Abstract
Objective
We tested independent and interactive effects of Apolipoprotein E (ApoE) and pulse pressure (PP) concurrently and longitudinally across 9 years (3 waves) of episodic (EM) and semantic memory (SM) data from the Victoria Longitudinal Study.
Method
We assembled a sample of older adults (n=570, Baseline M age=71, Age range=53–95) and used latent growth modeling to test four research goals.
Results
First, the best fitting memory model was two single latent variables for EM and SM, each exhibiting configural, metric, and partial scalar invariance. This model was analyzed as a parallel process model. Second, baseline level of PP predicted EM performance at centering age (75) and rate of 9-year EM change. Third, we observed no main effects of ApoE on EM or SM. Fourth, EM was affected by higher PP but differentially less so for carriers of the ApoE ε2 allele than the ε3 or ε4 alleles.
Conclusions
PP is confirmed as a risk factor for concurrent and changing cognitive health in aging, but the effects operate differently across risk and protective allelic distribution of the ApoE gene.
Keywords: Aging, Apolipoprotein E, Episodic Memory, Pulse Pressure, Victoria Longitudinal Study
Individual differences in cognitive performance (e.g., declarative memory) and trajectories of change are substantial (Anstey, 2012; Dixon, Small, MacDonald, & McArdle, 2012; Hertzog, 2008). These individual differences reflect a variety of influences representing a continuum from biological (e.g., genetic) to health and environmental (e.g., lifestyle) factors (Harris & Deary, 2011; Mitnitski, Song, & Rockwood, 2013; Plassman, Williams Jr., Burke, Holsinger, & Benjamin, 2010; Song, Mitnitski, & Rockwood, 2011). Whether these factors serve “risk” or “protective” roles, they may operate both independently and interactively in determining the level and shaping of trajectories of the phenotypic expression, including cognitive performance (e.g., Fotuhi, Hachinski, & Whitehouse, 2009; Harris & Deary, 2011; Josefsson, de Luna, Rudas, Nilsson, & Nyberg, 2012; Kalpouzos & Nyberg, 2012; Lindenberger et al., 2008). Accordingly, we adopt a gene x health approach to examining potential mechanisms of individual differences in concurrent level and 9-year change in declarative memory performance among older adults. Performance and change patterns of declarative memory, which consists of episodic (EM) and semantic (SM) memory systems, are known to be variable and influenced by selected neural, biological, and health factors (e.g., Nyberg et al., 2012). In the present study, we test the effects of a key genetic polymorphism (i.e., Apolipoprotein E [ApoE]) and an established vascular health indicator (i.e., pulse pressure [PP]) for interactive effects on both EM and SM performance and change among normally aging adults.
Vascular status is among the prominent health influences on normal memory aging. Arterial stiffening has been found to have an independent effect on cardiovascular disease (Dart & Kingwell, 2001; Mitchell et al., 2007; Schiffrin, 2004) and cognitive performance in non-demented older adults (Bender & Raz, 2012; Dahle, Jacobs, & Raz, 2009; McFall et al., 2014; Waldstein et al., 2008). Arterial stiffness is measured directly by pulse wave velocity, but PP is considered a good proxy of pulse wave velocity in aging research (Waldstein et al., 2008). PP is calculated as systolic minus diastolic blood pressure. Typically, PP shows a steep age-related increase in older adults (Franklin et al., 1997; Mattace-Raso et al., 2006, Raz, Dahle, Rodrigue, Kennedy, & Land, 2011) and is considered a better predictor of declining vascular health than systolic or diastolic blood pressure (Raz et al., 2011).
Several researchers have reported PP (and other vascular health) associations with memory deficits in typically aging older adults (Waldstein et al., 2008), associations with subclinical cerebrovascular disease (Waldstein et al, 2012), increased β-amyloid (Aβ) burden (Rodrigue et al., 2013), increased levels of phosphorylated tau (P-tau; Nation et al., 2013), and an increased risk of Alzheimer’s disease or dementia (Qui, Winblad, Viitanen, & Fratiglioni, 2003). Memory deficits associated with poor vascular health (i.e., as measured by high systolic or diastolic blood pressure) were observed for EM (Elias, Elias, Sullivan, Wolf & D’Agostino, 2003; Saxby, Harrington, McKeith, Wesnes, & Ford, 2003). However, Raz and colleagues (2011) reported that when age, sex, and genetic variants were taken into account, PP and EM correlations were no longer significant. Higher levels of PP were negatively associated with level of EM performance in a group of middle-aged adults (Pase et al., 2010), supporting the idea that midlife vascular health may be more strongly associated with older adult memory decrements than late-life vascular health (Nation et al., 2013; Waldstein et al., 2012). In addition, persons with high PP exhibited accelerated EM decline in comparison to their counterparts with lower PP (Waldstein et al., 2008). In contrast, other researchers observed no group differences between hypertensive and normotensive adults on tasks of working memory, associative memory, and free recall (Dahle et al., 2009). Bender and Raz (2012) found no main effects of PP on EM. Researchers have reported that vascular health has no effect on SM performance in older adults (Elias, Elias, Robbins, & Budge, 2004).
ApoE is a widely verified risk factor for late onset AD (Bertram, McQueen, Mullin, Blacker, & Tanzi, 2007), Aβ burden (Rodrigue et al., 2013), and mild cognitive impairment (Brainerd, Reyna, Petersen, Smith, & Taub, 2011; Dixon et al., 2014). As such, growing interest has focused on associations with normal cognitive aging and potential interactive effects with other polymorphisms or health factors (e.g., Jochemsen, Muller, van der Graaf, & Geerlings, 2012; Wisdom, Callahan, & Hawkins, 2011). ApoE consists of three isoforms, ApoE2, ApoE3, and ApoE4, and the corresponding ε2, ε3, and ε4 alleles. A gene that codes for a lipid-carrying protein known to be involved in cell maintenance and repair, ApoE modulates the efficiency of neuronal repair and plasticity (Lind & Nyberg, 2010; Mahley, 1988; Mahley, Weisgraber, & Huang, 2009). The ε3 allele is the most common. The ε2 allele has been identified as being associated with lower levels of cholesterol, heart disease, and risk of dementia and Alzheimer’s disease (Berlau, Corrada, Head & Kawas, 2009; Corder et al., 1993; Fotuhi et al., 2009; Mahley & Rall, 2000). It has also been associated with better cognitive performance in non-demented populations (Anstey & Christensen, 2000; Deary et al., 2004; Lindahl-Jacobsen et al., 2012; Small, Rosnick, Fratiglioni, & Bäckman, 2004; Wilson, Bienias, Berry-Kravis, Evans, & Bennett, 2002; Wisdom et al., 2011). The protective effect of the ε2 variant may be associated with the presence of cysteine at position 158, whereas the ε3 and ε4 variants have arginine at position 158 (Zlokovic, 2013). For ε2, this difference implies both a lower facility with binding to low-density lipoprotein receptor (LDLR) sites and better facilitation of lipoprotein clearance through other pathways (i.e., Heparan sulfate proteoglycans; Deane et al., 2008; Mahley et al., 2009). This lowered binding of LDLR with ε2 may account for the increased levels of apolipoprotein in the brain for ε2 carriers (Sullivan et al., 2011). In addition, LDLRs, with a marked preference for ε3 and ε4, act mainly as influx and not efflux receptors at the blood-brain-barrier (BBB). This allows lipids to cross from blood to brain and not from brain to blood, resulting in higher levels of Aβ in the brain (Deane et al., 2008).
The ε4 variant is the largest known risk factor for mild cognitive impairment and sporadic AD (Brainerd et al., 2011), but has also been linked to decreased vascular health (Bennet et al., 2007; Cattin et al., 1997; Smith, 2002) and increased mortality risk (Lindahl-Jacobsen et al., 2012), as well as cognitive decrements in global functioning, memory, executive functioning, and perceptual speed (Laukka et al., 2013; Small et al., 2004; Wisdom et al., 2011). Specifically, although the patterns are mixed, some studies have shown that ε4 carriers may have greater EM decrements in normal aging when compared to non-ε4 carriers (Anstey & Christensen, 2000; Caselli et al., 2011; Laukka et al., 2013; Lee et al., 2008; Nilsson et al., 2006; Schiepers et al., 2012; Small et al., 2004; Sternäng et al., 2009). Risk associated with the ε4 variant may be due to arginine at position 112, in comparison to ε2 and ε3 with cysteine at this position (Mahley et al., 2009). This difference may account for the increased risk the ε4 allele has with hyperphosphorylation of tau, reduced Aβ clearance, and neurodegenerative changes associated with toxic effects on the cerebrovascular system (Zlokovic, 2013). The arginine difference at position 112 accounts for the ε4 preference to bind to large lower-density lipoproteins. In contrast, the ε3 and ε2 bind to smaller, cholesterol-rich, high-density lipoproteins (Hatters, Peters-Libeu, & Weisgraber, 2006). These patterns result in a ε4 association with thicker arterial walls and lower vascular flexibility, resulting in reduced vascular health. Accordingly, researchers have found that the interaction of ApoE with selected health factors explained substantial variance associated with EM performance. For example, ApoE ε4 carriers have poorer memory performance when they also have poorer vascular health (Bender & Raz, 2012; Caselli et al., 2011; Ferencz et al., 2013; Sternäng et al., 2009; Yasuno et al., 2012; Zade et al., 2010). In contrast, the limited literature of the effect of ApoE on SM has resulted in small or non-significant findings (Nilsson et al., 2006; Reynolds, Gatz, Berg, & Pedersen, 2007). However, ApoE effects for SM have been reported in interaction analyses: Sternäng and colleagues (2009) observed poorer cognitive performance for older women with a ε4 allele and high cholesterol.
Although the precise pathways linking vascular disruption with ApoE status and declarative memory performance are not fully established, the two-hit vascular hypothesis of AD proposed by Zlokovic (2011) contributes to the current research. Briefly, this hypothesis assumes two pathways that lead to the eventual development of AD. The first vascular “hit” reflects the damage associated with early vascular risk factors that contribute to BBB dysfunction. This dysfunction allows accumulation of neurotoxins and diminished blood flow, resulting in a neurodegeneration cascade preceding onset of dementia. ApoE may play a role as follows. ApoE isoform-specific differentiation has been reported in middle adulthood in regard to the development of atherosclerosis and cardiovascular disease (Cattin et al., 1997). Specifically, adult ε4 carriers exhibited the highest level of carotid intima-media thickness (IMT; i.e., carotid artery wall thickness) followed by adults with a ε3. In contrast, ε2 carriers exhibited the lowest level of IMT. This is an indication of pre-symptomatic thickening and therefore reduced vascular flexibility. The second vascular “hit” relates to compromised clearance of Aβ at the BBB and increased production of Aβ as a result of vascular damage. Isoform-dependent levels of apolipoprotein E (ε4 < ε3 < ε2) have been implicated in BBB breakdown (Bell et al., 2014). Specifically, in mouse models the lower levels of apolipoprotein E associated with ε4 have been shown to increase susceptibility to injury. This can cause an accumulation of Aβ, perhaps due to limitations in influx and efflux of lipids and lipid-Aβ complexes at the BBB (Deane et al., 2008; Mahley & Huang, 2012) and amplification of neuronal dysfunction (Zlokovic, 2011).
A growing emphasis in the study of both normal cognitive aging and neurodegenerative diseases has been on examining risk and protective factors that may operate independently and interactively in producing variations in phenotypic trajectories and clinical outcomes. Many of these factors, evaluated independently, are not sufficient or necessary for producing normal cognitive decline, mild cognitive impairment, or dementia (e.g., Anstey & Christensen, 2000; Gomar, Bobes-Bascaran, Conejero-Goldberg, Davies, & Goldberg, 2011; Sapkota et al., 2014). However, interactions of ApoE with lifestyle characteristics (e.g., body mass index, physical fitness, smoking status, and education) have been reported to affect select cognitive phenotypes in non-demented older samples (Josefsson et al., 2012; Plassman et al., 2010; Raz, Rodrigue, Kennedy, & Land, 2009; Zade et al., 2013) as well as risk of dementia or Alzheimer’s disease (Kivipelto et al., 2008; Luck et al., 2013). For this reason, we adopt a gene x health approach to examining interactive influences on memory performance and change.
In summary, given the fact of declarative memory declines with aging, the enduring questions of when, how, and why point to several relevant research directions (Anstey, 2012; Dixon et al., 2012; Nyberg et al., 2012). First, do the two primary declarative memory domains (i.e., EM and SM) follow similar or different age-related performance and change patterns? Second, to what extent is the variability associated with performance in these two declarative memory domains produced by independent and interactive effects of genetic and health factors? Understanding the interactions of genes and health conditions in the aging of memory may (a) account for unexplained variance associated with performance and change, (b) lead to the detection of theoretically relevant effects that appear less independently due to real inter-dependence among multiple factors, and (c) promote further insights into the underlying mechanisms of memory performance and change in normal aging.
Research Goals
The overarching goal of the current study was to examine the independent and interactive effects of PP and ApoE on latent variables representing EM and SM performance and change in a group of typically aging older adults. We used a relatively large sample of genotyped older adults between 53 and 95 years of age (n = 570 at baseline) to explore four research goals. For the first two research goals we used confirmatory factor analysis and latent growth modeling within a structural equation modeling context. Research goal 1 (RG1) was to determine how EM and SM were best represented in regard to latent variable models and invariance testing across three waves. RG1 was twofold: (a) to use six declarative memory measures to estimate two latent variables (i.e., EM, SM) and (b) to test these models for longitudinal measurement invariance across three waves. Research goal 2 (RG2) was to determine how EM, SM, and PP change across time by utilizing the best fitting latent growth models. Using conditional growth models we explored two additional research goals. Research goal 3 (RG3) was to determine how EM and SM performance patterns in older adults were affected independently by PP and ApoE. Research goal 4 (RG4) was to determine if PP and ApoE interactively affected EM and SM. Based on previous findings, we expected there to be independent effects of PP and ApoE on EM but not on SM. We further predicted EM decrements for ε4 carriers and protection against EM deficits and decline associated with the ε2 allele. We also expected that PP would exacerbate the effect that ApoE has on EM for ε4, but not for ε2 carriers.
Method
Participants
Participants were community-dwelling adults (initially aged 53–95 years) drawn from the Victoria Longitudinal Study (VLS). The VLS is a longitudinal sequential study designed to examine older adult development in relation to biomedical, genetic, health, cognitive and neuropsychological aspects (see Dixon & de Frias, 2004). The VLS and all present data collection procedures were in full and certified compliance with prevailing human research ethics guidelines and boards. Informed written consent was provided by all participants. Using standard procedures (e.g., Dixon et al., 2012; Small, Dixon, & McArdle, 2011), we assembled a selected longitudinal data set consisting of three samples with up to three available waves collected in the period beginning in the early 2000s. Specifically, this data set consisted of participants from (a) Sample 1 (S1) Waves 6 and 7, (b) Sample 2 (S2) Waves 4 and 5, and (c) Sample 3 (S3) Waves 1, 2, and 3. The mean intervals between the waves of data collection were 4.45 (W1–W2) and 4.49 (W2–W3) years. For terminological efficiency, the respective earliest wave of each sample became Wave 1 (W1 or baseline), the respective second wave became Wave 2 (W2), and the respective third wave became Wave 3 (W3). The design stipulated that whereas S3 participants could contribute data to all three waves, S1 and S2 participants contributed data to W1 and W2 (the third wave not available). Accordingly, the present W3 sample has a relatively larger representation of participants in their 60s and 70s and a relatively smaller representation of those in their 80s and 90s. This consideration is balanced by the advantage of testing genetic-health associations for memory across an accelerated longitudinal period of nearly 9 years (M = 8.9 years). Demographic information is presented in Table 1.
Table 1.
Participant Characteristics Categorized by Time Point
| W1 | W2 | W3 | |
|---|---|---|---|
| N Sample 1 | 54 | 45 | na |
|
| |||
| Sample 2 | 164 | 128 | na |
|
| |||
| Sample 3 | 352 | 295 | 272 |
|
| |||
| Total | 570 | 468 | 272 |
| Gender (% Women) | 65.3 | 64.7 | 67.6 |
| Age | 70.6 (8.69) | 74.7 (8.58) | 74.9 (7.30) |
| Range | 53.2–95.2 | 57.3–94.5 | 62.4–94.9 |
| Years between waves | - | 4.45 (.55) | 4.45 (.71) |
| Education | 15.3 (3.01) | 15.5 (3.05) | 15.5 (3.10) |
| Health to perfecta | 1.79 (.723) | 1.83(.719) | 1.84 (.814) |
| Health to peersb | 1.57 (.688) | 1.63 (.652) | 1.67 (.747) |
| Pulse Pressure (mm Hg) | 52.1 (11.4) | 55.3 (12.5) | 55.2 (12.4) |
| Range | 32.1 – 171.4 | 26.2 – 102.6 | 33.0 – 95.5 |
| Correlation with age | .441† | .417† | .362† |
| BMI (kg/m2) | 26.9 (4.25) | 26.6 (4.32) | 26.7 (4.50) |
| Range | 15.0 – 48.6 | 16.2 – 41.0 | 10.0 – 39.5 |
| Correlation with age | −.047 | −.051 | −.086 |
| ApoE (ε2ε2, ε2ε3) n (%) | 72 (12.6) | 59 (12.6) | 38 (14.0) |
| (ε3ε3) | 355 (62.3) | 297 (63.5) | 166 (61.0) |
| (ε4ε3, ε4ε4) | 143 (25.1) | 112 (23.9) | 68 (25.0) |
| Smoking Status (%) | |||
| Present | 4.2 | 3.0 | 1.1 |
| Previous | 53.0 | 53.4 | 53.6 |
| Never | 42.8 | 43.6 | 45.3 |
| Alcohol Use (%) | |||
| Presently | 88.8 | 89.5 | 89.6 |
| Previous | 4.0 | 8.0 | 8.6 |
| Never | 7.2 | 2.4 | 1.9 |
Note. Results presented as Mean (Standard Deviation) unless otherwise stated. Age and education presented in years. Smoking and drinking status are reported in percentages of participants who responded to the question.
Self-reported health relative to perfect.
Self-reported health relative to peers. Self-report measures are based on 1 “very good” to 5 “very poor”.
na = data not yet collected.
p < .01.
Given the necessity for both genetic and longitudinal data in this study, these factors defined the initial opportunity in sample recruitment. VLS genotyping occurred in the 2009–2011 period and was limited by funding arrangement to about 700 continuing VLS participants. After initial evaluations, the eligible source sample consisted of 683 participants with genetic data. Several exclusionary criteria were then applied to this source sample: (a) a diagnosis of Alzheimer’s disease or any other dementia, (b) a Mini-Mental Status Exam (MMSE; Folstein, Folstein, & McHugh, 1975) score of less than 24, (c) a self-report of “severe” for potential comorbid conditions (e.g., epilepsy, head injury, depression), (d) a self-report of “severe” or “moderate” for potential comorbid diseases such as neurological conditions (e.g., stroke, Parkinson’s disease), and (e) EM or SM data missing from two or more waves. The remaining sample with full genetic data at W1 consisted of 600 adults. Due to the conflict between the reported protective effect of ε2 on memory and the reported risk associated with ε4, we wished to assess the independent effect of ε2 and ε4. Therefore, adults with ApoE genotype ε2/ε4 (n = 30) were removed. Consequently, W1 consisted of 570 adults, including 372 women and 198 men, (M age = 70.6 years, SD = 8.69, range 53.2 – 95.2). W2 consisted of 468 adults, including 303 women and 165 men, (M age = 74.7 years, SD = 8.58, range 57.3 – 94.5). W3 consisted of 272 adults, including 184 women and 88 men, (M age = 74.9 years, SD = 7.30, range 62.4 – 94.9). In this accelerated longitudinal design, a total of 257 adults contributed data to all three waves, 211 adults contributed to W1 and W2 only, 15 adults contributed to W1 and W3 only, and 87 adults contributed to W1 only. The retention rates for each available and defined interval are as follows (a) S1 W1-W2 = 83%; (b) S2 W1-W2 = 78%; (c) S3 W1-W2 = 84%, (d) S3 W2-W3 = 92%, and (e) S3 W1-W3 = 77%. Attrition effect analyses were conducted based on the sample for each of the four adjoining waves on nine background and outcome variables. Demographic and cognitive characteristics were similar between returning and non-returning participants. Specifically, of the 36 analyses conducted, two (6%) were associated with better health outcomes for the non-returning participants and five (14%) were associated with poorer cognitive outcomes for the non-returning participants. As noted, defined intervals are determined by availability, which in this instance is limited only by data collected and processed in this ongoing longitudinal study. For these analyses list-wise deletion was not used; instead, all missing data for pulse pressure, age, and EM and SM factor scores were estimated by multiple imputations using Mplus 7 (Muthén & Muthén, 2010). As per practice in the VLS lab, 50 imputations of the data set were generated and pooled for further analyses (for further description of imputations and pooling (see Enders, 2011; Graham, Olchowski, Gilreath, 2007; Little, 2013; Muthén & Muthén, 2010; Rubin, 1987).
Memory Measures
The EM and SM tests used for the current study have been widely used and documented within the VLS (and other studies), with established measurement and structural characteristics and demonstrated sensitivity to health and neurocognitive factors in various older adult populations (e.g., Anstey, 2012; Dixon et al., 2012; Dixon et al., 2004; Josefsson et al., 2012; Kalpouzos & Nyberg, 2012; MacDonald, DeCarlo, & Dixon, 2011).
Word recall
This EM task consisted of immediate free recall of two lists of 30 English words selected from the total set of six structurally equivalent (but content diverse) lists (Dixon et al., 2004). The word recall task was administered in a rotated design so as to eliminate context-related practice effects. Over three waves no participant sees the same list twice. Each list consisted of 6 words from each of five taxonomic categories (e.g., birds, flowers) typed on a single page in unblocked order. Participants were given 2 min to study each list and 5 min to write as many words as they could recall. The number of correctly recalled words averaged across the two lists was used for analysis.
Rey auditory verbal learning (REY)
This EM task was used to assess verbal learning and memory (Lezak, 1983; Vakil & Blachstein, 1993). The participant listened to 15 nouns read aloud and immediately after recalled aloud as many of these nouns as possible. This was repeated for 5 trials with the same list (A1–A5). Then a second list (B1) of 15 unrelated nouns was read aloud to the participant and immediate recall was required. Finally, the participant was asked to recall the first list (A6). List B1 was used to measure free recall and A6 was used to measure recall after interference. The number of nouns recalled from B1 and A6 were used.
Fact recall
This SM task consisted of six sets of 40 equivalent but different general information questions (e.g., What is the last name of the author of the book 1984?) that were content balanced in terms of science, history, art, sports, geography and entertainment (Nelson & Narens, 1980). The fact recall task was administered in a rotated design so as to eliminate context-related practice effects. Over three waves no participant sees the same list twice. Participants answered two sets of questions per testing session and the task was self-paced. The correct responses from each of the two tests (fact recall 1, fact recall 2) were used for analysis.
Vocabulary
This SM task used 54 multiple choice items (recognition) taken from the Kit of Factor Referenced Cognitive Tests (Ekstrom, French, Harman, & Dermen, 1976). Participants were given 15 minutes to choose the word that most closely matched the meaning of the presented word. The number of correctly recognized words was used for analyses.
Pulse Pressure (PP)
PP, a reliable proxy of the arterial stiffness aspect of vascular health, is calculated as follows: PP = systolic blood pressure – diastolic blood pressure. For all analyses PP was used as a continuous variable and was centered at the sample mean of 52.0 mm Hg. Increases in PP are considered to be an indication of decrease in vascular health. The current study was designed to examine typically aging adults and therefore we included those adults reporting relatively high blood pressure and blood pressure medication use. However, cases of each were relatively rare. Serious high blood pressure was reported at baseline by 4 participants (0.7% of the sample) and blood pressure medication use was reported by 153 adults (28% of the sample). More than 90% of participants’ actual blood pressure levels were considered normal or pre-hypertensive. (See Table 2 for a comparison of actual blood pressure levels in this sample.)
Table 2.
Blood pressure levels across time
| W1 | W2 | W3 | ||
|---|---|---|---|---|
| Systolic Blood Pressure
|
||||
| Hypotension | < 90 mm Hg | 1 (.2%) | 1 (.2%) | 0 |
| Normal and Prehypertension | 90 – 139.9 mm Hg | 453 (82.4) | 360 (78.4%) | 209 (80.7%) |
| Stage 1 Hypertension | 140 – 159.9 mm Hg | 87 (15.8%) | 88 (19.2%) | 43 (16.6%) |
| Stage 2 Hypertension | ≥ 160 mm Hg | 9 (1.6%) | 10 (2.2%) | 7 (2.7%) |
| Diastolic Blood Pressure
|
||||
| Hypotension | < 60 mm Hg | 32 (5.8%) | 39 (8.5%) | 21 (8.1%) |
| Normal and Prehypertension | 60 – 89.9 mm Hg | 485 (88.2%) | 404 (88.0%) | 231 (89.2%) |
| Stage 1 Hypertension | 90 – 99.9 mm Hg | 28 (5.1%) | 14 (3.1%) | 7 (2.7%) |
| Stage 2 Hypertension | ≥ 100 mm Hg | 5 (.9%) | 2 (.4%) | 0 |
DNA Genotyping
As described in previous studies (e.g., McFall et al., 2013), the VLS collects saliva according to DNA Genotek technology, including all recommended practices for biofluid collection, stabilization, and preparation. Specifically, saliva was stored at room temperature in the Oragene® disks until DNA extraction. DNA was manually extracted from 0.8 ml of saliva sample mix using the manufacturer’s protocol with adjusted reagent volumes. All standard preparation DNA extraction procedures were strictly followed. Genotyping was carried out by using a PCR-RFLP strategy to analyze the allelic status for ApoE (determined by the combination of the SNPs rs429358 and rs7412). All specified amplification, RFLP analysis, and confirmation procedures were applied. For genetic analyses, the ApoE genotypes were categorized according to the presence or absence of either the ε2 or the ε4 allele to test for protection or risk effects. Specifically, three groups were used: (a) ε2+, consisting of ε2/ε2, ε2/ε3; (b) ε3, consisting of ε3/ε3; and (c) ε4+, consisting of ε4/ε4, ε3/ε4. The number of adults in each group was 72 for ε2+, 355 for ε3, and 143 for ε4+. APOE genotypes were clustered according to the presence of the risk ε4 genotype for Hardy-Weinberg equilibrium analyses (i.e., ε4+/ε4+, ε4+/ε4−, ε4−/ε4−). Results of these analyses showed that the frequency distribution was well within population norms (χ2 = .5 (1), p > .05).
Statistical Analyses
Analyses pertaining to our research questions included confirmatory factor analysis and latent growth modeling. Statistical model fit for all analyses was determined using standard indexes: (a) χ2 for which a good fit would produce a non-significant outcome (p > .05) indicating that the data are not significantly different from the estimates associated with the model, (b) the comparative fit index (CFI) for which fit is judged by a value of ≥ .95 as good and ≥ .90 as adequate, (c) root mean square error of approximation (RMSEA) for which fit is judged by a value of ≤ .05 as good and ≤ .08 as adequate, and (d) standardized root mean square residual (SRMR) for which fit is judged by a value of ≤ .08 as good (Kline, 2011). Specific applications are described in the following research goal sections.
Analyses for RG 1: How are EM and SM best represented in regard to latent variable models and invariance testing across three waves?
We used Mplus 7 (Muthén & Muthén, 2010) to conduct confirmatory factor analysis. Using six declarative memory measures, we tested two models (a) a two-factor declarative memory model consisting of EM (word recall, REYB1 [free recall], REYA6 [recall after interference]) and SM (fact recall 1, fact recall 2, vocabulary), (b) a single EM and a single SM model outside the latent declarative memory construct. We then tested standard longitudinal (three-wave) measurement invariance for an EM model and an SM model. First, in order to determine if the same declarative memory measures represent the latent variables at each wave of data collection, we tested configural invariance (Con), for which the same indicator variables load onto the latent variable used to test the model across time. Second, in order to determine if the same construct is being measured we tested metric invariance (Met), for which factor loadings are constrained to be equal for each latent variable. Third, in order to determine if we can compare mean differences evident at the latent level we tested scalar invariance (Scal), for which indicator intercepts are constrained to be equal. Fourth, in order to determine if group differences are based on common variability we tested residual invariance (Res), for which indicator residuals are constrained to be equal accounting for error variability. We estimated factor scores for EM and SM in Mplus, which were then used in subsequent latent growth models. In addition, for all further analyses, we used multiple imputations to estimate missing values for PP, age, EM, and SM factor scores. By VLS procedures, 50 datasets were generated and pooled before analyses were conducted.
Analyses for RG 2: How do EM, SM, and PP change across time?
In order to examine change patterns for these three latent variables we used latent growth modeling. We coded age as a continuous variable and computed latent growth models with age as the metric of change rather than wave. All growth model analyses are therefore based on individual-varying age across a maximum of 9 years and a resulting accelerated longitudinal design that spans approximately 40 years. Age was centered at 75 years of age, as this was the approximate center point of the 40-year band of data (i.e., 53–95 years) and because 75 is a meaningful time point in cognitive aging (Dixon et al., 2012; Small et al., 2011). To identify the functional form of change, we determined the best-fitting unconditional growth model by testing in sequence: (a) a fixed intercept model, which assumes no inter- or intraindividual variation; (b) a random intercept model, which models interindividual variability in overall level, but no intraindividual change; (c) a random intercept fixed slope model, which allows interindividual variation in level, but assumes that all individuals change at the same rate; and (d) a random intercept random slope model, which models interindividual variation in initial level and change; (Singer & Willett, 2003).
Analyses for RG 3 and RG 4: How are the EM and SM performance patterns in older adults affected independently by PP and ApoE (RG3) and interactively by ApoE x PP (RG4)?
Using the best unconditional growth models identified for EM and for SM, we ran EM and SM as parallel processes in order to examine the differences our predictors might have on these two declarative memory domains. First, we tested two independent effect models by adding ApoE genotype and then PP, measured at W1, each as a predictor of EM and SM intercept and slope. Second, in order to test the interactive effects of ApoE and PP on EM and SM, we added PP as a predictor using three ApoE (ε2+, ε3, ε4+) genotype groups (see McArdle & Prescott, 2010). Based on the outcome of the three genotype group model, we wished to test a model consisting of a group of participants with at least one ε2 allele and a group of participants with no ε2 allele. Therefore, we tested the three-group constrained model that constrained the PP regression estimates to be equal for the ε3 and the ε4+ groups. If the three-group constrained model was not significantly different from the previous three-group unconstrained model, we could infer that the ε3 and ε4+ group profiles were similar and resulted in a model testing ε2+ versus ε2−.
Results
RG 1: How are EM and SM best represented in regard to latent variable models and invariance testing across time?
For simplicity of reporting, all model goodness of fit indices and abbreviations are in Table 3. The two-factor declarative memory model fit the data adequately. We tested a single-factor EM model consisting of word recall, REYA6, and REYB1 and then a single-factor SM model consisting of fact recall 1, fact recall 2, and vocabulary (see Table 3). Both of these models fit the data adequately. We then conducted invariance testing for the EM-Con model and the SM-Con model based on Δχ2 tests. Overall, we observed configural and metric invariance for the single-factor EM model and configural and metric invariance for the single-factor SM model (see EM-Con, EM-Met, SM-Con, SM-Met models in Table 3). Both models had partial scalar invariance by methodological design (i.e., to calculate factor scores for EM, word indicator intercepts were constrained; for SM, fact recall 1 intercepts were constrained), indicating that EM (except for word) and SM (except for fact recall 1) exhibited indicator mean differences across time outside of the latent differences. Across time the resulting models measured the same EM and SM construct, the same manifest variables marked EM and marked SM, and due to partial scalar invariance, latent variable means were comparable. According to the standard invariance testing protocol, because full scalar invariance was not observed, we did not test residual invariance.
Table 3.
Goodness of Fit Indexes for Memory Confirmatory Factor Analysis Models and Measurement Invariance Testing
| Model | AIC | BIC | χ2 | df | p | RMSEA | CFI | SRMR | Δχ2 | Δdf |
|---|---|---|---|---|---|---|---|---|---|---|
| Two-factor Memory | 46718.74 | 46995.74 | 456.77 | 126 | <.001 | .066 (.060–.073) | .941 | .100 | ||
| EM-Con | 24409.70 | 24581.17 | 16.13 | 15 | .373 | .011 (.000–.041) | .999 | .016 | ||
| EM-Met Equal indicator loadings |
24415.06 | 24568.95 | 29.50 | 19 | .059 | .030(.000–.051) | .994 | .041 | 13.37 | 4* |
| EM-Scal Equal intercepts |
24666.80 | 24794.31 | 293.24 | 25 | <.001 | .134 (.120–.148) | .858 | .108 | 263.74 | 6 |
| EM-PScalab Partial equal intercepts |
24488.23 | 24633.33 | 106.67 | 21 | <.001 | .082 (.067–.098) | .955 | .082 | 77.18 | 2 |
| SM-Con | Not positive definite | |||||||||
| SM-Cona | 22308.21 | 22457.70 | 120.35 | 20 | <.001 | .091 (.076–.108) | .971 | .080 | ||
| SM-Met b Equal indicator loadings |
22303.77 | 22435.68 | 123.91 | 24 | <.001 | .083 (.069–.098) | .971 | .073 | 3.56 | 4 |
| SM-Scal Equal intercepts |
22311.46 | 22425.78 | 139.60 | 28 | <.001 | .082 (.068–.095) | .968 | .082 | 15.69 | 4* |
| SM-PScal Partial equal intercepts |
22313.98 | 22437.09 | 138.12 | 26 | <.001 | .085 (.071–.099) | .968 | .080 | 14.21 | 2 |
Note. AIC = Akaike information criteria. BIC = Bayesian information criteria. RMSEA = Root Mean Square Error of Approximation. CFI = Comparative Fit Index. SRMR = Standardized Root Mean Square Residual. EM = episodic memory. SM = semantic memory. Con = configural. Met = metric. Scal = scalar. PScal = partial scalar.
First indicator intercepts constrained for this and all consequent models.
Best fitting model used for Factor Score Analysis.
p < .05.
p < .001.
RG 2: How do EM, SM, and PP change across time?
EM and SM
In order to determine how EM and SM changed across time we used latent growth modeling independently for EM and SM. Using age as the metric of change, latent growth models were tested using the estimated EM and SM factor scores. The best fitting unconditional growth model for EM was established as a random intercept, random slope latent growth model (see Table 4). The best fitting unconditional growth model for SM was established as a random intercept, fixed slope latent growth model. We then tested the best fitting models for EM and SM as a parallel process model. This model fit the data well and was used for all subsequent models (see Table 4). Specifically, adults varied significantly in their EM (b = 1.203, p < .001) and SM performance at age 75 (b = 1.034, p < .001). There was significant 9-year decline in EM performance (M = −.012, p < .001) and subjects exhibited significant individual differences in rate of EM decline (b = .001, p < .001). There was no significant 9-year decline in SM performance (b = −.005, p >.05) and this pattern was the same for all adults, as indicated by the non-significant random slope model. Due to the potential modification of SM by PP and ApoE, as evidenced by the observed variability around SM performance at age 75, we include SM in all further analyses.
Table 4.
Goodness of Fit Indexes for Memory and Pulse Pressure Latent Growth Models
| Model | −2LL | AIC | BIC | D | Δdf | |
|---|---|---|---|---|---|---|
| EM | Fixed intercept | 3920.24 | 3928.25 | 3945.84 | - | |
| Random intercept | 2013.42 | 2023.42 | 2045.41 | 1906.82 | 1╪ | |
| Random intercept Fixed slope | 1941.12 | 1953.12 | 1979.50 | 72.30 | 1╪ | |
| Random intercept Random slopea | 1416.36 | 1432.36 | 1467.36 | 524.76 | 2╪ | |
| SM | Fixed intercept | 3850.78 | 3834.78 | 3843.57 | - | |
| Random intercept | 477.62 | 483.62 | 496.81 | 7373.16 | 1╪ | |
| Random intercept Fixed slopea | 320.84 | 328.83 | 346.42 | 156.78 | 1╪ | |
| Random intercept Random slope | −420.32 | −408.31 | −381.93 | 741.16 | 2╪ | |
| EM PAR SMb | 1314.22 | 1346.22 | 1415.76 | |||
|
| ||||||
| PP | Fixed intercept | 5517.56 | 5522.56 | 5539.95 | - | |
| Random intercept | 4693.43 | 4914.13 | 4935.86 | 710.82 | 1╪ | |
| Random intercept Fixed slopea | 4608.36 | 4620.36 | 4646.44 | 295.76 | 1╪ | |
| Random intercept Random slope | 4567.86 | 4583.86 | 4618.62 | 40.50 | 2╪ | |
Note. −2LL = −2 log likelihood. AIC = Akaike information criterion. BIC = Baysian information criterion. D = deviance statistic. df = degrees of freedom. EM = episodic memory. SM = semantic memory. PP = pulse pressure.
Best fitting model.
Best fitting EM and SM model as parallel processes.
p < .001.
Pulse Pressure (PP)
To determine how PP changed across time we used latent growth modeling. The best fitting unconditional growth model for PP was established as a random intercept, fixed slope latent growth model (see Table 4). Specifically, PP levels were significantly different from the group mean at age 75 (M = .295, p < .001) and subjects differed significantly in their PP levels at age 75 (b = .811, p < .001). There was significant 9-year increase in PP (M = .055, p < .001) for all adults, as indicated by the non-significant random slope model.
RG 3: How are the EM and SM performance patterns in older adults affected independently by PP and ApoE?
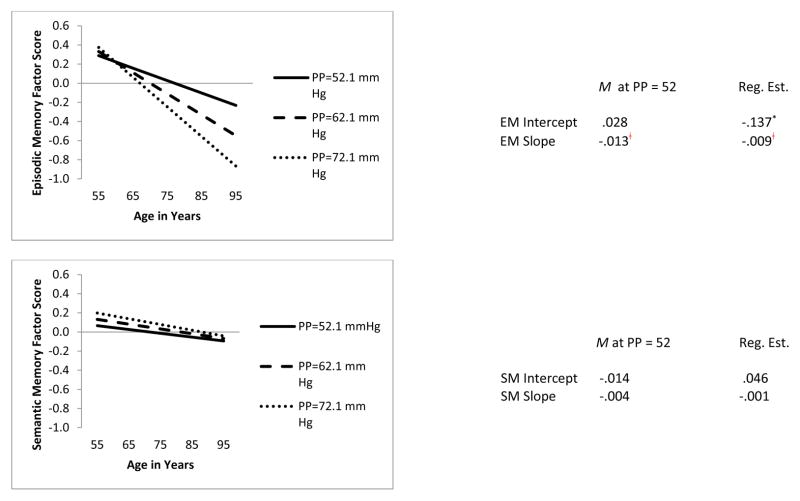
To determine the independent effects of PP and ApoE on EM and SM performance patterns, we tested two conditional growth models with PP as a predictor of EM and SM level and change and one conditional growth model with ApoE as a predictor of EM and SM level and change. First, we tested the effect of time-varying PP on time-varying EM and SM. This time-varying model was not identified. Second, we tested a model in which baseline level of PP (at W1) was used as predictor of time-varying EM and SM (see Figure 1). The baseline PP model resulted in two significant findings for EM. Baseline level of PP significantly predicted both (a) level of EM performance at age 75 (b = -.125, p < .05) and (b) rate of 9-year EM change (b = -.008, p < .001). Specifically, persons with the mean level of baseline PP (i.e., PP = 52.0 mm Hg) performed better on EM tasks (M = .033) at age 75 than persons with PP 10 mm Hg and higher (M = -.092). In addition, those with the mean level of PP at baseline exhibited less 9-year decline (M = -.013) than those with PP 10 mm Hg and higher (M = -.021). In contrast, baseline PP did not predict level of SM performance at age 75 (p > .05). Third, we tested a growth model using ApoE (ε2+, ε3, ε4+) and observed patterns of EM and SM level at age 75 and rate of EM change. This model did not significantly predict EM or SM performance at age 75 years (p > .05) nor did it significantly predict rate of 9-year EM change (p > .05).
Figure 1.
Predicted growth curve for declarative memory factor scores (episodic memory, semantic memory) using pulse pressure (PP, measured in mm Hg) at W1 as a predictor with age as a continuous variable centered at 75 years. −2 Log Likelihood = 1276.38. Akaike Information Criteria = 1316.37, Bayesian Information Criteria = 1403.28, Parameters Free = 20.
* p < .05. ╪ p < .001.
RG 4: How are the EM and SM performance patterns in older adults affected interactively by ApoE x PP?
In order to examine our moderation hypothesis, we tested two models. First, the three-group unconstrained model used baseline PP to predict (a) level of EM and SM at age 75, and (b) 9-year change in EM. The predictions were again based on three ApoE groupings: (a) ε2+, consisting of ε2ε2, ε2ε3; (b) ε3, consisting of ε3ε3; and (c) ε4+, consisting of ε4ε4, ε3ε4. Second, the three-group constrained model was nested within the first model and used baseline PP to predict (a) level of EM and SM at age 75, and (b) 9-year change in EM based on the same three ApoE groupings, but with PP regression estimates constrained to be equal for the ε3 and ε4+ groups. This second model was tested based on the observed EM performance and change patterns. Specifically, we observed similarities between the ε3 and the ε4+ groups and distinctly different pattern associated with the ε2+ group.
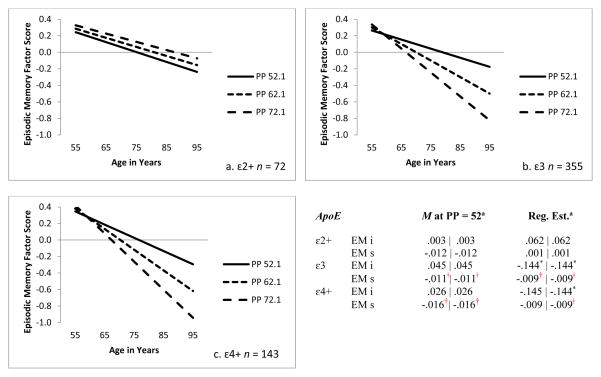
For the three-group unconstrained model, we observed differential EM patterns within the three ApoE groups, confirming the moderation hypothesis. First, for the ε2+ group PP did not significantly predict either level of EM performance at age 75 years or 9-year change in EM (ps > .05). Second, for the ε3 group, baseline level of PP significantly predicted both level of EM performance at age 75 (b = -.144, p < .05) and 9-year change in EM (b = -.009, p < .001). Third, for the ε4+ group PP did not significantly predict either level of EM at age 75 (b = -.145, p >.05) or 9-year change in EM (b = -.009, p >.001). Based on the observation of similar regression weight values for ε3 group and the ε4+ group we next tested the three-group constrained model. As expected, the constrained model was not significantly different from the unconstrained model (Δ-2LL = .194, Δdf = 2, p > .10). However, the constrained model also resulted in differential ApoE group findings. First, for the ε2+ group, PP did not significantly predict level of EM performance at age 75 years (p > .05) or 9-year change in EM (p > .05). Second, for the ε3 group, baseline level of PP significantly predicted both level of EM performance at age 75 (b = -.144, p < .05) and 9-year change in EM (b = -.009, p < .001). Third, for the ε4+ group baseline level of PP similarly predicted both level of EM performance at age 75 years (b = -.144, p < .05) and 9-year change in EM (b = -.009, p < .001). Taken together, these results indicate that PP at baseline moderates the relationship ApoE has with EM for ε3 or ε4+ carriers, but not for ε2+ carriers. This interaction, demonstrating moderation by PP for the ApoE genotype-EM association, is displayed in Figure 2. As can be seen in the figure (2a), EM performance for ε2 carriers is not affected by PP levels: There are no detectable memory performance differences and the change patterns across PP level are relatively modest. For ε3 carriers (2b), the lower PP level subgroup shows better mean performance and more shallow change patterns than the two higher vascular risk subgroups. For the ε4 carriers (2c), a similar pattern is observed; although each of the subgroups appears to have somewhat steeper slopes than the corresponding ε3 groups, the differences between the two patterns are not significant. Finally, regarding SM, baseline level of PP did not significantly predict level of SM at age 75 for any ApoE subgroup (p > .05).
Figure 2.
Predicted growth curve for episodic memory factor scores by ApoE genotype group using baseline pulse pressure (mmHg) as a predictor. ApoE grouping = ε2+ (ε 2ε2, ε2ε3), ε3 (ε3ε3), ε4+ (ε4ε3, ε4ε4). ApoE grouping Model 2 = ε2 + (ε2ε2, ε2ε3), ε (ε3ε3), ε4+ (ε4ε3, ε4 ε4) with ε3 constrained to be equal to ε4+. 2 Log Likelihood = 1237.92; Akaike Information Criteria = 1357.92; Bayesian Information Criteria = 1618.66. a Model 1 | Model 2. i = intercept. s = slope.
* p < .05. † p < .01. ╪ p < .001.
Discussion
The aim of this research was to examine independent and interactive effects of a prominent and modifiable vascular health indicator (PP) and a key genetic polymorphism (ApoE) on performance and change patterns of memory across three waves (9 years) of longitudinal data for a group of older adults (spanning a 40-year age band). For Research Goal 1 (i.e., EM and SM latent variable models and invariance testing across three waves), we observed two main findings. First, two single-factor models of EM and SM provided the best fit for the three waves of data (see Table 3). These results confirm that declarative memory can be usefully characterized in terms of two separate but related systems at the latent variable level, and that this might in part account for the frequently observed different performance patterns across adulthood (Nyberg et al., 2003, 2012; see also Tulving, 1987). Second, both EM and SM demonstrated configural, metric and partial scalar invariance. Establishing invariance across waves for EM and SM formally permitted us to conduct longitudinal analyses.
For Research Goal 2 (i.e., latent growth models for EM, SM, and PP), we observed several interesting findings. First, EM and SM exhibited different patterns of variability and change (Figure 1). Regarding the growth of EM, subjects exhibited (a) significant variability in EM performance around the centering point of 75 years, (b) significant 9-year EM decline, and (c) significant individual differences in rate of EM decline (Nyberg et al., 2012; Small et al., 2011). Regarding the growth of SM, subjects exhibited (a) significant variability in SM performance around the centering point of 75 years, (b) no significant 9-year SM decline, and (c) a consistent pattern for all subjects. Although relatively few studies of the aging of declarative memory have included latent variables of both EM and SM (Dixon et al., 2012; Nyberg et al., 2003; Wilson et al., 2002), the observed patterns are consistent with these and are similar to those observed with other approaches (e.g., Small et al., 2011). The SM patterns observed are consistent with other research in regard to interindividual differences associated with SM (MacDonald et al., 2011) and less decline than for EM (Nilsson et al., 2006). The contrasting longitudinal patterns for these two domains suggest that declarative memory variability may be differentially dependent on primary or secondary aging factors such as protective- or risk-related factors of biological vulnerability, health burden, or lifestyle choices (Anstey, 2012; Josefsson et al., 2012; Nilsson et al., 2006; Nyberg et al., 2012). Specifically, EM may be affected not only directly by biological factors, but also indirectly by health factors such as PP that exacerbate the extent of deleterious influence from the declining neurobiological substrate. SM is more dependent on secondary aging factors and may be protected by accumulating and supported environmental factors (e.g., education, cognitive activities), many of which may decline in effectiveness with aging.
For this research goal we also tested a growth model related to PP, known to reflect arterial aging. We found that older adults (a) differed significantly in their levels of PP at age 75 years, (b) exhibited a significant increase in PP across the 9-year follow-up period, and (c) showed the same pattern of change over the three waves. This aging-related increase in PP is consistent with other research, indicating general vascular health decline with aging (Dahle et al., 2009; Dart & Kingwell, 2001; Davenport, Hogan, Eskes, Longman, & Poulin, 2012; Franklin et al., 1997; Morra, Zade, McGlinchey, & Milberg, 2013; Raz et al., 2011).
For Research Goal 3, we tested conditional growth models to determine the independent effects of PP and ApoE on EM and SM performance and change. Several key results were observed. First, higher baseline level of PP was associated with both (a) lower levels of EM performance at age 75, and (b) more EM decline over the 9-year period. Second, baseline PP had no effect on either centering level of SM or change in SM, although the latter may be due to the fact that no reliable 9-year change was found for SM. This supports past research demonstrating relatively small effects of health factors on SM performance (e.g., Elias et al., 2004; Nilsson et al., 1997; see also Sternäng et al., 2009) Third, although time-varying PP exhibited significant 9-year increase, there was no effect of PP change on time-varying EM. Instead, initial level of PP accounted for the differential effect on EM change. Fourth, ApoE exhibited no main effects on either EM or SM level or 9-year change. Previous research on ApoE and memory performance in aging has produced somewhat inconclusive patterns. Our findings support studies that have reported no independent effects of ApoE on EM (Bender & Raz, 2012; Bunce, Anstey, Burns, Christensen, & Easteal, 2011; Ferencz et al., 2013; Raz et al., 2009; Sternäng et al., 2009). In contrast, other research has reported independent ApoE effects on EM performance (Laukka et al., 2013: Wilson et al., 2002). Wilson and colleagues (2002) found that ε2 carriers exhibited consistent (or even improved) EM performance over three years, whereas ε3 carriers exhibited slight EM decline, and ε4 carriers exhibited the steepest decline. These inconsistent findings may be a function of differences between study designs. First, ApoE may independently affect performance on some memory tasks and not others, making the inconsistencies dependent on task differences across studies (Wisdom et al., 2011). Second, independent effects of ApoE may be influenced by other study-specific factors such as participant age (i.e., heritability may increase with age), health or environmental factors that may have previously influenced risk allele carriers, or differences in other risk alleles in combination with ApoE (Harris & Deary, 2011; Plassman et al., 2010; Wisdom et al., 2011).
For Research Goal 4, we tested the hypothesis that the ApoE x PP interaction would have an effect on EM and SM, indicating a moderation effect. Although there were no main effects of ApoE on EM and SM, the gene x health interactions based on three ApoE groups (i.e., ε2+, ε3, and ε4+) and PP level showed differential effects on EM (see Figure 2). In general, adults with centering level of PP (i.e., 52 mm Hg) for any of the three ApoE groups (but especially the non-ε2+ groups) were similar in level of EM at age 75 and experienced more shallow negative change slopes than their genotype counterparts with higher levels of PP. The patterns of EM change across level of vascular health were differentiated by ApoE status. First, for the ε2+ group, EM performance and 9-year pattern showed some decline over the 9-year period, but neither performance at age 75 nor rate of decline was affected by level of PP. Second, the ε3 and ε4+ groups performed at significantly lower average levels of EM at age 75 and displayed more 9-year decline in EM, increasingly so as PP levels were elevated. In fact, the fan patterns demonstrated exacerbating effects of worsening PP on EM change, and these were in contrast to the tight parallel patterns for the ε2+ group.
Overall, these results support and extend past work regarding the protective ε2 allele, showing that it may also moderate memory deficits and decline associated with increases in vascular risk represented by elevated PP (Deary et al., 2004; Fotuhi et al., 2009; Small et al., 2004; Verghese, Castello, & Holtzman, 2011). In contrast, those adults who do not have the ε2 allele continue to be at risk – in fact increased risk – with higher levels of PP. Less directly, perhaps, the present results may have implications for recent reports concerning the synergistic negative effect of ApoE ε4 and decreased cardiovascular health (Bender & Raz, 2012;). Specifically, these authors reported no main effects of ApoE ε4 or PP on EM but that higher PP in a group of ε4+ carriers resulted in lower levels of EM. Other researchers reported that any cardiovascular risk factor (e.g., hypercholesterolemia, type 2 diabetes, high systolic blood pressure) in the presence of the ε4 allele resulted in exacerbated age-related memory decline (Caselli et al., 2011; Zade et al., 2010). Similarly, Yasuno and colleagues (2012) found that ε4 carriers who were hypertensive experienced more decline for a cognitive composite scale of attention, memory, language, and reasoning. Research with older ε2 carriers is limited, likely due to the low prevalence of ε2 carriers in the normal population (see Sternäng & Wahlin, 2011). Our data indirectly support the hypothesis of ε2 as a protective allele in that the lack of an effect associated with PP for the ε2 group was quite robust across elevated risk levels of vascular health, which, under other allelic conditions, had pernicious effects on episodic memory in aging. The present results also support intervention studies that report improvements for ε4 carriers, but not for ε2 carriers, when exposed to increased levels of docosahexaenoic acid (found in fish oil; Vandal et al., 2014) or increased physical activity (Ferencz et al., 2014).
Along with the possibility of preserved brain function for older ε2 carriers, the potentially protective effects of the ε2 allele, even in the presence of high PP, may be due to increased levels of the protein apolipoprotein E and therefore an increased ability to repair neuronal damage associated with neurobiological aging and decreased vascular health. Carriers of the ε3 or ε4 alleles may have the added disadvantage of less apolipoprotein E to repair neural damage associated with aging, including reduced hippocampal integrity and increased amyloid burden (Raz et al., 2005; Rodrigue et al., 2013). Mechanisms to explain the relationship of apolipoprotein E to cognition and AD are being investigated. As noted, delineating origins of both the deleterious effects of ε4 and the protective effects of ε2 is of paramount importance. Decreased levels of brain apolipoprotein E associated with ε4 cause an initial vulnerability (i.e., BBB breakdown) in a cognitive decline cascade (Bell et al., 2014; Mahley et al., 2009). This cascade can be perpetuated or exacerbated by genetic, metabolic, or environmental risks, including aging, inflammation, or arterial stiffening. The presence of a ε2 allele, with higher levels of apolipoprotein E in the brain, has less such vulnerability and would therefore confer protection. Our findings are concordant with these possibilities. We showed that the EM performance was sustained at relatively high levels for ε2 carriers even in the presence of higher levels of PP. This may be associated with the thinner, and therefore more flexible, arterial walls that are observed in ε2 carriers. The vascular flexibility decreases the deleterious effects of arterial stiffening and increases the permeability at the BBB allowing a more beneficial exchange of lipids and Aβ-lipid complexes.
There are several limitations and strengths associated with this study. First, although a direct measure of arterial stiffness (pulse wave velocity) is not available in the VLS, a well-established proxy (PP) is based on measures of systolic and diastolic blood pressures. Despite the significant ApoE x PP interaction showing differential effects on EM, future research could examine a broader representation of normal and clinical vascular health measures. Second, the present sample is relatively large and covers three waves over about 9 years, but a design characteristic mentioned earlier should be noted again, as it affected sample size and age characteristics of W3. The design characteristic is that at the time of this study (a) S1 and S2 had not yet been tested on their corresponding W3, and (b) only S3 contributed to W3. Attrition rates for each definable interval (two waves of data on the same sample) were reported and excellent—and the accelerated longitudinal approach was successful—but a more complete design would have included some W3 participants from all three samples. Notably, however, this design characteristic did not affect the results: From the invariance testing to the change-related analyses, the 3-wave data were quite informative. Third, a 40-year band of aging is reported in this study, but individuals provide data across 9 years. However, the accelerated longitudinal design utilized for this study may alleviate some of the methodological issues (e.g., cohort effects) associated with examining cross-sectional data within older adults and, especially, across wider age group gaps. Fourth, this study is designed to evaluate the effects of a genetic and vascular health factor in a relatively normal older adult sample (see Table 2). To represent typical aging, we deliberately included older adults with varying levels of blood pressure and even self-reported hypertension medication. In general, at intake, the VLS samples are designed to be relatively healthy (e.g., free of known neurodegenerative disease), community dwelling, and broadly educated. Although this group may not be representative of all older adults, it may represent a conservative estimation of the moderation effects of health factors (i.e., aspects of vascular health) on gene-cognition relationships.
There are also several strengths associated with this study. First, we used contemporary statistical approaches to analyze a series of research goals that systematically built the case for the final set of analyses. Second, we examined the effect of continuously measured age in an accelerated longitudinal design that allowed us to determine the effects of PP and ApoE across three data collection points spanning about 9 years. Third, our sample was relatively large (i.e., W1 n = 570) and well-characterized. That this group comprised a band of 40 years is important to note. Fourth, we investigated two latent declarative memory variables, EM and SM, composed of six standard manifest neuropsychological variables.
In conclusion, the goal of this study was to examine the independent and interactive effects of vascular health, as measured by PP and ApoE on EM and SM level for both (a) a centering age of 75 years and (b) change across 9 years. Although decreased vascular health negatively influenced EM, the ApoE ε2 allele provided protection from decrements in EM associated with aging and decreased vascular health. In addition, in the absence of an ε2 allele, the effects of decreased vascular health are not mitigated. This suggests that maintenance of vascular health is even more important for adults who possess less favorable allelic combinations of ApoE.
Acknowledgments
The present research is supported by grants from (a) the National Institutes of Health (National Institute on Aging; R01 AG008235) to author RAD and (b) the Alberta Health Services (University Hospital Foundation) to authors DW, JJ, and RAD. Both RAD and DW are also supported by the Canada Research Chairs program. LB is supported by grants from the Swedish Research Council, the Swedish Research Council for Health, Working Life, and Welfare, Swedish Brain Power, an Alexander von Humboldt Research Award, and a donation from the af Jochnick Foundation. We thank the volunteer participants and the VLS staff for their many contributions. We acknowledge the University of Alberta Centre for Prions and Protein Folding Diseases for laboratory and technical support. We acknowledge the specific contributions of Correne DeCarlo, Stuart MacDonald, and Bonnie Whitehead to the VLS genetics initiative. More information about the VLS may be found at: http://www.ualberta.ca/~vlslab/.
References
- Anstey KJ. Biomarkers and memory aging: A life-course perspective. In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; 2012. pp. 349–372. [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure, and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2014;785:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in episodic memory: A synergistic contribution of genetic and physiological vascular risk factors. Neuropsychology. 2012;26:442–450. doi: 10.1037/a0028669. [DOI] [PubMed] [Google Scholar]
- Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Danesh J. Association of apolipoprotein E genotypes with lipid levels and coronary risk. Journal of the American Medical Association. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Corrada MM, Head E, Kawas CH. APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nature Genetics. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Taub ES. Is the Apolipoprotein E genotype a biomarker for mild cognitive impairment? Findings from a nationally representative study. Neuropsychology. 2011;25:679–689. doi: 10.1037/a0024483. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Burns R, Christensen H, Easteal S. Does possession of apolipoprotein E ε4 benefit cognitive function in healthy young adults? Neuropsychologia. 2011;49:1693–1697. doi: 10.1016/j.neuropsychologia.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DEC, Sabbagh MN, Ahern GL, Rapcsak SZ, Reiman EM. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin L, Fisicaro M, Tonizzo M, Valenti M, Danek GM, Fonda M, Baralle F. Polymorphism of the apolipoprotein E gene and early carotid atherosclerosis defined by ultrasonography in asymptomatic adults. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:91–94. doi: 10.1161/01.atv.17.1.91. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Pericak-Vance M. Gene dose of apolipoprotein E type 4 allele and the risk of alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: Blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging. 2009;24:154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart AM, Kingwell BA. Pulse pressure – a review of mechanisms and clinical relevance. Journal of the American College of Cardiology. 2001;4:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: The link between fitness and cognitive function? Exercise and Sports Science Reviews. 2012;40(3):153–158. doi: 10.1097/JES.0b013e3182553430. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Zlokovic BV. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. The Journal of Clinical Investigation. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Whalley LJ. Apolipoprotein E gene variability and cognitive functions at age 79: A follow-up of the Scottish Mental Survey of 1932. Psychology and Aging. 2004;19:367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- Dixon RA, DeCarlo CA, MacDonald SW, Vergote D, Jhamandas J, Westaway D. APOE and COMT polymorphisms are complementary biomarkers of status, stability, and transitions in normal aging and early mild cognitive impairment. Frontiers in Aging Neuroscience. 2014;6:236. doi: 10.3389/fnagi.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, McArdle JJ. Yes, memory declines with aging—but when, how, and why? In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; 2012. pp. 325–348. [Google Scholar]
- Dixon RA, Wahlin Å, Maitland SB, Hultsch DF, Hertzog C, Bäckman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory & Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Dolcos S, MacDonald SWS, Braslavsky A, Camicioli R, Dixon RA. Mild cognitive impairment is associated with selected functional markers: Integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012;26:209–223. doi: 10.1037/a0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman H, Derman D. Kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. rev. ed. [Google Scholar]
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: Does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham Heart Study. International Journal of Obesity. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Enders CK. Analyzing longitudinal data with missing values. Rehabilitation Psychology. 2011;56:267–288. doi: 10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- Ferencz B, Laukka EJ, Lövdén M, Kalpouzos G, Keller L, Graff C, Bäckman L. The influence of APOE and TOMM40 polymorphisms on hippocampal volume and episodic memory in old age. Frontiers in Human Neuroscience. 2013;7:1–9. doi: 10.3389/fnhum.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz B, Laukka EJ, Welmer AK, Kalpuzos G, Angleman S, Keller L, Bäckman L. The benefits of staying active in old age: Physical activity counteracts the negative influence of PICALM, BIN1, and CLU risk alleles on episodic memory functioning. Psychology and Aging. 2014;29:440–449. doi: 10.1037/a0035465. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nature Reviews Neurology. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Franklin S, Gustin W, Wong N, Larson M, Weber M, Kannel W, Levy D. Hemodynamic patterns of age-related changes in blood pressure - The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of General Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive aging in healthy older people. Trends in Cognitive Sciences. 2011;15:388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends in Biochemical Sciences. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Hertzog C. Theoretical approaches to the study of cognitive aging: An individual-differences perspective. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: SAGE; 2008. pp. 34–49. [Google Scholar]
- Jochemsen HM, Muller M, van der Graaf Y, Geerlings MI. APOE ε4 differentially influences change in memory performance depending on age. The SMART-MR study. Neurobiology of Aging. 2012;33:832.e15–832.e22. doi: 10.1016/j.neurobiolaging.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Josefsson M, de Luna X, Pudus S, Nilsson LG, Nyberg L. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. Journal of the American Geriatrics Society. 2012;60:2308–2312. doi: 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Nyberg L. Multimodal neuroimaging in normal aging: Structure-function interactions. In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; 2012. pp. 273–304. [Google Scholar]
- Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, Nissinen A. Apolipoprotein E ε4 magnifies lifestyle risks for dementia: A population-based study. Journal of Cellular and Molecular Medicine. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3. New York: Guilford; 2011. [Google Scholar]
- Laukka EJ, Lövdén M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, Bäckman L. Genetic effects on old-age cognitive functioning: A population-based study. Psychology and Aging. 2013;28:262–274. doi: 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, Schwartz BS. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. The American Journal of Psychiatry. 2008;165:1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York, NY: Oxford University Press; 1983. [Google Scholar]
- Lind J, Nyberg L. Imaging genomics: Brain alterations associated with APOE genotype. In: Bäckman L, Nyberg L, editors. Memory, aging and the brain: A festschrift in honour of Lars-Göran Nilsson. New York: Psychology Press; 2010. pp. 300–328. [Google Scholar]
- Lindahl-Jacobsen R, Tan Q, Mengel-From J, Christensen K, Nebel A, Christiansen L. Effects of the ApoE ε2 allele on mortality and cognitive function in the oldest old. Journals of Gerontology: A Biological Sciences and Medical Sciences. 2012;68:389–394. doi: 10.1093/gerona/gls192. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li S-C, Keekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2(2):234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD. Longitudinal structural equation modeling. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Luck T, Riedel-Heller SG, Luppa M, Wiese B, Köhler M, Jessen F, Maier W. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: Analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychological Medicine. 2013:1–11. doi: 10.1017/S0033291713001918. Advance online publication. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: Toward improving characterizations of developmental time. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011;66B:i59–i70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein E sets the stage: Response to injury triggers neuropathology. Neuron. 2012;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: Far more than a lipid transport protein. Annual Review of Genomics and Human Genetics. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. Journal of Lipid Research. 2009;50(supplement):S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Wotteman JC. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Prescott CA. Contemporary modeling of gene x environment effects in randomized multivariate longitudinal studies. Perspectives on Psychological Science. 2010;5:606–621. doi: 10.1177/1745691610383510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Jhamandas J, Westaway D, Dixon RA. IDE (rs6583817) and pulse pressure are independently and interactively associated with level and change in executive function in older adults. Psychology and Aging. 2014;29:418–430. doi: 10.1037/a0034656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Dixon RA. IDE (rs6583817) polymorphism and type 2 diabetes differentially modify executive function in older adults. Neurobiology of Aging. 2013;34:2208–2216. doi: 10.1016/j.neurobiolaging.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. Journal of the American Medical Association. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- Mitnitski A, Song X, Rockwood K. Assessing biological aging: The origin of deficit accumulation. Biogerontology. 2013;14:709–717. doi: 10.1007/s10522-013-9446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra L, Zade D, McGlinchey RE, Milberg WP. Normal aging and cognition: The unacknowledged contribution of cerebrovascular risk factors. Aging, Neuropsychology, and Cognition. 2013;20:271–297. doi: 10.1080/13825585.2012.693905. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. American Academy of Neurology. 2013;81:2024–2027. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Norms of 300 general-information questions: Accuracy of recall, latency of recall, and feeling-of-knowing ratings. Journal of Verbal Learning and Verbal Behavior. 1980;19:338–368. [Google Scholar]
- Nilsson LG, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Winblad B. The Betula prospective cohort study: Memory, health, and aging. Aging, Neuropsychology, and Cognition. 1997;4:1–32. [Google Scholar]
- Nilsson LG, Adolfsson R, Bäckman L, Cruts M, Nyberg L, Small BJ, Van Broechoven C. The influence of APOE status on episodic and semantic memory: Data from a population-based study. Neuropsychology. 2006;20:645–657. doi: 10.1037/0894-4105.20.6.645. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends in Cognitive Sciences. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Maitland SB, Rönnlund M, Bäckman L, Dixon RA, Wahlin Å, Nilsson LG. Selective adult age differences in an age-invariant multifactor model of declarative memory. Psychology and Aging. 2003;18:149–160. doi: 10.1037/0882-7974.18.1.149. [DOI] [PubMed] [Google Scholar]
- Pase MP, Pipingas A, Kras M, Nolidin K, Gibbs AL, Wesnes KA, Stough C. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. Journal of Hypertension. 2010;28:1724–1729. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: A community-based, longitudinal study. Stroke; a Journal of Cerebral Circulation. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults. General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiology of Aging. 2011;32:1124–1137. doi: 10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Gatz M, Berg S, Pedersen NL. Genotype-environment interactions: Cognitive aging and social factors. Twin Research and Human Genetics. 2007;10:241–254. doi: 10.1375/twin.10.2.241. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors of β-amyloid deposition in healthy aging. JAMA Neurology. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputations for nonresponse in surveys. Hoboken, NJ: Wiley; 1987. [Google Scholar]
- Sapkota S, Vergote D, Westaway D, Jhamandas J, Dixon RA. Synergistic associations of COMT and BDNF with executive function in aging are selective and modified by. APOE Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2014.06.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxby BK, Harrington F, McKeith IG, Wesnes K, Ford GA. Effects of hypertension on attention, memory, and executive function in older adults. Health Psychology. 2003;22:587–591. doi: 10.1037/0278-6133.22.6.587. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, Deary IJ. APOE E4 status predicts age-related cognitive decline in the ninth decade: Longitudinal follow-up of the Lothian Birth Cohort 1921. Molecular Psychiatry. 2012;17:315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. American Journal of Hypertension. 2004;17:39S–48S. doi: 10.1016/j.amjhyper.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ. Tracking cognition-health changes from 55 to 95 years of age. The Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011;66B:i153–i161. doi: 10.1093/geronb/gbq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoproteins and aging: Emerging mechanisms. Ageing Research Reviews. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzeimer disease and dementia. Neurology. 2011;77(3):227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternäng O, Wahlin Å. What is the role of apolipoprotein E for cognitive functioning across the lifespan? Biological Psychiatry. 2011;70:109–110. doi: 10.1016/j.biopsych.2011.05.023. [DOI] [PubMed] [Google Scholar]
- Sternäng O, Wahlin Å, Adolfsson R, Sleegers K, Van Broeckhoven C, Nilsson LG. APOE and lipid level synergy effects on declarative memory functioning in adulthood. European Psychologist. 2009;14:268–278. [Google Scholar]
- Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Bales KR. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiology of Aging. 2011;32:791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Tulving E. Multiple memory systems and consciousness. Human Neurobiology. 1987;6:67–80. [PubMed] [Google Scholar]
- Vakil E, Blachstein H. Rey Auditory-Verbal Learning Test: Structure analysis. Journal of Clinical Psychology. 1993;49:883–890. doi: 10.1002/1097-4679(199311)49:6<883::aid-jclp2270490616>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem N, Jr, Calon F, Plourde M. Reduction of DHA transport to the brain of mice expressing human APOE4 compared to APOE2. Journal of Neurochemistry. 2014;129:516–526. doi: 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Wendell CR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Katzel LI. Interactive relations of blood pressure and age to subclinical cerebrovascular disease. Journal of Hypertension. 2012;30:2352–2356. doi: 10.1097/HJH.0b013e3283595651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Berry-Kravis E, Evans EA, Bennett DA. The apoliopoprotein E ε2 allele and decline in episodic memory. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiology of Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Tanimukai S, Sasaki M, Ikejima C, Yamashita F, Kodama C, Asada T. Effect of plasma lipids, hypertension and APOE genotype on cognitive decline. Neurobiology of Aging. 2012;33:2633–2640. doi: 10.1016/j.neurobiolaging.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, Milberg W. Interactive effects of apolipoprotein E type 4 genotype and cerebrovascular risk on neuropsychological performance and structural brain changes. Journal of Stroke and Cerebrovascular Diseases. 2010;19:261–268. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, Milberg W. Apolipoprotein epsilon 4 allele modifies waist-to-hip ratio effects on cognition and brain structure. Journal of Stroke and Cerebrovascular Diseases. 2013;22:119–125. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: Implications for Alzheimer disease. JAMA Neurology. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]