis. This was surgically resected and she remains disease-free. It was not clear whether her neuropathy was immune-mediated or related to prior surgery; she did not receive corticosteroids. Two other transient, potentially immune-related neurologic events occurred. In one case, a cranial nerve VII palsy (Bell’s Palsy) occurred nearly 3 weeks after the second dose of ipilimumab induction and rapidly resolved with corticosteroids. In another case, a patient who experienced hypophysitis subsequently developed peripheral neuropathy with patchy paresthesias and mild weakness in his extremities approximately 2 months after starting ipilimumab. Brain/cervical spine MRI were normal and electromyogram showed findings suggestive of mild chronic denervation. His findings completely resolved over 3 months with corticosteroid treatment (60mg daily with rapid taper).
Two patients treated with ipilimumab after receiving whole brain radiation for multiple brain metastases had significant functional decline over the next several years which was felt to be at least partially related to radiation therapy. Both of these patients had outstanding complete or near complete responses both intra- and extra-cranially after receiving ipilimumab. One patient developed progressive weakness, memory loss, and seizures; brain MRI demonstrated presumed radiation necrosis. Her symptoms and imaging findings progressed over the next several years despite treatment with dexamethasone and bevacizumab. She died suddenly of unknown causes nearly 5 years after receiving ipilimumab despite having no evidence of active disease. The second patient also received ipilimumab in 2008 but developed a left corona radiata lacunar infarct in 2012 associated with non-resolving aphasia and right lower extremity weakness. Pre-existing and ongoing poorly controlled blood pressure and medical non-compliance likely contributed to this event.
Other immune-related events of interest
No serious acute or chronic ocular disorders (e.g. uveitis, episcleritis), liver injury, or hematologic abnormalities were noted in this population.
Other health-related outcomes
To investigate whether ipilimumab is associated with other significant health events and morbidity in long-term survivors, we reviewed patient records for other adverse events that are not classically associated with immune therapy. Among these 33 patients, we did not observe any adverse cardiac, pulmonary, renal, hematologic, neoplastic, or hepatic events. One patient developed a transient, presumably non-malignant pericardial effusion while subsequently receiving an anti-PD-1 antibody although he was found to have hypothyroidism concurrently (attributed to anti-PD-1).
Several other events of interest occurred in this patient population that had unclear relation to ipilimumab. One patient who experienced a complete response to ipilimumab developed a chronic, non-healing ulcer at the site of a skin graft that had previously been radiated for a local recurrence; this complication occurred nearly 7 years following ipilimumab. Two patients developed fractures (rib and thoracic spine) associated with sites of metastatic disease. A 24 year old patient who had previously experienced colitis during her induction developed acute appendicitis 28 months following completion of ipilimumab; clinical and pathologic evaluation appeared consistent with a routine case of appendicitis without concurrent small bowel or colon inflammation.
Functional status and ongoing pharmacologic management
Among the 24 patients who were alive at last follow up, 14 (58%) were alive with no evidence of active disease and 10 patients (42%) were receiving subsequent lines of therapy. At the last available follow up, 8 patients (33% of surviving patients) continued to receive corticosteroids, and nine were on thyroid replacement therapy (38%) for endocrine complications. Although many patients required short-term, high-dose corticosteroids for acute events, all those requiring long-term steroid administration tolerated dose reduction to replacement level dosing of prednisone or hydrocortisone. Of the surviving patients, 23 of 24 had ECOG performance status of 0 or 1, with only 1 patient being more debilitated (the patient who experienced the cerebrovascular accident above; Table 2). Many patients returned to work and nearly half (11) had no symptoms or functional limitations attributable to melanoma, ipilimumab, or other health conditions. This included two patients with multiple brain metastases treated in 2007 and 2012, demonstrating the possible long-term benefit of ipilimumab even among patients with previously grim outcomes.
Table 2.
Status at most recent follow up
| Number | Percent | |
|---|---|---|
| Alive, no active disease | 14 | 42 |
| Alive, receiving treatment | 10 | 30 |
| Died of melanoma | 7 | 21 |
| Died of other causes | 2 | 6 |
| Functional status (N=24) | ||
| 0 | 11 | 46 |
| 1 | 12 | 50 |
| 2+ | 1 | 4 |
| Ongoing steroid replacement | 8 | 33 |
| Ongoing thyroid replacement | 9 | 38 |
Discussion
In this study, we characterized the effects of ipilimumab in a cohort of patients who survived ≥2 years following start of treatment. We found that the majority of these survivors maintained an excellent performance status with minimal chronic sequelae attributable to ipilimumab. Hypophysitis and other endocrinopathies are the primary immune-related adverse events that require ongoing pharmacologic management with long-term hormone replacement. In addition, chronic radiation toxicities were problematic for a small subset of patients in this series. To our knowledge, this is the first study that comprehensively characterizes the long-term toxicities, health outcomes, and functional status of long-term survivors who received ipilimumab.
Delayed and chronic toxicities associated with a wide range of cytotoxic chemotherapy and molecularly targeted agents have been identified (18–23). Understanding the long-term effects and possible complications from immune therapies, a completely distinct class of therapeutics, is critical. As these agents produce durable benefits across an increasing number of cancer types and are investigated in the adjuvant setting, these efforts will become still more important. Our study takes the first step by reporting long-term health outcomes from a series of melanoma patients receiving ipilimumab.
Our study provides several other critical insights. First, the long-term survival associated with ipilimumab also may unmask delayed toxicities related to radiation therapy. Two patients who received whole brain radiotherapy experienced cognitive and functional decline over a period of 4–5 years despite experiencing a durable antitumor response. Another patient, now 7 years from ipilimumab initiation, developed a delayed, non-healing ulcer at the site of radiation for a previous local recurrence that required surgical intervention. Confirmation of this phenomenon in a larger cohort may be prudent. Oncologists should be vigilant for potential delayed effects of radiation, particularly whole brain radiation therapy, among this patient population with possible long-term survival.
Second, we failed to observe any new concerning safety signals in most other organ systems, including cardiac, pulmonary, gastrointestinal, cutaneous, neoplastic, ocular, or hematologic systems. This is a critical finding in that chronic, multisystem immune-related toxicities are prominent features of many models of aberrant immune activation (e.g. graft vs. host disease, rheumatoid arthritis, systemic lupus erythematosus, etc.) (24, 25). Furthermore, a growing body of literature implicates T-cell dysregulation as critical to the pathogenesis of conditions like coronary artery disease and type 2 diabetes (26–28). Our sample size is relatively small and follow up is limited to <5 years in many patients. Long-term safety data for ipilimumab and other immune therapies, therefore, is needed from larger cohorts with prolonged follow-up.
Third, we provided additional support for the chronic nature of ipilimumab-induced endocrinopathies (13, 29). This finding is important when counseling patients regarding expected adverse events, particularly if ipilimumab is used as an adjuvant therapy in the future. Despite the protracted nature of these toxicities, patients remained largely asymptomatic when receiving adequate hormone replacement.
Finally, we observed that corticosteroid administration did not adversely influence survival among this select group of prolonged survivors. This observation provides additional support that judicious use of corticosteroids for immune-related adverse events does not adversely impact overall survival. A larger cohort of unselected patients (short-term and long-term survivors) would be required to answer this question conclusively.
Our study contains several important limitations. First, the sample size is relatively small and therefore infrequent outcomes may not be identified. Second, we focused on only long-term survivors, limiting the predictive value of our clinical observations for unselected patients; this has been examined in other investigations (5). Finally, we report only data extracted from the medical record. Patient-reported outcomes including quality of life and psychosocial well-being are important and should be considered in the design of a prospective study.
In conclusion, the increasing proportion of durable tumor regressions induced by ipilimumab and other immune therapies is a major therapeutic breakthrough in melanoma. Attendant to this welcome advance though, will arise new management challenges. In our experience, ipilimumab was associated with excellent functional outcomes in patients with extended survival with only a few exceptions. Evaluating the long-term complications of these agents and identifying survivorship concerns will grow in importance as these agents are used increasingly.
Acknowledgments
Research Support: This work was supported by NIH K12 CA 0906525 (DBJ).
Footnotes
The other authors have no conflicts to disclose.
Conflicts of Interest: CML has served on an advisory board for Novartis and has received research funding from Astra Zeneca and Novartis.
References
- 1.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–13. [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S, Investigators MDX. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20) Ann Oncol. 2013;24:2694–8. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 6.McDermott D, Lebbe C, Hodi FS, Maio M, Weber JS, Wolchok JD, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40:1056–64. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 9.Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol. 2011;22:991–3. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 10.Delyon J, Mateus C, Lambert T. Hemophilia A induced by ipilimumab. N Engl J Med. 2011;365:1747–8. doi: 10.1056/NEJMc1110923. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DB, Saranga-Perry V, Lavin PJ, Burnette WB, Clark SW, Uskavitch DR, et al. Myasthenia gravis induced by ipilimumab in metastatic melanoma patients. J Clin Oncol. 2014 Apr 28; doi: 10.1200/JCO.2013.51.1683. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients with Metastatic Melanoma. J Clin Endocrinol Metab. 2014;99:4078–85. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma. J Clin Oncol. 2013;31:9010. [Google Scholar]
- 17.Lovly CM, Dahlman KB, Fohn LE, Su Z, Dias-Santagata D, Hicks DJ, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan MK, Rampal R, Harding JJ, Klimek VM, Chung YR, Merghoub T, et al. Progression of RAS-Mutant Leukemia during RAF Inhibitor Treatment. N Engl J Med. 2012;367:2316021. doi: 10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 21.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantarjian HM, Keating MJ, Walters RS, Smith TL, Cork A, McCredie KB, et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4:1748–57. doi: 10.1200/JCO.1986.4.12.1748. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur Urol. 2014 Aug 2; doi: 10.1016/j.eururo.2014.07.010. pii: S0302-2838(14)00650-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Gabriel SE. Heart disease and rheumatoid arthritis: understanding the risks. Ann Rheum Dis. 2010;69 (Suppl 1):i61–4. doi: 10.1136/ard.2009.119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhushan V, Collins RH., Jr Chronic graft-vs-host disease. JAMA. 2003;290:2599–603. doi: 10.1001/jama.290.19.2599. [DOI] [PubMed] [Google Scholar]
- 26.Strissel KJ, Denis GV, Nikolajczyk BS. Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Curr Opin Endocrinol Diabetes Obes. 2014;21:330–8. doi: 10.1097/MED.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolbus D, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Bjorkbacka H, et al. Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med. 2013;274:41–51. doi: 10.1111/joim.12038. [DOI] [PubMed] [Google Scholar]
- 28.Bergstrom I, Backteman K, Lundberg A, Ernerudh J, Jonasson L. Persistent accumulation of interferon-gamma-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis. 2012;224:515–20. doi: 10.1016/j.atherosclerosis.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]