Abstract
Brain-derived neurotrophic factor (BDNF), the most abundant neutrophin in the mammalian central nervous system, is critically involved in synaptic plasticity. In both rodents and humans, BDNF has been implicated in hippocampus- and amygdala-dependent learning and memory and has more recently been linked to fear extinction processes. Fifty-nine healthy participants, genotyped for the functional BDNFval66met polymorphism, underwent a fear conditioning and 24h-delayed extinction protocol while skin conductance and blood oxygenation level dependent (BOLD) responses (functional magnetic resonance imaging) were acquired. We present the first report of neural activation pattern during fear acquisition ‘and’ extinction for the BDNFval66met polymorphism using a differential conditioned stimulus (CS)+ > CS− comparison. During conditioning, we observed heightened allele dose-dependent responses in the amygdala and reduced responses in the subgenual anterior cingulate cortex in BDNFval66met met-carriers. During early extinction, 24h later, we again observed heightened responses in several regions ascribed to the fear network in met-carriers as opposed to val-carriers (insula, amygdala, hippocampus), which likely reflects fear memory recall. No differences were observed during late extinction, which likely reflects learned extinction. Our data thus support previous associations of the BDNFval66met polymorphism with neural activation in the fear and extinction network, but speak against a specific association with fear extinction processes.
Keywords: amygdala, vmPFC, CBT, anxiety, fear recall, therapygenetics
INTRODUCTION
Brain-derived neurotrophic factor (BDNF) is the most abundant neutrophin in the mammalian central nervous system, and it is critically involved in synaptic plasticity (e.g. Bramham and Messaoudi, 2005). Initially, BDNF was implicated in hippocampus-dependent learning and memory in both rodents (for a review, see Tyler et al., 2002) and humans (Egan et al., 2003; Hariri et al., 2003). Later, a similar role of BDNF for Pavlovian amygdala-dependent fear conditioning was shown in animals (Rattiner et al., 2004, 2005; Ou and Gean, 2006). Also, human behavioural studies observed associations of a functional polymorphism in the BDNF gene (BDNFval66met) in a differential fear conditioning protocol (Lonsdorf et al., 2010; but see Torrents-Rodas et al., 2012), a fear generalization paradigm (Hajcak et al., 2009; but see Torrents-Rodas et al., 2012) as well as a context-fear generalization protocol (Mühlberger et al., 2013).
During ‘fear conditioning’, an initial neutral stimulus (conditioned stimulus, CS) is repeatedly paired with an aversive event (unconditioned stimulus, US), and thereby acquires the ability to elicit a fear reaction (conditioned reaction, CR), which is similar to the unconditioned reaction elicited by the US itself. In differential protocols, one CS is predictive of the US (the CS+), whereas another one is not (CS−) and serves as a control stimulus. After repeated presentation of the CS+ without being followed by the US, the CR gradually weakens, a process referred to as ‘extinction’.
More recently, a role for BDNF has been demonstrated in extinction (learning) in animals (Andero and Ressler, 2012 (for a review); Peters et al., 2010; Soliman et al., 2010; Psotta et al., 2013). In humans, the BDNFval66met polymorphism has been implicated in immediate fear extinction using functional magnetic resonance imaging (fMRI; Soliman et al., 2010) but not in delayed extinction using psychophysiological measures (Lonsdorf et al., 2010). Further, therapygenetic studies, which use genetic markers to predict the outcome of psychological treatment (for a review, see Lester and Eley, 2013), have reported associations of the met-allele with reduced responder rates in obsessive compulsive disorder patients (Fullana et al., 2012), and reduced response to cognitive behavioral therapy (CBT) in patients suffering from post-traumatic stress disorder (Felmingham et al., 2013).
In particular, the interdisciplinary study by Soliman and colleagues (Soliman et al., 2010) has drawn a lot of attention. They studied fear extinction in knock-in mice as well as the functional BDNFval66met polymorphism in healthy humans. In the human research part of the study, a paradigm, consisting of a conditioning, reversal learning and an extinction phase that followed immediately upon each other was used in the fMRI. During reversal learning, the stimulus that had served as CS+ during conditioning, now served as the CS− and vice versa. In extinction, both stimuli were unpaired. During extinction, met-carriers showed decreased activation in the ventromedial prefrontal cortex (vmPFC) and enhanced activation of the amygdala to CS+s (that is, the CS− in the preceding reversal phase) relative to a fixation baseline. In addition, they report generally heightened skin conductance responses (SCRs) in met-carriers during fear conditioning and extinction. While this study currently represents the only evidence from the human neuroimaging field with respect to BDNFval66met and fear extinction, data on neural activation pattern during fear acquisition are still lacking. As extinction performance is dependent on acquisition learning, comparing activations between groups during both phases will allow for a more unequivocal interpretation of extinction findings and prevent misinterpretation of findings. Thus, to date, it remains possible that the reported extinction-specific findings represent persistent group differences that arose during fear acquisition or preceding reversal learning and clarification is needed. In fact, fear acquisition processes have been linked to BDNF in animals (Rattiner et al., 2004, 2005; Ou and Gean, 2006) and to the BDNFval66met polymorphism in humans (Lonsdorf et al., 2010; but see Torrents-Rodas et al., 2012), while studies reporting neural activation patterns during both fear acquisition and fear extinction in humans are missing. The importance of this becomes clear in light of our previous findings of significant CS+ startle potentiation against the intertrial interval (ITI) as well as CS+/CS− discrimination in val-homozygotes but not met-carriers in late acquisition. This was reflected in 24 h later in early extinction, which most likely reflects recall of the fear memory that was acquired the day before. No differences were found in late extinction, which reflects the outcome of online extinction learning.
To elucidate these questions, we explored the neural activation pattern of fear conditioning and extinction between BDNFval66met met-carriers and val-homozygotes using a differential imaging contrast (CS+ > CS−). Based on our previous fear-potentiated startle data (Lonsdorf et al., 2010), we expected more pronounced neural discrimination in val-homozygotes in regions of the fear and extinction network (amygdala, insula, hippocampus, vmPFC) during acquisition (based on the results of Soliman et al., 2010), that carry over to early but not late extinction.
Methods
Participants
Sixty-seven participants were selected from a larger database of genotyped participants (N ∼ 500, mainly healthy young university students), but analyses are based on 59 participants (32 females, mean age: 24.0 years, s.d. : 0.4, range: 19–31 years). Eight participants were excluded from the experiment because of technical problems (N = 5), left-handedness (N = 2) and pathological anatomy (N = 1). In addition, four participants (one val-homozygote) were excluded for Day 1 because of artifacts, and three participants (two val-homozygotes) were excluded on Day 2 because of artifacts.
The majority of participants were selected for a different study arm (data to be published elsewhere and (same sample, but different experimental phase: Lonsdorf et al., 2011) based on other genotypes (5HTTLPR [s-carriers vs l/l], COMTval158met [val-carrier vs met/met], while an additional two participants were specifically selected based on the BDNFval66met genotype (N = 3 additional met/met genotype individuals) for the purpose of the study arm that is reported here. Participants of both study arms were scanned intermixed in a double-blind fashion. Therefore, genotypes of no interest for this article were included as covariates (5-HTTLPR and COMTval158met). In total, the sample consisted of 34 val-homozygotes and 25 met-carriers (whereof 5homozygotes). As previously done (e.g. Egan et al., 2003; Hajcak et al., 2009; Lonsdorf et al., 2010; Soliman et al., 2010; Torrents-Rodas et al., 2012), carriers of one and two met-alleles were grouped together because of the low frequency of met-homozygotes despite of the pre-selection of two additional individuals with the met/met genotype. Chi-square tests confirmed that BDNFval66met genotype groups were equally distributed within the genotype groups of these two polymorphisms (that is, neither 5-HTTLPR s-carriers nor COMT met-carriers were overrepresented within any of the BDNF genotype groups) both P’s > 0.13. Further, genotype frequencies (excluding the three specifically for this study arm invited participants) did not differ from Hardy-Weinberg-Equilibrium (HWE) (P = 0.46).
BDNFval66met genotype groups did not differ with respect to sex, age, State Trait Anxiety Inventory (STAI) state (Spielberger et al., 2014) prior to or after fear conditioning or extinction, ratings of US valence or US arousal, salivary cortisol concentrations prior or post-conditioning or change of cortisol concentrations during conditioning (methods reported in Lonsdorf et al., 2011), time of day the experiment was conducted, self-reported intake of oral contraceptives (for females) or menstrual cycle phase (for females not taking oral contraceptives; calculated by self-reported time since last menses and self-reported average cycle duration) all P’s > 0.11.
Exclusion criteria were self-reported non-Caucasian, lifetime psychiatric or neurological disorders, pregnancy and non-removable metal parts in the body.
Participants were instructed to refrain from eating, drinking (except water), smoking, chewing gum and exercising for 2 h prior to the experiment. The study was approved by the Ethics Committee at the Karolinska Institutet and performed in compliance with the Declaration of Helsinki; all participants provided written informed consent. After completing the whole study (involving two additional experiments after extinction (Golkar et al., 2012 and data to be reported elsewhere), participants were paid 400SEK. After complete description of the study, written informed consent was obtained.
Stimuli
Two angry male faces from the Karolinska Directed Emotional Faces (Lundqvist et al., 1998) served as CSs (mean duration 7 s, jittered 6–8 s to reduce collinearity of the CS+ and the US in this 100% reinforcement paradigm), and a white fixation cross on a black background was presented during the ITI (mean duration 13 s, jittered 10–16 s).
An individually adjusted monopolar 100 ms direct current (DC)-pulse electric stimulation (STM200; Biopac Systems Inc, www.biopac.com) served as the US. Importantly, final intensity did not differ between BDNFval66met genotype groups, F(1,57) < 1.
Stimuli were presented via Presentation® (Neurobehavioral Systems Inc., Albany, CA) using fMRI-compatible goggles (NordicNeuroLab, Bergen, Norway).
Experimental Protocol
Day 1. Before fear conditioning, both pictures were presented six times to avoid that orienting responses to the stimuli affect neural activation during conditioning (habituation), and participants were explicitly informed that there would not be any US presentation yet. During fear conditioning, there were 15 presentation of each CS, and assignment of the two face picture to the CS+ and CS− was counterbalanced between participants. A reinforcement ratio of 100% was used. Participants were not instructed about the contingencies or the learning element of the experiment beforehand.
After conditioning, participants underwent a structured interview to assess CS–US awareness. All participants but one (val/val genotype) were able to correctly report the conditioning contingencies (“aware”). In our previous study (Lonsdorf et al. 2010), a higher percentage of unawares was observed despite of a nearly identical experimental protocol. It can only be speculated that differences in arousal (fMRI vs non-fMRI environment) might underlie this difference.
Day 2 (extinction). Participants returned for an extinction session ∼24 h later during which they were presented with each CS 24 times (whereof 12 CS assigned to an early and late extinction phase, respectively). No other experimental task took place in between the conditioning and the extinction phase, and recall of the CS–US contingencies was not assessed again prior to the extinction session on Day 2. Participants were informed that they would continue the experiment from the previous day.
Genotyping
Genetic material was collected as 20 ml whole blood or as saliva samples using the Oragene®DNA SelfCollection Kit (DNA Genotek Inc., Kanata, Kanada). DNA extraction was performed as described earlier from either whole blood (Jensen et al., 2009) or from saliva using the protocol and reagents supplied by Oragene®. Genotyping was performed as described earlier for 5HTTLPR/rs25531 (Lonsdorf et al., 2009a) and COMTval158met (Lonsdorf et al., 2009b) and BDNFval66met (Lonsdorf et al., 2010).
Data acquisition, response definition and data analysis
SCRs. SCRs were acquired with a BIOPAC MP150 digital converter (Biopac Systems, Goleta, CA) and fed into AcqKnowledge 4.0 software. SCRs were sampled at 250 Hz, and a 1 Hz lowpass filter was applied during acquisition. SCR responses were scored in AcqKnowledge 4.0 as the largest increase in SCR occurring 0.9–4 s post-stimulus onset with a minimal amplitude of 0.03 m Siemens.
A mixed model analysis of variance with stimulus (2) and time (2) as repeated factors and BDNFval66met genotype as between-subject factors was calculated. 5-HTTLPR and COMTval158met genotypes were entered as covariates. The significance level for all analyses was set at P < 0.05.
fMRI. An anatomical scan and fMRI data were obtained using a GE Signa Echo Speed 1.5T scanner and an 8 channel headcoil. Functional wholebrain images were acquired using a gradient echo T2*-weighted echoplanar imaging scan, echo time = 40 ms, repetition time = 2.5 s, flip angle of 90°, 32 axial slices (thickness = 3 mm with 1 mm gap) and a field of view = 22 cm × 22 cm. The first scans were defined as dummy scans to allow for longitudinal T1-equilibrization, and these were not included in the analysis.
Pre-processing [SPM8 (www.fil.ion.ucl.ac.uk/spm) on MatlabR2009b (The MathWorks, Natick, MA)] involved realignment, unwarping and normalization to a sample-specific template, using DARTEL (Ashburner, 2007). A general linear model [for details, see (Friston et al., 2006)] was set up for statistical first-level (single-subject) analysis. CS onsets were modeled as an event using a “stick” function. During acquisition, three regressors per CS type [one for the habituation, as well as a categorical and a parametric regressor (modulated with a linear decreasing function to capture responses over time)] were included as well as two nuisance regressors for the US and the ITI (eight regressors in total). Note that parametric regressors capture responses over time (Büchel et al., 1998; Marschner et al., 2008; Lonsdorf et al., 2014) and higher parameter estimates are indicative of faster decaying responses. During extinction, a model including nine regressors (CS+early, CS+late, CS−early, CS−late for both categorical and parametric, ITI) was set up. An additional extinction model captured the complete extinction phase through five regressors, two per CS type (categorical and parametric) and the ITI. All regressors were convolved with a canonical hemodynamic response function.
On the second level, random effects full factorial models included regressors for the CS+ and the CS− during conditioning and extinction, respectively, and a group variable (BDNFval66met genotype). 5-HTTLPR and COMTval158met genotypes were entered as covariates. Our contrast of interest was the differential contrast CS+ > CS−. To make sure that both genotype groups did not differ in neural activation pattern elicited by angry male faces prior to conditioning, an additional second-level model including categorical regressors for picture presentations during the habituation phase was set up. Again, 5-HTTLPR and COMTval158met genotypes were entered as covariates, and BDNFval66met genotype was entered as group variable.
All analyses were restricted to pre-defined regions of interest (ROIs) using small volume correction (SVC) based on Gaussian random field theory [family-wise error rate method (FWE; Friston et al., 2006)] at an α-level of P < 0.05. A priori ROI selection was based on expected genotype differences in regions ascribed to the fear network and previously linked to the BDNFval66met polymorphism such as the amygdala (Montag et al., 2008; Soliman et al., 2010), the anterior cingulate/vmPFC (Soliman et al., 2010) as well as the (anterior) insula (Mukherjee et al., 2011) and the hippocampus (e.g. Hariri et al., 2003; Hashimoto et al., 2008; Dennis et al., 2011). Probabilistic anatomical masks (0.7 probability) derived from the Harvard–Oxford cortical and subcortical structural atlases were used (http://www.cma.mgh.harvard.edu; threshold 0.7; Desikan et al., 2006). The provided insula masks were cut at y = 0 to form anterior insula ROIs.
Parameter estimates plots from peak voxels were created using RFXplot (Gläscher, 2009). In addition, beta values were extracted from peak voxels and correlations were performed in Matlab.
RESULTS
SCRs
Prior to conditioning, SCRs to the pictures of angry males did not differ between both genotype groups (F < 1.6, P > 0.2).
During conditioning and extinction, no main or interaction effect involving the factor BDNFval66met genotype was observed, all Fs < 2.0, all Ps > 0.16 (see Figure 1A and B) either when considering the whole phase or when separating extinction into early and late phases (all Fs < 1.4, all Ps > 0.25, data not reported in detail).
Fig. 1.
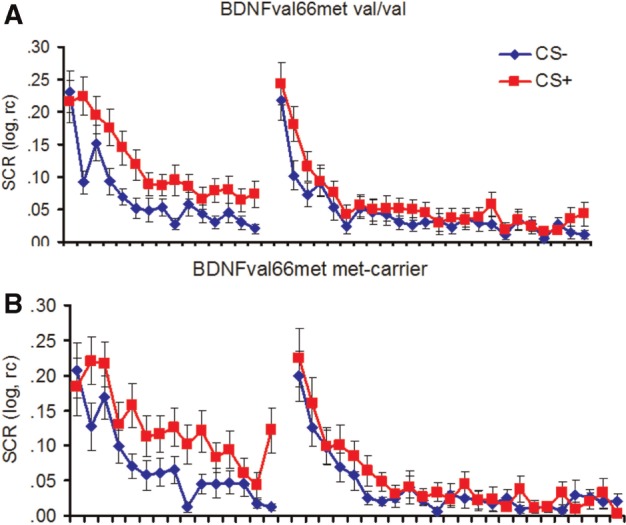
SCRs to the CS+ and the CS− during fear acquisition and extinction in BDNF val-homozygotes (A) and met-carriers (B). Error bars represent s.e.m.
During acquisition, generally successful acquisition was indicated by a significant main effect of stimulus, F(1,51) = 15.75, P < 0.001, Eta2 = 0.24. In addition, a main effect of time indicates habituating SCRs over time, F(1,51) = 18.61, P < 0.001, Eta2 = 0.27, in the absence of a significant stimulus x time interaction, F(1,51) = 1.41, P = 0.24, reflecting rapid acquisition (see Figure 1).
During extinction, a main effect of stimulus indicated generally successful recall of conditioning contingencies learned 24 h before, F(1,52) = 14.55, P < 0.001, Eta2 = 0.22, and a main effect of time indicated habituating SCRs over time, F(1,52) = 68.59, P < 0.001, Eta2 = 0.57. In addition, a stimulus x time interaction indicated faster decreasing responses for the CS− than for the CS+, F(1,52) = 3.48, P = 0.024 Eta2 = 0.06. CS+/CS− discrimination weakened over the course of time and was only significant in the first block, F(1,52) = 14.05, P < 0.001 Eta2 = 0.22, trend level in Blocks 2 and 3, F(1,52) = 3.93, P = 0.053 Eta2 = 0.07 and F(1,52) = 3.30, P = 0.075, Eta2 = 0.06, respectively, had disappeared in the last block, F(1,52) = 1.93, P = 0.17 Eta2 = 0.04.
fMRI - Fear conditioning
Prior to conditioning (i.e. during the preceding habituation phase), no genotype-dependent differences in any of the ROIs were observed in response to pictures of angry males.
During fear conditioning, CS discrimination (CS+ > CS−) differed between genotype groups as reflected in significantly higher left amygdala reactivity (categorical) in met-carriers as opposed to val-homozygotes (P = 0.002 FWE(SVC); see Table 1, Figure 2A), mainly driven by more pronounced amygdala reactivity to the CS+ in met-carriers (see Figure 2A). An exploratory analysis taking all three genotype groups into account (val/val, val/met and met/met) finds the same result (P = 0.011 FWE(wholebrain), see Supplementary Material) and suggests an allele-dose effect (see Supplementary Material and Supplementary Figure S1). No differences in the right amygdala were observed even at more lenient thresholds.
Table 1.
Imaging results
| Phase | Contrast | cat/pm | Comparison | Region | x,y,z | Z(SVC) | k(SVC) | p(SVC) | p(uc) |
|---|---|---|---|---|---|---|---|---|---|
| Conditioning | CSP>CSM | cat. | met-carrier > val/val | L amygdala | −20,0,−18 | 3.94 | 17 | 0.002 | <0.001 |
| val/val > met-carrier | R ACC/vmPFC | 2,40,−2 | 3.35 | 15 | 0.045 | <0.001 | |||
| pm. | val/val > met-carrier | R amygdala | 26,−4,−12 | 3.37 | 27 | 0.023 | <0.001 | ||
| R ACC/vmPFC | 8,42,6 | 3.67 | 34 | 0.023 | <0.001 | ||||
| L ant. hippocamp | −22,−10,−24 | 3.32 | 30 | 0.06 | 0.001 | ||||
| Extinction | CSP>CSM | cat. | met-carrier > val/val | R amygdala | 22 −4 −14 | 3.59 | 24 | 0.013 | <0.001 |
| (1st half) | L ant Insula | −36, 6, 0 | 3.43 | 83 | 0.023 | <0.001 | |||
| R ant Insula | 36, 4, 8 | 3.68 | 33 | 0.012 | <0.001 | ||||
| 36,2,−12 | 3.41 | 34 | 0.028 | <0.001 | |||||
| L hippocampus | −26 −10 −20 | 3.57 | 22 | 0.023 | <0.001 | ||||
| R ACC | 2 12 40 | 3.27 | 25 | 0.089 | 0.001 | ||||
| val/val > met-carrier | none | ||||||||
| pm. | val/val > met-carrier | L ant. Insula | −34 14 −10 | 3.66 | 41 | 0.012 | <0.001 | ||
| −38 4 −12 | 3.08 | 4 | 0.070 | 0.001 | |||||
| Extinction | CSP>CSM | cat. | met-carrier > val/val | none | |||||
| (2nd half) | val/val > met-carrier | none |
Note. cat. = categorial regressor; pm. = parametric (linear decreasing) regressor; L = left; R = right cluster detection threshold of 0.01.
Fig. 2.
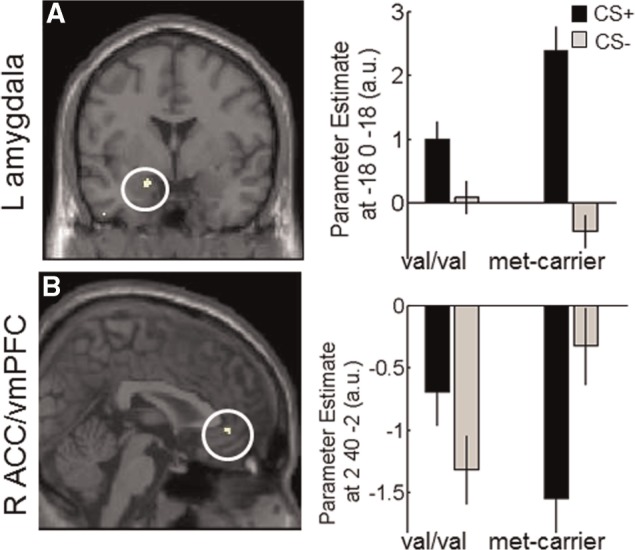
Activation in the contrast CS+> CS− (categorical) for met-carriers > val/val in the left amygdala (A) and for val/val > met-carriers in the vmPFC (B). Images are thresholded at P < 0.001(UC) for illustrative purposes. Error bars represent s.e.m. and beta estimates are derived from peak coordinates.
No time x stimulus effect (linear decreasing parametric regressor) was observed for met-carriers> val-homozygotes.
In val-homozygotes CS discrimination (CS+ > CS−) was reflected in more reactivity (categorical) in the right (perigenual) ACC/vmPFC (P = 0.045 FWE(SVC); see Table 1, Figure 2B) as compared with met-carriers, mainly driven by reduced deactivation to the CS+ than to the CS− in this area in met-carriers while the opposite pattern (reduced deactivation the CS− as compared to the CS+) was found in val-homozygotes (Figure 2A). An exploratory analysis taking all three genotype groups into account demonstrates this pattern for both carriers of one and two met-alleles, suggesting a dominant effect of the met-allele on vmPFC activation (Supplementary Material and Supplementary Figure S1).
A Stimulus × Time interaction (parametric regressors) indicated faster decaying responses (higher parameter estimates) for the CS+ as compared with the CS− in val-homozygotes as compared with met-carriers in the right amygdala, right perigenual ACC/vmPFC and trend-wise in the left (anterior) hippocampus (P = 0.023, P = 0.023 and P = 0.06, respectively, all P’s FWE(SVC); see Table 1).
Amygdala activation was observed in both genotype groups but with different temporal profiles and laterality, categorical in met-carriers and decreasing over time in val-homozygotes.
In any of the two genotype groups the discrimination index ([CS+] − [CS−]), derived from beta estimates for the CS+ and the CS− extracted from the peak voxels for the amygdala (Table 1) were correlated with beta estimates extracted from the subgenual anterior cingulate cortex/vmPFC peak voxel after exclusion of one outlier, both P’s > 0.38.
See Supplementary Table S1 for exploratory whole brain analyses.
fMRI—fear extinction
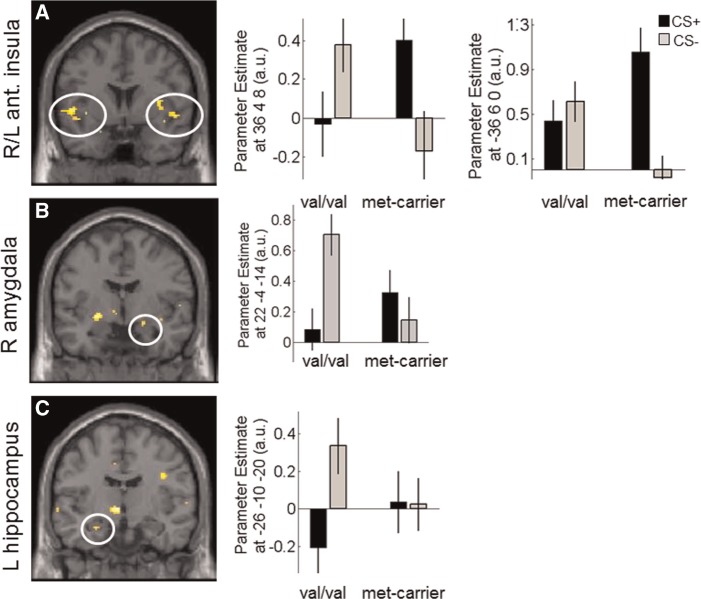
During early extinction (first half), which can be taken to reflect fear memory recall 24 h after conditioning CS discrimination, met-carriers showed enhanced (categorical) responses in areas previously implicated in the fear network such as the bilateral insula (both P’s < 0.028 FWE(SVC); see Table 1, Figure 3A), right amygdala (P = 0.013 FWE(SVC); see Table 1, Figure 3B), left hippocampus (P = 0.023 FWE(SVC); see Table 1, Figure 3C) and trendwise in the (right) dorsal ACC (Table 1), while no areas were more activated in val-homozygotes as compared with met-carriers, suggesting enhanced activation in brain areas associated with fear memory recall in met-carriers than val-homozygotes. While only group differences in the insula reflected CS+ > CS− discrimination in met-carriers, all other significant activation clusters were driven by larger responses to the CS− than the CS+ in val-homozygotes (see parameter estimates Figure 3). A Time × Stimulus interaction as observed in the (left) anterior insula that showed faster declining responses in val-homozygotes as compared with met-carriers. In late extinction in turn, there were no more differences (categorical) between the genotype groups.
Fig. 3.
Activation in the contrast CS+> CS− for met-carriers > val/val in the bilaterial anterior insula (A) right amygdala (B) and left hippocampus (C). Images are thresholded at P < 0.001(UC) for illustrative purposes. Error bars represent s.e.m. and beta estimates are derived from peak coordinates.
Collapsing extinction data across early and late extinction phases, revealed enhanced bilateral activation of the anterior insula in met-carriers as compared with val-homozygotes (both Ps < 0.039 FWE(SVC)) during CS discrimination.
See Supplementary Table S1 for exploratory whole brain analyses.
DISCUSSION
Our results add an important new piece of the puzzle in understanding the involvement of the human BDNFval66met polymorphism in fear and extinction-related processes and thereby complements and extends prior human work. First, we provide the first data on neural activation pattern during fear conditioning depending on the BDNFval66met polymorphism and second, report on the stability of this effect by investigating fear recall and extinction 24 h later.
Mechanistically, activity-dependent elevations of BDNF levels during/after fear conditioning may mediate emotion-induced synaptic plasticity and thereby restructuring of synapses at critical sites of the fear network. The BDNFval66met polymorphism has been shown to affect activity-dependent BDNF secretion (e.g. Egan et al., 2003) and dendritic arborization and thus has the capacity to affect learning and memory-dependent processes.
The present fMRI results, as our previous results from fear potentiated startle (FPS), suggest an association of BDNFval66met with fear acquisition (Lonsdorf et al., 2010; but see Torrents-Rodas et al., 2012) and 24 h-delayed fear memory recall (as indicated by early extinction) but not (delayed) extinction processes (as indicated by late extinction phases) (Lonsdorf et al., 2010).
The observed differences in neural activation pattern, namely enhanced activation of the left amygdala in met-carriers and enhanced perigenual ACC/vmPFC activation in val-homozygotes, nicely mirrors the genotype group differences observed by Soliman et al. (2010). While this replicates an association of BDNFval66met with reactivity in these brain areas, the present study observed these genotype group differences during ‘fear acquisition’, while results are derived from the extinction phase in the Soliman et al. study (2010), where fMRI data from either the preceding fear acquisition or the reversal learning phases were not reported. Thus, it cannot be excluded that Soliman’s results reflect the continuation of genotype group differences that emerged during acquisition or reversal learning, as might be suggested by our results. In addition, the reversal manipulation immediately preceding the extinction phase may have significantly impacted the results. Further complicated by this design, the critical contrast for differential fear conditioning studies CS+ > CS− could not be reported and results are based on the CS+ > fixation baseline contrast, which cannot control for orienting responses and sensitization effects, which affect both CS+ and CS− likewise and thus generally heightened CS-responsivity in met-carriers might be an additional alternative explanation.
In addition to the regions already implicated in genotype-dependent differences by Soliman et al. we also observed higher activation in the bilateral anterior insula and the left hippocampus to the CS+ (>CS−), which are regions implicated in the fear network during early extinction, which likely reflects fear recall. These genotype group differences were largely (with the exception of the anterior insula) driven by an enhanced response to the CS− in val-homozygotes. This is in line with previous work, as genotype differences in insula activation during threat processing have been reported previously (Mukherjee et al., 2011). As no genotype group differences were observed during late extinction, our data speak in favour of a more general association of the BDNFval66met polymorphism and emotional learning beyond extinction processes. As extinction occurs rapidly in this paradigm, future studies should investigate genotype-dependent differences in paradigms designed to slow down extinction (e.g. through the use of a lower reinforcement ratio during acquisition).
Previously, we had observed attenuated FPS CS+/CS− discrimination and CS+ potentiation during fear acquisition in contingency aware met-carriers. Based on these previous results and the assumption that FPS is a fear-specific measure and represents an immediate output of the amygdala (Davis and Whalen, 2001), we had expected heightened amygdala responses in val-homozygotes during fear acquisition and fear recall 24 h later. However, contrary to our expectations, we observed heightened left amygdala responses to the CS+ as compared with the CS− in met-carriers. Amygdala activation in val-homozygotes displayed a different temporal profile as evident from faster decaying responses during acquisition. During fear recall 24 h later, we again observed enhanced, however, right amygdala activation in met-carriers as opposed to val-homozygotes. Enhanced activation to the CS+ in brain areas associated with emotional responding in met-carriers is in line with animal work showing that BDNF met knock-in mice show intact and possibly enhanced cue-dependent fear conditioning (Chen et al., 2006) and human work showing stronger amygdala activation in the right hemisphere in response to emotional stimuli in met-carriers (Montag et al., 2008) and in anxious and depressed adolescents to emotional faces (Lau et al., 2010).
At first glance, these results for fear acquisition are, however, at odds with attenuated CS+ potentiated startle reactions (Hajcak et al., 2009; Lonsdorf et al., 2010) and this requires clarification. It is generally accepted that the startle reflex is potentiated during aversive motivational states, while it is attenuated during appetitive motivational states (Lang et al., 1990). Recently however, this clear distinction has been challenged by accumulating reports about paradoxically diminished and not potentiated startle reactions in situations of enhanced autonomic arousal during imminent or prolonged threat (Löw et al., 2008; Richter et al., 2012; Dunning et al., 2013). For example, patients suffering from anxiety disorders characterized by ‘discrete’ fear (e.g. specific phobias) display ‘enhanced’ FPS, while disorders characterized by longer lasting ‘diffuse anxiety’ display ‘diminished’ FPS (McTeague and Lang, 2012). Such paradoxically reduced startle reactivity has been interpreted as deficient threat mobilization. As the organisms’ defensive behaviour is known to depend on threat proximity (predator-imminence model; Fanselow, 1994; Lang et al., 1997), it is tempting to speculate that the observation of enhanced amygdala activation to the CS+ in met-carriers (this study), co-occurring with attenuated CS+ specific FPS in a nearly identical paradigm (Lonsdorf et al., 2010) might be explained though such paradoxical deficiencies in threat reflex mobilization in the face of imminent threats (e.g. it can be speculated that a 100% reinforcement ratio may represent an imminent threat). Future studies should use experimental paradigms explicitly designed to test this model (e.g. Mobbs et al., 2007). If this speculation would be correct, the interpretation of our previous results would need to be revised. In this case, the lack of fear-potentiated startle reactions in met-carriers may not indicate deficient amygdala-dependent fear learning (as discussed in Lonsdorf et al., 2010) but might rather reflect deficient threat reflex mobilization. This would be indicative of ‘enhanced’ diffuse anxiety-like behaviour, which in turn would be in line with enhanced amygdala reactivity during fear conditioning.
As in previous work (Lonsdorf et al., 2010; Torrents-Rodas et al., 2012), we did not observe any genotype group differences in SCRs, which might not be sensitive and fear-specific enough to detect small group differences in fear conditioning and extinction, while FPS (Lonsdorf et al. 2010 but see Torrents-Rodas et al. 2012) taps a basic affective level of fear conditioning, largely independent of higher cognition (Hamm and Weike, 2005; Sevenster et al., 2014). This interpretation is supported by other studies that find dissociations between results for SCRs and fMRI in Imaging Genetics studies (e.g. Lonsdorf et al., 2011a; Klucken et al., 2012; Klumpers et al., 2012) and findings that SCRs can be dissociated from amygdala activation during human fear conditioning (Tabbert et al., 2006) and largely reflects contingency awareness (Hamm and Weike, 2005).
Having discussed our present findings in the context of the literature and previous work, it becomes evident that the work on BDNFval66met and fear conditioning and extinction processes is paved by divergent findings (for a review see Lonsdorf and Kalisch, 2011b). While Torrents-Rodas et al. (2012) did not observe any associations, two fMRI studies report associations with fear conditioning (this study) and fear extinction (Soliman et al., 2010). It may be possible that the aversive fMRI environment may facilitate that genotype-dependent differences become evident. On the other hand, two small behavioural studies also observed genotype-dependent differences in FPS (Hajcak et al., 2009; Lonsdorf et al., 2010), which renders this interpretation unlikely. It is possible that subtle methodological variations (e.g. reinforcement ratio, neutral or emotional CSs) may underlie these differences.
In closing, some limitations of the current study should be mentioned: First, life-time mental disorders and corresponding exclusion of participants was based on the participants self-report, which has to be considered somewhat imprecise. Second, angry male faces served as CSs. It remains therefore unexplored if results would be identical with neutral CSs [e.g. geometrical symbols as in (Hajcak et al., 2009; Soliman et al., 2010; Torrents-Rodas et al., 2012)]. Related to this, it cannot be completely excluded that social events related to angry male individuals occurring in the 24 h between the conditioning and the extinction session may impact extinction results in a genotype-dependent fashion. However, this is rather unlikely. Future studies using delayed extinction tasks should account for this by acquiring information about experiences in between both experimental sessions. Third, most of the participants included in the current study were selected a priori based on other genotypes (5-HTTLR/rs25531 and COMTval158met) for another study arm, while only a minority was selected specifically for this study arm. Even though, these genotypes were included as covariates, it cannot be excluded that this might affect the results slightly. Fourth, while the genotype differences in the amygdala in acquisition and early extinction/fear recall as well as the left insula activation during early extinction are strong and would even survive a Bonferroni correction for the number of ROIs used [for conditioning and for extinction 4 ROIs each yielding significance threshold of P = 0.012 (0.05/4)], all other genotype-dependent differences as well as lateralization of the findings in neural activation pattern should be considered preliminary. Last, but not least, owing to restrictions on acquiring EMG signals inside the scanner, it was not possible to measure FPS in the current study, which would be necessary to directly compare the results with those of our prior work. However, recent technical developments recently resulted in first attempts of simultaneously assessing FPS and fMRI during a fear conditioning experiment, suggesting startle discrimination to be reflected in amygdala and dorsal PFC activation (van Well et al., 2012). As FPS was not measured, that the behavioural relevance of the present findings remains unclear until future studies indexing both neural and behavioral differences between BDNFval66met genotype groups have validated the interpretations put forth here.
In sum, the present data support a general association of BDNFval66met with a broader range of emotional memory processes than merely or selectively for fear extinction. Our preliminary data on all three genotype groups suggest that both an allele-load and a dominant effect of the met-allele may be observed depending on the brain region and function studied. The exact role for the BDNFval66met polymorphism in fear conditioning and extinction processes, however, as well as in general emotional memory formation still requires further investigation.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank the KIgene core facility at the Center for Molecular Medicine (CMM). The authors report no competing interests.
This work was supported by grants from Swedish Research Council, Karolinska Institutet, Stockholm County Concil and Karolinska University Hospital as well as Hjärnfonden (Swedish Brain Foundation). TBL was partly supported by the SFB TRR58 (subproject B07).
REFERENCES
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes, Brain, and Behavior. 2012;11(5):503–12. doi: 10.1111/j.1601-183X.2012.00801.x. doi:10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. doi:10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in Neurobiology. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. doi:10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–57. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–3. doi: 10.1126/science.1129663. doi:10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R, Need AC, Waters-Metenier S, Goldstein DB, LaBar KS. Brain-derived neurotrophic factor val66met polymorphism and hippocampal activation during episodic encoding and retrieval tasks. Hippocampus. 2011;21(9):980–9. doi: 10.1002/hipo.20809. doi:10.1002/hipo.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. doi:10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dunning JP, DelDonno S, Hajcak G. The effects of contextual threat and anxiety on affective startle modulation. Biological Psychology. 2013;94(1):130–5. doi: 10.1016/j.biopsycho.2013.05.013. doi:10.1016/j.biopsycho.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin and Review. 1994;1(4):429–38. doi: 10.3758/BF03210947. doi:10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA. The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biological Psychiatry. 2013;73(11):1059–1063. doi: 10.1016/j.biopsych.2012.10.033. doi:10.1016/j.biopsych.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; 2006. [Google Scholar]
- Fullana MA, Alonso P, Gratacòs M, et al. Variation in the BDNF Val66Met polymorphism and response to cognitive-behavior therapy in obsessive-compulsive disorder. European Psychiatry: The Journal of the Association of European Psychiatrists. 2012;27(5):386–90. doi: 10.1016/j.eurpsy.2011.09.005. doi:10.1016/j.eurpsy.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Gläscher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7(1):73–82. doi: 10.1007/s12021-008-9042-x. doi:10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012;7(11):e48107. doi: 10.1371/journal.pone.0048107. doi:10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Castille C, Olvet D, Dunning J, Roohi J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain and Behavior. 2009;8(1):80–5. doi: 10.1111/j.1601-183X.2008.00447.x. doi:10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2005;57(1):5–14. doi: 10.1016/j.ijpsycho.2005.01.006. doi:10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(17):6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Moriguchi Y, Yamashita F, et al. Dose-dependent effect of the Val66Met polymorphism of the brain-derived neurotrophic factor gene on memory-related hippocampal activity. Neuroscience Research. 2008;61(4):360–7. doi: 10.1016/j.neures.2008.04.003. doi:10.1016/j.neures.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PloS One. 2009;4(6):e6016. doi: 10.1371/journal.pone.0006016. . doi:10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, et al. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Human Brain Mapping. 2012;34:2549–60. doi: 10.1002/hbm.22085. doi:10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Heitland I, Oosting RS, Kenemans JL, Baas JMP. Genetic variation in serotonin transporter function affects human fear expression indexed by fear-potentiated startle. Biological Psychology. 2012;89(2):277–282. doi: 10.1016/j.biopsycho.2011.10.018. doi:10.1016/j.biopsycho.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. Attention and Orienting: Sensory and Motivational Processes. 1997:97–135. [Google Scholar]
- Lau YF, Goldman D, Buzas B, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53(3):952–61. doi: 10.1016/j.neuroimage.2009.11.026. doi:10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester KJ, Eley TC. Therapygenetics: using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biology of Mood and Anxiety Disorders. 2013;3:4. doi: 10.1186/2045-5380-3-4. doi:10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstöm K, et al. 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biological Psychology. 2011a;87:106–12. doi: 10.1016/j.biopsycho.2011.02.014. doi:10.1016/j.biopsycho.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Kalisch R. Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: a reinstatement study in two independent samples. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu018. nsu018. doi:10.1093/scan/nsu018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Translational Psychiatry. 2011b;1:e41. doi: 10.1038/tp.2011.36. doi:10.1038/tp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf T, Ruck C, Bergstrom J, et al. The symptomatic profile of panic disorder is shaped by the 5-HTTLPR polymorphism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009a;33(8):1479–83. doi: 10.1016/j.pnpbp.2009.08.004. doi:10.1016/j.pnpbp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T, Weike A, Golkar A, Schalling M, Hamm A, Ohman A. Amygdala-dependent fear conditioning in humans is modulated by the BDNFval66met polymorphism. Behavioral Neuroscience. 2010;124(1):9–15. doi: 10.1037/a0018261. doi:10.1037/a0018261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf T, Weike A, Nikamo P, Schalling M, Hamm A, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009b;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. doi:10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. 1998 ISBN 91-630-7164-9. [Google Scholar]
- Löw A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: emotional arousal in threat and reward. Psychological Science. 2008;19(9):865–73. doi: 10.1111/j.1467-9280.2008.02170.x. doi:10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience. 2008;28(36):9030–6. doi: 10.1523/JNEUROSCI.1651-08.2008. doi:10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depression and Anxiety. 2012;29(4):264–81. doi: 10.1002/da.21891. doi:10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science (New York, N.Y.) 2007;317(5841):1079–83. doi: 10.1126/science.1144298. doi:10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: evidence from a genetic imaging study. Neuroimage. 2008;42(4):1554–9. doi: 10.1016/j.neuroimage.2008.06.008. doi:10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Andreatta M, Ewald H, et al. The BDNF Val66Met polymorphism modulates the generalization of cued fear responses to a novel context. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2013;39(5):1187–95. doi: 10.1038/npp.2013.320. doi:10.1038/npp.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Whalley HC, McKirdy JW, et al. Effects of the BDNF Val66Met polymorphism on neural responses to facial emotion. Psychiatry Research. 2011;191(3):182–8. doi: 10.1016/j.pscychresns.2010.10.001. doi:10.1016/j.pscychresns.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31(2):287–96. doi: 10.1038/sj.npp.1300830. doi:10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science (New York, N.Y.) 2010;328(5983):1288–90. doi: 10.1126/science.1186909. doi:10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psotta L, Lessmann V, Endres T. Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiology of Learning and Memory. 2013;103:34–8. doi: 10.1016/j.nlm.2013.03.003. doi:10.1016/j.nlm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learning and Memory (Cold Spring Harbor, N.Y.) 2004;11(6):727–31. doi: 10.1101/lm.83304. doi:10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. The Neuroscientist. 2005;11(4):323–33. doi: 10.1177/1073858404272255. doi:10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Richter J, Hamm AO, Pané-Farré CA, et al. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biological Psychiatry. 2012;72(6):512–20. doi: 10.1016/j.biopsych.2012.03.035. doi:10.1016/j.biopsych.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. Frontiers in Behavioral Neuroscience. 2014;8:32. doi: 10.3389/fnbeh.2014.00032. doi:10.3389/fnbeh.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science (New York, N.Y.) 2010;327(5967):863–66. doi: 10.1126/science.1181886. doi:10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 2014. [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage. 2006;32(2):761–70. doi: 10.1016/j.neuroimage.2006.03.038. doi:10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Torrents-Rodas D, Fullana MA, Arias B, et al. Acquisition and generalization of fear conditioning are not modulated by the BDNF-val66met polymorphism in humans. Psychophysiology. 2012;49(5):713–19. doi: 10.1111/j.1469-8986.2011.01352.x. doi:10.1111/j.1469-8986.2011.01352.x. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learning and Memory (Cold Spring Harbor, N.Y.) 2002;9(5):224–37. doi: 10.1101/lm.51202. doi:10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cognitive, Affective and Behavioral Neuroscience. 2012;12(3):499–512. doi: 10.3758/s13415-012-0089-7. doi:10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.