Abstract
Post-traumatic stress disorder (PTSD) is a disorder that involves impaired regulation of the fear response to traumatic reminders. This study tested how women with male-perpetrated interpersonal violence-related PTSD (IPV-PTSD) differed in their brain activation from healthy controls (HC) when exposed to scenes of male–female interaction of differing emotional content. Sixteen women with symptoms of IPV-PTSD and 19 HC participated in this study. During magnetic resonance imaging, participants watched a stimulus protocol of 23 different 20 s silent epochs of male–female interactions taken from feature films, which were neutral, menacing or prosocial. IPV-PTSD participants compared with HC showed (i) greater dorsomedial prefrontal cortex (dmPFC) and dorsolateral prefrontal cortex (dlPFC) activation in response to menacing vs prosocial scenes and (ii) greater anterior cingulate cortex (ACC), right hippocampus activation and lower ventromedial prefrontal cortex (vmPFC) activty in response to emotional vs neutral scenes. The fact that IPV-PTSD participants compared with HC showed lower activity of the ventral ACC during emotionally charged scenes regardless of the valence of the scenes suggests that impaired social perception among IPV-PTSD patients transcends menacing contexts and generalizes to a wider variety of emotionally charged male–female interactions.
Keywords: Parental PTSD, emotion regulation, fMRI, human interaction, interpersonal violence
INTRODUCTION
Among adult women, intimate partner violence (IPV) is a leading trigger of post-traumatic stress disorder (PTSD) (Pico-Alfonso, 2005). PTSD is characterized by symptoms of reexperiencing, avoidance/numbing and hyperarousal in response to having experienced a traumatic event (American Psychiatric Association, 2000). In the Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV-TR), PTSD had been classified as an anxiety disorder (American Psychiatric Association, 2000), but has recently been reclassified as a form of ‘stress and trauma related disorder’ in the DSM-V (American Psychiatric Association, 2013). This reclassification is consistent with specific neural activation patterns in response to environmental triggers and corresponding stress physiology. Meta-analysis has linked PTSD to increased activity in structures such as the amygdala and insula, similar to what has been found for other anxiety disorders (Etkin and Wager, 2007; Hayes et al., 2012). PTSD, more specifically, was also associated with less activation of the ventromedial prefrontal cortex (vmPFC) (Dickie et al., 2008, 2011; Bluhm et al., 2012) and rostral anterior cingulate cortex (ACC) (Kim et al., 2008; Offringa et al., 2013). Broad evidence suggests that the dorsal–caudal regions of the ACC and mPFC are involved in the appraisal of negative emotion (Kalisch et al., 2006; Maier et al., 2012), while ventral–rostral portions of the ACC and mPFC have a regulatory function in relation to the limbic regions that are involved in generating emotional responses (Etkin et al., 2011; Motzkin et al., 2015).
Previous studies have shown that increased activation in limbic regions such as the amygdala is often inversely related to vmPFC activation (Sripada et al., 2012; Stevens et al., 2013; Killgore et al., 2014). This suggests that not only isolated brain regions but circuits that are related to emotion regulation processes are altered among individuals suffering from PTSD as compared with non-PTSD healthy controls (HC). Behavioral studies have also linked PTSD to diminished empathic resonance (Nietlisbach et al., 2010), diminished mentalizing and emotion recognition (Poljac et al., 2011; Mazza et al., 2012) as well as greater levels of alexithymia (Declercq et al., 2010). In all, PTSD has been shown to affect brain regions that are related to both emotion expression and regulation.
PTSD is characterized by difficulties in emotion regulation particularly when new experiences remind patients of aspects of their trauma. Among patients with PTSD, there has been a multitude of imaging studies looking at PTSD and emotionally charged stimuli, which are both trauma (Shin et al., 2004; Frewen et al., 2008) and non-trauma related (Felmingham et al., 2010; Offringa et al., 2013; Thomaes et al., 2013). No study to our knowledge has used stimuli involving human interaction, though. Steuwe et al. (2014) have shown that the orientation of gaze within faces in still photographs, and in particular, whether the gaze is directed toward the participant-as-observer, moderates how interpersonal violence-related PTSD (IPV-PTSD) is related to neural activity. PTSD patients with a history of childhood abuse showed increased activation in the superior colliculus and the periaqueductal gray, as well as increased subcortical activity to direct gaze as compared with averted gaze. This pattern differed from HC who showed a top-down (dorsomedial prefrontal, temporal junction and temporal pole) pattern of activation.
Among PTSD imaging studies that have considered the perception of different facial expressions of emotion, only one study focused on an IPV-PTSD sample (Fonzo et al., 2010). This study found that IPV-PTSD patients displayed greater activation in the dorsal ACC and mPFC when they performed an emotional face-matching task for male compared with female targets. Their ventral ACC (vACC) activation was decreased in this condition—an effect that did not arise in controls. This suggested that female experience of male-perpetrated violence may lead to alternative neural processing of male–female interaction.
Given the particular importance of intimate-partner violence among women with IPV-PTSD, it is important to go beyond static stimuli and to consider dynamic male–female interactions. Women with IPV-PTSD may have a specific reaction that is particularly attuned to cues for male aggression. These cues are often manifested as non-verbal dynamic gestures that intimidate, and that are unpredictable and crescendoing in intensity so as to suggest escalation of threat or impending violence. Thus, a film stimulus that reflects the interplay of human interaction and emotion is compelling for individuals suffering from IPV-PTSD. Therefore, this study tested how women with male-perpetrated IPV-PTSD differed in their brain activation from HC when exposed to motion-picture scenes of male–female interactions of differing emotional content. We used video stimuli of neutral, menacing and prosocial interactions. These stimuli were selected with the goal of extending the validity of previous findings on emotion perception and regulation in IPV-PTSD; they were specifically chosen because video stimuli displaying menacing male–female interactions might function as a traumatic reminder, thereby, interfering with emotion and arousal regulation.
We were interested in responses to scenes that depicted men intending to do harm to women (menacing scenes) compared with positively valenced high-arousal male–female interactions (prosocial/romantic scenes). We thus hypothesized that when watching menacing as compared with prosocial scenes, women with IPV-PTSD compared with HC would show greater activation of limbic system structures, including dACC (Burgdorf and Panksepp, 2006; Etkin and Wager, 2007; Hayes et al., 2012). This would echo prior results in response to still images of negative vs positive affective valence (Fonzo et al., 2010).
Second, we wanted to know whether there would be a more generalized emotion-processing deficit related to IPV-PTSD. For an individual who has repeatedly experienced IPV, the interpretation of emotional communication may be negatively biased (Plana et al., 2014). Individuals suffering from IPV-PTSD may misread emotional communication in high-arousal male–female interactions regardless of whether the scene is positively (i.e. prosocial/romantic scene) or negatively (i.e. menacing scene) valenced. This misreading is linked to their hypervigilance for and overestimation of the likelihood of aggression in high-arousal situations. Thus, some neural responses should differentiate between high-arousal (both menacing and prosocial scenes, regardless of their valence) vs low-arousal interactions (neutral scenes).
METHOD
Participants
Recruitment. Participants were recruited via flyers posted at the University of Geneva Hospitals and the University of Geneva as well as at community centers, daycares, schools and domestic violence agencies and shelters at which mothers would have access to the study information. This magnetic resonance imaging (MRI) study was nested within an ongoing study of interpersonal violence and intergenerational transmission of related trauma and psychopathology (Schechter and Rusconi, 2014). Thirty-nine mothers of young children (12–42 months of age) who were eligible for an MRI scan participated. Two participants were excluded from analysis due to excessive motion in the scanner. Of the remaining participants, 16 were diagnosed with IPV-PTSD (mean age = 32.3 years; s.d. = 6.5 years), 19 were HC (mean age = 34.5 years; s.d. = 5.6 years) and 5 mothers did not belong in either group (i.e. had subthreshold IPV-PTSD; mean age = 32.4; s.d. = 5.2 years). Results for these latter participants are thus not presented here. Groups did not differ significantly from each other with respect to age [F(2, 39) = 0.65, P = 0.526]. All participants of the IPV-PTSD group had experienced traumatic life events (i.e. domestic violence) as adults, while 42% of HC had experienced at least one type of physical or sexual form of domestic violence.
Diagnosis. During an initial videotaped interview, which was part of the pre-MRI protocol, IPV-PTSD participants and HC underwent a variety of psychometrics including the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995) to assess lifetime PTSD and the Post-traumatic Symptom Checklist-short version (PCL-S) to assess current PTSD symptoms.
Participants were diagnosed as having IPV-PTSD if their CAPS score was ≥55 and their PCL-S score was ≥40, and as HC if their CAPS score was <30, and their PCL-S score was <25. If one of their scores was in between those values they were classified as subthreshold IPV-PTSD. All IPV-PTSD participants would also be diagnosed with PTSD by an experienced clinician, in accordance with the DSM-IV-TR (American Psychiatric Association, 2000).
Compared with HC (mean = 4.26; s.d. = 2.00), IPV-PTSD participants (mean = 6.07, s.d. = 2.19) had a significantly lower socioeconomic status (SES); t(32) = −2.51, P = 0.017). SES was assessed with the Geneva Sociodemographic Questionnaire (Sancho Rossignol et al., 2010) and measured by the scale endorsed by the Swiss Society of Neonatology and based on a paper by Largo et al. (1989).
Procedure
Procedures for Visits Before MRI. The pre-MRI protocol consisted of an hour-long screening plus two 2 h visits. The latter included structured and semi-structured clinical interviews and a behavioral protocol with participant mothers and children. Before their participation, mothers gave informed consent. The study procedure had been approved by the University of Geneva Hospital institutional review board in accordance with the Helsinki Declaration of Human Rights (World Medical Association, 1999).
MRI Procedure. Participants saw 23 movies in pseudorandomized order. After each movie, participants were asked to rate the valence of the predominant emotion that the movie elicited on a scale of 1 (very negative) to 7 (very positive) and then to rate the arousal level of that emotion again on a scale of 1 (no arousal) to 7 (maximum arousal). The time limit for those judgments was 4 s. Participants practiced the rating procedure before entering the MRI scanner.
After scanning, we asked participants whether they had previously seen the movies from which the excerpts were taken. To ensure that participants had actually looked at the stimuli, we also filmed participants’ eye gaze with an eye tracker (Eye-Trac 6, Applied Science Laboratories, Bedford, Massachusetts, USA) during the scan. Because of space limitations, image acquisition and preprocessing are described in the supplementary data.
Stimuli
Twenty-three silent 20 s video excerpts that displayed male–female interactions were extracted from feature films. These excerpts were grouped into three conditions: eight excerpts displayed menace and high levels of negative affect, eight displayed prosocial/romantic interaction and moderately to highly positive affect and seven displayed neutral affect. The categorization of those excerpts was done by an unpublished rating study, the details of which can be found in the supplementary data.
Hypothesis-driven analyses
To verify whether there were any differences in how the two groups judged the arousal and valence of the respective movies, we performed two analyses of variance (ANOVAs). Both had group (IPV-PTSD vs HC) as a between-participants factor and film condition (i.e. menacing, prosocial and neutral) as a within-participants factor.
Imaging analysis at the first level was focused on two main conditions: menacing and prosocial. Neutral scenes were considered as the control-condition of comparison. We thus produced a contrast between the average activation in response to menacing as compared with neutral scenes. We also measured the difference between average activation in response to prosocial as compared with neutral scenes. These two contrasts represent our conditions. Images acquired while participants rated the valence and arousal of the movie scenes were modeled, but not analyzed.
Second-level analysis then focused on group differences between IPV-PTSD participants and HC and analyzed these via a general linear model (GLM) using each of the specified contrasts of interest of the first-level analysis as a within-participants factor and group as a between-participants factor. A main effect of group could thus be attributed to a group difference when comparing all arousing scenes (i.e. menacing and prosocial) independent of valence to neutral scenes, while a group × film-condition interaction could be attributed to the difference between menacing and prosocial scenes being different between the groups. While beyond the focus of this article, main effects of film condition are also listed in Table 1.
Table 1.
Regions that show significant differences in activation between mothers with IPV-PTSD and HC when watching scenes depicting menace and prosocial scenes combined (emotional scenes) contrasted with neutral scenes
| Cluster size | xyz | Region | This cluster also includes | Mean menace vs neutral | Mean prosocial vs neutral | Main effect group | Main effect film condition | Group × film-condition interaction | Tukey test on significant interactions | R-value of the correlation of PTSD symptom severity and activation within IPV-PTSD group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
F(1, 33) | P | F(1, 33) | P | F(1, 33) | P | P values; only values <0.05 given | P value | |||||||
| Main effect group | Emotion vs neutral contrast | |||||||||||||||
| IPV-PTSD > controls | ||||||||||||||||
| 28 | 30 | −10 | −17 | Right Hippocampus |
|
|
12.499 | .001 | 0.612 | 0.440 | 0.018 | 0.895 |
|
|
||
| Controls > IPV-PTSD | ||||||||||||||||
| 51 | −15 | 41 | −14 | Left vmPFC | ACC |
|
|
15.549 | <0.001 | 1.781 | 0.191 | 0.015 | 0.905 |
|
|
|
| 338 | −33 | −34 | 58 | Left postcentral gyrus | Left SPL |
|
|
15.948 | <0.001 | 0.003 | 0.960 | 0.509 | 0.481 |
|
|
|
| 33 | 42 | −49 | 46 | Right IPL |
|
|
13.433 | 0.001 | 0.522 | 0.475 | 1.510 | 0.228 |
|
|
||
| 30 | −60 | −16 | 40 | Left precentral gyrus |
|
|
12.737 | 0.001 | 0.847 | 0.364 | 0.117 | 0.734 |
|
|
||
| 105 | 30 | −37 | −29 | Right cerebellum anterior lobe |
|
|
19.475 | <0.001 | 17.725 | <0.001 | 2.466 | 0.126 |
|
|
||
| Interaction group × film condition | Menacing vs prosocial contrast | |||||||||||||||
| 709 | 36 | 50 | 16 | Right dlPFC |
|
|
|
0.101 | 0.752 | 5.913 | 0.021 | 26.348 | <0.001 |
|
|
|
| 33 | 36 | 26 | 43 | Right dlPFC |
|
|
3.313 | 0.078 | 4.245 | 0.047 | 16.745 | <0.001 |
|
|
||
| 99 | −18 | 26 | 55 | Left dlPFC |
|
|
0.005 | 0.943 | 0.000 | 0.994 | 16.378 | <0.001 |
|
|
||
| 33 | 39 | −31 | 19 | Right insula (operculum) |
|
|
0.037 | 0.848 | 0.064 | 0.802 | 14.20 | 0.001 | 0.047A |
|
||
| 32 | −54 | 11 | 34 | Left precentral gyrus |
|
|
0.074 | 0.788 | 12.830 | 0.001 | 23.082 | <0.001 | <0.001A |
|
||
| 48 | 48 | −58 | 52 | Right IPL |
|
|
2.079 | 0.159 | 7.752 | 0.009 | 14.188 | 0.001 |
|
|
||
| 66 | 3 | −58 | −5 | Right cerebellum anterior lobe |
|
|
1.017 | 0.321 | 6.411 | 0.016 | 13.806 | 0.001 |
|
|
||
| Main effect film condition | ||||||||||||||||
| Menacing > prosocial | ||||||||||||||||
| 501 | −9 | 50 | 28 | dmPFC |
|
|
|
3.235 | 0.081 | 37.042 | <0.001 | 11.540 | 0.002 |
|
||
| 1744 | 27 | 8 | 55 | Right dlPFC |
|
|
|
0.731 | 0.399 | 50.565 | <0.001 | 0.188 | 0.668 |
|
||
| 32 | 3 | 17 | 22 | dACC |
|
|
0.001 | 0.972 | 21.858 | <0.001 | 1.253 | 0.271 | 0.002A | |||
| 573 | −51 | 35 | −2 | Left OFC | Left precentral gyrus |
|
|
0.218 | 0.644 | 31.856 | <0.001 | 0.471 | 0.497 |
|
||
| 16099 | 42 | −76 | 19 | Right Occipital lobe |
|
|
|
0.497 | 0.486 | 376.080 | <0.001 | 0.269 | 0.608 |
|
||
| 667 | −21 | 8 | 64 | Left Superior frontal gyrus |
|
|
|
1.028 | 0.318 | 42.579 | <0.001 | 3.257 | 0.080 |
|
||
| Prosocial > menacing | ||||||||||||||||
| 176 | −36 | −22 | 19 | Left insula | Left rolandic operculum |
|
|
3.175 | 0.084 | 14.787 | 0.001 | 2.292 | 0.140 |
|
||
| 45 | 39 | −19 | 19 | Right insula | Right rolandic operculum |
|
|
3.058 | 0.090 | 14.778 | 0.001 | 3.088 | 0.88 |
|
||
| 82 | 9 | −7 | 28 | (mid) cingulate | Limbic lobe |
|
|
3.561 | 0.068 | 12.947 | 0.001 | 0.596 | 0.446 |
|
||
| 254 | −6 | −22 | 64 | Paracentral lobule |
|
|
|
4.811 | 0.035 | 22.128 | <0.001 | 2.549 | 0.120 |
|
||
| 38 | 51 | −28 | 1 | Right STG |
|
|
0.586 | 0.450 | 13.795 | 0.001 | 0.814 | 0.373 | 0.009F | |||
| 32 | −45 | −70 | 46 | Left angular gyrus |
|
|
3.296 | 0.079 | 17.902 | <0.001 | 11.727 | 0.002 |
|
|||
| 40 | 45 | −64 | 49 | Right angular gyrus |
|
|
0.063 | 0.804 | 15.896 | <0.001 | 11.328 | 0.002 | <0.001F | |||
| 36 | 60 | −1 | 7 | Right precenral gyrus |
|
|
0.466 | 0.500 | 9.878 | 0.004 | 2.136 | 0.153 | 0.009F | |||
Legend for interaction tests: A = IPV-PTSD menacing-neutral vs IPV-PTSD prosocial-neutral; B = IPV-PTSD menacing-neutral vs HC menacing-neutral; C = IPV-PTSD menacing-neutral vs HC prosocial-neutral; D = IPV-PTSD prosocial-neutral vs HC menacing-neutral; E = IPV-PTSD prosocial-neutral vs HC menacing-neutral; F = HC menacing-neutral vs HC prosocial-neutral. Abbreviations: ACC = Anterior Cingulate Cortex; dACC dorsal Anterior Cingulate Cortex; dlPFC = dorsolateral Prefrontal Cortex; dmPFC = dorsomedial Prefrontal Cortex; IPL = Inferior Parietal Lobule; OFC = Orbitofrontal Cortex; PCC = Posterior Cingulate Cortex; SMA = Supplementary Motor Area; STG = Superior Temporal Gyrus; vmPFC = ventromedial Prefrontal Cortex.
A Monte Carlo Simulation with 10 000 iterations indicated that the probability of finding an arbitrarily significant effect is reduced to an alpha of 0.05 when implementing the condition that a cluster of at least 27 contiguous voxels displays an effect of P < 0.005. Application of this threshold corrects for the multiple comparisons produced by the high number of voxels considered. For our whole-brain analysis, the threshold of significance was thus defined as an uncorrected P < 0.005 with cluster size of at least 27 contiguous voxels. In addition, we also performed an ROI analysis for each amygdala with an uncorrected threshold of P < 0.05.
Post hoc tests
For each significant cluster identified in the GLM, we subsequently extracted beta-values of the peak voxel and performed a Tukey test to define the origins of possible group × condition interactions. Because there was a significant group difference in SES (i.e. IPV-PTSD < HC), we entered SES as a covariate in our statistical analyses. Because it did not alter results significantly, SES was not included in any reported analysis. We also tested whether areas displaying group-related effects additionally showed continuous changes related to IPV-PTSD severity within the IPV-PTSD group. For all clusters revealing a significant main effect of group, we therefore calculated a Pearson correlation coefficient of IPV-PTSD symptom severity with the contrast of mean activation of all emotional (prosocial and menacing) conditions compared with the neutral condition. This was done only within the IPV-PTSD group. Similarly, for all clusters that had revealed significant group × condition effects, we calculated a Pearson correlation coefficient of IPV-PTSD symptom severity with the menacing vs prosocial contrast. IPV-PTSD symptom severity was determined as the average of the z-standardized PCL-S and CAPS scores.
RESULTS
Behavioral ratings
An ANOVA comparing IPV-PTSD and HC groups, as to their appraisal of emotional valence evoked by the film stimuli resulted in a significant effect of film condition [F(2, 30) = 120.1, P < 0.001]. Results were similar to those of the aforementioned validation study in that prosocial scenes were rated as having more positive valence followed by neutral scenes. Menacing scenes were rated as having the most negative valence. There was no significant main effect of group [F(1, 31) = 2.3, P = 0.137] or group × film-condition interaction [F(2, 30) = 1.0, P = 0.371].
An ANOVA comparing IPV-PTSD and HC participants with respect to appraisal of arousal also found an effect of film condition [F(2, 30) = 37.4, P < 0.001]. This was in accordance with results of the previous validation study as well, i.e. menacing scenes were the most arousing followed by prosocial and finally neutral stimuli, respectively. There was no significant main effect of group [F(1, 31) = 0.5, P = 0.494] or group × film-condition interaction [F(2, 30) = 1.5, P = 0.241].
IPV-PTSD participants did not significantly differ from HC with respect to the number of movies they had previously seen (prosocial films seen previously: PTSD mean = 31%, HC mean = 28%, t(30) = −0.79, P = 0.72; neutral films seen previously: PTSD mean = 39%, HC mean = 32%, t(30) = −0.94, P = 0.36; menacing films seen previously: PTSD mean = 35%, HC mean = 29%, t(30) = −0.65, P = 0.52).
fMRI analysis
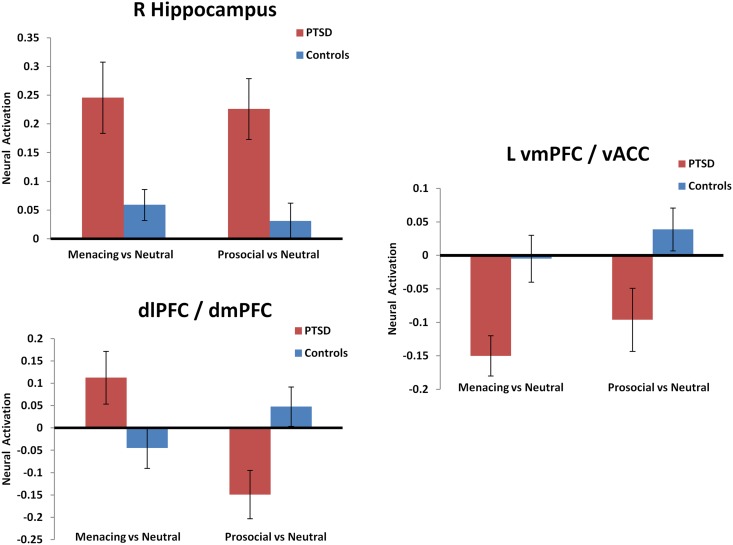
Main effects of group: The whole-brain analysis showed a main effect of group (IPV-PTSD participants compared with HC) in the right hippocampus. Post hoc analysis showed this to be mainly due to the IPV-PTSD participants’ greater activation response to arousing stimuli (both prosocial and menacing) compared with neutral stimuli (Table 1). HC showed higher activation than IPV-PTSD participants in a cluster that included the vmPFC and vACC. This effect was driven by an IPV-PTSD within-group contrast that showed significantly less activity during all emotional vs neutral scenes Figure 1). Furthermore, HC displayed higher activation than the IPV-PTSD group in a cluster that included the left postcentral gyrus and superior parietal lobe, as well as in the right inferior parietal lobe, and in the left precentral gyrus. These differences were driven by the IPV-PTSD group, which showed deactivation during emotional scenes (i.e. lower activity than during neutral scenes). The reverse was observed in HC (Table 1). Correlations of IPV-PTSD symptom severity with neural activation in the clusters showing a main effect of group—while mostly pointing into the expected direction—were almost altogether non-significant, the only exception concerning the cluster in the left precentral gyrus (r = −0.52, P = 0.045).
Fig. 1.
Mean difference in peak voxel activation when participants watched menacing vs neutral film conditions and prosocial vs neutral film conditions. Shown are selected clusters that showed significant group differences. The mean difference for participants diagnosed with IPV-PTSD is shown in red, and the mean difference for HC is shown in blue. Error bars indicate the standard error. The clusters shown are the right hippocampus (top left) dorso-lateral and dorsomedial prefrontal cortex (dlPFC and dmPFC, botttom left) and the left ventromedial prefrontal cortex (vmPFC)/ventral anterior cingulate (vACC; right side).
Group-by-condition interactions: The comparison of menacing with prosocial scenes revealed significant group differences in a cluster in the right insula as well as in a cluster that encompassed parts of the bilateral dorsolateral prefrontal cortex (dlPFC) and dorsomedial prefrontal cortex (dmPFC). These cluster differences were driven by the IPV-PTSD group having shown greater deactivation than HC when watching prosocial scenes and at the same time greater activation than HC when watching menacing scenes (see Table 1 and Figures 1 and 2). Another interaction within the right inferior parietal lobule was driven by activation in the HC when watching prosocial scenes; deactivation was observed in all other instances. Correlations of IPV-PTSD symptom severity with activations in all clusters revealing group-by-condition interactions failed to reach significance.
Fig. 2.
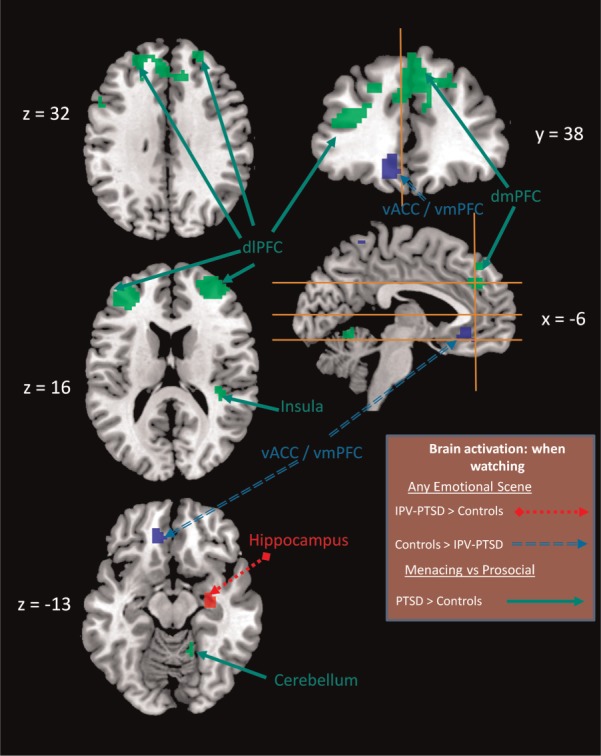
Significant main effects of group and group × condition interactions from an ANOVA comparing participants with IPV-PTSD with HC in two contrasts of film conditions (menacing vs neutral and prosocial vs neutral). Slice placement is according to the atlas by the Montreal Neurological Institute. Red areas indicate a main effect of group for menacing and prosocial film conditions, where participants with IPV-PTSD showed greater activation than HC. Blue areas indicate a main effect of group for menacing and prosocial film conditions, where participants with IPV-PTSD showed less activation than HC. Green areas indicate a group × condition interaction, where participants with IPV-PTSD showed greater activation than HC during menacing compared with prosocial scenes (see also Table 1). Abbreviations: dlPFC = dorsolateral prefrontal cortex, dmPFC = dorsomedial prefrontal cortex, vACC = ventral anterior cingulate cortex, vmPFC = ventromedial prefrontal cortex.
Results of the ROI analyses regarding amygdala activity did not reach significance either [right amygdala: menacing vs neutral condition, PTSD: mean = 0.179, s.d. = 0.222, HC: mean = 0.064, s.d. = 0.185; prosocial vs neutral condition: PTSD: mean = 0.147, s.d. = 0.238, HC: mean = 0.079, s.d. = 0.152; intercept: F(1, 33) = 14.812, P = 0.001; main effect of condition: F(1, 33) = 0.084, P = 0.774; main effect of group: F(1, 33) = 2.273, P = 0.141; interaction group × condition: F(1, 33) = 0.63, P = 0.434; left amygdala: menacing vs neutral condition, PTSD: mean = 0.049, s.d. = 0.229, HC: mean = 0.065, s.d. = 0.194; prosocial vs neutral condition: PTSD: mean = −0.026, s.d. = 0.306, HC: mean = 0.065, s.d. = 0.194; intercept: F(1, 33) = 2.005, P = 0.166; main effect of condition: F(1, 33) = 0.57, P = 0.45; main effect of group: F(1, 33) = 0.97, P = 0.332; interaction group × condition: F(1, 33) = 0.71, P = 0.405].
DISCUSSION
We studied whether women with IPV-PTSD differ from HC in their experience of specifically menacing and more general emotional human interactions.
Menacing compared with prosocial conditions
The dorsal areas of the mPFC and ACC showed a greater difference in activation in response to menacing vs prosocial scenes among IPV-PTSD participants as compared with HC; this difference in the dorsal mPFC and ACC was thus specific to the stimulus’ valence. The dACC and dmPFC have been linked to appraisal of negatively biased emotion (Etkin et al., 2011), and more generally to cognitive strategies of emotion control (Kalisch, 2009). IPV-PTSD participants as compared with HC also had greater dlPFC activation when watching scenes depicting menacing vs prosocial scenes. The dlPFC plays a role in many executive functions and has been linked to emotion regulation of both positively and negatively valenced stimuli (Viinikainen et al., 2010; Vrticka et al., 2012).
We speculate that IPV-PTSD subjects recruited the dlPFC in addition to the dmPFC and dACC in an effort to attain additional regulatory control so as to counteract the potential dysregulating effect of the menacing stimulus-as-stressor. Individuals suffering from IPV-PTSD likely activate the dorsal PFC (both medial and lateral) to downregulate their own arousal in response to the perception of violent social interactions. We can imagine that if such an arousal response has already been triggered by the film stimuli, in real-life situations, these individuals’ cortical regulatory function may be strained by the increased demands of the situation to a level beyond that which can be regulated by the dorsal PFC. The latter would possibly impair the implementation of socially adaptive conflict-resolution strategies.
This study also found that the right posterior insula showed greater activation among IPV-PTSD participants when seeing menacing vs prosocial scenes. The latter is consistent with previous research, in which the insula showed increased activation among PTSD patients (Etkin and Wager, 2007; Hayes et al., 2012). The posterior insula has been demonstrated to be hyperactivated among PTSD participants on viewing emotionally negative scenes (Mazza et al., 2013). Consistent with this picture, insula activation has also been linked to flashbacks (Whalley et al., 2013) and pain sensitivity among PTSD subjects (Strigo et al., 2010).
More generally, the insula has been implicated in homeostasis involving salience and the default mode networks (Sripada et al., 2012). Even though the referenced study concentrated more on the anterior than posterior insula, our own finding seems consistent with it, suggesting that to women with IPV-PTSD, menacing male–female interactions are particularly threatening to the self-regulation of emotion and arousal.
Emotional compared with neutral scenes
In our study, IPV-PTSD participants compared with HC displayed lower activity in the vACC and vmPFC during all emotional (i.e. menacing and prosocial) scenes compared with neutral scenes. The ventral part of the mPFC and ACC has been linked to the regulation of limbic activation underlying emotional expression more generally (Etkin et al., 2011). This finding of corticolimbic dysregulation fits with a general alteration of arousal-related networks in PTSD (Felmingham et al., 2009). Studies using personalized traumatic scripts as stimuli have found similar results to this study, particularly in relation to the association of PTSD with an altered pattern of ACC and mPFC activation (e.g. Lanius et al., 2001; Britton et al., 2005). These studies consistently revealed specific activation patterns in response to stimuli involving traumatic triggers, such that PTSD patients displayed less rostral ACC and mPFC activity when subjected to negative personalized trauma-related scripts as compared with neutral scripts (Lanius et al., 2001, 2003; Britton et al., 2005). Our study supports these findings, as the stimuli that depict menace elicited similar group differences. Yet, our study also extends these earlier results by suggesting that it is the arousing or general emotional character of the stimuli rather than their specific negative valence, which evoked this group difference. We therefore interpret these findings to reflect a more general (i.e. not menacing-specific) IPV-PTSD emotion-processing deficit as elicited by stimuli involving male–female interactions.
Previous studies have found that PTSD patients manifest a diminished capacity for self-reflective functioning when stressed, as reflected by reduced dmPFC and vmPFC activation (Schechter et al., 2005, 2012; Bluhm et al., 2012). Alexithymia has also been associated with deactivation of the vACC and vmPFC (Frewen et al., 2008). Thus, to respond to one of our main questions, diminished vACC/vmPFC activity seems to be related to a generalized emotion processing deficit among IPV-PTSD individuals, and this deficit extends beyond questionnaires and comorbidities to the immediate perception of male–female interactions.
This emotion-processing deficit is also consistent with our observation that when IPV-PTSD participants watch emotional vs neutral scenes, they show greater activation of the right hippocampus as compared with HC. This result extends previous research on PTSD (Etkin and Wager, 2007), in which connectivity of the posterior hippocampus to the default-mode network (i.e. posterior cingulate cortex, precuneus and pregenual ACC) (Buckner et al., 2008) has been shown to differentiate PTSD patients from HC and from generalized anxiety disorder patients (Chen and Etkin, 2013). The increased difference in hippocampal activity between emotional and neutral stimuli among IPV-PTSD participants may represent a higher level of salience of the former stimuli due to their association with traumatic memory traces (Brohawn et al., 2010). The rationale for this is that any kind of emotionally charged male–female interaction, regardless of its valence, may be generalized to signify a potential threat to women with male-perpetrated IPV-PTSD because many of them previously had intimate relationships with men who had later become perpetrators. We speculate that HC—even those with a history of IPV—experience greater situation specificity and thus do not generalize male behavior in the same way as women with IPV-PTSD do. The hippocampal activation noted may also play a role in IPV-PTSD women’s routine appraisal of their partners’ (or other male) behavior.
While the hippocampus played a significant role in this study, the amygdala did not. Our data show that both groups similarly activated the right amygdala while viewing both the menacing and prosocial film clips, while the left amygdala differed in no group between arousing and neutral scenes.
Implications of our findings
This study, having used human dynamic interactive stimuli, supports the generalizability of previous studies that used still-image stimuli and found that mPFC and ACC activation is consistent with corticolimbic dysregulation (Bryant et al., 2008; Kim et al., 2008; Fonzo et al., 2010, 2013). Our findings provide further evidence that those studies can indeed be related to how IPV-PTSD impacts the participant’s perception of human interactions.
We saw a clear functional dissociation of IPV-PTSD-associated processing of the dorsal compared with the ventral part of both the mPFC and the ACC. IPV-PTSD participants showed a specificity of emotional valence in the dorsal PFC; the more negative the valence of presented films, the more IPV-PTSD participants activated the dorsal PFC. In the vACC/vmPFC on the other hand, their activity was better predicted by arousal (Figure 1). We esteem that when an individual suffers from IPV-PTSD, the dorsal and ventral parts of the mPFC and ACC change their specific function with respect to emotion perception and regulation. This may be related to a previous study that found that vmPFC activation and alexithymia (emotional awareness) were positively correlated among HC, and negatively correlated among PTSD participants (Frewen et al., 2008), suggesting that functionality in the vmPFC changes in relation to socioemotional content. We speculate that changes of vmPFC/vACC function may occur owing to an increased need for cortico-limbic downregulation in IPV-PTSD participants.
We did not find a difference as to how the groups assessed the valence and arousal levels among the film scenes by post hoc questionnaires. Group differences in fMRI activation, despite the absence of group differences on subjective report, suggests that women with IPV-PTSD differ from HC in their neural functioning and processes of emotion perception and regulation in response to human interaction in ways that are beyond conscious experience. We also found that most of the effects related to group differences or group × film-condition interactions we observed were not accompanied by a significant correlation with IPV-PTSD symptom severity within the IPV-PTSD group. This may be task specific, as several previous studies showed evidence for continuous effects of PTSD on brain activation (Shin et al., 2004; Simmons et al., 2008; Fonzo et al., 2010; Moser et al., 2013).
Because our study was part of a wider mother–child study, our participants were all mothers of toddlers, the latter being dependent on their caretaker’s help for emotion regulation. The majority of IPV-PTSD mothers had a history of childhood abuse and subsequent partner violence that had been frequently witnessed by their children. Children of women who witnessed and/or experienced IPV as children, have an elevated risk of experiencing IPV as adults (Cannon et al., 2009; McIntyre and Widom, 2011). How group differences in dlPFC, mPFC, ACC and insula activation relates to maternal sensitivity is thus a research area that we are additionally pursuing. Maternal sensitivity is related to mothers’ appraisal of their child’s emotional communication (Schechter and Rusconi, 2014). Maternal sensitivity among IPV-PTSD mothers may be linked to their emotion perception in response to adult male–female interaction stimuli in the present experiment. This is consistent with a generalization of a fear-conditioned response to their toddlers’ developmentally determined emotion dysregulation (Schechter et al., 2012, 2014; Moser et al., 2013); such a response might be adaptive to a violent environment, but may come at the expense of parental sensitivity (Schechter et al., 2010; Sturge-Apple et al., 2012).
Limitations
Because a sizeable part of the control group had not experienced traumatic domestic violence, we cannot exclude the possibility, that some of the group differences found in our analyses were due to differences in previous life events rather than participants having or not having developed IPV-PTSD.
Finding continuous effects within the IPV-PTSD group was not a primary goal of the article. The fact that we did not find continuous effects of symptom severity within the IPV-PTSD group may be due to the fact that the chosen methods in this study were better suited to finding discrete (i.e. categorical) rather than continuous effects of IPV-PTSD.
CONCLUSION
This study has shown that women with IPV-PTSD have a characteristic pattern of activation, specifically involving the dmPFC, dlPFC and dACC when seeing scenes of menacing compared with prosocial male–female interaction. This pattern suggests that emotion perception and appraisal, which are essential to social interaction, are significantly affected among women with IPV-PTSD when film-stimuli signal menace. Additionally, emotional male–female interaction scenes regardless of the valence of the emotions in IPV-PTSD are related to heightened right hippocampal activation, suggesting higher personal relevance of these stimuli (Zhu et al., 2012). IPV-PTSD mothers compared with HC also tended to deactivate the vACC during all emotional scenes, independent of their valence. This finding supports the likelihood of a more general emotion regulation deficit among IPV-PTSD patients for moderate- to high-arousal interactions.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This research was supported by the National Center of Competence in Research (NCCR) ‘SYNAPSY—The Synaptic Bases of Mental Diseases’ financed by the Swiss National Science Foundation (n° 51AU40_125759), the Gertrude von Meissner Foundation, the Oak foundation and la Fondation Prim’Enfance.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Frewen PA, Coupland NC, Densmore M, Schore AN, Lanius RA. Neural correlates of self-reflection in post-traumatic stress disorder. Acta Psychiatrica Scandinavica. 2012;125(3):238–46. doi: 10.1111/j.1600-0447.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57(8):832–40. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biological Psychiatry. 2010;68(11):1023–30. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping. 2008;29(5):517–23. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and Biobehavioral Reviews. 2006;30(2):173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Cannon EA, Bonomi AE, Anderson ML, Rivara FP. The intergenerational transmission of witnessing intimate partner violence. Archives of Pediatrics and Adolescent Medicine. 2009;163(8):706–8. doi: 10.1001/archpediatrics.2009.91. [DOI] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–98. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq F, Vanheule S, Deheegher J. Alexithymia and posttraumatic stress: subscales and symptom clusters. Journal of Clinical Psychology. 2010;66(10):1076–89. doi: 10.1002/jclp.20715. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46(5):1522–31. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49(7):1771–8. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119(1):241–7. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Research. 2009;173(1):59–62. doi: 10.1016/j.pscychresns.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, Paulus MP, Stein MB. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research. 2013;211(2):93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA, Dozois DJ, Neufeld RW, Pain C, Hopper JW, Densmore M, Stevens TK. Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Journal of Abnormal Psychology. 2008;117(1):171–81. doi: 10.1037/0021-843X.117.1.171. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33(8):1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30(4):1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL, Rosso IM, Simon NM, Pollack MH, Rauch SL. Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety. 2014;31(2):150–9. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon SJ, Jeong DU, Lyoo IK. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42(4):268–77. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. The American Journal of Psychiatry. 2001;158(11):1920–2. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological Psychiatry. 2003;53(3):204–10. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G. Significance of prenatal and postnatal factors in the development of AGA-preterm children at 5–7 years. Developmental Medicine and Child Neurology. 1989;31(4):440–56. doi: 10.1111/j.1469-8749.1989.tb04022.x. [DOI] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J, Philipsen A, van Elst LT, Kalisch R, Tuscher O. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One. 2012;7(11):e50120. doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Giusti L, Albanese A, Mariano M, Pino MC, Roncone R. Social cognition disorders in military police officers affected by posttraumatic stress disorder after the attack of An-Nasiriyah in Iraq 2006. Psychiatry Research. 2012;198(2):248–52. doi: 10.1016/j.psychres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Mazza M, Tempesta D, Pino MC, Catalucci A, Gallucci M, Ferrara M. Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(7):575–83. doi: 10.1007/s00406-013-0394-3. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Widom CS. Childhood victimization and crime victimization. Journal of Interpersonal Violence. 2011;26(4):640–63. doi: 10.1177/0886260510365868. [DOI] [PubMed] [Google Scholar]
- Moser DA, Aue T, Wang Z, Rusconi Serpa S, Favez N, Peterson BS, Schechter DS. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress. 2013;16(5):493–502. doi: 10.3109/10253890.2013.816280. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry. 2015;77(3):276–84. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietlisbach G, Maercker A, Rossler W, Haker H. Are empathic abilities impaired in posttraumatic stress disorder? Psychological Reports. 2010;106(3):832–44. doi: 10.2466/pr0.106.3.832-844. [DOI] [PubMed] [Google Scholar]
- Offringa R, Handwerger Brohawn K, Staples LK, Dubois SJ, Hughes KC, Pfaff DL, Vanelzakker MB, Davis FC, Shin LM. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biology of Mood and Anxiety Disorders. 2013;3(1):10. doi: 10.1186/2045-5380-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico-Alfonso MA. Psychological intimate partner violence: the major predictor of posttraumatic stress disorder in abused women. Neuroscience and Biobehavioral Reviews. 2005;29(1):181–93. doi: 10.1016/j.neubiorev.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Plana I, Lavoie MA, Battaglia M, Achim AM. A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. Journal of Anxiety Disorders. 2014;28(2):169–77. doi: 10.1016/j.janxdis.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Poljac E, Montagne B, de Haan EH. Reduced recognition of fear and sadness in post-traumatic stress disorder. Cortex. 2011;47(8):974–80. doi: 10.1016/j.cortex.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Sancho Rossignol A, Luthï Faivre F, Suardi F, Moser D, Cordero M, Rusconi Serpa S, Schechter D. Geneva Socio-demographic Questionnaire (GSQ) Geneva, Switzerland: Child and Adolescent Psychiatry Service, University of Geneva Hospitals; 2010. [Google Scholar]
- Schechter DS, Coots T, Zeanah CH, Davies M, Coates SW, Trabka KA, Marshall RD, Liebowitz MR, Myers MM. Maternal mental representations of the child in an inner-city clinical sample: violence-related posttraumatic stress and reflective functioning. Attachment and Human Development. 2005;7(3):313–31. doi: 10.1080/14616730500246011. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, McCaw JE, Myers MM. Autonomic functioning in mothers with interpersonal violence-related posttraumatic stress disorder in response to separation-reunion. Developmental Psychobiology. 2014;56(4):748–60. doi: 10.1002/dev.21144. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Social Cognitive and Affective Neuroscience. 2012;7(8):969–79. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Rusconi S. Understanding how traumatised mothers process their toddlers‘ affective communication under stress: towards preventive intervention for families at high risk for intergenerational violence. In: Emde R, Leuzinger-Bohleber M, editors. Early Parenting Research and Prevention of Disorder: Psychoanalytic Research at Interdisciplinary Frontiers. London: Karnac Books; 2014. [Google Scholar]
- Schechter DS, Willheim E, Hinojosa C, Scholfield-Kleinman K, Turner JB, McCaw J, Zeanah CH, Myers MM. Subjective and objective measures of parent-child relationship dysfunction, child separation distress, and joint attention. Psychiatry. 2010;73(2):130–44. doi: 10.1521/psyc.2010.73.2.130. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64(8):681–90. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine. 2012;74(9):904–11. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Pannasch S, Beblo T, Reiss J, Lanius RA. Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience. 2014;9(1):88–97. doi: 10.1093/scan/nss105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(10):1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, Paulus MP, Stein MB. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biological Psychiatry. 2010;68(5):442–50. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Interparental violence, maternal emotional unavailability and children's cortisol functioning in family contexts. Developmental Psychology. 2012;48(1):237–49. doi: 10.1037/a0025419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, Sjoerds Z, van Balkom AJ, Smit JH, Veltman DJ. Increased anterior cingulate cortex and hippocampus activation in Complex PTSD during encoding of negative words. Social Cognitive and Affective Neuroscience. 2013;8(2):190–200. doi: 10.1093/scan/nsr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M. Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Human Brain Mapping. 2010;31(7):1030–40. doi: 10.1002/hbm.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P, Bondolfi G, Sander D, Vuilleumier P. The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience. 2012;7(5):473–93. doi: 10.1080/17470919.2011.647410. [DOI] [PubMed] [Google Scholar]
- Whalley MG, Kroes MC, Huntley Z, Rugg MD, Davis SW, Brewin CR. An fMRI investigation of posttraumatic flashbacks. Brain and Cognition. 2013;81(1):151–9. doi: 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association (1999) Proposed revision of the Declaration of Helsinki. BME. 1999;147:18–22. [PubMed] [Google Scholar]
- Zhu L, Guo X, Li J, Zheng L, Wang Q, Yang Z. Hippocampal activity is associated with self-descriptiveness effect in memory, whereas self-reference effect in memory depends on medial prefrontal activity. Hippocampus. 2012;22(7):1540–52. doi: 10.1002/hipo.20994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.