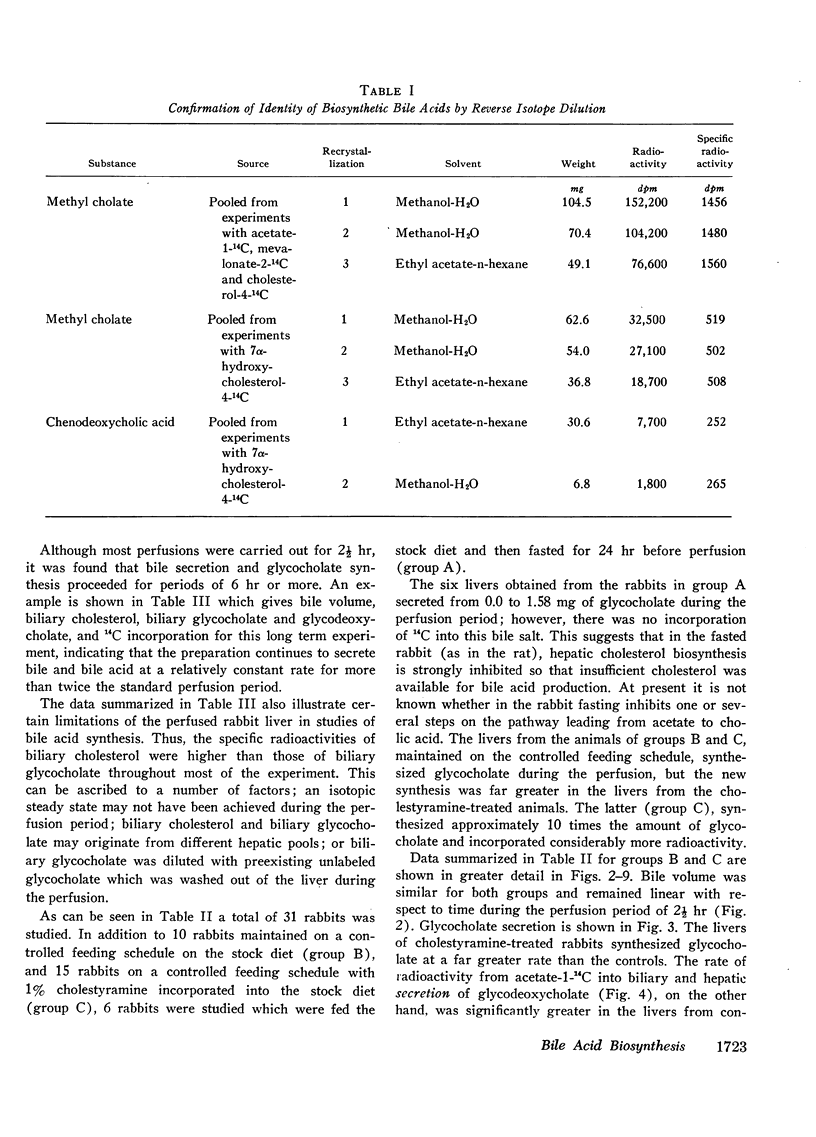
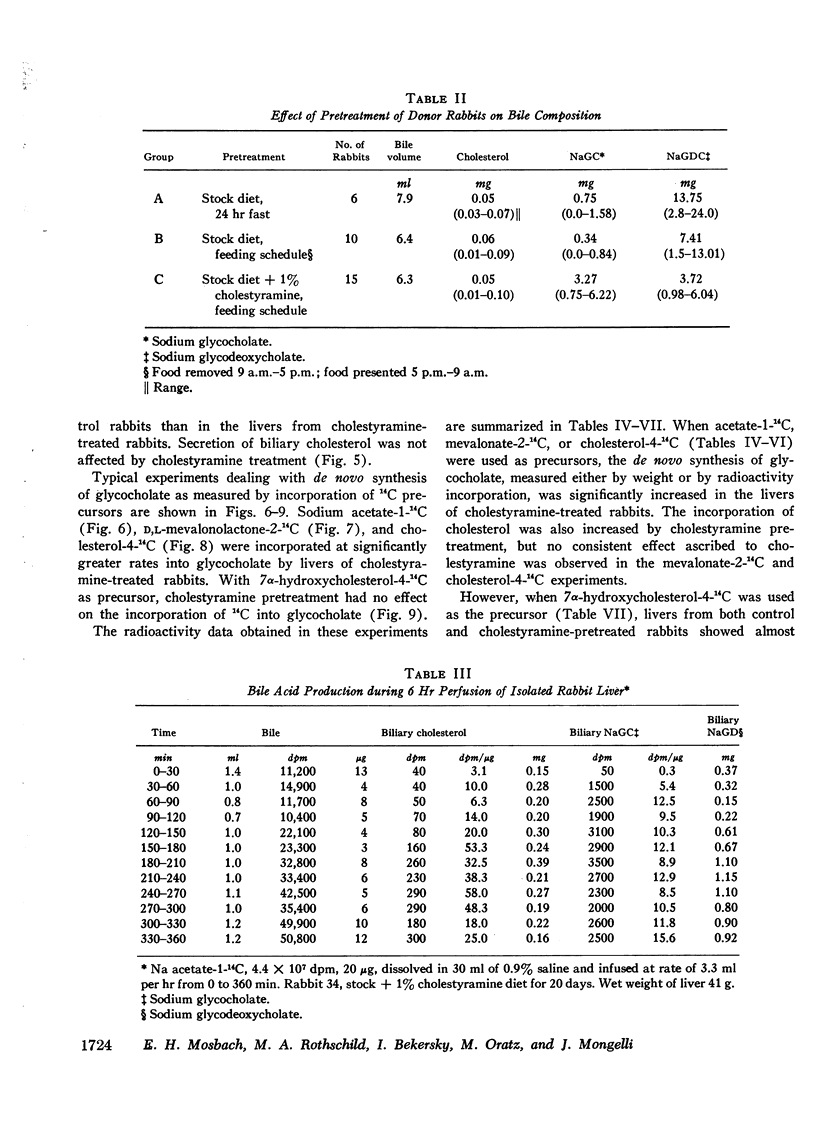
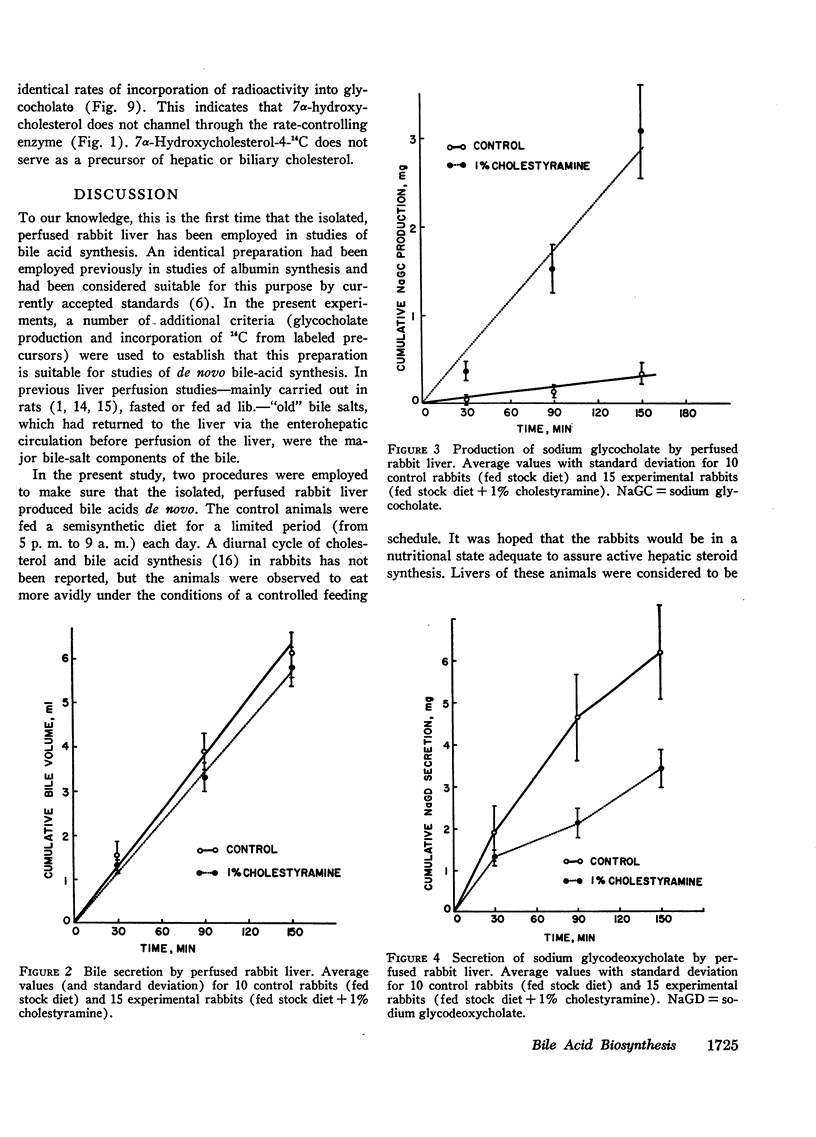
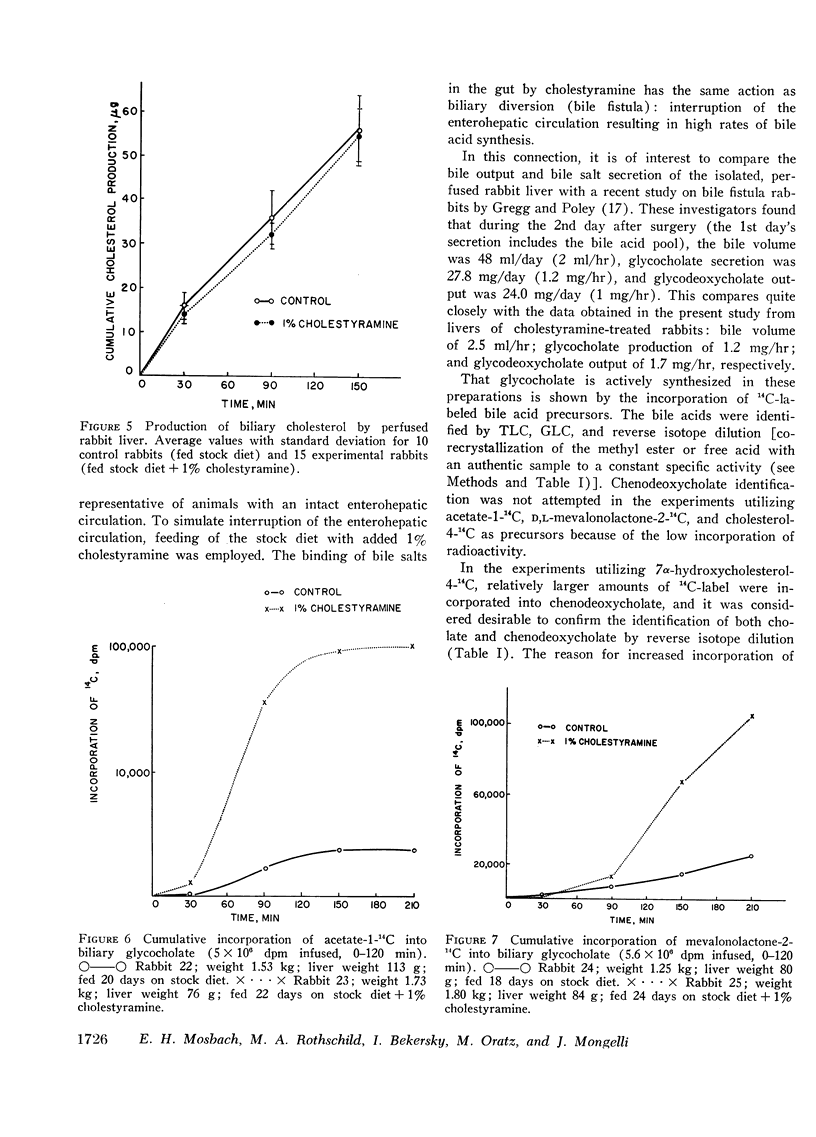
Abstract
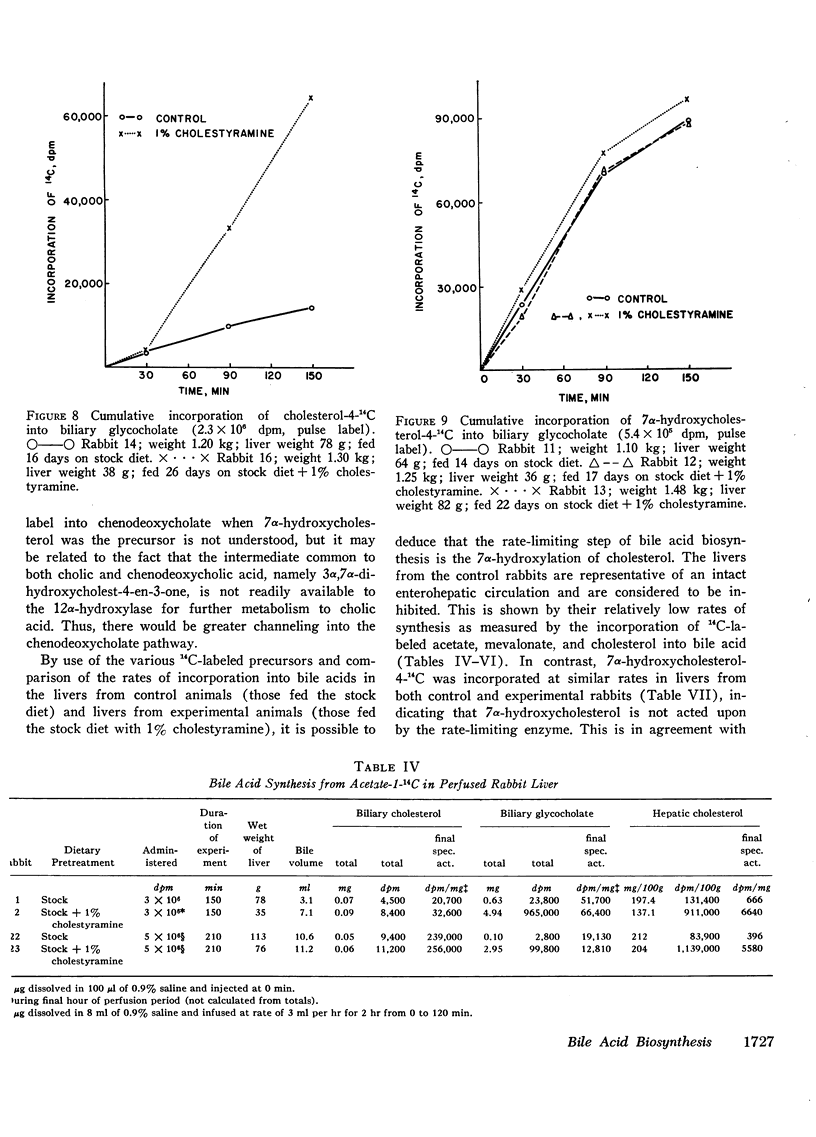
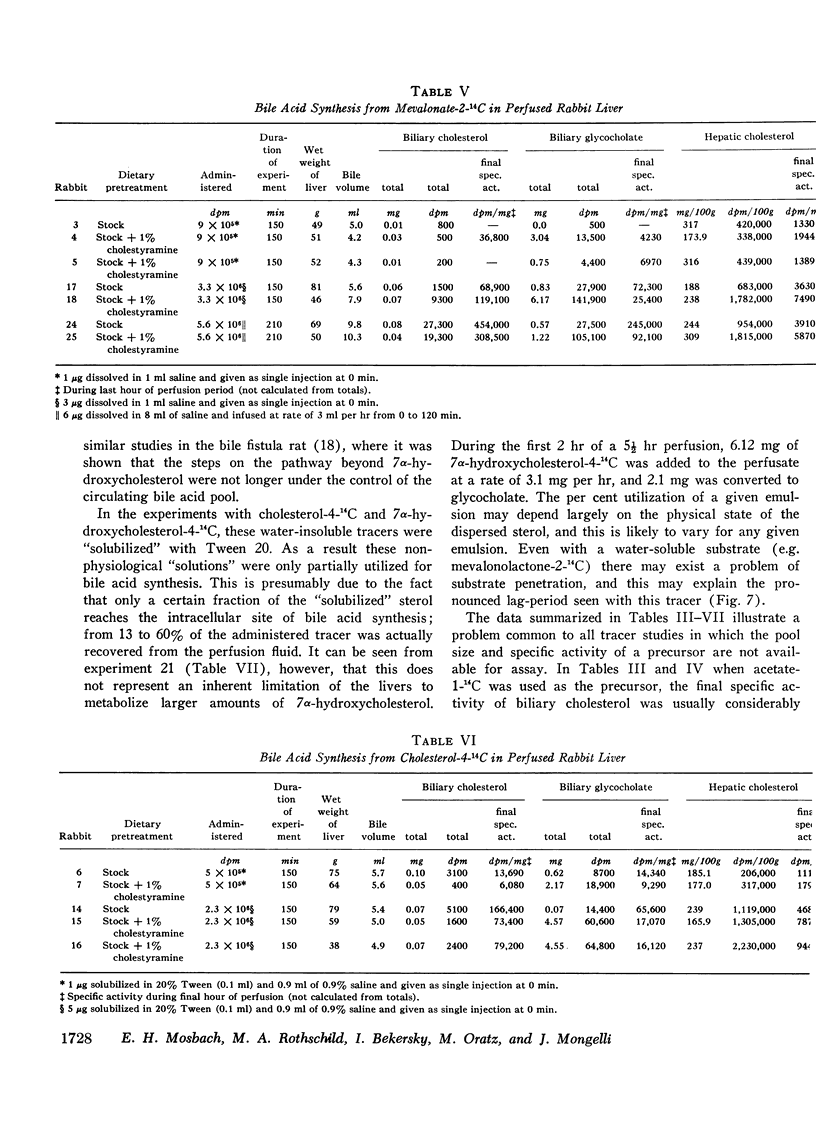
These experiments were carried out to demonstrate the usefulness of the perfused rabbit liver for studies of bile acid metabolism, and to determine the rate-limiting enzyme of bile acid synthesis. Rabbits were fed a semisynthetic diet, with or without the addition of 1% cholestyramine, under controlled conditions. At the end of 2-5 wk, the livers were removed and perfused for 2.5 hr employing various 14C-labeled precursors to measure de novo cholic acid synthesis. The livers were then analyzed for cholesterol, and the bile collected during the perfusion was analyzed for cholesterol and bile acids. Control bile contained, on the average, 0.34 mg of glycocholate, 7.4 mg of glycodeoxycholate, and 0.06 mg of cholesterol. After cholestyramine treatment of the donor rabbits, the bile contained 3.3 mg of glycocholate, 3.7 mg of glycodeoxycholate, and 0.05 mg of cholesterol. It was assumed that in cholestyramine-treated animals the enterohepatic circulation of the bile acids had been interrupted sufficiently to release the feedback inhibition of the rate-controlling enzyme of bile acid synthesis. Therefore, a given precursor should be incorporated into bile acids at a more rapid rate in livers of cholestyramine-treated animals, provided that the precursor was acted upon by the rate-controlling enzyme. It was found that the incorporation of acetate-14C, mevalonolactone-14C, and cholesterol-14C into cholate was 5-20 times greater in the livers of cholestyramine-treated animals than in the controls. In contrast, there was no difference in the incorporation of 7α-hydroxycholesterol-14C into cholate regardless of dietary pretreatment. It was concluded that given an adequate precursor pool, the 7α-hydroxylation of cholesterol is the rate-limiting step in bile acid formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back P., Hamprecht B., Lynen F. Regulation of cholesterol biosynthesis in rat liver: diurnal changes of activity and influence of bile acids. Arch Biochem Biophys. 1969 Aug;133(1):11–21. doi: 10.1016/0003-9861(69)90482-2. [DOI] [PubMed] [Google Scholar]
- Bartsch G. G., Gerber G. B. Incorporation of acetate-1-14C into lipids by the perfused liver of normal, X-irradiated, or partially hepatectomized rats. J Lipid Res. 1966 Mar;7(2):204–209. [PubMed] [Google Scholar]
- ERIKSSON S. Biliary excretion of bile acids and cholesterol in bile fistula rats; bile acids and steroids. Proc Soc Exp Biol Med. 1957 Mar;94(3):578–582. doi: 10.3181/00379727-94-23018. [DOI] [PubMed] [Google Scholar]
- Evrard E., Janssen G. Gas-liquid chromatographic determination of human fecal bile acids. J Lipid Res. 1968 Mar;9(2):226–236. [PubMed] [Google Scholar]
- Gerber G. B., Remy-Defraigne J. Studies on detoxication in the isolated perfused liver. 3. Synthesis and conjugation of bile acids. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;10(2):141–149. doi: 10.1080/09553006614550191. [DOI] [PubMed] [Google Scholar]
- Gregg J. A., Poley J. R. Excretion of bile acids in normal rabbits. Am J Physiol. 1966 Nov;211(5):1147–1151. doi: 10.1152/ajplegacy.1966.211.5.1147. [DOI] [PubMed] [Google Scholar]
- HOFMANN A. F., MOSBACH E. H. IDENTIFICATION OF ALLODEOXYCHOLIC ACID AS THE MAJOR COMPONENT OF GALLSTONES INDUCED IN THE RABBIT BY 5-ALPHA-CHOLESTAN-3-BETA-OL. J Biol Chem. 1964 Sep;239:2813–2821. [PubMed] [Google Scholar]
- Klevay L. M., Hegsted D. M. Effect of dietary fat on bile acid excretion by the isolated, perfused rat liver. J Atheroscler Res. 1968 Mar-Apr;8(2):329–341. doi: 10.1016/s0368-1319(68)80067-5. [DOI] [PubMed] [Google Scholar]
- MOSBACH E. H., BLUM J., ARROYO E., MILCHS A new method for the determination of dihydrocholesterol in tissues. Anal Biochem. 1963 Feb;5:158–169. doi: 10.1016/0003-2697(63)90022-8. [DOI] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Mongelli J., Schreiber S. S. Effects of a short-term fast on albumin synthesis studied in vivo, in the perfused liver, and on amino acid incorporation by hepatic microsomes. J Clin Invest. 1968 Dec;47(12):2591–2599. doi: 10.1172/JCI105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., CHAIKOFF I. L. C14-Cholesterol. III. Excretion of carbons 4 and 26 in feces, urine, and bile. J Biol Chem. 1952 Sep;198(1):93–104. [PubMed] [Google Scholar]
- Schoenfield L. J., Sjövall J. Bile acids and cholesterol in guinea pigs with induced gallstones. Am J Physiol. 1966 Nov;211(5):1069–1074. doi: 10.1152/ajplegacy.1966.211.5.1069. [DOI] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Biochemical site of regulation of bile acid biosynthesis in the rat. J Lipid Res. 1970 Sep;11(5):404–411. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Feedback regulation of bile acid biosynthesis in the rat. J Lipid Res. 1969 Nov;10(6):646–655. [PubMed] [Google Scholar]
- TENNENT D. M., SIEGEL H., ZANETTI M. E., KURON G. W., OTT W. H., WOLF F. J. Plasma cholesterol lowering action of bile acid binding polymers in experimental animals. J Lipid Res. 1960 Oct;1:469–473. [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Failure of bile acids to control hepatic cholesterogenesis: evidence for endogenous cholesterol feedback. J Clin Invest. 1969 Dec;48(12):2398–2408. doi: 10.1172/JCI106206. [DOI] [PMC free article] [PubMed] [Google Scholar]


