Abstract
The variable response to therapy in multiple sclerosis (MS) suggests a need for personalized approaches based on individual genetic differences. GWAS have linked CBLB gene polymorphisms with MS and recent evidence demonstrated that these polymorphisms can be associated with abnormalities in T cell function and response to interferon-β therapy. Cbl-b is an E3 ubiquitin ligase that regulates T cell activation and Cbl-b-deficient (Cbl-b−/−) mice show T cell abnormalities described in MS patients. We now show that Cbl-b−/− T cells demonstrate significant lymph node trafficking abnormalities. We thus asked whether the MS-approved drug, FTY720, postulated to trap T cells in lymphoid tissues, is less effective in the context of Cbl-b dysfunction. We now report that FTY720 significantly inhibits EAE in Cbl-b−/− mice. Our results newly document a role for Cbl-b in T cell trafficking but suggest nevertheless that MS patients with Cbl-b abnormalities may still be excellent candidates for FTY720 treatment.
Keywords: Multiple sclerosis, Casitas-B lineage lymphoma-b, Experimental autoimmune encephalomyelitis, sphingosine-1-phosphate receptor 1, FTY720, T cell trafficking
1. Introduction
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) primarily affecting individuals 20 to 50 years old. The cause of MS is unknown and the disease in incurable, though a number of therapeutic drugs are available. Unfortunately, all presently available therapeutic options are effective only in the relapsing remitting (RR) subset of patients, and for each treatment option a proportion of these patients remain resistant to the therapeutic effects. The variable response of MS patients to the different drugs available suggests that a personalized treatment approach based on individual clinical and genetic differences may be desirable. However, how genetic variations in MS patients influence treatment outcomes is unclear.
It is known that both genetic and environmental factors are involved in the etiology of MS. In identifying genetic factors potentially underlying the cause of MS, genome wide association studies (GWAS) have revealed a number of single nucleotide polymorphisms (SNPs) that are potentially relevant in the pathogenesis of MS. One of the SNPs demonstrated in some, but not all, of such MS GWAS studies involves the CBLB gene [1–3]. The CBLB gene encodes for Cbl-b (Casitas B-lineage lymphoma-b), an E3 ubiquitin ligase that negatively regulates T cell activation [4–6]. Cbl-b has been shown to be relevant in regulating T cell responses in models of human disease such as allergic airway inflammation [7] and Cbl-b has been shown to be essential for TGF-β receptor signaling through direct inhibition of SMAD7 [8]. Importantly, Cbl-b deficiency in mice (Cbl-b−/− mice) leads to multi-organ cellular infiltration associated with T cell hyper-reactivity [4], co-stimulation independence in T cell activation [5], and T cell resistance to regulatory T cell (Treg)-mediated suppression [9, 10]. These abnormalities in Cbl-b−/− mice have also been documented in MS patients [11–15]. Consistent with this, Cbl-b−/− mice have been described to show increased susceptibility to experimental autoimmune encephalomyelitis (EAE), the murine model of MS [5, 16]. Recently, one of three described MS-associated CBLB SNPs was reported to alter T cell Cbl-b expression levels and T cell function in both MS patients and healthy individuals carrying this SNP [17]. Importantly, this alteration in T cell function was found to interfere with the normal immune-regulatory function of type I IFN, a commonly used drug to treat MS [17]. These findings suggest that this CBLB SNP could potentially be important in predicting therapeutic effectiveness of type I IFN in this subset of patients. Thus, there is a potentially significant functional role for Cbl-b in at least a subset of MS patients and this in turn suggests that Cbl-b−/− mice could prove useful both for studying pathogenic mechanisms in MS and for predicting personalized therapeutic approaches in this subset of MS patients.
The various therapeutic approaches available for the treatment of MS mediate their effects through different physiologic mechanisms. FTY720 (Fingolimod/Gilenya), an FDA-approved orally administered drug for relapsing remitting MS (RRMS), targets the sphingosine-1-phosphate receptors, S1P1, S1P3, S1P4 and S1P5 [18]. Though still controversial, FTY720 theoretically mediates its therapeutic effect in MS by causing degradation of the lymphocyte homing receptor S1P1 [19]. This blocks the egress of T and B cells from lymph nodes resulting in lymph node trapping of these cells and an inability of the immune system to mount an attack on self-antigens in the CNS [20]. As with all the treatment options in MS, FTY720 is effective only in a proportion of patients with RRMS [21], but which patients will fare better with which specific treatment option is not yet predictable. In the present study, our goal was to use Cbl-b−/− mice as a new model for analyzing the efficacy of FTY720 in the context of altered Cbl-b function. Moreover, the efficacy of FTY720 had been demonstrated in studies using EAE in wild-type (WT) mice [22–25], but had never been tested in mice such as Cbl-b−/− mice that have both an MS-relevant genetic alteration and hyperactive T cells.
We now report for the first time that Cbl-b plays a role in regulating T cell trafficking and expression of trafficking related molecules, thus extending our knowledge of the involvement of Cbl-b in the regulation of T cell function. However, despite this role of Cbl-b in regulating T cell trafficking, FTY720 treatment was highly effective in inhibiting EAE in Cbl-b−/− mice. Overall, our findings document a novel role for Cbl-b in regulating T cell trafficking, but suggest, nevertheless, that MS patients with Cbl-b abnormalities may still be excellent candidates for FTY720 treatment.
2. Material and methods
2.1. Mice
Female C57BL/6 (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Cbl-b−/− mice on a C57BL/6 background were a gift from Dr. H. Gu (Columbia University, New York, NY). RAG-1−/− mice were purchased from the Jackson Laboratory and bred and maintained in our facility. All mice were maintained and bred under specific pathogen-free conditions in accordance with the guidelines of the Center for Laboratory Animal Care at the University of Connecticut Health Center (Farmington, CT).
2.2. Adoptive transfer of CD4+ CD25− effector T cells to RAG-1−/− mice
CD4+ CD25− effector T cells (Teffs) were isolated via magnetic bead purification (Miltenyi Biotec, Auburn, CA) from spleens of 6–8 weeks old female C57BL/6 WT and Cbl-b−/− mice. Viability of Teffs was determined via trypan blue exclusion prior to adoptive transfer. 0.9–1.4 × 106 Teffs were given intraperitoneally (i.p.) to 6–8 weeks old female RAG-1−/− mice. On day 4–11 post transfer, mesenteric lymph nodes (mLNs) and peripheral lymph nodes (pLNs; axillary, cervical, and inguinal LNs pooled) of the recipient RAG-1−/− mice were harvested and LN cells were stained with anti-CD4-APC (Biolegend, San Diego, CA), anti-CD44-Pacific Blue (Biolegend) and anti-CD62L-APC eFluor780 (eBioscience, San Diego, CA) antibodies and analyzed by flow cytometry. The data were analyzed using Flowjo software.
2.3. CFA/MOG35–55 immunization and S1P1/CD69/CCR7 expression assay
6–8 weeks old female C57BL/6 WT and Cbl-b−/− mice were immunized subcutaneously (s.c.) in the footpads with 80 µg of myelin oligodendrocyte glycoprotein peptide (35–55) (MOG35–55) emulsified with complete Freund’s adjuvants (CFA) containing 150 µg of H37RA mycobacteria (Difco BD Diagnostics, Sparks, MD). On day 4–5 post immunization, draining popliteal LNs and non-draining mesenteric LNs were harvested and LN cells stained with rat anti-S1P1 monoclonal antibody (R&D Systems, Minneapolis, MN), biotin-SP-congugated AffiniPure F(ab’)2 fragment donkey anti-rat IgG (H+L) (Jackson Immunoresearch, West Grove, PA), streptavidin-PE (Life Technologies, Norwalk, CT), anti-CD4-APC, anti-CD8α-FITC (Biolegend), anti-CD44-Pacific Blue, anti-CD69-PerCP-Cy.5.5 (Biolegend), and analyzed by flow cytometry. CCR7 staining was performed using human CCL19-Ig (generously provided from the Dr. Lefrançois lab, University of Connecticut Health Center) and goat anti-human IgG-Alexa Fluor 488 (Life Technologies).
2.4. Lymphopenia assay after administration of the S1P lyase inhibitor, THI
6–8 weeks old female C57BL/6 WT and Cbl-b−/− mice were administered 200 µg of THI (2-acetyl-5-hydroxybutyl imidazole) (Cayman Chemical, Ann Arbor, MI) or vehicle (sterile water) via oral gavage. At 24 and 48 hours post treatment, these mice were bled, red blood cells lysed with ACK buffer, total peripheral blood leukocytes stained with anti-CD4-APC and anti-CD8α-FITC antibodies (Biolegend) and samples analyzed by flow cytometry.
2.5. Lymphopenia assay after administration of the S1P1-selective agonist, SEW2871
6–8 weeks old female C57BL/6 WT and Cbl-b−/− mice were administered 20 mg/kg of SEW2871 (Cayman Chemical) or vehicle (50% DMSO/25% Tween-20, v/v) via oral gavage. These mice were bled at 14, 22, and 38 hours post treatment, red blood cells lysed with ACK buffer, total peripheral blood leukocytes stained with anti-CD4-APC and anti-CD8α-FITC antibodies (Biolegend), and samples analyzed by flow cytometry.
2.6. Induction of experimental autoimmune encephalomyelitis (EAE) and FTY720 treatment
6–8 weeks old female C57BL/6 WT and Cbl-b−/− mice were immunized s.c. in the back with 165 µg of MOG35–55 emulsified with CFA containing 300 µg of H37RA mycobacteria (Difco BD). 150 ng of Pertussis toxin (PTX) (List Biological Laboratories, Campbell, CA) was administered i.p. on day 0 and 2. On the day of EAE induction (day 0), these mice were also administered either 0.375 mg/kg of FTY720 (Cayman Chemical) or vehicle (2% (2-hydroxypropyl)-β-cyclodextrin) (Sigma-Aldrich, St. Louis, MO). FTY720 (0.375 mg/kg) or vehicle was given every five days after the immunization up to day 20. Mice were evaluated daily for signs of EAE for approximately 30 days. EAE was scored as: grade 1, tail paralysis; grade 2, weakness of hind limbs with an altered gait; grade 3, hind limb paralysis; grade 4, front limb paralysis; and grade 5, death.
2.7. Statistical Analysis
Unless noted otherwise, values reported in all analyses are expressed as the mean ± SEM. Differences between groups were analyzed using an unpaired two-tailed Student’s t-test. For EAE, the Mann-Whitney test was used to determine statistical significance. Statistical significance was accepted at p<0.05.
3. Results
3.1. Cbl-b−/− CD4+ T cells show impaired mLN accumulation after adoptive transfer into RAG-1−/− mice
In prior unpublished studies, we had noted a decreased mesenteric lymph node (mLN) recovery of Cbl-b−/− compared to wild type C57BL/6 (WT) CD4+ T cells after adoptive transfer into RAG-1−/− mice. Despite this abnormal mLN accumulation, Cbl-b−/− CD4+ T cells are efficient mediators of disease in the adoptive transfer model of colitis (unpublished observations). These findings suggested a potential abnormality in Cbl-b−/− CD4+ T cell trafficking or lymph node (LN) accumulation. To examine this question, we analyzed mLN and peripheral lymph node (pLN) recovery of WT and Cbl-b−/− CD4+ T cells at various time points after adoptive transfer into RAG-1−/− mice.
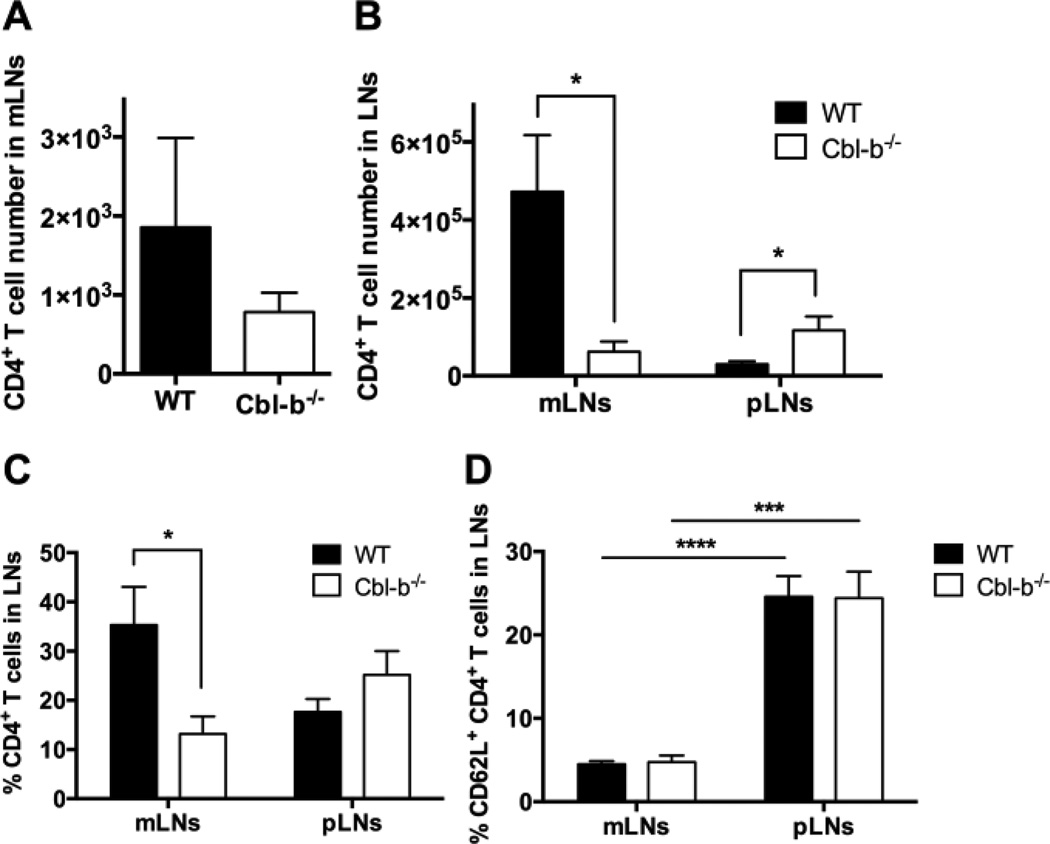
WT and Cbl-b−/− CD4+ CD25− effector T cells (Teff) were transferred into C57BL/6 RAG-1−/− mice. On day 4 post transfer only very low numbers of cells could be recovered from the mLNs of the RAG-1−/− recipients. However, at this early time point, the number of transferred WT and Cbl-b−/− CD4+ T cells found in mLNs of the recipient RAG-1−/− mice was not statistically different (Figure 1A). In contrast, on day 8–11 post transfer, in confirmation of our preliminary results, both the absolute number and the frequency of Cbl-b−/− CD4+ T cells recovered in mLNs were significantly lower than WT cells (Figure 1B, 1C). In addition, the total mLN cell number recovered from mice receiving Cbl-b−/− Teff was also significantly lower than in those receiving WT cells (data not shown). On day 15–18 post transfer, comparable number of Cbl-b−/− CD4+ T cells and WT cells were found in the mLNs (Supplemental Figure 1). However, at this later time point, RAG-1−/− recipients are developing severe colitis and the mLNs are draining a site of active colitic inflammation. Thus, the interpretation of the mLN T cell recovery in terms of T cell trafficking and accumulation becomes complex.
Figure 1.
Decreased accumulation of Cbl-b−/− CD4+ T cells in mLNs of RAG-1−/− mice after adoptive transfer. 0.9–1.4 × 106 WT or Cbl-b−/− CD4+ CD25 T cells were injected i.p. into RAG-1−/− mice and mLNs and pLNs were harvested on day 4 and day 8–11 for analysis. (A and B) Absolute number of transferred WT or Cbl-b−/− CD4+ T cells in LNs of recipient RAG-1−/− mice on day 4 (A), and day 8–11 (B) post transfer. (C) Frequency of transferred WT or Cbl-b−/− CD4+ T cells in LNs of recipients on day 8–11 post transfer. (D) Frequency of WT and Cbl-b−/− CD62L+ CD4+ T cells in mLNs and pLNs of recipients on day 8–11 post transfer. *p<0.05, ***p=0.0001, ****p<0.0001 by Student’s t-test. n=7–9/group for A, B, and C. n=6/group for D. The data shown are from three (A), five (B and C) and two (D) independent experiments.
To understand whether the decreased recovery seen with Cbl-b−/− T cells at day 8–11 was specific for the mLNs, we also examined peripheral lymph node (pLN) recovery of WT and Cbl-b−/− CD4+ T cells at various time points after adoptive transfer into RAG-1−/− mice. The pLNs examined were the inguinal, the axillary, and the cervical lymph nodes. Because the recovery of CD4+ T cells from the mLNs at day 4 was extremely low, we did not study the pLNs at this time point. When the recovery of CD4+ T cells from the pLNs was examined at day 8–11, we found that, in contrast to the mLNs, recovery of Cbl-b−/− CD4+ T cells was actually higher than that of WT CD4+ T cells (Figure 1B and C). At day 15–18 post transfer, comparable numbers of Cbl-b−/− CD4+ T cells and WT cells were found in the pLNs (data not shown). These results suggest that the decreased accumulation seen with transferred Cbl-b−/− CD4+ T cells was specific for the mLNs.
Because spontaneous colitis develops in RAG-1−/− mice adoptively transferred with Teff, we postulated that the day 8–11 mLN-specific decrease in accumulation noted with Cbl-b−/− CD4+ T cells may be related to an increase in CD4+ T cell activation occurring in the mLNs but not the pLNs. To examine this possibility, we first analyzed CD44 expression on the transferred CD4+ T cells. We found that greater than 90% of both WT and Cbl-b−/− CD4+ T cells expressed high levels of CD44 in both mLNs and pLNs (data not shown). This high level of CD44 expression is likely a result of the systemic activation induced under lymphopenic conditions.
To further pursue the postulate that there is more activation occurring in the transferred CD4+ T cells in the mLNs as compared to the pLNs, we next measured CD62L expression on the transferred WT and Cbl-b−/− CD4+ T cells. At day 8–11 after adoptive transfer, the time point at which a significant decrease in recovery of Cbl-b−/− CD4+ T cells was seen, we found that both WT and Cbl-b−/− CD4+ T cells showed a lower percentage of CD62L expression in the mLNs compared to the pLNs (Figure 1D). These results suggest that, post-transfer for both WT and Cbl-b−/− CD4+ T cells, there is a higher frequency of activated cells in the mLNs compared to the pLNs at this time point. This difference in activation between the mLNs and the pLNs likely relates to the colitis which is beginning to develop at that time. These results, in turn, suggest that the difference in accumulation of Cbl-b−/− versus WT CD4+ T cells in the mLNs is dependent on the colitis-related, foreign antigen-induced activation occurring in the mLNs. Specifically, we postulated that the observed difference between the mLN accumulation of Cbl-b−/− versus WT CD4+ T cells is dependent on the transferred T cells being highly activated and furthermore, that this activation affects Cbl-b−/− T cells differently than WT T cells. These transferred CD4+ T cells express molecules, including S1P1, CD69, and CCR7, which are all likely relevant to the mechanism proposed for the effect of FTY720 in MS. Thus, we next analyzed the effect of Cbl-deficiency on these molecules.
3.2. Cbl-b−/− CD4+ T cells show enhanced S1P1 expression and decreased CD69 expression in draining LNs after CFA/MOG35–55 immunization
To further characterize the decreased LN accumulation of Cbl-b−/− T cells, we next analyzed the expression of lymph node ingress/egress-related molecules on activated WT and Cbl-b−/− T cells. We began by characterizing the expression of the sphingosine-1-phosphate receptor 1 (S1P1). Although S1P1 is documented to be a major regulator of T cell egress from lymph nodes and peripheral tissues [26, 27], there have been no prior reports of the relationship between S1P1 expression and Cbl-b, or of the S1P1 expression status in mice with Cbl-b deficiency. Moreover, characterizing T cell S1P1 expression was particularly relevant in light of our goal of using Cbl-b−/− mice as a new model for analyzing the MS-treatment efficacy of the S1P1-modulating drug FTY720 (Fingolimod) in the context of altered Cbl-b function.
Given our finding of decreased LN accumulation of Cbl-b−/− CD4+ T cells in the context of activation and our goal of establishing a model for studying therapeutic responses relevant to MS, we induced T cell activation by utilizing an immunization protocol similar to EAE. Cbl-b−/− and WT mice were immunized subcutaneously in the footpads with myelin oligodendrocyte glycoprotein peptide (35–55) (MOG35–55) emulsified with complete Freund’s adjuvant (CFA). On day 4 and 5 post immunization, draining popliteal LNs (dLNs) and mLNs (as non-draining controls) were harvested and T cell expression of S1P1 was analyzed.
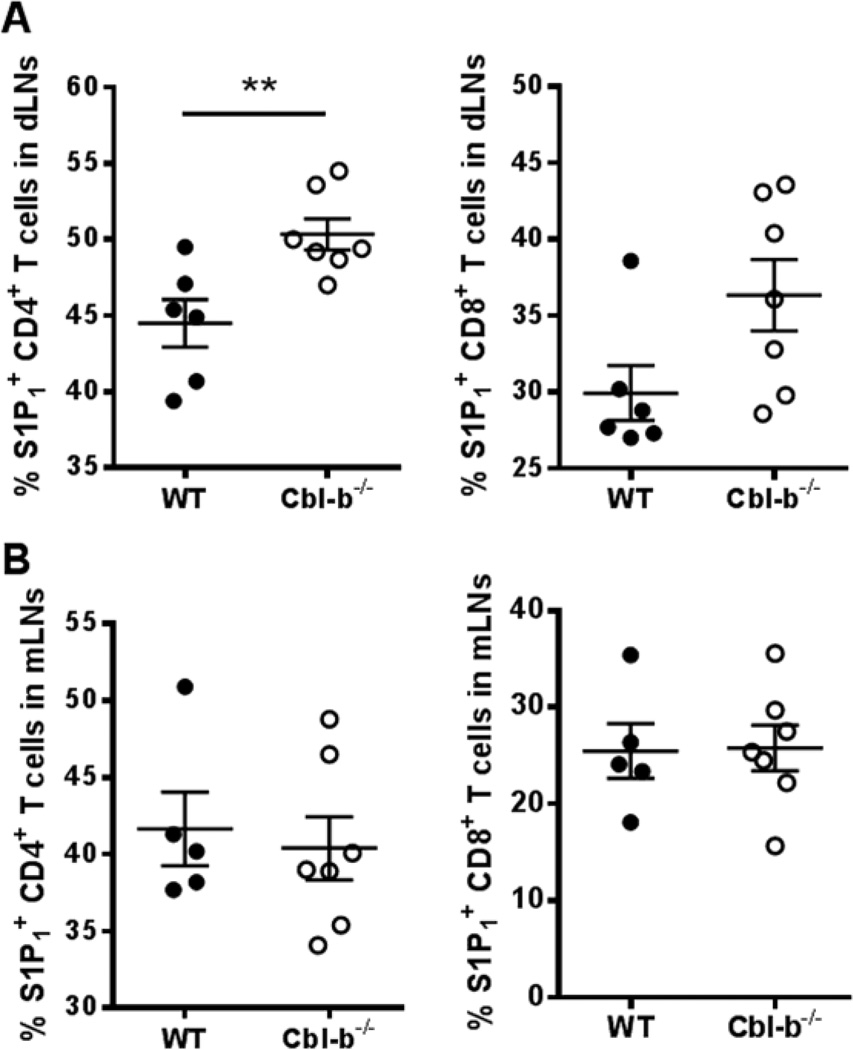
In dLNs, the frequency of Cbl-b−/− CD4+ T cells expressing S1P1 was significantly increased compared to WT CD4+ T cells (Figure 2A). In addition, Cbl-b−/− CD8+ T cells also showed the trend of increased frequency of S1P1+ cells compared to WT, although this did not reach statistical significance (Figure 2A). Mean fluorescence intensity (MFI) analysis also indicated a statistically significant increase in S1P1 MFI of Cbl-b−/− CD4+ T cells compared to WT cells in dLNs. For CD8+ T cells, there was no statistical difference in S1P1 MFI between WT and Cbl-b−/− mice (Supplemental Figure 2A). In contrast, in the mLNs (i.e., non-draining), the frequency of S1P1+ Cbl-b−/− CD4+ and CD8+ T cells did not differ from that of WT (Figure 2B). MFI analysis also showed no difference in S1P1 expression between WT and Cbl-b−/− CD4+ and CD8+ T cells in the mLNs (Supplemental Figure 2B). These results represent the first demonstration of increased S1P1 expression in Cbl-b−/− T cells and indicate that this increase correlates with the enhanced T cell activation status seen in the dLNs.
Figure 2.
Increased frequency of S1P1+ CD4+ T cells in draining LNs of Cbl-b−/− mice after CFA/MOG35–55 immunization. WT and Cbl-b−/− mice were immunized s.c. in the footpads with 150 µg of CFA and 80 µg of MOG35–55 and the popliteal LNs were harvested 4–5 days later. (A) Percentage of CD4+ T cells (left) and CD8+ T cells (right) that are S1P1+ in the popliteal LNs of WT and Cbl-b−/− mice. (B) Percentage of CD4+ T cells (left) and CD8+ T cells (right) that are S1P1+ in the mLNs of WT and Cbl-b−/− mice. **p<0.01 by Student’s t-test. The data shown are from three independent experiments.
To further characterize the increased S1P1 expression in activated Cbl-b−/− T cells, we analyzed the relationship between S1P1 expression and CD44 expression. We noted that the frequency of CD44hi CD4+ and CD8+ T cells were comparable between Cbl-b−/− and WT T cells in the dLNs. Surprisingly, we found that the increased S1P1 expression seen in Cbl-b−/− CD4+ T cells versus WT CD4+ T cells in the dLNs was restricted to the CD44lo populations, with the CD44hi populations showing no difference in S1P1 expression between Cbl-b−/− T cells versus WT T cells (Supplemental Figure 3). This may suggest that the enhanced S1P1 expression on Cbl-b−/− T cells occurs very early in the activation process and might account for an early egress from the LNs by Cbl-b−/− CD4+ T cells.
3.3. Expression of CD69 and CCR7 on Cbl-b−/− T cells after CFA/MOG35–55 immunization
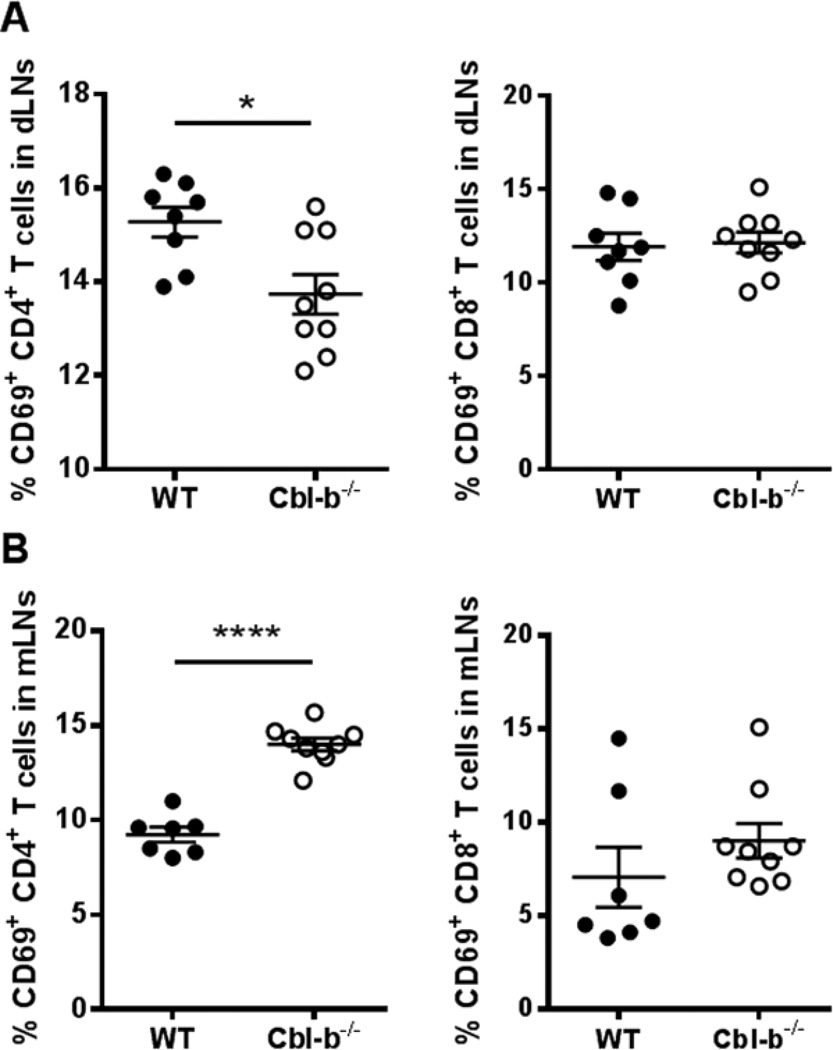
It has been documented that T cell expression of CD69 plays a role in the down-regulation of T cell S1P1 expression [28, 29]. We next analyzed the CD69 expression on WT and Cbl-b−/− T cells after immunization with CFA/MOG35–55. We found that the frequency of Cbl-b−/− CD4+ T cells expressing CD69 was significantly decreased compared to WT CD4+ T cells in the dLNs. Surprisingly, this was not the case with the dLN CD8+ T cells (Figure 3A). MFI analysis revealed no statistical difference in CD69 expression levels between WT and Cbl-b−/− CD4+ T cells (Supplemental Figure 4A), suggesting that the frequency of CD4+ T cells that express CD69, rather than the CD69 expression levels, is decreased in dLNs of Cbl-b−/− mice. In the non-draining mLNs, the frequency of CD69+ Cbl-b−/− CD4+ T cells, rather than being decreased, was significantly increased compared to WT CD4+ T cells (Figure 3B). The frequency of CD69+ Cbl-b−/− CD8+ T cells in the mLNs was again comparable to that of WT CD8+ T cells (Figure 3B). Despite the increased frequency of Cbl-b−/− CD4+ T cells expressing CD69 in mLNs, MFI analysis did not show any difference in CD69 expression between Cbl-b−/− and WT CD4+ and CD8+ T cells in mLNs (Supplemental Figure 4B). Since it is known that CD69 expression down-regulates the expression of S1P1, the decreased CD69 expression in Cbl-b−/− CD4+ T cells in the dLNs may play a role in the increase in S1P1 expression seen in these cells. Both the decreased frequency of CD69-expressing Cbl-b−/− CD4+ T cells in the dLNs, along with the increased S1P1 expression on these cells, may potentially contribute to their abnormal LN accumulation in vivo.
Figure 3.
Decreased frequency of CD69+ CD4+ T cells in draining LNs of Cbl-b−/− mice after CFA/MOG35–55 immunization. WT and Cbl-b−/− mice were immunized as in Figure 2 and the popliteal LNs were harvested 4–5 days later. (A) Percentage of CD4+ T cells (left) and CD8+ T cells (right) that are CD69+ in the popliteal LNs of WT and Cbl-b−/− mice. (B) Percentage of CD4+ T cells (left) and CD8+ T cells (right) that are CD69+ in the mLNs of WT and Cbl-b−/− mice. *p<0.05, ****p<0.0001 by Student’s t-test. The data shown are from three independent experiments.
In parallel to the CD44lo-restricted increased S1P1 expression in Cbl-b−/− CD4+ T cells, the decreased frequency of Cbl-b−/− CD4+ T cells expressing CD69 versus that of WT in the dLNs was again restricted to the CD44lo populations, with the CD44hi populations showing no difference in the frequency of CD69+ CD4+ T cells (Supplemental Figure 5). This may suggest that the further downregulation of CD69-expressing Cbl-b−/− CD4+ T cells, along with enhanced expression of S1P1 on these cells, occurs very early in the activation process and might account for an early egress from the LNs by Cbl-b−/− CD4+ T cells.
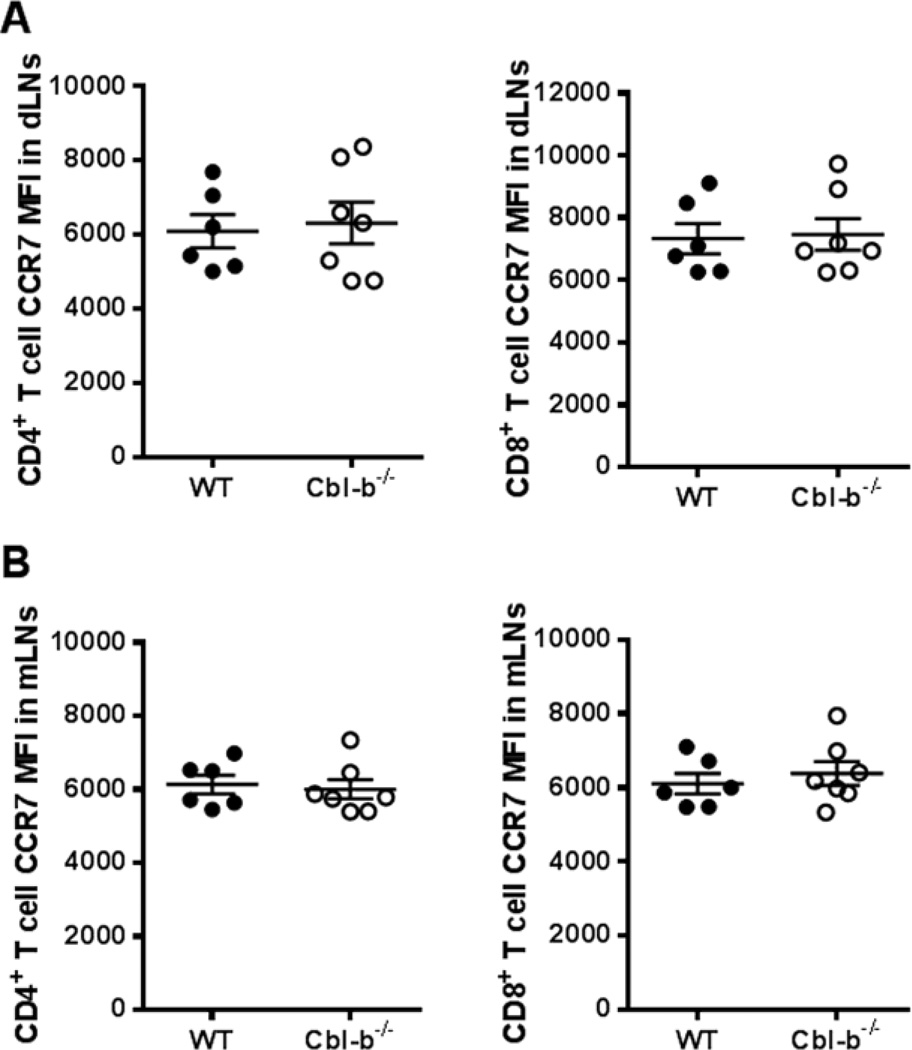
In addition to CD69, CCR7 has been shown to promote T cell entry and retention in lymph nodes [30, 31]. We next examined the status of CCR7 expression in WT and Cbl-b−/− T cells in dLNs and non-draining mLNs after immunization with CFA/MOG35–55. We found no significant difference in frequency of CCR7 expression between the WT and Cbl-b−/− T cells for both CD4+ and CD8+ T cells (data not shown). However, because approximately 80% of both WT and Cbl-b−/− T cells expressed CCR7, we analyzed CCR7 expression using mean fluorescence intensity (MFI). As seen in Figure 4A and B, there was no significant difference in CCR7 MFI between WT and Cbl-b−/− T cells in both dLNs and non-draining mLNs. Our results suggest that the abnormal LN accumulation noted for Cbl-b−/− CD4+ T cells is not related to alterations in CCR7 expression.
Figure 4.
CCR7 expression is unaltered in Cbl-b−/− T cells after CFA/MOG35–55 immunization. WT and Cbl-b−/− mice were immunized, the popliteal LNs harvested as in Figure 2, and stained with human CCL19-Ig and anti-human IgG-Alexa Fluor 488. (A) MFI of CCR7 expression on CD4+ T cells (left) and on CD8+ T cells (right) in draining popliteal LNs of WT and Cbl-b−/− mice. (B) MFI of CCR7 expression on CD4+ T cells (left) and on CD8+ T cells (right) in the mLNs of WT and Cbl-b−/− mice. Results were analyzed using Student’s t-test. The data shown are from three independent experiments.
3.4. Normal response of Cbl-b−/− mice to T cell lymphopenia induced by S1P lyase inhibitor, THI
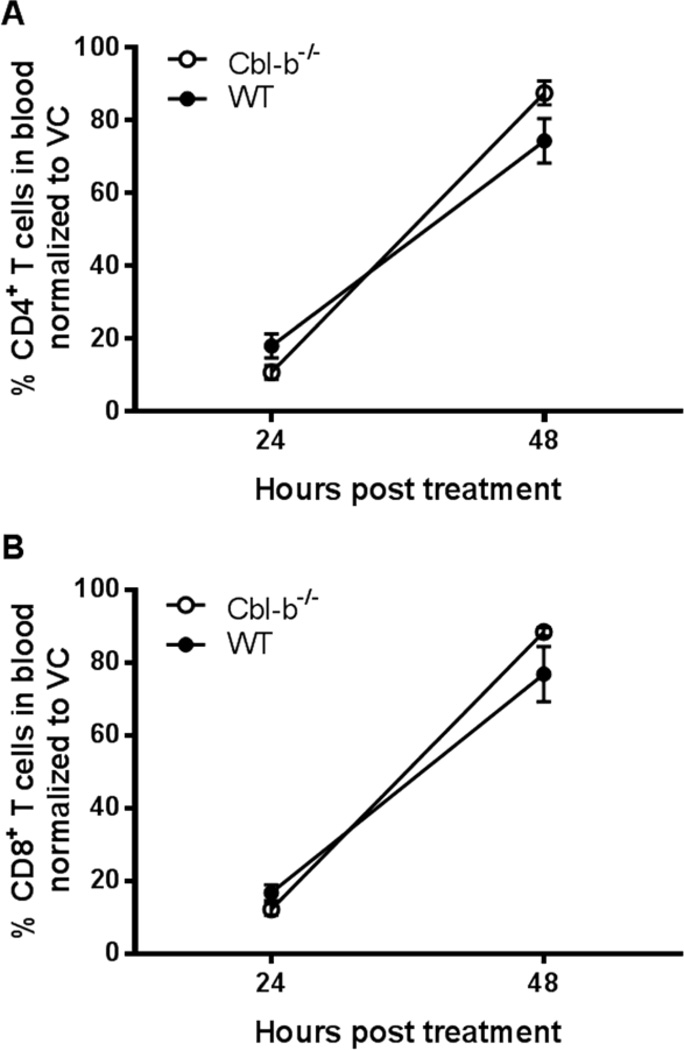
Our finding of the significantly increased frequency of Cbl-b−/− CD4+ T cells expressing S1P1 compared to WT CD4+ T cells in the dLNs, prompted us to more directly understand the impact of Cbl-b deficiency on T cell S1P1 physiology. We next compared S1P1 functional status in WT versus Cbl-b−/− T cells using two approaches. In the first approach, we tested the induction of T cell lymphopenia mediated through the enhancement of sphingosine-1-phosphate (S1P), the endogenous ligand of S1P1. For this, we utilized the S1P lyase inhibitor, THI (2-acetyl-4-tetrahydroxybutyl imidazole) [32]. S1P lyase normally degrades S1P and is highly expressed in the lymph nodes and other tissues compared to the blood, resulting in a S1P gradient between the tissues and the blood. This S1P gradient actively promotes the egress of lymphocytes from the LNs to the blood/lymph. The inhibition of S1P lyase by THI disrupts this S1P gradient. This results in internalization and loss of S1P1 expression on lymphocytes with entrapment of these cells in LNs and a significant decrease in blood lymphocytes [32]. Thus, we measured the response to THI by analyzing the decrease in the frequency of CD4+ and CD8+ T cells in the blood of the THI-treated mice.
Prior to THI treatment, the frequencies of CD4+ and CD8+ T cells in the blood did not differ between WT and Cbl-b−/− mice (data not shown). Of note, the absolute numbers of CD4+ and CD8+ T cells in the blood of Cbl-b−/− mice were higher than the absolute numbers of CD4+ and CD8+ T cells in the blood of WT mice prior to THI treatment (mean WT CD4+ T cells = 3.53 × 105/ml; Cbl-b−/− CD4+ T cells = 5.23 × 105/ml; WT CD8+ T cells = 1.98 × 105/ml; Cbl-b−/− CD8+ T cells = 3.00 × 105/ml). Twenty-four hours after treatment with THI, the frequency of blood CD4+ and CD8+ T cells of WT and Cbl-b−/− mice decreased significantly but equally (Fig. 5A and B). Forty-eight hours after THI treatment, the frequency of blood CD4+ and CD8+ T cells returned significantly towards pre-treatment levels and this return was also equal between WT and Cbl-b−/− mice (Figure 5A and B). Thus, we found that the T cell lymphopenia resulting from administration of the S1P lyase inhibitor, THI, was comparable between Cbl-b−/− and WT mice. This suggests that the functional status of S1P1 in Cbl-b−/− T cells is normal under noninflammatory, naïve condition.
Figure 5.
Equal lymphopenic response of WT and Cbl-b−/− mice induced by the S1P lyase inhibitor, THI. WT and Cbl-b−/− mice were administered 10 mg/kg of THI or vehicle (VC) via gavage, and percent of CD4+ and CD8+ T cells in the total blood leukocytes was determined by flow cytometry. (A) Percentage of blood CD4+ T cells and (B) Percentage of blood CD8+ T cells of WT or Cbl-b−/− mice, 24 and 48 hrs after administration of VC or THI. Percentages represent mean percentage of T cells in THI-treated mice / mean percentage of T cells in VC-treated mice x100 at each time point. Results were analyzed using Student’s t-test. n=3–4/group. The data shown are from two independent experiments.
3.5. Comparable response of Cbl-b−/− and WT mice to T cell lymphopenia induced by a selective S1P1 agonist, SEW2871
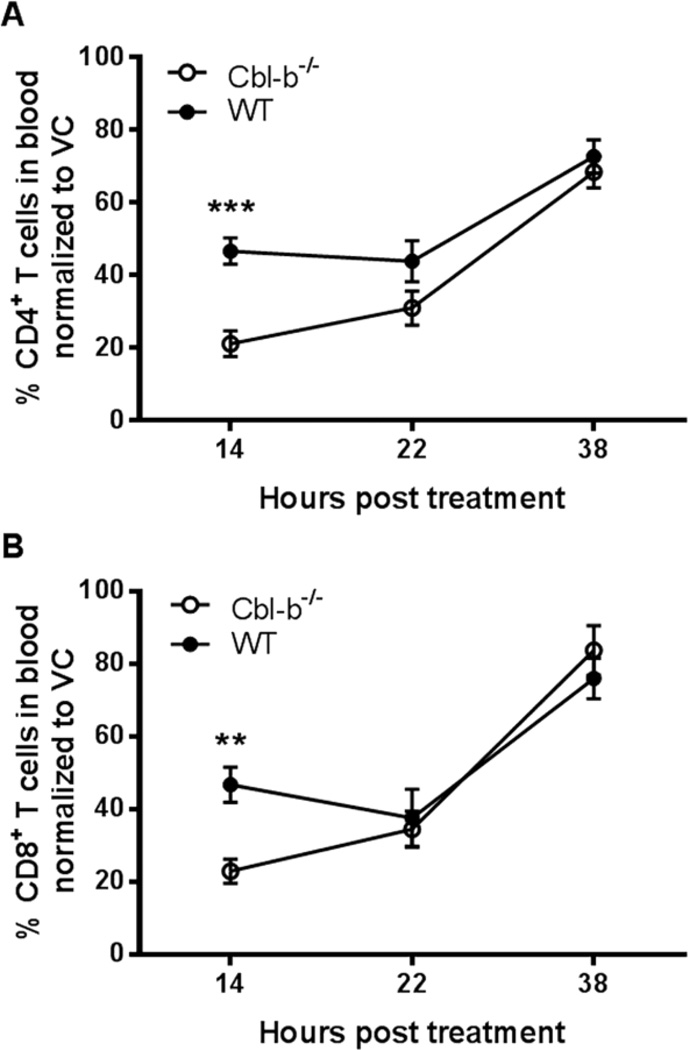
In the second functional approach, we tested the induction of T cell lymphopenia mediated through the administration of an exogenous S1P1-selective agonist, SEW2871 [33]. SEW2871 directly binds to S1P1 on T cells, resulting in S1P1 internalization and subsequent entrapment of T cells in LNs [33]. While Cbl-b−/− mice showed more CD4+ and CD8+ T cell lymphopenia compared to WT mice in response to SEW2871 at 14 hours post treatment, we found comparable responses in WT and Cbl-b−/− mice at 22 and 38 hours (Figure 6A and B). The reasons for the enhanced early response to SEW2871 by Cbl-b−/− mice T cells in a non-inflammatory, naïve condition is unclear. However, taking into account the later time points, at which time there is no difference between the SEW2871-induced T cell lymphopenia in WT and Cbl-b−/− mice, overall our results with THI and SEW2871 suggest no significant alteration in the S1P1 functional status of Cbl-b−/− T cells in a non-activated milieu.
Figure 6.
Equal lymphopenic response of WT and Cbl-b−/− mice induced by the selective S1P1 agonist, SEW2871. WT and Cbl-b−/− mice were administered 20 mg/kg of SEW2871 or vehicle (VC) via gavage, and percentage of CD4+ and CD8+ T cells in the total blood leukocytes was determined by flow cytometry. (A) Percentage of blood CD4+ T cells and (B) Percentage of blood CD8+ T cells of WT or Cbl-b−/− mice treated with VC or SEW2871 at 14, 22 and 38 hrs after treatment. Percentages represent mean percentage of T cells in SEW2871-treated mice / mean percentage of T cells in VC-treated mice x100 at each time point. **p<0.01, ***p<0.001 by Student’s t-test. n=4–7/group. The data shown are from five independent experiments.
3.6. Exacerbated EAE yet high efficacy of FTY720 in Cbl-b−/− mice during EAE
Our results to this point suggested that under activating conditions, Cbl-b−/− CD4+ T cells demonstrate enhanced expression of S1P1 and decreased expression of CD69 and that these characteristics potentially result in enhanced LN egress. By extension, these results also suggested that MS patients with CBLB SNPs might have abnormalities in T cell trafficking and that these could impinge on the efficacy of treatment with FTY720. To address this possibility, we next asked whether FTY720 would be less effective in treating EAE in Cbl-b−/− versus mice WT mice. Theoretically, this approach would, for the first time test the efficacy of FTY720 in the context of Cbl-b dysfunction and T cell hyper-reactivity – both characteristics associated with MS.
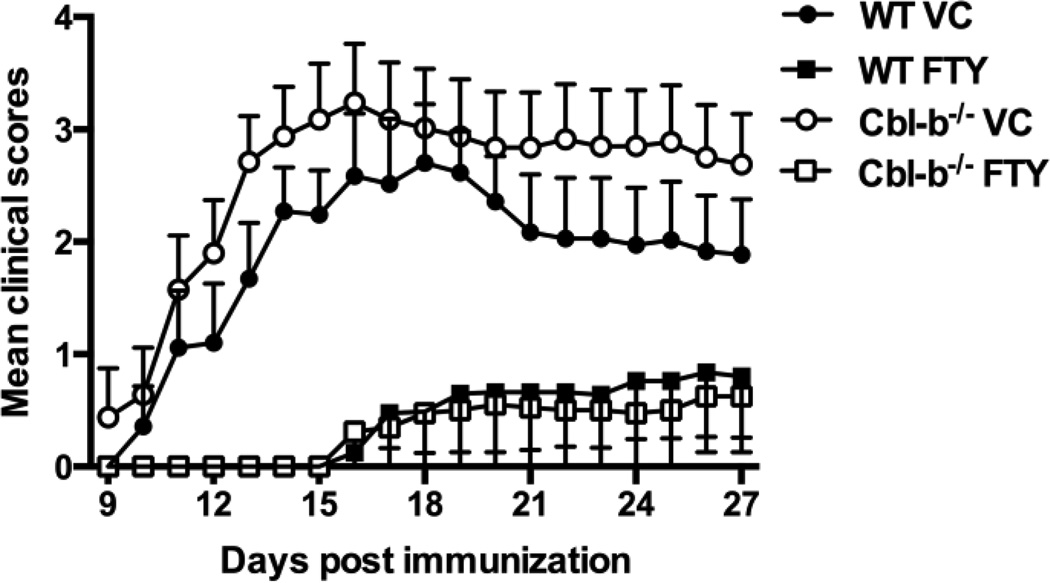
We induced EAE in WT and Cbl-b−/− mice using CFA and MOG35–55, and on the same day began treating these mice with either 0.375 mg/kg of FTY720 or the vehicle control (VC). Based on previous published studies in which FTY720 was used in treating EAE in WT mice, we chose an intermediate dosage of FTY720 but administered it only every 5 days rather than daily as in most of the earlier studies [23, 25, 34]. In the vehicle-treated mice, we found an enhancement of EAE in Cbl-b−/− mice compared to WT mice (Mann-Whitney test: ***p<0.0004, Figure 7) as previously reported by other investigators [5, 16]. Despite our use of a restricted FTY720 treatment regimen (i.e., administered only every 5th day), we noted significant inhibition of EAE by FTY720 in WT mice compared to VC-treated WT mice (****p<0.0001) (Figure 7). Most importantly however, despite the enhanced baseline EAE and the evidence for both decreased CD69 and enhanced S1P1 expression in activated T cells in Cbl-b−/− mice, we also found significant inhibition of EAE by FTY720 in Cbl-b−/− mice compared to VC-treated Cbl-b−/− mice (****p<0.0001) (Figure 7). These results suggest that FTY720 can inhibit EAE in the context of both Cbl-b deficiency and hyperactive T cell responses.
Figure 7.
Efficacy of FTY720 in inhibiting EAE in Cbl-b−/− mice. EAE was induced in WT and Cbl-b−/− mice by s.c. immunization with 165 µg of MOG35–55 and 300 µg of CFA. 150 ng of Pertussis toxin was also given i.p. on day 0 and 2. On the day of EAE-induction, the mice were administered either 0.375 mg/kg of FTY720 (FTY) or vehicle (VC) via gavage. FTY720 or vehicle was then administered every five days until day 20. Clinical scores: 1 = tail flaccidity, 2 = abnormal gait, 3 = hind leg paralysis, 4 = front leg paralysis, 5 = death. The disease severity differed significantly between the VC-treated WT group vs. the VC-treated Cbl-b−/− group, ***p=0.0004. In addition, the disease severity differed significantly between the VC-treated WT group vs. the FTY720-treated WT group, ****p<0.0001, and also between the VC-treated Cbl-b−/− group vs. the FTY720-treated Cbl-b−/− group, ****p<0.0001 by Mann-Whitney test. The data shown are from two independent experiments (n = 7–8/treatment group in total).
4. Discussion
A number of therapeutic options are now available for patients with MS. Unfortunately, almost all of these agents are associated with severe side effects and are limited in their efficacy to only a proportion of patients with RRMS. This has spurred interest in a “personalized medicine” approach involving individualization of treatment regimens for MS patients based not only on clinical sub-types of MS but also on an individual’s genetic profile. In identifying genetic factors potentially involved in the pathogenesis of MS, GWAS have revealed a number of potentially MS-relevant genetic polymorphisms or SNPs. While understanding the role of these SNPs in the disease pathogenesis is a primary research goal, another goal has emerged, namely to identify whether these SNPs can associate with (and potentially predict) differential responses to the various therapeutic approaches.
One gene identified as being associated with MS is CBLB. GWAS have identified an association of MS with three different polymorphisms in the CBLB gene [1–3]. As recently discussed, while not all GWAS have identified these MS-associated CBLB gene SNPs, the different outcomes of the various GWAS may be related to differing ethnic origins and geographical locations of the populations analyzed [35]. Interestingly, decreased Cbl-b mRNA and decreased Cbl-b protein expression in PBMCs of patients with RRMS have also previously been described [36]. The CBLB gene encodes for the E3 ubiquitin ligase Cbl-b. Cbl-b is known to be involved in regulating a number of cellular processes but has been most characterized as a regulator of T cell activation. In Cbl-b−/− mice, T cells have been shown to be hyperactive [4, 5], co-stimulation-independent [5], have a lower activation threshold [4, 5], and to be resistant to Tregs [9, 10], TGF-β [37], and the induction of anergy [38]. Interestingly, many of these abnormalities have also been documented in MS patients [11–15]. In addition, Cbl-b−/− mice have been shown to have increased susceptibility to EAE [5, 16].
Sturner et al. recently studied T cell functional abnormalities associated with the CBLB SNP – MS-risk allele, rs12487066 [17]. This specific CBLB SNP was studied because it had previously been identified in a GWAS of individuals with MS recruited from the U.K. and the US. These authors believed their patient cohort, derived from Northern Germany, would be similar to the U.K./U.S. cohort. In this study, it was reported that in RRMS patients this risk allele altered both T cell Cbl-b expression levels and T cell function, and specifically interfered with the normal regulatory function of type I IFN on T cells [17]. Thus, this recent study for the first time associated a specific CBLB SNP with both an alteration in immunological function and an abnormal response to an MS treatment option.
Although Cbl-b−/− mice and MS patients demonstrate many similar T cell abnormalities, Cbl-b−/− mice have not been specifically used as a model for studying MS until now. Given the immunological similarities between Cbl-b−/− mice and a subset of patients with MS, these mice may provide a better model than wild type C57BL/6 mice for studying many aspects of the pathophysiology of MS. Moreover in the present study we postulated that Cbl-b−/− mice, because of their T cell hyper-reactivity, may prove to be a relevant new tool for studying personalized treatment approaches in MS, and specifically for a subset of MS patients with polymorphisms in the CBLB gene.
FTY720 (Fingolimod/Gilenya), an FDA-approved orally available drug for treating RRMS, is postulated to target the sphingosine-1-phosphate receptors, S1P1, S1P3, S1P4 and S1P5 [18]. Though controversial, FTY720 theoretically mediates its therapeutic effect in MS by causing the degradation of the lymphocyte homing receptor S1P1 [19], which then blocks the egress of T and B cells from lymph nodes resulting in an inability of the immune system to mount an attack on self-antigens in the CNS [20]. As with all present treatment choices for MS, FTY720 is not effective in all of RRMS patients [21]. Importantly, pre-clinical studies demonstrating the efficacy of this drug were carried out in C57BL/6 mice [22–25], and thus not in mice with either known genetic alterations related to MS or in mice with MS-like hyper-reactive cellular immune responses.
Our specific interest in studying the potential inter-relationship between abnormalities in Cbl-b and FTY720 initially arose from two sources. First, it is known that Cbl-b normally regulates the PI3K-Akt pathway [39] and thus affects many targets downstream of this pathway including the S1P1-regulating transcription factor KLF2 [40, 41]. Second, we had preliminary results indicating that T cells from Cbl-b−/− mice demonstrated impaired LN accumulation when adoptively transferred into RAG-1−/− mice, suggesting a potential abnormality in T cell S1P1 function in Cbl-b−/− mice. We thus postulated that Cbl-b−/− mice, and by extension MS patients carrying CBLB gene SNPs, would be refractory to the EAE/MS-inhibiting effect of FTY720.
We now report for the first time that Cbl-b−/− T cells demonstrate: 1) abnormal accumulation in the mLNs upon adoptive transfer into RAG-1−/− mice; 2) enhanced S1P1 expression in dLN CD4+ T cells after immunization in vivo and; 3) decreased CD69 expression in dLN CD4+ T cells after immunization in vivo. Thus, in addition to the well-documented list of altered T cell functions resulting from deletion of Cbl-b, we now add altered expression of trafficking-related molecules and altered trafficking after adoptive transfer. Importantly, these newly defined abnormalities are associated with activated Cbl-b−/− T cells. In non-activated scenarios, Cbl-b−/− T cells show no difference from WT T cells in expression of trafficking-related molecules and no functional differences in response to exogenous agents that modulate S1P1-dependent T cell trafficking.
In T cells, activation of the PI3K-Akt pathway downstream of the TCR and co-stimulatory receptors such as CD28 has been shown to negatively regulate S1P1 expression by inactivating the Foxo1-KLF2 axis [42, 43]. Cbl-b normally negatively regulates the PI3K-Akt pathway [39, 44, 45] and the deletion of Cbl-b is known to enhance the activation of this pathway [44, 45]. This enhanced activation of the PI3K-Akt pathway in Cbl-b−/− mice leads to increased inactivation of Foxo1 [44], which theoretically inhibits KLF2 activity and should result in decreased expression of S1P1. Our finding of enhanced S1P1 expression in activated Cbl-b−/− CD4+ T cells suggests the involvement of a more complex mechanism, or at least more complex kinetics, involved in the regulation of S1P1 in Cbl-b−/− CD4+ T cells. Given that T cell expression of CD69 is known to result in the decreased expression of S1P1 [28], our findings of enhanced expression of S1P1 by Cbl-b−/− CD4+ T cells may be related to the decreased expression of CD69 by these cells after immunization.
Because of our findings of abnormal mLN accumulation after adoptive transfer, enhanced expression of S1P1 and decreased expression of CD69 by Cbl-b−/− CD4+ T cells under activating conditions, we postulated that a characteristic of activated Cbl-b−/− CD4+ T cells might be enhanced lymph node egress. By extension, these results suggested that MS patients with CBLB SNPs might also have abnormalities in CD4+ T cell trafficking and that these could impinge on the efficacy of treatment with FTY720. As such, our primary goal was to utilize Cbl-b−/− mice as a new model for studying the efficacy of FTY720 in this subset of MS patients.
In these studies, we opted to be on the low end of published effective doses of FTY720 in EAE by administering a middle range dose, but utilized an every fifth day, rather than the usual, every-day dosing schedule. In inducing EAE in vehicle-treated WT and Cbl-b−/− mice, we found the expected enhancement of EAE in Cbl-b−/− mice compared to WT mice. However, despite enhanced EAE in vehicle-treated Cbl-b−/− mice, as well as enhanced S1P1 and decreased CD69 expression in activated Cbl-b−/− CD4+ T cells, FTY720 treatment nevertheless resulted in a significant inhibition of EAE in both WT mice and Cbl-b−/− mice.
It is not clear at this time why Cbl-b−/− mice are sensitive to FTY720 despite enhanced S1P1 and decreased CD69 T cell expression under activating conditions. It is possible that the degree of alteration in S1P1 and CD69 expression is not sufficient to overcome the immune-regulatory effects mediated by FTY720. In this regard, it is of interest that Thangada et al. reported that knock-in mice expressing an internalization-defective S1P1 were still sensitive to the lymphopenic effects of FTY720 [46]. Importantly, there are also suggestions that FTY720 may inhibit EAE/MS through mechanisms other than lymph node trapping of T cells. These may include effects on endothelial cells, the blood brain barrier, or resident cells of the CNS [47]. While this will be a topic of future study in our laboratory, our finding of note in the present study is the efficacy of FTY720 in inhibiting EAE in the context of Cbl-b dysfunction.
In sum, in these studies we have identified novel characteristics of T cell trafficking related to Cbl-b deficiency and these characteristics are potentially also relevant to this subset of MS patients. Moreover, we have documented for the first time the efficacy of FTY720 in inhibiting EAE in the context of both Cbl-b deficiency and MS-like T cell hyper-reactivity. Consistent with the report of Sturner et al. [17], our present results suggests that Cbl-b−/− mice may prove to be a valuable new model both for studying pathogenic mechanisms in MS and for testing personalized treatment approaches in a subset of MS patients. Finally, our results suggest for the first time that those MS patients with aberrations in Cbl-b function are still likely to be excellent candidates for treatment with FTY720.
Supplementary Material
Highlights.
Polymorphisms in the CBLB gene have been associated with MS
Cbl-b−/− T cell lymph node accumulation is abnormal after transfer into RAG-1−/− mice
Activated Cbl-b−/− T cells express enhanced S1P1 and decreased CD69
Despite trafficking abnormalities the MS-approved drug FTY720 significantly inhibits EAE in Cbl-b−/− mice
Cbl-b−/− mice may represent a new model for testing therapeutic approaches in MS
Acknowledgements
This work was supported by the National Multiple Sclerosis Society, RG4070B7/2 (RBC) and the National Institute of Health, AI097375 (KMK). The authors would like to thank Dr. Alexander Bénéchet, Ms. Leigh Maher, Dr. Shobha Thangada, and Dr. Evan Jellison for their advice and technical assistance with these studies.
Abbreviations
- CBLB/Cbl-b
Casitas-B lineage lymphoma-b
- Cbl-b−/−
Cbl-b-deficient
- RRMS
relapsing remitting MS
- S1P
sphingosine-1-phosphate
- S1P1
sphingosine-1-phosphate receptor 1
- THI
2-acetyl-4-tetrahydroxybutyl imidazole
- SEW2871
5-[4-phenyl-5-(trifluoromethyl)-2-thienyl]-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The author (s) declare that there are no conflicts of interest.
References
- 1.International Multiple Sclerosis Genetics, C. Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 2.International Multiple Sclerosis Genetics, C., C. Wellcome Trust Case Control. Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanna S, Pitzalis M, Zoledziewska M, Zara I, Sidore C, Murru R, Whalen MB, Busonero F, Maschio A, Costa G, et al. Variants within the immunoregulatory CBLB gene are associated with multiple sclerosis. Nat Genet. 2010;42:495–497. doi: 10.1038/ng.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 5.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 6.Paolino M, Thien CB, Gruber T, Hinterleitner R, Baier G, Langdon WY, Penninger JM. Essential role of E3 ubiquitin ligase activity in Cbl-b-regulated T cell functions. J Immunol. 2011;186:2138–2147. doi: 10.4049/jimmunol.1003390. [DOI] [PubMed] [Google Scholar]
- 7.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, Li Z, Solway J, Tao E, Chiang YJ, et al. E3 ubiquitin ligase Cbl-b suppresses proallergic T cell development and allergic airway inflammation. Cell Rep. 2014;6:709–723. doi: 10.1016/j.celrep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruber T, Hinterleitner R, Hermann-Kleiter N, Meisel M, Kleiter I, Wang CM, Viola A, Pfeifhofer-Obermair C, Baier G. Cbl-b mediates TGFbeta sensitivity by downregulating inhibitory SMAD7 in primary T cells. J Mol Cell Biol. 2013;5:358–368. doi: 10.1093/jmcb/mjt017. [DOI] [PubMed] [Google Scholar]
- 9.Adams CO, Housley WJ, Bhowmick S, Cone RE, Rajan TV, Forouhar F, Clark RB. Cbl-b(−/−) T cells demonstrate in vivo resistance to regulatory T cells but a context-dependent resistance to TGF-beta. J Immunol. 2010;185:2051–2058. doi: 10.4049/jimmunol.1001171. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 11.Kofler DM, Marson A, Dominguez-Villar M, Xiao S, Kuchroo VK, Hafler DA. Decreased RORC-dependent silencing of prostaglandin receptor EP2 induces autoimmune Th17 cells. J Clin Invest. 2014;124:2513–2522. doi: 10.1172/JCI72973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28- costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–1194. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M, Buckner JH. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci Transl Med. 2013;5:170ra115. doi: 10.1126/scitranslmed.3004970. [DOI] [PubMed] [Google Scholar]
- 15.Scholz C, Patton KT, Anderson DE, Freeman GJ, Hafler DA. Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J Immunol. 1998;160:1532–1538. [PubMed] [Google Scholar]
- 16.Gruber T, Hermann-Kleiter N, Hinterleitner R, Fresser F, Schneider R, Gastl G, Penninger JM, Baier G. PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation. Sci Signal. 2009;2:ra30. doi: 10.1126/scisignal.2000046. [DOI] [PubMed] [Google Scholar]
- 17.Sturner KH, Borgmeyer U, Schulze C, Pless O, Martin R. A Multiple Sclerosis-Associated Variant of CBLB Links Genetic Risk with Type I IFN Function. J Immunol. 2014;193:4439–4447. doi: 10.4049/jimmunol.1303077. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 19.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 21.Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 22.Chiba K, Kataoka H, Seki N, Shimano K, Koyama M, Fukunari A, Sugahara K, Sugita T. Fingolimod (FTY720), sphingosine 1-phosphate receptor modulator, shows superior efficacy as compared with interferon-beta in mouse experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2011;11:366–372. doi: 10.1016/j.intimp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, Chiba K. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–448. [PubMed] [Google Scholar]
- 24.Quancard J, Bollbuck B, Janser P, Angst D, Berst F, Buehlmayer P, Streiff M, Beerli C, Brinkmann V, Guerini D, et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol. 2012;19:1142–1151. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Rossi S, Lo Giudice T, De Chiara V, Musella A, Studer V, Motta C, Bernardi G, Martino G, Furlan R, Martorana A, et al. Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br J Pharmacol. 2012;165:861–869. doi: 10.1111/j.1476-5381.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 27.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 28.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 30.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 33.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Brieland JK, Kim JH, Chen YJ, O'Neal J, O'Neil SP, Tu TW, Trinkaus K, Song SK. Diffusion tensor imaging detects treatment effects of FTY720 in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2013;26:1742–1750. doi: 10.1002/nbm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon-Sanchez J, Singleton A. Genome-wide association studies in neurological disorders. Lancet Neurol. 2008;7:1067–1072. doi: 10.1016/S1474-4422(08)70241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou WB, Wang R, Deng YN, Ji XB, Huang GX, Xu YZ. Study of Cbl-b dynamics in peripheral blood lymphocytes isolated from patients with multiple sclerosis. Neurosci Lett. 2008;440:336–339. doi: 10.1016/j.neulet.2008.05.089. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 38.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 40.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 41.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 42.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 43.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, Zhang J. T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells. J Immunol. 2013;191:632–639. doi: 10.4049/jimmunol.1202068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.