Abstract
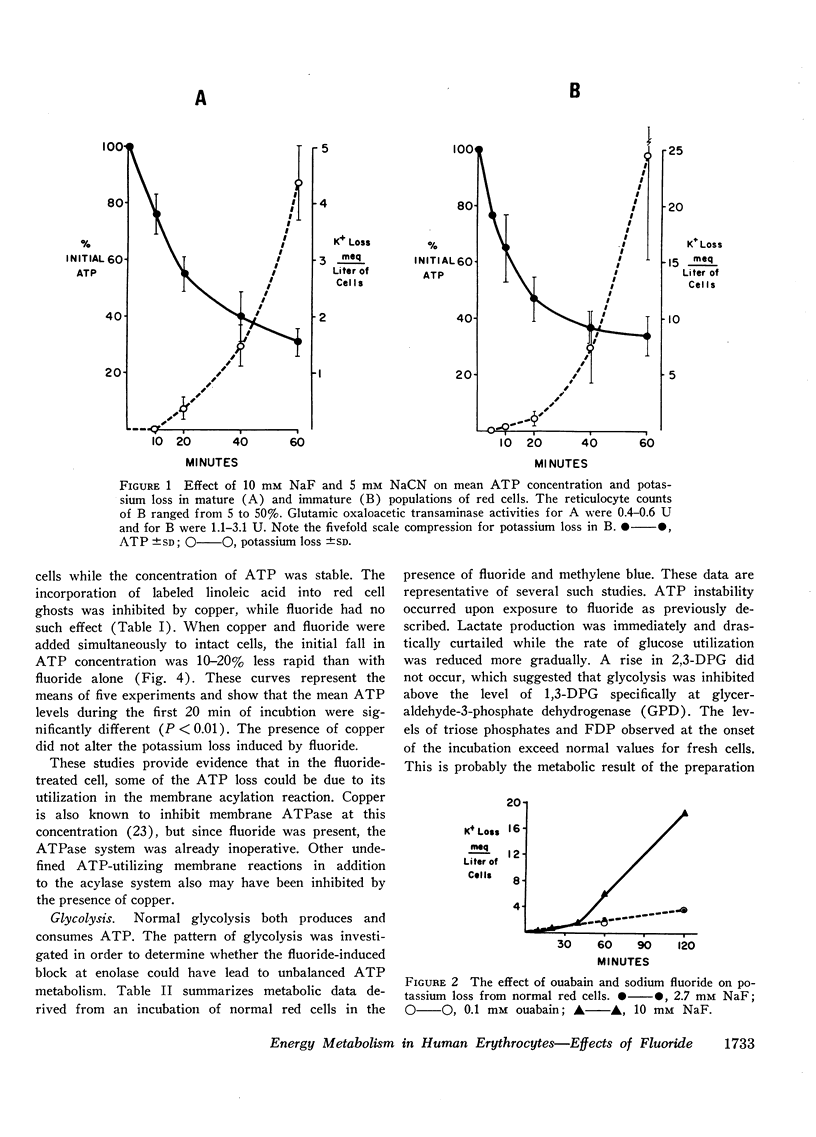
Exposure of red cells to fluoride produces a variety of metabolic alterations, most of which are based upon the secondary effects of enolase inhibition, which reduces pyruvate synthesis and interferes with the regeneration of diphosphopyridine nucleotide (NAD). Adenosine triphosphate (ATP) is consumed in the hexokinase and phosphofructokinase reactions but is not regenerated since the deficiency of NAD limits glyceraldehyde phosphate dehydrogenase. ATP depletion in the presence of fluoride and calcium induces a massive loss of cations and water.
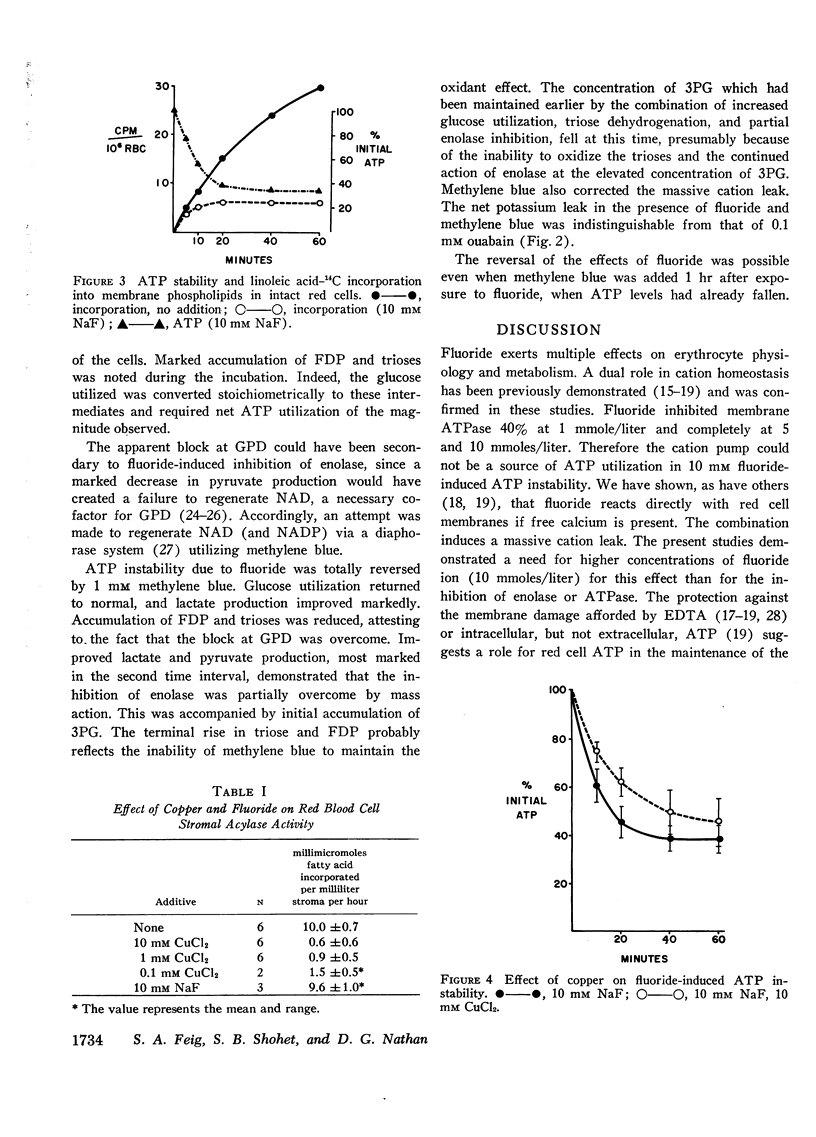
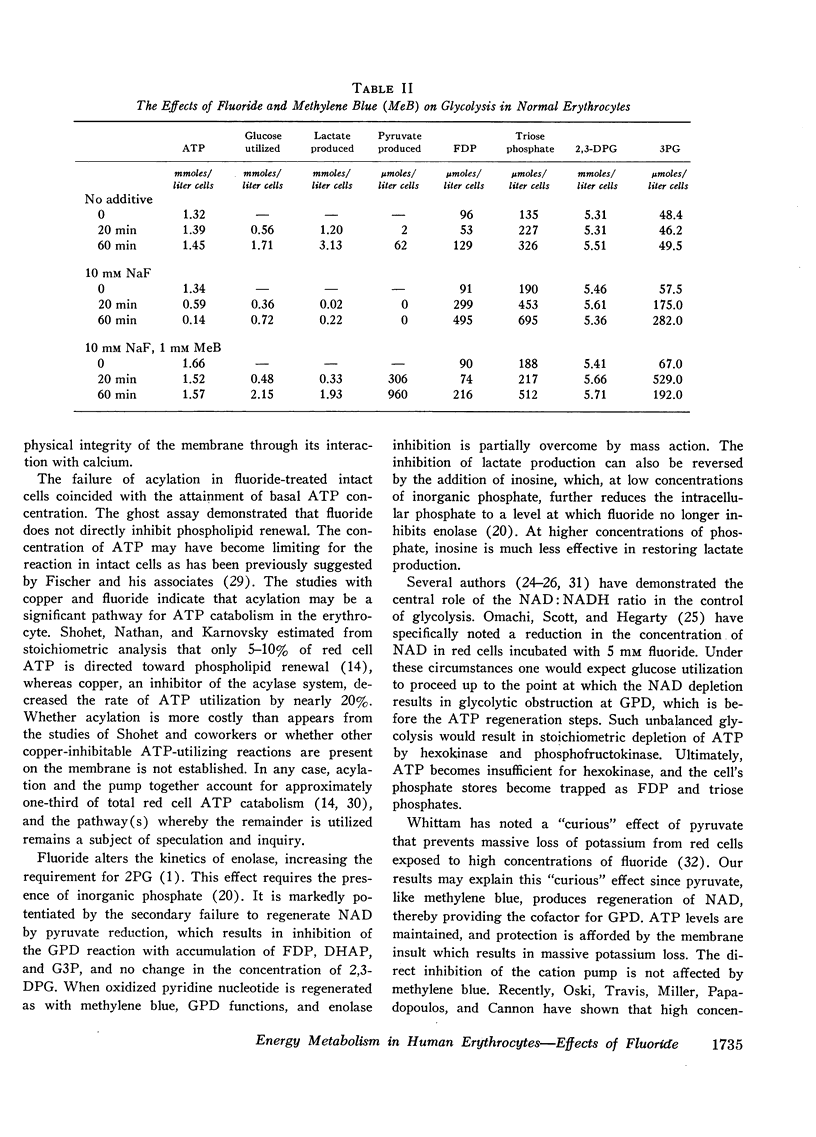
Of the other known sites of ATP utilization, membrane-bound ATPase is inhibited by fluoride, but the incorporation of fatty acids into membrane phospholipids is unaffected until ATP is depleted.
The addition of methylene blue to fluoride-treated red cells regenerates NAD, permitting triose oxidation and the generation of 3-phosphoglycerate and 2,3-diphosphoglycerate. Enolase inhibition is then partially overcome by mass action, and sufficient glycolysis proceeds to maintain the concentration of ATP. This in turn prevents the massive cation and water loss, and permits membrane phospholipid renewal to proceed. Membrane ATPase activity is not restored by the oxidant so that normal cation leakage remains unopposed by cation pumping in red cells exposed to the combination of fluoride and methylene blue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowler K., Duncan C. J. The effect of copper on membrane enzymes. Biochim Biophys Acta. 1970 Jan 6;196(1):116–119. doi: 10.1016/0005-2736(70)90174-4. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- GARDOS G. Effect of ethylenediaminetetraacetate on the permeability of human erythrocytes. Acta Physiol Acad Sci Hung. 1958;14(1):1–5. [PubMed] [Google Scholar]
- GARDOS G., STRAUB F. B. Uber die Rolle der Adenosintriphosphorsäure (ATP) in der K-Permeabilität der menschlichen roten Blutkörperchen. Acta Physiol Acad Sci Hung. 1957;12(1-3):1–8. [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The role of calcium in the potassium permeability of human erythrocytes. Acta Physiol Acad Sci Hung. 1959;15(2):121–125. [PubMed] [Google Scholar]
- GLOSTER J. A., HARRIS P. Observations on an enzymic method for the estimation of pyruvate in blood. Clin Chim Acta. 1962 Mar;7:206–211. doi: 10.1016/0009-8981(62)90011-6. [DOI] [PubMed] [Google Scholar]
- KARMEN A., WROBLEWSKI F., LADUE J. S. Transaminase activity in human blood. J Clin Invest. 1955 Jan;34(1):126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCHNER L. B. FLUORIDE INHIBITION OF SODIUM EXTRUSION FROM SWINE ERYTHROCYTES AND ITS METABOLIS CORRELATES. Arch Biochem Biophys. 1964 Jul 20;106:57–64. doi: 10.1016/0003-9861(64)90156-0. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Mills G. C. The physiologic regulation of erythrocyte metabolism. Tex Rep Biol Med. 1969 Fall;27(3):773–786. [PubMed] [Google Scholar]
- Nakao K., Kurashina S., Nakao M. Adenosinetriphosphatase activity of erythrocyte membrane in hereditary spherocytosis. Life Sci. 1967 Mar 15;6(6):595–600. doi: 10.1016/0024-3205(67)90094-x. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA M. M., VAUGHAN M. INCORPORATION OF FATTY ACIDS INTO PHOSPHOLIPIDS OF ERYTHROCYTE MEMBRANES. J Lipid Res. 1964 Apr;5:156–162. [PubMed] [Google Scholar]
- Omachi A., Deuticke B., Gerlach E. Fluoride inhibition of erythrocyte metabolism as a function of cellular P. Biochim Biophys Acta. 1966 Aug 24;124(2):421–423. doi: 10.1016/0304-4165(66)90213-3. [DOI] [PubMed] [Google Scholar]
- Omachi A., Scott C. B., Hegarty H. Pyridine nucleotides in human erythrocytes in different metabolic states. Biochim Biophys Acta. 1969 Jun 17;184(1):139–147. doi: 10.1016/0304-4165(69)90108-1. [DOI] [PubMed] [Google Scholar]
- Omachi A., Scott C. B., Parry T. E. Influence of glycolysis on NADH content in human erythrocytes. Am J Physiol. 1969 Mar;216(3):527–530. doi: 10.1152/ajplegacy.1969.216.3.527. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Baughan M. A., Miller D. R., Reed C. F., McIntyre O. R. An inherited molecular lesion of erythrocyte pyruvate kinase. Identification of a kinetically aberrant isozyme associated with premature hemolysis. J Clin Invest. 1968 Aug;47(8):1929–1946. doi: 10.1172/JCI105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Control of glycolysis in the human red blood cell. J Biol Chem. 1966 Nov 10;241(21):4848–4854. [PubMed] [Google Scholar]
- Sass M. D., Caruso C. J., Axelrod D. R. Accumulation of methylene blue by metabolizing erythrocytes. J Lab Clin Med. 1967 Mar;69(3):447–455. [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]



