Abstract
Previous studies have shown that depressed individuals have difficulty directing attention away from negative distractors, a phenomenon known as affective interference. However, findings are mixed regarding the neural mechanisms and network dynamics of affective interference. The present study addressed these issues by comparing neural activation during emotion-word and color-word Stroop tasks in participants with varying levels of (primarily subclinical) depression. Depressive symptoms predicted increased activation to negative distractors in areas of dorsal anterior cingulate cortex (dACC) and posterior cingulate cortex (PCC), regions implicated in cognitive control and internally directed attention, respectively. Increased dACC activity was also observed in the group-average response to incongruent distractors, suggesting that dACC activity during affective interference is related to overtaxed cognitive control. In contrast, regions of PCC were deactivated across the group in response to incongruent distractors, suggesting that PCC activity during affective interference represents task-independent processing. A psychophysiological interaction emerged in which higher depression predicted more positively correlated activity between dACC and PCC during affective interference, i.e. greater connectivity between cognitive control and internal-attention systems. These findings suggest that, when individuals high in depression are confronted by negative material, increased attention to internal thoughts and difficulty shifting resources to the external world interfere with goal-directed behavior.
Keywords: depression, executive function, emotion, default network, connectivity
Clinical and subclinical depression are associated with cognitive biases toward negative emotional information (Gotlib and Joormann, 2010; Joormann, 2010). Such biases purportedly arise from the match between negative information and the depressed individual’s mood (Dalgleish and Watts, 1990) and beliefs about the self and future (Beck, 1967, 2008). When negative material should be ignored, such as in executive function (EF) tasks that require control of attention (Banich, 2009; Miller and Cohen, 2001), performance impairments in depressed individuals have been attributed to the interfering effects of these biases (Gotlib and Cane, 1987; Joormann, 2004; Levin et al., 2007; Levens and Gotlib, 2010). In theory, the personal and emotional salience of negative information makes it especially distracting for depressed people and thus likely to tax cognitive control. Ultimately, the distracting influence of negative emotional information (affective interference) may launch ruminative self-referent thinking and interfere with goal-directed thinking and behavior.
In support of this theory, research aimed at identifying the neural mechanisms of affective interference has implicated brain regions involved in cognitive control, including lateral prefrontal cortex (lPFC), dorsal anterior cingulate cortex (dACC) and anterior insula (Mitterschiffthaler et al., 2008; Wang et al., 2008; Dichter et al., 2009; Engels et al., 2010; Herrington et al., 2010; Foland-Ross et al., 2013). Although the precise functions and network boundaries of these regions are unclear (see Seeley et al., 2007; Vincent et al., 2008; Power et al., 2011; Yeo et al., 2011), they can be broadly divided into a central executive or ‘frontoparietal’ network (FPN; Dosenbach et al., 2008; Vincent et al., 2008) and a ‘salience’ network (SN; Seeley et al., 2007). The SN and FPN are thought to work in concert to integrate information about stimulus salience with task goals, allocate attentional resources to other brain networks involved in external (i.e. ‘dorsal attention network’) or internal (i.e. ‘default network’, DN) attention and select and evaluate task-relevant behavior (Vincent et al., 2008; Menon and Uddin, 2010; Menon, 2011; Andrews-Hanna et al., 2014). The finding that affective interference alters activity of FPN and/or SN suggests that dysfunction in these systems may underlie the disruptive effects of affective biases on executive control.
However, ambiguity persists regarding the neural systems that are involved in affective interference in depression. First, despite brain systems involved in cognitive control being broadly implicated, evidence is mixed regarding which specific regions are involved, the function of such regions and the direction of effects (discussion in Murrough et al., 2011; Diener et al., 2012). Although several factors may contribute to such discrepancies, such as differences in the clinical or subclinical characteristics of the samples (Levin et al., 2007), these mixed findings are nevertheless difficult to interpret. In particular, it is not always easy to distinguish those aspects of abnormal neural response that represent dysfunctional or overtaxed cognitive control exerted to achieve task goals, vs task-irrelevant processing of salient negative material. One strategy to address these issues is to administer, in the same sample, a non-affective task that challenges cognitive control and has the same goals as the affective version of the task (Compton et al., 2003; Mohanty et al., 2007). This approach can localize brain regions that are recruited when cognitive control is taxed by task demands, regardless of the nature of distraction.
Second, evidence is mixed regarding the neural systems involved in biased affective processing in depression. Several studies have detected increased activity in regions of the DN when depressed individuals attempt to disengage from (Johnson et al., 2009) or ignore (Mitterschiffthale et al., 2008) emotional material. The DN has been shown to play an important role in internally directed thinking ranging from autobiographical memory to self-reflection (Buckner and Carroll, 2007; Andrews-Hanna et al., 2010, 2014); therefore, this pattern is consistent with the idea that affective biases stem from increased sensitivity to the personal salience of negative material and difficulty disengaging from self-focused rumination that may be evoked by negative material (Koster et al., 2011; Holtzheimer and Mayberg, 2011). However, other studies investigating affective bias have failed to implicate DN, instead linking depression to altered activity in brain systems implicated in basic emotional processing (Fales et al., 2008) or other regions (Diener et al., 2012). Together, these mixed results suggest that additional investigation of the neural mechanisms of affective bias is warranted.
Third, the dynamic nature by which brain systems involved in top-down control or in affective bias relate to one another has largely been inferred from patterns of co-activation rather than directly tested. Increasingly, researchers propose that depression is related to dysfunction within and between large-scale brain networks (Hamilton et al., 2011, 2013; Marchetti et al., 2012; Whitfield-Gabrieli and Ford, 2012), suggesting the importance of examining interactions between neural systems in addition to magnitude of activation. One method for investigating relationships between brain regions is through psychophysiological interaction (PPI) analysis, in which correlations between activity in a seed region and activity in other regions of the brain are compared between task conditions. If affective interference were related to the interaction between regions involved in top-down control, and regions that process emotional or personally salient information, one would predict altered functional connectivity between such regions in individuals with elevated depression.
The present study aimed to address these issues by investigating the neural systems involved in affective interference in individuals with varying levels of depressive symptoms, from minimal (non-depressed) to severe (current depression). Participants were scanned with functional magnetic resonance imaging (fMRI) while performing an emotion-word Stroop task (Williams et al., 1996), instructing individuals to direct their attention to the color of ink in which an emotional word is printed while ignoring the meaning of the word (e.g. ‘lonely’ written in blue ink). Brain regions in which response to negative words is correlated with depression may be mechanisms of affective interference.
To localize brain regions recruited for top-down control in this sample, a color-word Stroop (Stroop, 1935) was also administered. The color-word Stroop has the same task goal as the emotion-word Stroop, i.e. attend to ink color, but incongruent words are distracting owing to their conflicting color meaning (e.g. ‘red’ written in blue ink) rather than affective content. These tasks differ in several aspects (e.g. semantic conflict), but their shared task goal makes them suitable for comparison to identify regions involved in top-down control in the service of that goal (Compton et al., 2003; Mohanty et al., 2007). Hence, the comparison of brain regions that are active for incongruent words across people, and those that are active for negative words at higher levels of depression, can help to isolate those top-down control systems that are challenged by negatively valenced affective interference. In contrast, brain regions in which response to negative words is associated with depression, but that fail to overlap with those that are responsive to incongruent words, may represent biased processing related to affective content. Finally, patterns of connectivity between regions involved in cognitive control and those involved in affective bias may provide unique insight regarding the network dynamics that underlie affective interference.
METHOD
Recruitment and sample characteristics
The sample consisted of 92 right-handed native English-speaking participants (ages 18–25 years, mean 19.03 years; 58% female; 80% European American), recruited from introductory psychology classes at the University of Illinois at Urbana-Champaign. Analyses performed with an overlapping sample, but which address research questions distinct from those of the present study, are reported elsewhere (Engels et al., 2010; Silton et al. 2011).
Participants were pre-screened and selected for high variance in severity of current depressive symptoms, as assessed with the 8-item anhedonic depression subscale (MASQ-AD8) of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995a, b; Nitschke et al., 2001). Participants were also screened and selected for low covariance between symptoms of depression and symptoms of anxiety, to minimize potential confounding effects of anxiety (see Supplementary Material).
Participants were screened to exclude: (i) use of psychoactive medications, (ii) abnormal color vision, (iii) previous loss of consciousness that exceeded 10 min, (iv) claustrophobia, (v) recent drug or alcohol use, (vi) excessive caffeine intake or (vii) recent lack of sleep. In addition, of 106 participants enrolled in the study, 14 were excluded from the present analyses for excessive motion in the scanner (N = 6), equipment malfunction (N = 7) or missing questionnaire data (N = 1). Hence, the final sample for the present analyses consisted of 92 participants.
MR data acquisition
A 3T Siemens Allegra scanner with a quadrature headcoil was used for data acquisition. For functional scans, 370 functional images were acquired with the following echoplanar image (EPI) parameters: 2000 ms TR, 25 ms TE, flip angle 80°, FOV = 22 cm. Thirty-eight oblique axial slices (3.4375 mm × 3.4375 mm in-plane resolution, 3 mm slice thickness, 0.3 mm gap between slices) were acquired parallel to the anterior and posterior commissures. These parameters were identical across functional runs. After the functional scans, a high-resolution T1-weighted image with the same slice prescription was acquired to provide anatomical data to register each participant’s functional data to standard space. For anatomical scans, a T1-weighted 160-slice MPRAGE sequence was acquired (1 × 1 mm in-plane resolution, 1 mm slice thickness, sequence parameters of 1700 ms TR, 3.5 ms TE, 900 ms inversion time). In addition, a multi-echo gradient-echo field map scan (TEs of 10 and 12.46 ms) was acquired before the EPI scans with a slice prescription identical to the functional slices for correction of geometric distortions.
Procedures
The present study consisted of two research sessions. During session 1, participants were informed of study procedures, provided written consent and completed psychosocial measures. During session 2, fMRI data were collected during two Stroop tasks, with task order counterbalanced across participants. Participants also completed an electroencephalography (EEG) session (see Silton et al., 2011).
Assessment of depression
At session 1, participants completed the MASQ (see Recruitment and sample characteristics section) (Table 1). Session 1 MASQ-AD8 (Nitschke et al., 2001) scores provided the primary measure of depressive symptom severity used for all subsequent analyses. Using a continuous measure of depression is consistent with the view that depression is often better treated as a dimensional phenomenon (Hankin et al., 2005; Widiger and Samuel, 2005) and provides improved statistical power (Irwin and McClelland, 2003).
Table 1.
Sample characteristics
| Demographics | M (s.d.) |
|---|---|
| Age | 19.07 (1.07) |
| MASQ-AD8 | 17.01 (6.08) |
| MASQ-AA | 27.13 (7.49) |
| PSWQ | 48.00 (18.65) |
| Clinical diagnoses | Percentage with Dx (%) |
| Current MDD | 6.52 |
| Definite current MDD | 3.26 |
| Provisional current MDD | 3.26 |
| Past MDD | 26.09 |
| Definite past MDD | 17.39 |
| Provisional past MDD | 6.52 |
| Dysthymia | 2.17 |
| Definite Dysthymia | 1.09 |
| Provisional Dysthymia | 1.09 |
| Depression NOS | 0.00 |
| Bipolar disorder I or II | 0.00 |
| Anxiety disorder | 36.96 |
| Substance/alcohol dependence | 8.70 |
| Eating disorder | 2.17 |
Note. MASQ = Mood and Anxiety Symptom Questionnaire; -AD8 = Anhedonic Depression Subscale; -AA = Anxious Arousal Subscale; PSWQ = Penn State Worry Questionnaire; MDD = major depressive disorder; diagnoses = meets criteria for definite (full symptom criteria) or provisional (one symptom short of full criteria) disorder as revealed by a Structured Clinical Interview for Axis 1 Disorders (see ‘Methods’ section).
In addition, a graduate student in clinical psychology with extensive diagnostic training with the Structured Clinical Interview for Axis I Disorders, Non-Patient edition (SCID-NP; First et al., 2007) administered the SCID-NP. A second experienced interviewer and a clinical faculty supervisor reviewed written case summaries detailing each criterion symptom, and assessed lifetime DSM-IV-TR diagnoses of depressive disorders (major depressive disorder, dysthymia or depressive disorder not otherwise specified) on the following scale: 1 = absent, 2 = features (≥2 symptoms), 3 = provisional (one symptom short of full criteria) and 4 = definite.
Emotion-word Stroop task (e-Stroop)
The e-Stroop consisted of blocks of positive or negative emotion words alternating with blocks of neutral (non-emotional, e.g., ‘carpet’) words (Williams et al., 1996). Word stimuli, each presented one time per session, were selected from the set of Affective Norms for English Words (Bradley and Lang, 1999) on the basis of established norms for valence, arousal and frequency of usage. Affective words were selected and matched for arousal and word length; neutral words were selected for low arousal and neutral valence.
The e-Stroop included 16 blocks (4 positive, 4 negative and 8 neutral blocks; 32 s each), each consisting of 16 trials, for a total of one run of 256 trials. Also included were four fixation blocks (32 s each) in which a fixation cross was presented, and five periods of rest (6–34 s) in which participants viewed written instructions to relax with their eyes open. Each participant was randomly assigned to one of eight orders of stimulus presentation, optimized to control for stimulus order effects. Each trial consisted of a word presented in red, yellow, green or blue ink for 1500 ms followed by a fixation cross presented for 275–725 ms (onset-to-onset intertrial interval of 2000 ± 225 ms). Word presentation and recording of behavioral responses were controlled by STIM software (James Long Company, Caroga Lake, NY). Words were presented in capital letters via back projection onto a screen outside the scanner bore and a mirror fixed to the head coil. Participants responded using both hands (middle and index fingers), with a specific and unchanging response mapping of color to finger; 32 practice trials presented before the first Stroop task allowed the participant to acquire the stimulus-response mapping.
Color-word Stroop task (c-Stroop)
The c-Stroop consisted of blocks of congruent or incongruent words alternating with blocks of neutral (non–color-related, e.g. ‘divide’) words, for 256 trials presented in 16 blocks (4 congruent, 4 incongruent, 8 neutral) (Stroop, 1935). Within congruent and incongruent blocks, 50% of trials were neutral to prevent reliance on word-reading strategies. Block counterbalancing, stimulus presentation parameters and color-response mapping were identical to that described above.
Neuroimaging data analysis
Preprocessing
Image processing and analyses used tools from the FMRIB Software Library analysis package (http://www.fmri-b.ox.ac.uk/fsl), AFNI (http://afni.nimh.nih.gove/afni) and Matlab (Mathworks, Inc., Natick, MA). First, each fMRI time series was motion-corrected with FMRIB’s Linear Image Registration Tool (Jenkinson et al., 2002). Participants demonstrating <3.3 mm absolute motion or 2 mm relative motion were included in the analysis. Second, spikes (artifactual sudden intensity shifts) were corrected with the AFNI tool 3dDespike. Third, each time series was corrected for geometric distortions caused by inhomogeneity in the magnetic field. These distortions were corrected using Fugue in FSL with a field map collected before the EPI sequence, with the same slice prescription as the functional scans. The remaining preprocessing steps were conducted using FMRIB’s Expert Analysis Toolbox. The first three volumes of each data set were discarded, retaining volumes collected when the magnetic resonance signal was at a steady state, yielding 367 images per task. Each time series was temporally filtered with a high-pass filter (212 s) to remove drift in signal intensity, and spatially smoothed with a three-dimensional Gaussian kernel (full-width half maximum = 8 mm). Functional and structural data were registered into Montreal Neurological Institute 152 stereotaxic space with FMRIB’s Linear Image Registration Tool.
Lower-level single-subject analysis
FMRIB’s Improved Linear Model was used to perform regression analyses on each participant’s time series for each Stroop task separately, and statistical maps were created with a regression analysis performed at each intra-cerebral voxel (Woolrich et al., 2001). Regressors were created for each condition, with fixation blocks left as the unmodeled baseline. Each regressor was convolved with a double-gamma response function, yielding a per-voxel effect-size parameter estimate (β) map representing the magnitude of activity associated with that condition compared with baseline.
Conditions of interest for the present study were negative or incongruent words. For consistency, neutral words were selected as the baseline comparison in each task, yielding two contrasts of interest: negative–neutral and incongruent–neutral. Because the present study focused on distractors that were expected to elicit affective interference, and previous studies have shown that depressed individuals fail to exhibit interference with positive distractors (Gotlib and McCann, 1984; Gotlib and Cane, 1987; Elliott et al., 2002; Clasen et al., 2013), positive words were not considered a condition of interest. However, because depression has been associated with deficits in other aspects of cognitive control with positive information (e.g. selecting or approaching positive material, Levens and Gotlib, 2010), individual difference analyses were also performed to investigate whether depressive symptoms predicted altered response to positive distractors (see Supplementary Material).
Group-average and individual difference analyses
Higher-level statistical analyses were carried out with FMRIB’s Local Analysis of Mixed Effects. Monte Carlo simulations were performed with AFNI’s AlphaSim program (Ward, 2000) to correct for multiple comparisons at an overall familywise error rate of P < 0.05. Intrinsic smoothing of the functional data was calculated using AFNI’s 3dFWHMx. Voxels that survived a voxelwise threshold of z > 2.58 (P < 0.01) and an accompanying cluster-extent threshold of P < 0.05 were considered significant.
Four independent group-level analyses were performed. First, two group averages were computed to identify brain regions commonly recruited across the full sample in response to each contrast of interest. Second, two whole-brain correlation analyses were conducted to identify regions in which individual differences in depressive symptoms predicted activation to each contrast of interest.
Conjunction analyses
A conjunction analysis was performed to isolate regions in which independent significant effects were detected both in the depressive (correlation) response to the negative distractor contrast and in the group (average) response to the incongruent distractor contrast. This conjunction was expected to identify regions involved in cognitive control when such control is challenged. To be complete, a second conjunction was performed between the former and the group (average) response to the negative distractor contrast.
The conjunction procedure entailed comparing the P value of each voxel between thresholded group maps (Nichols et al., 2005). If both P values exceeded the threshold of < 0.01 and represented the same direction of effect (i.e. positive values signifying greater activity, or negative values signifying reduced activity), the voxel was set to 1; otherwise, the voxel was set to 0. Conjunction maps were cluster-corrected based on the more conservative threshold of the two component maps.
PPI analyses
PPI analyses (Friston et al., 1997; O’Reilly et al., 2012) were conducted to examine patterns of functional connectivity during affective interference. Specifically, PPI tested whether individual differences in depressive symptoms predicted connectivity between neural regions generally involved in cognitive control (i.e. regions implicated in conjunction analysis by both the depressive response to the negative distractor contrast, and the group-average response to the incongruent distractor contrast) and regions that may be involved in affective bias (i.e. regions implicated only in the depressive response to the negative distractor contrast, but not in the group response to the incongruent distractor contrast).
To accomplish the PPI analysis, at the peak of any cortical cluster identified by the conjunction of interest, a 5-voxel spherical seed region was created in MNI space. The seed region was transformed to native space, and the timecourse extracted for the e-Stroop. Lower-level models were rerun (using pre-processed data, O’Reilly et al., 2012), including all task regressors and three new regressors: the seed timecourse, the interactions between the seed timecourse and the negative distractor condition and the interaction between the seed timecourse and the neutral distractor conditions. The contrast of the latter two regressors provided a measure of the difference in functional connectivity of the seed region for negative vs neutral distractors. Group-level models were rerun as described above. To investigate patterns of connectivity within the set of brain regions implicated in affective interference, the PPI analysis was restricted to regions that were previously identified in the whole-brain correlation between depressive symptoms and neural response to negative distractors. To be complete, whole-brain PPI results are also included.
A second PPI analysis was performed using the same seed region(s), but examining average connectivity across the group for incongruent as compared with neutral distractors in the c-Stroop. This PPI investigated the normative patterns of functional connectivity of brain systems involved in cognitive control during a non-affective EF task.
Behavioral analyses
Average reaction time (RT) and percent accuracy were computed for each trial type, and comparison between conditions was performed by repeated-measures analysis of variance (ANOVA). Percent interference scores were calculated for negative [(negative RT-neutral RT)/neutral RT] and incongruent distractors [(incongruent RT-neutral RT)/neutral RT], a method that controls for scaling effects (Lansbergen et al., 2007). To examine the influence of depressive symptoms, mean-deviated MASQ-AD8 scores were added to ANOVAs as a covariate (Miller and Chapman, 2001; Verona and Miller, in press), and for any significant interactions follow-up correlations were performed.
Neuroimaging analyses were repeated including performance measures as covariates. Because the inclusion of such covariates failed to affect the pattern or significance of effects, simple analyses are reported here.
RESULTS
Behavioral results
Performance across the group
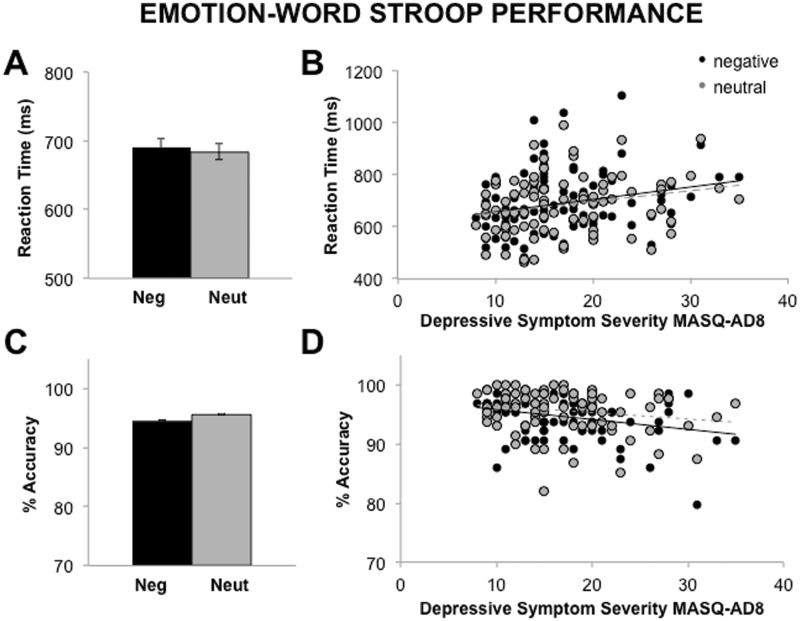
Significant differences in percent accuracy, F(3,91) = 60.68, P < 0.01, response speed, F(91) = 68.63, P < 0.01, and RT interference, F(91) = 134.99, P < 0.01, were observed between distractor types. Follow-up t-tests revealed that on average across the group, participants were slower and less accurate in responding to incongruent distractors than any other distractor type, (Ps < 0.05; Figure 1 and Supplementary Figure S1).
Fig. 1.
Emotion-word Stroop performance. (A) On average, there were no differences in RT detected across the group for negative as compared with neutral distractors (P > 0.10). However, (B) increased severity of current symptoms of depression was related to slower RT for both negative and neutral distractors (Ps < 0.05). Similarly, (C) although participants on average exhibited comparable accuracy for negative and neutral distractors (P > 0.1; standard error within negative or neutral trial conditions < 1%), (D) higher severity of depression predicted poorer accuracy for negative distractors (P < 0.05), and marginally poorer accuracy for neutral distractors (P < 0.10). Note: MASQ-AD8 = scores for the Mood and Anxiety Symptom Questionnaire Anhedonic Depression subscale.
Effects of depression on performance
Trends emerged in which depressive symptoms moderated differences in accuracy, F(1,91) = 2.96, P = 0.09, response speed, F(1,91) = 3.81, P = 0.05, and RT interference, F(1,91) = 2.85, P = 0.09, between distractors. Follow-up correlations indicated that higher levels of depression predicted poorer accuracy, r(91) = -0.30, P < 0.01, and slower RT, r(91) = 0.24, P = 0.02, for negative distractors. However, depression also predicted somewhat poorer accuracy, r(91) = -0.18, P = 0.08, and slower RT, r(91) = 0.23, P = 0.03, for neutral distractors in the e-Stroop, and depression failed to predict RT interference to negative compared with neutral distractors, r(91) = 0.08, P = 0.46. Together, these results indicate that participants high in depression showed impaired performance on the e-Stroop for negative, but also to some extent for neutral, distractors (Figure 1). Depression was not significantly correlated with accuracy, RT or RT interference in the c-Stroop (Ps > 0.11; Supplementary Figure S1).
Neuroimaging results
Group-average response to negative distractors
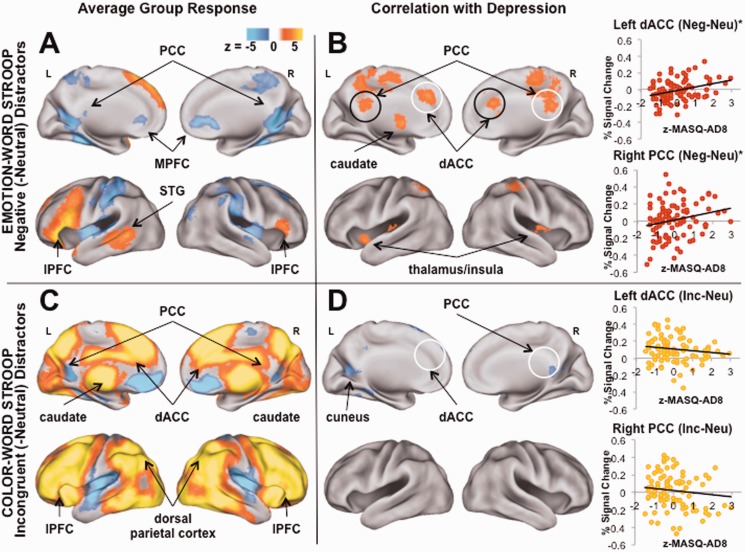
Figure 2A shows regions in which, across the full sample, activity differed for negative compared with neutral distractors. Although this analysis was not directly relevant to the hypotheses tested in the present study, it shows the normative response to the task of ignoring negative information. Regions implicated included activation in bilateral areas of lPFC, and deactivation in midline regions including posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC) and bilateral parahippocampal gyrus (PHG; Table 2).
Fig. 2.
Group and depression-related responses to negative or incongruent distractors. (A) Across the group in response to negative (compared with neutral) distractors, increased activity was detected in regions of lateral prefrontal cortex (lPFC) likely involved in aspects of cognitive control such as maintaining task goals; decreased activity was observed in medial cortical and parahippocampal regions of the default network (DN) implicated in internally directed attention. (B) Higher severity of depressive symptoms predicted increased activation to negative (-neutral) distractors in regions of DN including posterior cingulate cortex (PCC); and in a region of dorsal anterior cingulate cortex (dACC) involved in cognitive control and mediating the allocation of resources to other brain networks. (Regions in which activity was extracted for each task and plotted here are circled in white). (C) Across the group in response to incongruent (-neutral) distractors, increased activity was observed in lPFC, dACC and regions involved in attending to the external world, e.g. dorsal parietal cortex. (D) Higher severity of depressive symptoms predicted decreased activity to incongruent (-neutral) distractors in regions of cuneus and areas of DN. Note: z-MASQ-AD8 = z-transformed scores for the Mood and Anxiety Symptom Questionnaire Anhedonic Depression subscale; *P < 0.05.
Table 2.
Neural response to negative distractors
| Region | Cluster size (voxels) | Max z | COI location MNI |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Negative > Neutral | |||||
| Left inferior frontal gyrus | 3495 | 7.08 | −48 | 26 | −4 |
| Left middle temporal gyrus | 952 | 5.41 | −58 | −38 | −4 |
| Left medial frontal gyrus | 1559 | 4.71 | −6 | 48 | 44 |
| Right inferior frontal gyrus | 242 | 3.85 | 58 | 32 | 8 |
| Negative < Neutral | |||||
| Bilateral parahippocampal cortex extending to PCC | 20 210 | −5.82 | −28 | −42 | −18 |
| Right angular gyrus | 283 | −4.54 | 40 | −76 | 30 |
| Right mPFC | 1067 | −4.16 | 10 | 40 | 0 |
P < 0.01, cluster size > 94.
Relationships between depressive symptoms and response to negative distractors
Figure 2B shows brain regions in which depressive symptoms predicted response to the negative distractor contrast. Critical to the present study goals, this analysis was conducted to identify neural systems involved in affective interference. Higher depression predicted increased activity in a region of dACC positioned at the intersection of the FPN and SN (Yeo et al., 2011), which has been suggested to play a role in cognitive control (Dosenbach et al., 2006). Elevated depression also predicted increased activity in regions involved in internal attention and autobiographical memory, including PCC and PHG. Finally, increased depressive symptoms severity predicted greater activity in subcortical regions responsive to salience and arousal (e.g. brain stem, caudate, left thalamus; Table 3). Notably, depressive symptom severity failed to predict response to positive compared with neutral distractors, but did predict increased activity to negative compared with positive distractors in several regions that emerged for the above negative vs neutral distractor contrast (see Supplementary Material), together suggesting that the present pattern of affective interference is unlikely to be explained by general effects of arousal.
Table 3.
Affective interference: depressive symptoms predict neural response to negative distractors
| Region | Cluster size (voxels) | Max z | COI location MNI |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Negative > Neutral | |||||
| Right caudate extending to right dACC | 771 | 3.68 | 14 | 18 | 10 |
| Left caudate | 452 | 3.63 | −16 | 16 | 12 |
| Brain stem extending to right PHG | 384 | 3.54 | −2 | −26 | −24 |
| Left thalamus extending to left insula | 246 | 3.34 | −22 | −14 | 14 |
| Bilateral PCC extending to precuneus and mid cingulate | 1522 | 3.33 | 0 | −22 | 46 |
| Left dACC cortex | 323 | 3.31 | −12 | 32 | 30 |
| Right postcentral gyrus | 262 | 3.29 | 34 | −32 | 54 |
| Right posterior insula | 338 | 3.22 | 44 | −2 | 10 |
| Negative < Neutral | |||||
| (None) | |||||
P < 0.01, cluster size > 198.
Group-average response to non-affective incongruent distractors
Figure 2C shows regions in which activity differed for incongruent compared with neutral distractors across the full sample. Critically, this analysis identified neural systems involved in non-affective cognitive control across participants. Activation was observed in regions involved in cognitive control (Vincent et al., 2008), e.g. prefrontal cortex with local maxima in dACC, dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex and dorsal parietal cortex. Significant deactivation was detected in regions of DN, e.g. PCC and mPFC (Table 4).
Table 4.
Neural response to incongruent distractors
| Region | Cluster size (voxels) | Max z | COI location MNI |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Incongruent > Neutral | |||||
| Bilateral lPFC local maxima in dorsal and ventral areas; right and left dorsal anterior cingulate; dorsal parietal cortex | 112 704 | 11.6 | −48 | 10 | 34 |
| Incongruent < Neutral | |||||
| Left mPFC | 2879 | −7.34 | −6 | 44 | −16 |
| Left posterior insula | 3826 | −7.21 | −38 | −20 | 18 |
| Right insula | 3100 | −6.38 | 40 | −14 | 16 |
| Left PHG | 1447 | −5.42 | −12 | −52 | 4 |
| Subcortical | 520 | −5.03 | 26 | −44 | 12 |
| Left precentral gyrus | 283 | −4.69 | −12 | −32 | 70 |
| Right mPFC | 664 | −3.77 | 8 | −22 | 58 |
P < 0.01, cluster size > 200.
Relationships between depressive symptoms and response to incongruent distractors
Although this analysis was not directly relevant to the hypotheses tested in the present study, it was conducted to investigate whether generally altered neural response to demands for cognitive control was exhibited at higher levels of depression. Elevated depression symptoms did not predict greater activity to the incongruent contrast in any region, but predicted reduced activity in regions of cuneus and DN (Figure 2D and Supplementary Materials).
Comparing brain systems involved in affective interference with brain systems involved in top-down control
The first key goal of the present study was to isolate top-down control systems that are challenged by affective interference, by comparing the depression-related neural response to negative distractors to the group (average) response to incongruent distractors. Conjunction analyses revealed a single prefrontal region in dACC that was active both when participants high in depression responded to negative (-neutral) distractors and when participants as a group responded to incongruent (-neutral) distractors. Additional subcortical regions were also activated by both contrasts, e.g. caudate, brain stem and left thalamus (subcortical cluster extending to insula).
The second key goal of the present study was to identify neural regions implicated by affective interference that are outside the set of areas involved in top-down control, and in which activity may relate to biased processing of the emotional content of negative distractors. The above conjunction analysis indicated that PCC regions, in which depression-related activation to the negative distractor contrast was detected, failed to show activation to the incongruent distractor contrast across the group (indeed, overlapping regions of PCC were deactivated for the latter contrast). Together, these results suggest that PCC regions implicated in affective interference may be involved in biased emotional processing.
The results of the second conjunction analysis (conducted to be complete, but not the subject of the present hypotheses), between the depression-related and the group (average) responses to negative (-neutral) distractors, failed to reveal any regions of overlapping effects.
Task-related changes in functional connectivity
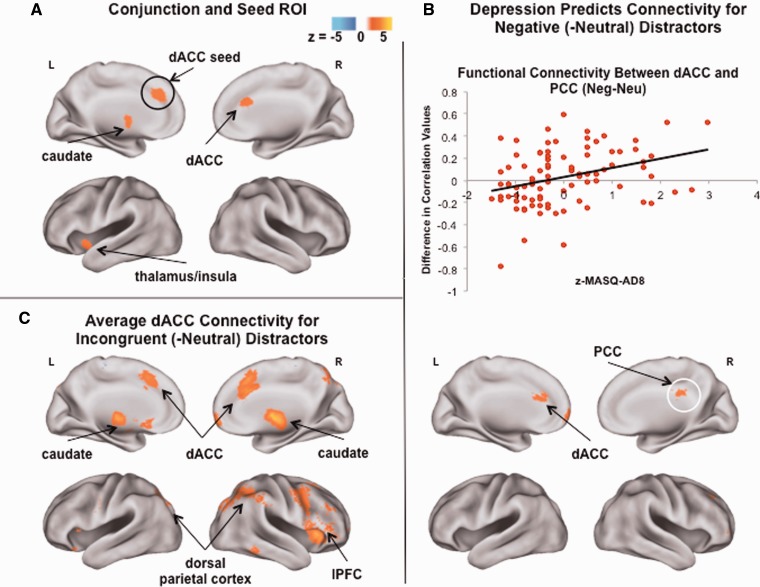
The third key goal of the present study was to examine patterns of connectivity between regions involved in cognitive control and those involved in affective bias, either during affective interference (as predicted by depression) or during non-affective cognitive control (across the group). A seed region in dACC (Figure 3A) was selected for PPI analyses, based on the finding that this cortical region was implicated in conjunction analysis both by the depression-related response to negative distractors and the group response to incongruent distractors (suggesting that this region was involved in top-down control in both cases). Also, a region-of-interest (ROI) mask was created for the set of cortical regions implicated in affective interference, i.e. those that were identified by the whole-brain correlation between depression and response to negative (-neutral) distractors.
Fig. 3.
Conjunction of activation and functional connectivity of dorsal anterior cingulate cortex (dACC) during affective interference and non-affective cognitive control. (A) The conjunction of the depressive response to negative (-neutral) distractors, and the group-average response to incongruent (-neutral) distractors, revealed activation in dACC in both contrasts, and activation in subcortical regions including caudate, brainstem and thalamus (extending to insula). A 5-voxel spherical seed was created in dACC (at the peak defined by depressive response to the negative distractor contrast). (B) Higher severity of depressive symptoms predicted greater functional connectivity (more positively correlated activity) between dACC and a region of posterior cingulate cortex (PCC) for negative (compared with neutral) distractors. (The region in which activity was extracted and plotted here is circled in white). (C) Across the group, increased functional connectivity was observed between dACC and regions of lateral prefrontal cortex (lPFC) and dorsal parietal cortex for incongruent (-neutral) distractors. Note: z-MASQ-AD8 = z-transformed scores for the Mood and Anxiety Symptom Questionnaire Anhedonic Depression subscale; *P < 0.05.
The first PPI analysis tested whether depression predicted changes in functional connectivity for negative compared with neutral distractors between the dACC seed and other regions implicated by affective interference. Results showed that, at higher levels of depression, dACC was more positively correlated with activity in PCC for negative than neutral distractors (Figure 3B). This finding is consistent with the idea that neural mechanisms of affective interference involve dynamic interactions between regions involved in cognitive control and those involved in affectively biased cognition.
To ensure that the above ROI approach was not overly restrictive, the PPI described above was expanded to the whole brain. No additional regions were detected in which functional connectivity with dACC was moderated by depression for the negative distractor contrast. (Whole-brain PPI results did not survive cluster-extent correction and should be viewed as exploratory).
The second PPI analysis tested whether, across the group, functional connectivity between the seed dACC and the affective-interference ROI differed for incongruent and neutral distractors. Within this ROI, activity in dACC was more positively correlated with activity in regions of caudate for incongruent than for neutral distractors. In addition, whole-brain PPI showed that dACC was more positively correlated with activity in other regions of FPN, e.g. dlPFC, and regions involved in attention to the external environment, e.g. dorsal parietal cortex, for incongruent than for neutral distractors (Figure 3C).
DISCUSSION
This study provides evidence that severity of depressive symptoms predicts affective interference to negative words, and predicts activity in brain regions involved in cognitive control or in internally directed thinking during affective interference. High functional connectivity between these systems may represent dynamic interactions between overtaxed or dysfunctional cognitive control and internal thoughts evoked by negative content. Together, these results suggest that affective interference stems from the increased salience of negative emotional information, coupled with impairments in allocating resources away from internal thought and toward the external environment.
Overtaxed or dysfunctional cognitive control in depression
Neural correlates of affective interference that represent dysfunctional or overtaxed cognitive control were identified in the present study by comparing the depression-related response to negative distractors with the normative response to incongruent distractors. Higher severity of depression predicted increased activity to negative distractors in a region of dACC that was also active in the group response to incongruent distractors, and which has been shown to be involved in cognitive control in previous research (Dosenbach et al., 2006). This pattern of hyperactivity converges with other studies examining affective interference (Mitterschiffthaler et al., 2008; Wang et al., 2008) and is consistent with the idea that the task of ignoring negative task-irrelevant information is especially taxing at higher levels of depression. It may be that, because highly depressed individuals experience greater interference by negative material, they must recruit dACC to ‘pick up the slack’ in top-down control (e.g. Banich, 2009; Silton et al., 2011).
Another interpretation is that dACC hyperactivity represents abnormalities in how cognitive control is exerted in the face of negative information, e.g. not only overtaxed top-down regulation of other brain systems but also qualitatively different regulatory effects (Johnstone et al., 2007). As an SN ‘hub’ that interacts with FPN and DN, dACC has been proposed to play an active role in allocating resources to external- or internal-attention systems (Sridharan et al., 2008; Menon and Uddin, 2010). Such network interactions are believed to enable dynamic adjustments to changing environmental demands and support adaptive behavior (Corbetta et al., 2008). Hence, in the case of affective interference, hyperactivity in dACC may represent abnormalities in top-down control for appropriate allocation of resources: attention should be allocated toward perceptual features of stimuli, but instead may be allocated to internal thoughts. Note that this interpretation does not require that dACC is responsible for determining how resources should be allocated (a process that may be supported by other regions, e.g. lPFC), but suggests that dACC may mediate such allocation.
Negative affective biases and autobiographical thought in depression
Important differences emerged in the set of regions that co-activated with (and were functionally related to) dACC in the case of affective interference vs non-affective cognitive control. For example, when individuals across the group responded to incongruent distractors, activation was observed in dorsal parietal regions involved in attending to the external environment (Vincent et al., 2008), and increased functional connectivity was detected between dACC and such external-attention systems. In contrast, elevated depression was related to increased activity in DN regions in response to negative distractors, and greater functional connectivity was observed between dACC and PCC, an important ‘hub’ of the DN whose subdivisions ‘echo’ neural signals from other brain networks, including the SN (Buckner et al., 2009; Leech et al., 2012; Leech and Sharp, 2014). How might such DN hyperactivity relate to affective bias?
Putative functions of the DN include introspective, self-referent and autobiographical thinking (Buckner and Carroll, 2007; Spreng et al., 2008; Andrews-Hanna et al., 2014). Activity in the DN tends to decrease during externally focused tasks, when resources should be allocated away from introspection and toward task-relevant processing (Buckner et al., 2008). Indeed, in the present study, DN regions were broadly deactivated across the group in response to both tasks. Critically, this pattern was reversed for individuals high in depression, yet only when depressed individuals were confronted by negative distracting information. In such cases, increased severity of depression predicted greater activity in key DN regions including PCC and PHG, consistent with prior research (Northoff et al., 2011; Anticevic et al., 2012; Marchetti et al., 2012; Whitfield-Gabrieli and Ford, 2012). Hyperactivity of key DN regions involved in autobiographical thought supports the theory that depressed individuals have a tendency to engage in ruminative styles of thinking (Holtzheimer and Mayberg, 2011; Koster et al., 2011), particularly pertaining to negative and self-referential material (Andrews-Hanna et al., 2013). Together, this pattern suggests that DN hyperactivity may represent task-independent thinking prompted by the personal salience of negative cues.
Dynamic relationships between default and control/SNs in depression
The finding that affective interference is related not only to increased activity in but also amplified connectivity between dACC and PCC is consistent with the idea that depressed individuals exhibit abnormal network dynamics (Hamilton et al., 2013). Depression is conceptualized as a disorder characterized by difficulty shifting out of internally directed and ruminative thinking (Holtzheimer and Mayberg, 2011); the present results suggest that altered dACC function may contribute to such cognitive tendencies. Specifically, increased functional connectivity between dACC and regions of DN may signify deficits in the capacity to switch between networks: when emotionally salient but task-irrelevant information is detected, individuals high in depression may be unable to shift away from internal thoughts and toward the external world. Together, these findings are consistent with evolving theories about the nature of pathological cognition in depression and suggest that brain systems involved in switching between large-scale networks may play a critical role in such pathology.
Limitations and future directions
The present study focused primarily on subclinical depression. This approach is designed to complement affective interference research conducted in clinical populations (see Supplementary Material), but the extent to which these findings would generalize to those with more severe symptoms is unknown.
The present study did not explicitly test the potential influence of anxiety in moderating neural mechanisms of affective interference. Symptoms of depression and anxiety are highly comorbid (Kessler et al., 2003), and anxiety has been linked to abnormal activity in brain regions that are also implicated in depression (Heller and Nitschke, 1998; Nitschke et al., 2001). Controlling for anxiety post hoc did not alter present results (see Supplementary Material), and the present sample was selected for low covariance between symptoms of depression and anxiety. Hence, it is unlikely that comorbid anxiety was responsible for the effects observed here.
CONCLUSIONS
In conclusion, the present study suggests that affective interference in subclinical depression is related to overtaxed or dysfunctional cognitive control and disrupted allocation of cognitive resources to internal- or external-attention systems. This finding is consistent with previous research, and critically extends prior studies by directly testing functional relationships between brain systems. Future studies that examine such dynamics with other types of personally salient or affective material, or during rumination, may yield further insights into the mechanisms of cognitive dysfunction in depression.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Laura Crocker and members of the P50 center on Executive Function and Dysfunction for valuable discussion. This research was supported by grants from the National Institutes of Health (P50 MH079485, R01 MH61358 and T32 MH19554).
REFERENCES
- Andrews-Hanna JR, Kaiser RH, Turner AE, et al. A Penny for your thoughts: dimensions of thought content and relationships with individual differences in emotional well-being. Frontiers in Psychology. 2013;4:1–13. doi: 10.3389/fpsyg.2013.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Executive function: the search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. New York: 1967. Hoeber. Republished as Depression: Causes and Treatment. Philadelphia: University of Pennsylvania Press. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. Technical report C-1. 1999 The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Buckner RL, Andrews-Hanna J, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–58. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Ellis AJ, Beevers CG. Attentional biases and the persistence of sad mood in Major Depressive Disorder. Journal of Abnormal Psychology. 2013;122:74–85. doi: 10.1037/a0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, et al. Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective and Behavioral Neuroscience. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T, Watts FN. Biases of attention and memory in disorders of anxiety and depression. Clinical Psychology Review. 1990;10:589–604. [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. Journal of Affective Disorders. 2009;114:131–42. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-network architecture of top-down control. Trends in Cognitive Science. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, et al. Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective and Behavioral Neuroscience. 2010;10:141–56. doi: 10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 2007 Structured clinical interview for DSM-IV-TR Axis I Disorders-Non-Patient Edition (SCID-N/P, 1/2007 revision) [Google Scholar]
- Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychological Science. 2013;24:334–44. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buchel C, Fink GR, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Cane DB. Construct accessibility and clinical depression: a longitudinal investigation. Journal of Abnormal Psychology. 1987;96:199–204. doi: 10.1037//0021-843x.96.3.199. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Reviews in Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, McCann CD. Construct accessibility and depression: an examination of cognitive and affective factors. Journal of Personality and Social Psychology. 1984;47:427–39. doi: 10.1037//0022-3514.47.2.427. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of Disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: multivariate granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16:763–72. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin B, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. Journal of Abnormal Psychology. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cognition and Emotion. 1998;12:421–44. [Google Scholar]
- Herrington JD, Heller W, Mohanty A, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47:442–54. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends in Neurosciences. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JR, McClelland GH. Negative consequences of dichotomizing continuous predictor variables. Journal of Marketing Research. 2003;40:366–71. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Attentional bias in dysphoria: the role of inhibitory processes. Cognition and Emotion. 2004;18:125–47. [Google Scholar]
- Joormann J. Inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19:161–6. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–45. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21:251–62. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. Journal of Neuroscience. 2012;32:215–22. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain: A Journal of Neurology. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology: General. 2010;139:654–64. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–33. [Google Scholar]
- Marchetti I, Koster EHW, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychological Review. 2012;22:229–51. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Williams SCR, Walsh ND, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine. 2008;38:247–56. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiological Learning and Memory. 2011;96:553–63. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishingdimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. [Google Scholar]
- Northoff G, Wiebking C, Feinberg T, Pankseppe J. The resting-state hypothesis of major depressive disorder: a translational subcortical-cortical framework for a system disorder. Neuroscience and Biobehavioral Reviews. 2011;35:1929–45. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7:604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Engels AS, Towers DN, Spielberg JM, Edgar JC, et al. Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. Journal of Abnormal Psychology. 2011;120:272–85. doi: 10.1037/a0023204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2008;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin D, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- Verona E, Miller GA. Analysis of covariance and related strategies. In: Cautin R, Lilienfeld S, editors. Encyclopedia of Clinical Psychology. New York: Wiley-Blackwell; in press. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research, Neuroimaging. 2008;163:143–55. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for fMRI Data. 2000. Available: afni.nimh.nih.gov/afni/doc/manual/AlphaSim. [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition. Journal of Abnormal Psychology. 2005;114:494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Reviews in Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.