Abstract
It is unclear whether reflective awareness of emotions is related to extent and intensity of implicit affective reactions. This study is the first to investigate automatic brain reactivity to emotional stimuli as a function of trait emotional awareness. To assess emotional awareness the Levels of Emotional Awareness Scale (LEAS) was administered. During scanning, masked happy, angry, fearful and neutral facial expressions were presented to 46 healthy subjects, who had to rate the fit between artificial and emotional words. The rating procedure allowed assessment of shifts in implicit affectivity due to emotion faces. Trait emotional awareness was associated with increased activation in the primary somatosensory cortex, inferior parietal lobule, anterior cingulate gyrus, middle frontal and cerebellar areas, thalamus, putamen and amygdala in response to masked happy faces. LEAS correlated positively with shifts in implicit affect caused by masked happy faces. According to our findings, people with high emotional awareness show stronger affective reactivity and more activation in brain areas involved in emotion processing and simulation during the perception of masked happy facial expression than people with low emotional awareness. High emotional awareness appears to be characterized by an enhanced positive affective resonance to others at an automatic processing level.
Keywords: emotional awareness, implicit affect, automatic emotion processing, neuroimaging
INTRODUCTION
Trait emotional awareness is a cognitive skill reflecting individual differences in the ability to recognize and describe emotion in oneself and others (Lane et al., 1990). Lane and Schwartz (1987) proposed a model of emotional-cognitive development that differentiates between five levels of emotional development. The five levels in ascending order are awareness of physical sensations (level 1), action tendencies (level 2), single emotions (level 3), blends of emotions (level 4) and blends of ‘blends of emotions’ (level 5, the capacity to appreciate complexity in the experiences of self and other persons). A fundamental tenet of this model is that individual differences in emotional awareness reflect variations in the degree of differentiation and integration of schemata used in emotional processing. Fundamental information for such emotional processing schemata can be derived from the external world or the internal world through introspection (Lane and Schwartz, 1987). A performance-based task, the Levels of Emotional Awareness Scale (LEAS; Lane et al., 1990) has been developed to measure trait emotional awareness.
The five levels of emotional awareness are hierarchically related, in that, functioning at each level adds to and modifies the function of previous levels but, importantly, does not eliminate them (Lane, 2008). For example, blends of emotion (Level 4 experiences), compared with action tendencies (Level 2), are assumed to be associated with more differentiated representations of somatic sensations (Level 1). The feelings associated with a given emotional response can be viewed as a construction consisting of each of the levels of awareness up to and including the highest level attained (Lane and Pollermann, 2002; Lane and Garfield, 2005). The trait level of function is the level at which a given individual typically functions (Lane, 2008).
The levels of emotional awareness can be mapped onto the distinction between implicit and explicit processes (Lane, 2000). Level 1 (bodily sensations) and Level 2 (action tendencies) phenomena are critical components of emotional responses but, viewed in isolation, cannot necessarily be considered indicators of emotions. The peripheral physiological arousal and action tendencies associated with emotion are ‘implicit’ in the sense that they occur automatically and do not require conscious processes to be executed efficiently. Levels 3, 4 and 5 consist of conscious (or explicit) emotional experiences at different levels of complexity. The levels of emotional awareness theory puts implicit and explicit processes on the same continuum. However, relatively little research has been completed that explores the details of the interactions across levels.
Research addressing the neural correlates of emotional awareness has focused exclusively on controlled or explicit processing of emotional information. Lane et al. (1998) found evidence that individual differences in trait emotional awareness may at least in part be a function of the degree to which the anterior cingulate cortex (ACC) participates in the experiential processing and response to emotional cues. Similarly, McRae et al. (2008) found positive correlations between emotional awareness and activity in the dorsal ACC, middle frontal gyrus, primary somatosensory cortex, inferior frontal gyrus and insula during the processing of highly arousing, as opposed to less arousing, emotional stimuli. The findings of both studies support the idea that individual differences in emotional awareness are associated with increased ACC activity, which according to McRae et al. (2008) may reflect greater attentional processing of emotional information. They also suggest that individuals with higher emotional awareness are better able to process highly arousing emotional stimuli. Thus, research on controlled emotion processing suggests heightened activity in several cortical areas implicated in attention allocation and arousal as a function of trait emotional awareness.
Evidence that higher emotional awareness is associated with top-down modulation of lower-level function comes from two different sources. Evidence for modulation of level 1 (somatic sensation) function comes from Lane et al. (2011), which demonstrated that patients at risk for sudden cardiac death owing to the Long QT Syndrome with higher levels of emotional awareness had more differentiated reporting of somatic symptoms in an ecological momentary assessment study. Evidence for modulation of level 2 (action tendency) function comes from Bréjard and colleagues (2012) who observed that adolescents with higher levels of emotional awareness were less impulsive. The latter finding is consistent with expectations, in that, level 2 function is action-oriented, whereas higher levels of emotional awareness include consideration of long-term consequences in addition to satisfaction of immediate needs. In these contexts, the correlates of trait emotional awareness were outcomes (symptoms, behaviors) that involved conscious reflection and attention, particularly among those who were more emotionally aware.
Less well studied is the bottom-up or input side of cross-level communication. To do so requires examination of the relation between trait emotional awareness and indices of lower-level functions at the moment of confrontation with emotional stimuli. Based on the findings by McRae et al. cited above it could be hypothesized that individuals who are more emotionally aware are more sensitive to emotional information and have more intense responses to emotion stimuli. Supporting evidence for this comes from research showing that trait emotional awareness is positively correlated with skin conductance responses to emotional stimuli (Lane et al., 2000a; McRae et al., 2008). This means that greater trait emotional awareness is characterized by stronger peripheral physiological arousal in response to emotional stimuli. In an affective priming study (Suslow et al., 2001), higher trait emotional awareness was associated with prolonged processing times during the automatic perception of lexical and facial emotion stimuli. These findings indicate that individuals with high trait emotional awareness automatically allocate more attention to emotional stimuli and react to them with greater physiological arousal. It could be that greater arousal amplifies the input signal and greater attentional processing enables the detection of greater complexity in that signal. This would be consistent with the theory that greater emotional awareness is associated with more complex emotion information processing due to the differentiation and integration of the schemata that participate in such processing, and would begin to explain the mechanisms by which individuals who are more aware are able to extract more information from exteroceptive emotional stimuli.
According to Zajonc’s (1980) affective primacy hypothesis, initial responses to emotional stimuli are automatic and do not necessarily require conscious awareness. It is plausible that individuals with higher emotional awareness of their own and others’ feelings could be characterized by stronger automatic reactions to emotional stimuli. The effortless production of responses in basal emotion processing systems of the brain such as the amygdala appears to provide an important basis of information for higher processing systems. In case higher-order neocortical areas receive poor input from the limbic and somatosensory system, mental representation and differentiation of emotional responses could be reduced. High automatic emotion responsivity could thus be an important factor in promoting the emotional development and differentiation of individuals.
To elicit unconscious affective reactions, researchers have used subliminal presentations of emotional facial expressions (Zajonc, 2000). It has been shown that masked happy and masked angry faces can cause positive or negative reactions that are not accessible to conscious awareness (Winkielman et al., 2005). Subliminal perception of emotional facial expression is a complex process that implicates an interactive network of brain areas. Central neural structures underpinning automatic emotion perception from the face are the amygdalae, thalamus, occipito-temporal visual cortical regions (including the fusiform gyrus), the inferior frontal gyrus, the insula and the somatosensory cortices (e.g. Killgore and Yurgelun-Todd, 2004; Phillips et al., 2004; Liddell et al., 2005; Duan et al., 2010; Suslow et al., 2010). It has been shown that facial mimicry occurs even during subliminal perception of facial emotion (Dimberg et al., 2000; Rotteveel et al., 2001). Observation of others’ facial expression of emotions activates brain regions involved in experiencing similar emotions. The amygdala, insula and anterior cingulate gyrus participate in processes of emotion simulation (Bastiaansen et al., 2009; Molenberghs et al., 2012).
In this study, we examined for the first time the relationship between trait emotional awareness and automatic brain reactivity to positive and negative facial emotions in healthy adults. It was hypothesized that high trait emotional awareness is related to increased reactivity in brain structures implicated in the processing of masked facial emotions. Moreover, we hypothesized that LEAS is positively related to affective priming effects (i.e. high emotional awareness should be associated with more shifts in implicit affect due to masked facial emotion compared with low emotional awareness). By administering the affective priming paradigm, we examined the relationship between trait emotional awareness and affective resonance at an automatic processing level. Affective resonance can be defined as a person's tendency to resonate and experience the same affect in response to viewing a display of that affect by another person. Affective resonance can be considered to be the original basis for interpersonal communication (Tomkins, 1962).
To increase the probability of occurrence of behavioral affective priming effects, we reduced visibility of primes compared with other studies (e.g. Kugel et al., 2008; Reker et al., 2010) by using forward and backward masking. To examine the effects of emotional primes on implicit positive and negative affect separately, we combined affective priming based on facial expression with items of the ‘Implicit Positive and Negative Affect Test’ (IPANAT; Quirin et al., 2009). The IPANAT has been shown to be a valid measure of implicit positive and negative affect (Quirin et al., 2009, 2011). To evaluate the success of the masking procedure, participants took part in a forced-choice detection task outside the scanner.
METHOD
Participants
Fifty-two right-handed healthy volunteers participated in this functional magnetic resonance imaging (fMRI) study. Six participants had to be excluded from further analysis, five because of excessive head motion in the scanner (>3 mm translation) and one because of high depression score (Beck Depression Inventory, BDI ≥ 14). The final sample consisted of 46 participants (23 women; mean age = 23.5 years, s.d. = 2.7). None of the participants had current psychiatric illness or any lifetime history of psychiatric condition according to the criteria of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 1994), as diagnosed with the Structured Clinical Interview for DSM-IV Axis I disorders (Wittchen et al., 1997). Exclusion criteria of this study were any neurological abnormalities, head trauma or loss of consciousness, psychotropic medication and the standard magnetic resonance imaging contraindications. Handedness of subjects was measured by the Handedness Questionnaire (Raczkowski et al., 1974). Visual acuity was tested before inclusion in the study. The local ethics committee approved the procedure of the experiment. All participants gave their written consent to participate in the study and were financially compensated on study completion.
The LEAS (Lane et al., 1990; Subic-Wrana et al., 2001) was administrated as a measure of trait emotional awareness. The LEAS is a written performance task. The subject is asked to describe his or her feelings and those of other people. In this study, we used the short version of the LEAS with 10 vignettes. The mean LEAS total score was 35.9 (s.d. = 4.9). Cronbach’s alpha for the LEAS total score was α = 0.73. Higher scores on LEAS indicate higher emotional awareness. The BDI-II (Beck and Steer, 1987; Hautzinger et al., 1994) was administered to assess current symptoms of depression. The mean BDI score was 3.0 (s.d. = 3.5; α = 0.78). The State-Trait-Anxiety Inventory (STAI; Spielberger et al., 1970; Laux et al., 1981) was applied to assess trait anxiety. In this sample, the mean trait anxiety score was 33.7 (s.d. = 8.2; α = 0.89). The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988; Krohne et al., 1996) was used to measure trait positive affect (P) and trait negative affect (N). The mean score for PANAS-P was 16.2 (s.d. = 2.5; α = 0.83). The mean score for PANAS-N was 6.22 (s.d. = 0.9; α = 0.73).
fMRI experiment: stimulus materials and procedure
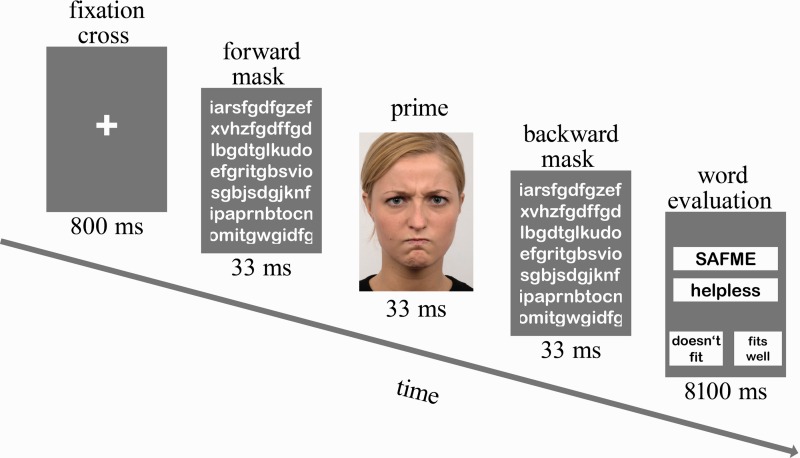
Colored photographs of 20 different individuals (Langner et al., 2010) served as stimuli for the fMRI experiment. Four different emotional facial expressions (i.e. happy, angry, fearful and neutral) were applied as primes. Primes were shown with a sandwich masking technique using letter strings (see Figure 1). The letter strings were drawn randomly on each trial. The durations of the masks were chosen based on the results of a pilot study (n = 10) conducted in our laboratory. The experiment consisted of 80 trials: 20 per prime condition. Primes were presented with restriction of no repetition of an individual and no more than one repetition of a prime condition on consecutive trials. The lexical material for the evaluation of the fit between artificial word and emotion adjective was taken from the Implicit Positive and Negative Affect Test (IPANAT; Quirin et al., 2009). We used five artificial words (SAFME, VIKES, TUNBA, TALEP and BELNI) and four adjectives: two were positive (happy, cheerful), two were negative (helpless, inhibited). The duration of each trial was 9 s. The trial started with a fixation cross lasting for 800 ms. Then, the prime stimulus was presented (33 ms) forward and backward masked by the letter mask (duration 33 ms each). During the following 8.1 s, participants had to evaluate the fit between artificial and emotion word on a four-point Likert scale by pressing one of four buttons (0, 1, 2 or 3). For half of the subjects, higher number indicated higher fitting between artificial and emotion word. The other half of the subjects indicated higher fittings by pressing lower numbers. The shifts in implicit positive affect were calculated by subtracting implicit positive affect score for the neutral face condition from those of the happy face condition. Similarly, the shifts in implicit negative affect were calculated by subtracting implicit negative affect score for the neutral face condition from those of the angry or fearful face conditions.
Fig. 1.
Sequence of events within trials in the affective priming task. In our example, a trial with an angry prime is shown.
During the fMRI session, participants lay supine in the scanner with extended arms holding fiber optic response pads with two buttons in each hand. Half of the subjects indicated better fit between artificial word and adjective by pressing the right buttons, half by pressing the left buttons. Word evaluations and reactions times were recorded. Subjects’ head position was stabilized with cushions.
fMRI data acquisition and data analysis
Participants underwent structural and functional MRI scanning on a 3 T scanner (Magnetom Verio, Siemens, Erlangen, Germany) using a standard 12-channel head coil. For each participant, structural images were acquired with a T1-weighted 3D MP-RAGE (Mugler and Brookeman, 1990). Magnetization preparation consisted of a non-selective inversion pulse. The imaging parameters were TI = 650 ms, TR = 1300 ms, TE = 3.5 ms, flip angle = 10°, isotropic spatial resolution of 1 mm3, two averages. Blood oxygen level-dependent (BOLD) sensitive images were collected using T2*-weighted echo-planar imaging (EPI) sequence [matrix 642; 30 slices; resolution 3 mm × 3 mm × 4 mm; gap 0.8 mm; repetition time (TR) = 2 s; echo time (TE) = 30 ms; flip angle (FA) = 90°; interleaved slice acquisition; 400 images]. Scanning planes were oriented parallel to a line through the posterior and anterior commissures. fMRI data were analyzed using Statistical Parametric Mapping SPM5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm/). The initial five functional volumes were discarded to allow longitudinal magnetization to reach equilibrium. Functional volumes were slice time-corrected (middle slice as reference), realigned to the temporally first image and corrected for movement-induced image distortions (six-parameter rigid body affine realignment). Functional and anatomical images were co-registered. Anatomical images were then segmented, including normalization to a standard stereotaxic space using the T1 template by the Montreal Neurological Institute (MNI) delivered with SPM. The normalization parameters were then applied to the functional EPI series. On the functional data, spatial smoothing was performed using a three-dimensional Gaussian filter of 6 mm full-width at half-maximum.
We used an event-related design. For each participant, trials were averaged for each prime condition (happy, angry, fearful and neutral) resulting in four average trials for each subject. First level t-contrasts were calculated by contrasting each emotional condition to the neutral one (i.e. happy vs neutral, angry vs neutral and fearful vs neutral). Then one-sample t-tests were performed on activation data for the three emotion conditions (compared with the neutral face condition) to obtain main effects of emotions. Finally, the relationship between trait emotional awareness and brain activation during processing of masked facial emotions was evaluated [controlling for positive affectivity (PANAS-P)] using multiple regression as implemented in SPM5. In contrast to positive affectivity, negative affectivity (PANAS-N) did not change the fMRI findings, so the latter results are not further presented and discussed. The general linear model was used to model the effects of interest and other confounding effects.
First, region-of-interest (ROI) analyses were carried out focusing on the amygdala. It has been repeatedly shown that the amygdala is activated during the subliminal perception of facial emotion (e.g. Whalen et al., 1998; Dannlowski et al., 2007). The amygdala was defined according to Tzourio-Mazoyer et al. (2002), and the amygdala mask was created by means of the Wake Forest University (WFU) pickatlas (Maldjian et al., 2003). In the ROI analyses, significant clusters are reported corrected for search volume (P < 0.05). In addition, exploratory whole-brain analyses were conducted for each contrast using a statistical threshold of P < 0.001 (uncorrected) and a spatial extent of 10 contiguous voxels.
Detection task
To evaluate the success of the masking procedure, a forced-choice detection task was administered outside the scanner after the fMRI experiment. The detection task had the same presentation conditions and the same stimuli as the fMRI experiment. The task consisted of 40 trials, 10 trials per emotion condition. Participants were presented with a photograph of an emotional face preceded and followed by pictures with letter strings (sandwich masking). Participants’ task was to label the expression of the face. Subjects were informed that they would see facial expressions depicting happy, angry, fearful, sad and neutral emotions (though sad faces were actually not shown) and asked to indicate which prime was presented between the two letter masks via button press. The non-parametric index of sensitivity A’ proposed by Aaronson and Watts (1987) was used to assess detection performance. It represents an extension of the sensitivity index defined by Grier (1971) and is appropriate for signal detection tasks in which the false alarm rate exceeds the hit rate. The A’ index takes into account hit and false alarm rates and indicates chance performance at A’ = 0.5. We calculated a sensitivity index for each facial expression condition (e.g. happy) relative to the other conditions (in this example angry, fearful and neutral).
RESULTS
Trait emotional awareness and measures of affectivity
The LEAS was not correlated with STAI and BDI (r = 0.05 and r = 0.09, P > 0.55, two-tailed) or PANAS-N (r = −0.01, P > 0.95, two-tailed). However, the LEAS was significantly related to positive affect as assessed by the PANAS-P (r = 0.38, P < 0.01, two-tailed).
Behavioral performance in the fMRI experiment: shifts in implicit affect
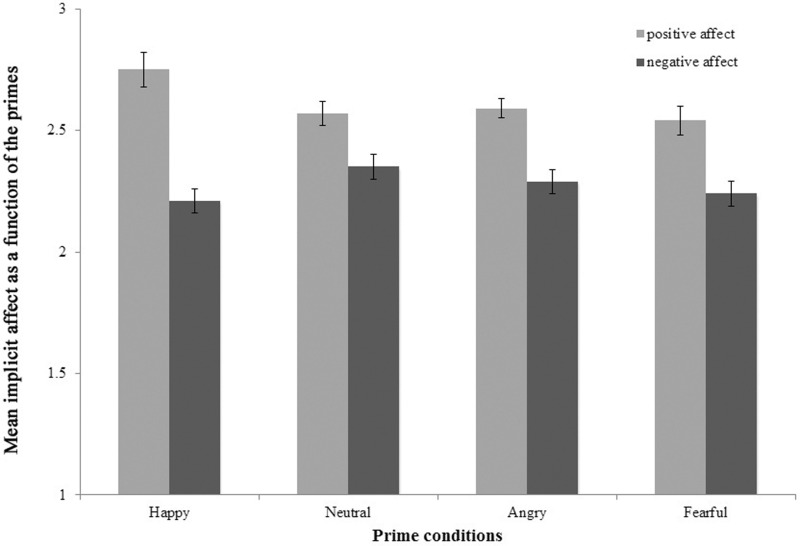
A repeated measures analysis of variance (ANOVAs) on the implicit affect scores with prime (happy, neutral, angry and fearful) and valence (positive and negative) as within-subject factors was conducted to examine whether changes in implicit affect differ as a function of prime condition and valence. The main effects prime [F (3,43) = 3.95, P < 0.05] and valence [F (1,45) = 31.6, P < 0.01] and the interaction prime × valence [F (1,45) = 5.65 P < 0.01] were significant. The mean changes for the different prime conditions are depicted in Figure 2.
Fig. 2.
Mean implicit positive and negative affect as a function of primes. Error bars represent standard error of the mean (SEM). The figure depicts mean implicit positive and negative affect following the presentation of masked happy, neutral, angry and fearful facial emotions.
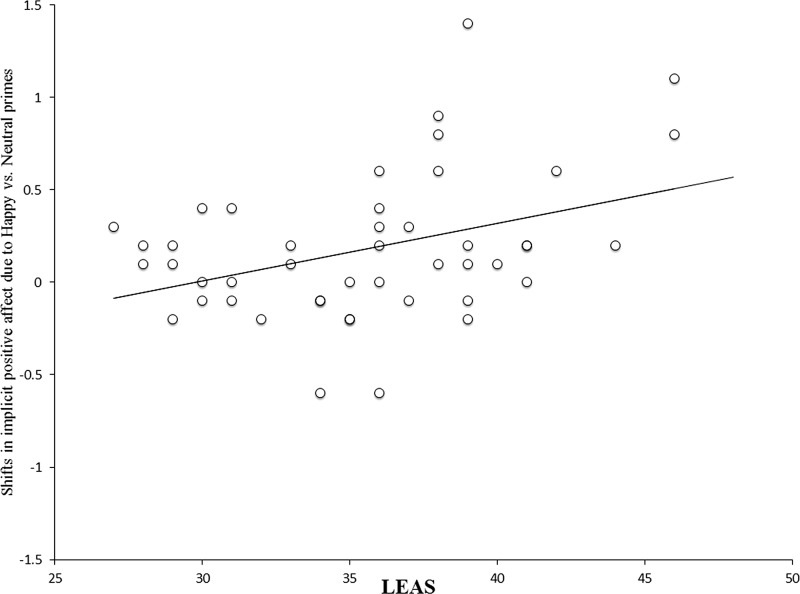
Post hoc comparisons revealed significant differences in the increase of implicit positive affect in the happy vs neutral prime condition [t (45) = 3.11 P < 0.01]. There were no significant differences for the implicit negative affect. We correlated the difference score for the implicit positive affect in the happy against neutral prime condition with trait emotional awareness. The LEAS was positively correlated with this score even after controlling for trait positive affect (r = 0.34, P < 0.05, two-tailed; see Figure 3). Hence, participants with more emotional awareness showed more prime valence congruent shifts in implicit positive affect.
Fig. 3.
Shifts in implicit positive affect due to masked happy as compared with masked neutral faces as a function of trait emotional awareness (LEAS) [r = .34, P < .05, two-tailed, controlled for positive affect (PANAS-P)]. The shifts were calculated by subtracting implicit positive affect scores for the neutral face condition from those of the happy face condition.
Detection task performance
All sensitivity values A’ were at chance-level performance: for happy faces 0.51 (se = 0.01), for angry faces 0.49 (se = 0.01), for fearful faces 0.52 (se = 0.01) and for neutral faces 0.46 (se = 0.03). None of the sensitivity values were significantly different from 0.5 (P > 0.05), suggesting chance-level performance of study participants.
Neuroimaging results
Main effects of masked emotional faces on amygdala activation
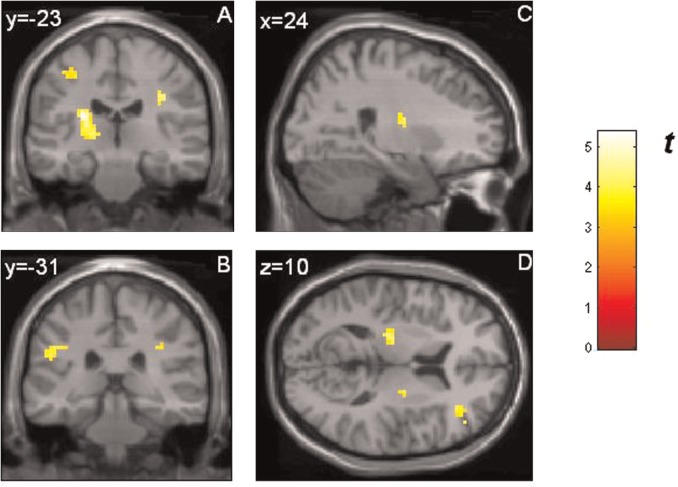
Masked presentation of happy faces (compared with neutral faces) activated the bilateral amygdala (see Figure 4). No amygdala activation was revealed for masked angry and masked fearful faces.
Fig. 4.
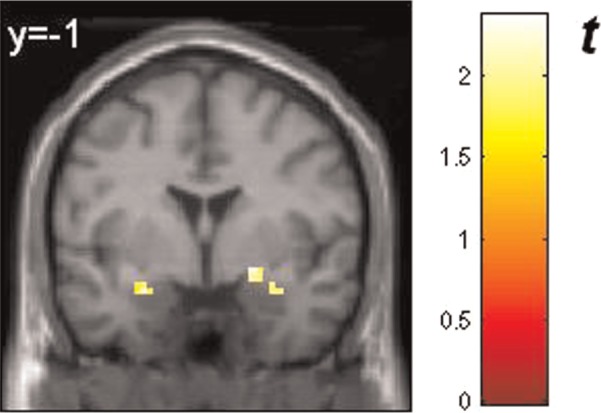
Response of the amygdalae to masked happy faces compared with masked neutral faces (ROI analysis): left amygdala [peak voxel xyz, −27, −1, −20 (MNI coordinates), cluster size: 8, Z-score = 2.03] and right amygdala (peak voxel xyz, 21, 2, −14, cluster size: 8, Z-score = 2.37, and xyz, 36, 2, −23, cluster size: 7, Z-score = 2.06). All activations are significant at P < 0.05, corrected for search volume. The color bar (t value) indicates the strength of activation. BOLD responses are superimposed on MNI standard brain template. Reader’s right is subjects’ right.
Main effects of masked emotion faces on brain activation: whole-brain analysis
There was significantly more neural activation in response to masked happy compared with masked neutral faces in several regions of the brain (see Table 1 for details). Masked presentation of happy expression activated inferior and middle frontal, superior temporal, inferior and superior parietal areas and middle occipital regions as well as cerebellar and insular areas (see Table 1 for details). Masked happy faces activated also the basal ganglia and thalamus.
Table 1.
Brain regions showing significantly increased activation to masked happy faces as compared with masked neutral faces
| Brain region | Hemisphere | Peak coordinates | Size | Z-score |
|---|---|---|---|---|
| Inferior frontal gyrus, BA47 | L | −18 29 −14 | 17 | 3.82 |
| Middle frontal gyrus, BA46 | L | −45 41 4 | 31 | 4.05 |
| Middle frontal gyrus, BA9 | R | 51 20 31 | 116 | 4.14 |
| to middle frontal gyrus, BA46 | R | 48 23 19 | 3.67 | |
| Middle frontal gyrus, BA6 | L | −30 14 52 | 10 | 3.56 |
| Superior temporal gyrus, BA22 | L | −51 −19 −8 | 30 | 4.13 |
| Superior temporal gyrus, BA22 | R | 54 −46 7 | 129 | 3.87 |
| to superior temporal gyrus, BA13 | R | 48 −49 13 | 3.84 | |
| Temporal lobe, subgyral, BA37 | R | 48 −43 −14 | 21 | 3.85 |
| Inferior parietal lobule, BA40 | L | −51 −52 46 | 16 | 3.67 |
| Superior parietal lobule, BA7 | L | −30 −64 52 | 17 | 3.37 |
| Lingual gyrus, BA18 | R | 33 −70 −14 | 41 | 3.96 |
| Middle occipital gyrus, BA19 | R | 24 −97 13 | 17 | 4.16 |
| Occipital lobe, cuneus, BA19 | R | 27 −85 34 | 59 | 3.73 |
| Insula, BA 13 | L | −42 17 10 | 54 | 4.33 |
| Putamen | R | 24 23 −11 | 25 | 4.03 |
| Caudate nucleus, caudate body | L | −9 8 7 | 38 | 3.86 |
| to thalamus | L | −9 −7 10 | 3.48 | |
| Caudate nucleus, caudate body | R | 12 5 7 | 28 | 3.68 |
| Cerebellum, pyramis | L | −6 −73 −35 | 20 | 4.01 |
| Cerebellum, pyramis | R | 12 −73 −38 | 28 | 4.36 |
Hemisphere, peak voxel coordinates in MNI space, cluster extent and the associated
Z-values are shown. The activations are significant at P < .001 (uncorrected), 10 voxel cluster threshold.
Masked fearful faces (compared with masked neutral faces) activated only areas in the right middle frontal gyrus [BA45, peak voxel xyz, 51, 20, 34 (MNI coordinates), cluster size: 12, Z-score = 4.03]. No brain activation was revealed for masked angry faces compared with masked neutral faces.
Correlations of trait emotional awareness with amygdala response to masked emotion faces controlling for trait positive affect (PANAS-P)
The voxel-wise ROI analysis of the amygdalae yielded significant positive correlations between LEAS and amygdala reactivity to masked happy faces (see Figure 5 for details). Importantly, there were no significant correlations in the opposite direction for happy faces. No positive (or negative) correlations between trait emotional awareness and amygdala response to masked fearful or masked angry faces were observed.
Fig. 5.
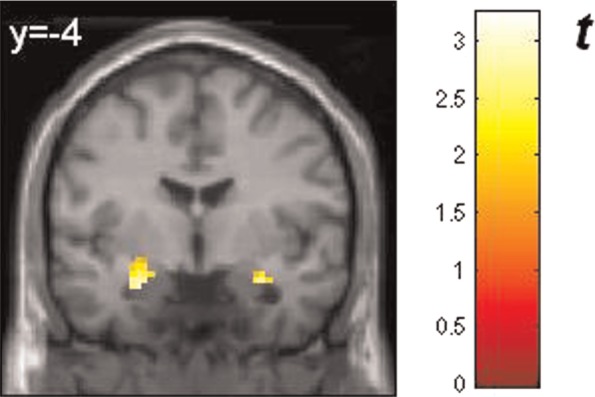
Regions within the amygdalae showing positive correlations between response to masked happy faces and trait emotional awareness (LEAS) controlling for trait positive affect (PANAS-P): left amygdala (peak voxel xyz, −27, −7, −17 (MNI coordinates), cluster size: 25, Z-score = 2.95) and right amygdala (peak voxel xyz, 30, −1, −23, cluster size: 20, Z-score = 3.05). Activations are significant at P < 0.005, corrected for search volume. The color bar (t value) indicates the strength of correlation. BOLD are superimposed on MNI standard brain template. Reader’s right is subjects’ right.
Correlations of trait emotional awareness with brain responses to masked happy faces controlling for trait positive affect (PANAS-P)
Trait emotional awareness was positively correlated with activation of the middle and medial frontal gyrus, the cingulate and lingual gyrus, precentral and postcentral regions in response to masked happy faces (see Table 2 and Figure 6 for details). Moreover, there were positive correlations between the LEAS and activation of the inferior parietal lobule, basal ganglia, thalamic and cerebellar regions in response to masked happy facial expression. There were no negative correlations between LEAS and brain response to masked happy faces.
Table 2.
Brain regions showing significantly increased activation to masked happy faces as a function of trait emotional awareness (LEAS) controlling for trait positive affect (PANAS-P)
| Brain region | Hemisphere | Peak coordinates | Size | Z-score |
|---|---|---|---|---|
| Medial frontal gyrus, BA32 | L | −15 8 49 | 16 | 4.18 |
| Middle frontal gyrus, BA46 | R | 36 35 13 | 19 | 3.63 |
| Cingulate Gyrus, BA24 | L | −18 −16 40 | 56 | 4.54 |
| to precentral gyrus, BA4 | L | −30 −19 46 | 3.59 | |
| to postcentral gyrus, BA3 | L | −39 −25 52 | 3.38 | |
| Postcentral gyrus, BA2 | R | 33 −22 34 | 43 | 4.08 |
| Postcentral gyrus, BA3 | L | −24 −40 52 | 13 | 3.72 |
| Inferior parietal lobule, BA40 | L | −51 −31 25 | 29 | 3.68 |
| to postcentral gyrus, BA2 | L | −39 −28 31 | 3.58 | |
| Occipital lobe, lingual gyrus | L | −12 −61 −8 | 15 | 3.64 |
| Claustrum | L | −27 −22 19 | 107 | 4.68 |
| to thalamus | L | −18 −19 4 | 4.05 | |
| Putamen | R | 24 −10 13 | 10 | 3.73 |
| Cerebellum, culmen | R | 12 −55 −14 | 19 | 3.63 |
Hemisphere, peak voxel coordinates in MNI space, cluster extent and the associated Z-values are shown. The activations are significant at P < .001 (uncorrected), 10 voxel cluster threshold.
Fig. 6.
Brain regions showing positive correlations between response to masked happy faces and trait emotional awareness (LEAS) controlling for trait positive affect (PANAS-P): (A) coronal view: activation in the left postcentral gyrus [BA3; extending to precentral and cingulate gyrus; peak voxel xyz, −39, −25, 52 (MNI coordinates), cluster size: 56, Z-score = 3.38], left thalamus (extending to claustrum; peak voxel xyz, −18, −19, 4, cluster size: 107, Z-score = 4.05) and right postcentral gyrus (BA2; peak voxel xyz, 33, −22, 34, cluster size: 43, Z-score = 4.08); (B) coronal view: activation in the left inferior parietal lobule (BA40, peak voxel xyz, −51, −31, 25, cluster size: 29, Z-score = 3.68) and right postcentral gyrus (BA2; peak voxel xyz, 33, −22, 34, cluster size: 43, Z-score = 4.08); (C) sagittal view: activation in the right putamen (peak voxel xyz, 24, −10, 13, cluster size: 10, Z-score = 3.73); (D) axial view: activation in the left thalamus (extending to claustrum; peak voxel xyz, −18, −19, 4, cluster size: 107, Z-score = 4.05), right putamen (peak voxel xyz, 24, −10, 13, cluster size: 10, Z-score = 3.73) and right middle frontal gyrus (BA46, peak voxel xyz, 36, 35, 13, cluster size: 19, Z-score = 3.63). All activations are significant at P < 0.001 (uncorrected). The color bar (t value) indicates the strength of correlation. BOLD responses are superimposed on MNI standard brain template. Reader’s right is subjects’ right.
Correlations of trait emotional awareness with brain responses to masked fearful and angry faces controlling for trait positive affect (PANAS-P)
Trait emotional awareness was positively correlated with response of the cingulate gyrus to masked fearful faces (BA31, peak voxel xyz, 24, −37, 34 (MNI coordinates), cluster size: 20, Z-score = 4.18; and BA24, peak voxel xyz, 18, 5, 34, cluster size: 12, Z-score = 3.70). There were positive correlations between LEAS and activation of parietal areas (BA40, peak voxel xyz, 33, −40, 31, cluster size: 12, Z-score = 4.26; and BA40, peak voxel xyz, −51, −31, 25, cluster size: 13, Z-score = 4.04) and nucleus caudatus (extending to putamen) in response to angry facial expression (peak voxel xyz, −21, 14, 22, cluster size: 23, Z-score = 3.79). There were no negative correlations between trait emotional awareness and brain response to masked fearful or masked angry faces.
DISCUSSION
In this study, the relationship between trait emotional awareness and automatic brain reactivity to positive and negative facial emotions was investigated in healthy adults. The results in the objective test of prime awareness indicate that subjects were unaware of the emotional primes presented during the fMRI experiment. Our hypotheses were confirmed in part. As expected, positive correlations were observed between trait emotional awareness and automatic reactivity in brain structures implicated in the processing of masked facial emotions and the development of implicit affect. However, these associations were found primarily for masked happy faces and, to a much lesser extent, for masked fearful and masked angry faces. Importantly, no negative correlations between trait emotional awareness and brain reactivity to masked facial emotions were found in our study. Thus, our data indicate that individuals with high emotional awareness show more activation in brain areas involved in emotion processing and simulation during the subliminal perception of happy facial emotion compared with individuals with low emotional awareness. These findings were independent of habitual positive affect.
The present fMRI data suggest that masked happy faces produced activation in many brain regions, which are known to be involved in the automatic processing of facial emotions (i.e. inferior and middle frontal, superior temporal and middle occipital gyri, insula, somatosensory cortex, cerebellar regions, basal ganglia, thalamus and amygdala; e.g. Killgore and Yurgelun-Todd, 2004; Rauch et al., 2007; Schutter et al., 2009; Suslow et al., 2009, 2010; Juruena et al., 2010). In contrast, masked fearful faces activated only the middle frontal gyrus, and masked angry faces showed no brain activation compared with masked neutral faces. This differential pattern of activation parallels the behavioral findings, which showed significant positive priming (based on happy facial expression) but no negative priming effects (based on fearful or angry facial expression). An explanation for the absence of amygdala response to fearful faces in our experiment could be the type of mask used. According to Kim et al. (2010), the amygdala response to fearful faces masked by pattern images substantially decreases compared with amygdala response to fearful faces masked by neutral faces. Diminished amygdala activation to fearful facial expression in the context of pattern masks might be caused by interactions of the amygdala with other neural systems. In our task, a sandwich-masking procedure was used consisting of randomly drawn letters. It is possible that the presentation of letter strings activated higher-order cortical structures, which inhibited the response of the amygdala. In sum, the present affective priming task produced activation and processing effects primarily for positive facial expression on a neural and behavioral level (see below for a detailed discussion of possible mood-congruent processing effects in our sample of healthy subjects). In the absence of a significant main effect of brain activation due to negative priming effects, an association with trait emotional awareness would be extremely difficult to detect.
Trait emotional awareness was found to be positively associated with activation in the primary somatosensory cortex, inferior parietal lobule, dorsal anterior cingulate gyrus, middle frontal and cerebellar areas, thalamus, putamen and amygdala in response to masked happy faces. This means, on the one hand, that high trait emotional awareness was associated with high responsivity to positive facial emotion in subcortical systems, which are relevant for the detection of biologically relevant stimuli. It is well-known that the amygdala is involved in the assessment of emotional significance (Davis and Whalen, 2001; Adolphs, 2010), the modulation of vigilance to enhance subsequent information processing throughout the brain and, like the pulvinar thalamus, the coordination of cortical networks during evaluation of visual stimuli (Pessoa and Adolphs, 2010). A close relationship between amygdala and striatal activity has been reported during the processing of reward relevant stimuli (Ousdal et al., 2012). The thalamus is part of a rapid subcortical path by which low-level visual information can reach the amygdala without conscious awareness (Phillips et al., 2004; Liddell et al., 2005). The thalamic nuclei serve a variety of functions in the brain beyond relaying sensory information, which comprise the initiation and coordination of motor behavior (Schmahmann, 2003). Interestingly, amygdala, thalamus and basal ganglia have been reported to be activated during imitation of happy facial expression (Pohl et al., 2013). In sum, according to our data, individuals with high levels of emotional awareness appear to be characterized by an enhanced reactivity in subcortical areas implicated in rapid stimulus evaluation and allocation of processing resources.
On the other hand, trait emotional awareness was also found to be positively related to response of several cortical areas such as the primary somatosensory cortex (SI) and inferior parietal lobule during the subliminal perception of happy faces. It has been shown repeatedly that the inferior parietal lobule is involved in the observation as well as in the imitation of emotional facial expression (Carr et al., 2003; Hennenlotter et al., 2005; van der Gaag et al., 2007). The primary somatosensory cortex (SI) is known to be critically involved in the processes of emotional mimicry and simulation (Adolphs et al., 2000). Primary somatosensory regions are implicated in simulating aspects of the body states associated with the viewed emotional state—including proprioceptive and somatovisceral sensations (Heberlein and Adolphs, 2007; Heberlein and Atkinson, 2009). According to the shared-substrates model of emotion recognition identification of other persons’ emotional states is mediated by internally generated somatosensory representations that simulate how the other individual feels when showing a certain facial expression (Heberlein and Adolphs, 2007). Such vicarious responding has also been discussed in the context of empathic responses (Preston and de Waal, 2002). Interestingly, previous psychological research has reported that trait emotional awareness is related to enhanced empathy (Ciarrochi et al., 2003; Igarashi et al., 2011). Lane (2008) has pointed out that the somatosensory and parietal cortex could participate in the experience of the somatic sensations associated with implicit responses.
Finally, trait emotional awareness was correlated with activation in areas of the ACC and cerebellum in response to masked happy faces. The ACC is an integral part of the limbic system and participates in emotion formation and processing. Specifically, dorsal regions of the ACC are involved in the appraisal of emotion (Etkin et al., 2011). The cerebellum is incorporated into distributed neural circuits subserving emotion perception and social interaction (Schmahmann, 2010). Increased cerebellar responses to masked happy faces could subserve processes of increased implicit attention for positive social stimuli (Schutter et al., 2009). All in all, the present neuroimaging data show that trait emotional awareness is positively associated with activation in brain areas responsible for rapid stimulus evaluation and emotion simulation during the subliminal perception of happy facial expression. These findings are in line with the idea that emotional awareness is characterized by an enhanced affective resonance to others at an automatic processing level (Ciarrochi et al., 2003; Igarashi et al., 2011).
It is interesting to note in this context that previous studies have demonstrated a positive relationship of level of emotional awareness with verbal and non-verbal recognition of facial emotions (Lane et al., 1996, 2000b). In the light of the present results, it appears possible that more spontaneous activation in brain areas implicated in emotion simulation could have been a factor promoting the identification of emotion from facial expression in high compared with low trait emotional awareness.
Our behavioral results partially confirm the hypothesis that the LEAS is positively related to shifts in implicit affect owing to masked facial emotion. High trait emotional awareness was associated with stronger shifts in implicit positive affect caused by happy facial expression. Importantly, in our experiment, presentation of masked happy faces induced significant increases in implicit positive affect compared with the presentation of neutral faces, but there was no indication that masked negative (i.e. angry and fearful) faces elicited implicit negative affect. The present data suggest that high emotional awareness is characterized by an enhanced resonance to others’ positive affects at an automatic processing level. Affective resonance refers to a person's tendency to experience the same affect in response to viewing a display of that affect by another person. Individuals with high levels of emotional awareness appear to develop stronger implicit positive affects in response to others’ brief facial expressions compared with those with low levels of emotional awareness. These results of an enhanced affective resonance are consistent with those of previous studies showing that individuals with high emotional awareness allocate involuntarily more attention to emotional stimuli (Suslow et al., 2001) and react with more physiological arousal to them (Lane et al., 2000a; McRae et al., 2008). Thus, there is evidence that functioning at high levels of emotional awareness (i.e. levels of explicit processes of emotional experience) goes along with pronounced implicit affects and reactions (bodily sensations and automatic attention allocation). This does not contradict assumptions of the levels of emotional awareness theory according to which functioning at a high level can modify but does not eliminate the function of previous levels (Lane, 2008).
It is possible that the positive mood state of our healthy study participants induced mood-congruent response biases favoring the processing of happy facial expression. This might have facilitated the detection of associations between emotional awareness and automatic brain response to happy facial expression in our sample. In our study, we found evidence for positive priming owing to masked happy faces (i.e. there was a positive prime valence congruent shift in implicit affect caused by happy faces compared with neutral faces) but our results did not provide any evidence for negative priming effects elicited by masked angry or fearful faces (i.e. no negative prime valence congruent shifts in implicit affect were found for angry or fearful faces compared with neutral faces). The results from the first studies examining shifts in affective or liking evaluations owing to masked facial emotions on the basis of the affective priming paradigm (Murphy and Zajonc, 1993; Murphy et al., 1995) suggested comparably pronounced effects of positive and negative facial expression on evaluations. Compared with neutral expression, angry facial expression led to more negative evaluations of subsequently presented stimuli, whereas happy facial expression elicited more positive evaluations of subsequently presented stimuli. However, subsequent behavioral studies on affective priming in which samples of healthy adults were examined frequently failed to find negative priming effects. There are four studies using sad and happy faces as emotional primes in which (valence congruent) affective priming effects were only observed for happy but not for sad faces (Suslow et al., 2003; Wong and Root, 2003; Dannlowski and Suslow, 2006; Donges et al., 2012). In addition, results from three other studies administering happy and angry faces as emotional primes indicate (valence congruent) affective priming only for happy but not for angry faces (Rotteveel et al., 2001, experiment 1; Dannlowski et al., 2007; Paul et al., 2012). Finally, in the study of Almeida et al. (2013), affective priming for happy faces was stronger than that for angry faces. In all of the aforementioned affective priming studies, participants were unaware of the primes as controlled by measures of subjective or objective awareness.
It can be concluded that our findings of significant priming based on happy faces but no priming based on negative faces are consistent with results from many previous investigations examining automatic influences of masked facial emotion on affective or evaluative processes. The healthy participants of our study tended to show mood-congruent priming effects. The selective susceptibility or responsiveness to positive social information at an automatic processing level as found in this study is similar to observations made in research on attention in depressed and normal individuals. According to these findings, healthy (as opposed to depressed) subjects are characterized by attentional biases favoring positive stimuli (Gotlib et al., 1988) and avoiding negative stimuli (McCabe and Gotlib, 1995; Karparova et al., 2007). This pattern of attention allocation has been termed positive or protective bias. There is evidence that protective biases away from negative information can operate at an early stage of information processing in healthy subjects (Leyman et al., 2009). It has also been shown in electrophysiological studies that normal individuals manifest an early response bias for positive information (Deldin et al., 2001). As noted above, the absence of a negative priming effect might explain the failure to detect associations between emotional awareness and negative evaluative shifts in our sample.
To further clarify the issue of a relationship between emotional awareness and negative affective priming, it appears advisable to include clinical subjects such as depressed, anxious or schizophrenia patients in future studies. These patients have shown (mood-congruent) negative affective priming effects in previous studies (Suslow et al., 2003, 2013; Dannlowski et al., 2006). It would be interesting to examine whether patients with higher trait emotional awareness manifest stronger negative priming. We assume that trait emotional awareness can basically affect the processing of negative as well as positive stimuli but that the detection of relationships may depend on the mood characteristics and processing biases of study participants.
These data suggest that the extent of reflective awareness of feelings may also derive from the extent and intensity of implicit affective reactions. High responsiveness of limbic and somatosensory areas to emotional signals of low intensity in everyday life could make the recognition of subtle emotions less difficult. People who develop automatically stronger bodily feelings to emotional stimuli might become more easily aware of these spontaneous affective reactions and make them more frequently the objects of conscious reflection, resulting in reports reflecting more complex processing of emotional information. Less detailed processing and somatosensory representation of facial emotional expressions could contribute to a decreased ability to draw inferences about the emotional and motivational significance of fundamental interpersonal signals. Reduced activation in limbic areas when attempting to feel other people’s feelings or retrieving their own emotional episodes has been reported previously for people with decreased emotional awareness (see Kano and Fukudo, 2013, for an overview). Low spontaneous cerebral responsivity to external emotional triggers as found in our study coincides with the empirical impression of clinicians that people with decreased affect awareness look unaffected and emotionally dull (Moriguchi and Komaki, 2013).
Previous research addressing the neural correlates of trait emotional awareness has focused exclusively on explicit emotion processing. Lane et al. (1998) and McRae et al. (2008) reported more activation primarily of the ACC during film- or recall-induced emotion and during viewing of emotional pictures in individuals with higher emotional awareness [see also Frewen et al.’s study (2008) in which similar results have been obtained in the control group]. Our data on early stages of emotion processing complement the previous findings by showing that subjects with high levels of emotional awareness activate brain regions implicated in evaluation and affective resonance more strongly during automatic emotion perception compared with those with low levels of emotional awareness. These data suggest that more emotionally aware individuals could have more neurally elaborated emotional reactions and representations even before they are consciously aware of the emotions. This could mean that the interoceptive information that forms the basis for reporting on experience is possibly more detailed or elaborated even before it is put into words in people who are more emotionally aware. Quirin and Lane (2012) have argued that the reflective construction of emotional experience requires the integration of implicit and explicit emotional processes. It appears that greater awareness does not just involve better ability to put emotions into words or appreciate complexity in one’s own experience and that of others; it seems to involve a more elaborated type of bottom-up emotion processing and an enhanced automatic affective resonance to others that creates the possibility for enhanced emotional awareness. This is consistent with emotional awareness theory (Lane and Schwartz, 1987; Lane, 2008), which states that more detailed schemata for processing emotional stimuli lead to more detailed emotional information processing independent of and preceding the reporting function itself.
Acknowledgments
This work was supported by grants from the German Research Foundation DFG to Thomas Suslow and Harald Kugel (SU 222/6-1).
We thank Sophie-Luise Lenk and Marc Rupietta for their help in data collection.
REFERENCES
- Aaronson D, Watts B. Extensions of Grier’s computational formulas for A’ and B’’ to below-chance performance. Psychological Bulletin. 1987;3:439–42. [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience. 2000;20:2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Pajtas PE, Mahon BZ, Nakayama K, Caramazza A. Affect of the unconscious: visually suppressed angry faces modulate our decisions. Cognitive Affective and Behavioral Neuroscience. 2013;13:94–101. doi: 10.3758/s13415-012-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Washington: American Psychiatric Association; 1994. [Google Scholar]
- Bastiaansen JACJ, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2009;364:2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory-Manual. San Antonio: The Psychological Association; 1987. [Google Scholar]
- Bréjard V, Bonnet A, Pedinielli J. The role of temperament and emotional awareness in risk taking in adolescents. Encéphale. 2012;38:1–9. doi: 10.1016/j.encep.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the Academy of Natural Sciences of Philadelphia. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrochi J, Caputi P, Mayer JD. The distinctiveness and utility of a measure of trait emotional awareness. Personality and Individual Differences. 2003;34:1477–90. [Google Scholar]
- Dannlowski U, Kersting A, Donges US, Lalee-Mentzel J, Arolt V, Suslow T. Masked facial affect priming is associated with therapy response in clinical depression. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:215–21. doi: 10.1007/s00406-005-0628-0. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, et al. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Research Neuroimaging. 2007;154:13–20. doi: 10.1016/j.pscychresns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Suslow T. Test-retest reliability of subliminal facial affective priming. Psychological Reports. 2006;98:153–8. doi: 10.2466/pr0.98.1.153-158. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Cognitive bias and emotion in neuropsychological models of depression. Cognition and Emotion. 2001;15:787–802. [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–9. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Donges US, Kersting A, Suslow T. Women’s greater ability to perceive happy facial emotion automatically: gender differences in affective priming. PLoS One. 2012;7:e41745. doi: 10.1371/journal.pone.0041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Dai Q, Gong Q, Chen H. Neural mechanism of unconscious perception of surprised facial expression. Neuroimage. 2010;52:401–7. doi: 10.1016/j.neuroimage.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P, Lane RD, Neufeld RWJ, Densmore M, Stevens T, Lanius R. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosomatic Medicine. 2008;70:27–31. doi: 10.1097/PSY.0b013e31815f66d4. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McLachlan A, Katz AN. Biases in visual attention in depressed and non-depressed individuals. Cognition and Emotion. 1988;2:185–200. [Google Scholar]
- Grier JB. Nonparametric indexes for sensitivity and bias: computing formulas. Psychological Bulletin. 1971;75:424–9. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depressions-Inventar. Bern: Huber; 1994. [Google Scholar]
- Heberlein AS, Adolphs R. Neurobiology of emotion recognition: current evidence for shared substrates. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience Integrating Biological and Psychological Explanations of Social Behavior. New York: Guilford Press; 2007. pp. 31–55. [Google Scholar]
- Heberlein AS, Atkinson AP. Neuroscientific evidence for simulation and shared substrates in emotion recognition: beyond faces. Emotion Review. 2009;1:162–77. [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–91. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Komaki G, Lane RD, et al. The reliability and validity of the Japanese version of the Levels of Emotional Awareness Scale (LEAS-J) BioPsychoSocial Medicine. 2011;5:2. doi: 10.1186/1751-0759-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena MF, Giampietro VP, Smith SD, et al. Amygdala activation to masked happy facial expression. Journal of the International Neuropsychological Society. 2010;16:1–5. doi: 10.1017/S1355617709991172. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S. The alexithymic brain: the neural pathways linking alexithymia to physical disorders. BioPsychoSocial Medicine. 2013;7:1. doi: 10.1186/1751-0759-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karparova SP, Kersting A, Suslow T. Deployment of attention in clinical depression during symptom remission. Scandinavian Journal of Psychology. 2007;48:1–5. doi: 10.1111/j.1467-9450.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–23. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Neta M, et al. Behind the mask: the influence of mask-type on amygdala response to fearful faces. Social, Cognitive and Affective Neuroscience. 2010;5:363–8. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne HW, Egloff B, Kohlmann CW, Tausch A. Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule” (PANAS) Diagnostica. 1996;42:139–56. [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, et al. Alexithymic features and automatic amygdala reactivity to facial emotion. Neuroscience Letters. 2008;435:40–4. doi: 10.1016/j.neulet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Lane RD. Neural correlates of conscious emotional experience. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford: Oxford University Press; 2000. pp. 345–70. [Google Scholar]
- Lane RD. Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine. 2008;70:214–31. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- Lane RD, Allen J, Schwartz G, Sechrest L. Emotional awareness in men is positively correlated with skin-conductance response magnitude during emotional arousal. Psychosomatic Medicine. 2000a;62:100. [Google Scholar]
- Lane R, Carmichael C, Reis H. Differentiation in the momentary rating of somatic symptoms covaries with trait emotional awareness in patients at risk for sudden cardiac death. Psychosomatic Medicine. 2011;73:185–92. doi: 10.1097/PSY.0b013e318203b86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Garfield DAS. Becoming aware of feelings: integration of cognitive-developmental, neuroscientific, and psychoanalytic perspectives. Neuro-Psychoanalysis. 2005;7:5–30. [Google Scholar]
- Lane RD, Pollermann BZ. Complexity of emotion representations. In: Barrett LF, Salovey P, editors. The Wisdom in Feeling. Psychological Processes in Emotional Intelligence. New York: Guilford Press; 2002. pp. 271–93. [Google Scholar]
- Lane RD, Quinlan DM, Schwartz GE, Walker PA, Zeitlin SB. The levels of emotional awareness scale: a cognitive-developmental measure of emotion. Journal of Personality Assessment. 1990;55:124–34. doi: 10.1080/00223891.1990.9674052. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwarz GE. Neural correlates of levels of emotional awareness: evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. American Journal of Psychiatry. 1987;144:133–43. doi: 10.1176/ajp.144.2.133. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Riedel R, Shapiro E, Kaszniak W. Pervasive emotion recognition deficit common to alexithymia and the repressive coping style. Psychosomatic Medicine. 2000b;62:492–501. doi: 10.1097/00006842-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Riedel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosomatic Medicine. 1996;58:203–10. doi: 10.1097/00006842-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus D, Hawk S, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition and Emotion. 2010;24:1377–88. [Google Scholar]
- Leyman L, de Raedt R, Koster EHW. Attentional biases for emotional facial stimuli in currently depressed patients with bipolar disorder. International Journal of Clinical and Health Psychology. 2009;9:393–410. [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger C. Das State-Trait-Angstinventar. Weinheim: Beltz; 1981. [Google Scholar]
- Liddell B, Brown K, Kemp A, et al. A direct brainstem–amygdala–cortical “alarm” system for subliminal signals of fear. Neuroimage. 2005;24:235–43. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCabe SB, Gotlib IH. Selective attention and clinical depression: performance on a deployment-of-attention task. Journal of Abnormal Psychology. 1995;104:241–5. doi: 10.1037//0021-843x.104.1.241. [DOI] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. Neuroimage. 2008;41:648–55. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews. 2012;36:341–9. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Komaki G. Neuroimaging studies of alexithymia: physical, affective, and social perspectives. BioPsychoSocial Medicine. 2013;7:8. doi: 10.1186/1751-0759-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–39. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB, Monahan JL. Additivity of nonconscious affect—combined effects of priming and exposure. Journal of Personality and Social Psychology. 1995;69:589–602. doi: 10.1037//0022-3514.69.4.589. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Three-Dimensional Magnetization-Prepared Rapid Gradient-Echo Imaging (3D MP RAGE) Magnetic Resonance in Medicine. 1990;15:152–7. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Reckless GE, Server A, Andreassen OA, Jensen J. Effect of relevance on amygdala activation and association with the ventral striatum. Neuroimage. 2012;62:95–101. doi: 10.1016/j.neuroimage.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Paul ES, Pope SAJ, Fennell JG, Mendl MT. Social anxiety modulates subliminal affective priming. PLoS One. 2012;7:e37011. doi: 10.1371/journal.pone.0037011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–96. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Pohl A, Anders S, Schulte-Rüther M, Mathiak K, Kircher T. Positive facial affect—an fMRI study on the involvement of insula and amygdala. PLoS One. 2013;8:e69886. doi: 10.1371/journal.pone.0069886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Quirin M, Bode R, Kuhl J. Recovering from negative events by boosting implicit positive affect. Cognition and Emotion. 2011;25:559–70. doi: 10.1080/02699931.2010.536418. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kazén M, Kuhl J. When nonsense sounds happy or helpless: the Implicit Positive and Negative Affect Test (IPANAT) Journal of Personality and Social Psychology. 2009;97:500–16. doi: 10.1037/a0016063. [DOI] [PubMed] [Google Scholar]
- Quirin M, Lane RD. The construction of emotional experience requires the integration of implicit and explicit emotional processes. Behavioral and Brain Sciences. 2012;35:159–60. doi: 10.1017/S0140525X11001737. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat W, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–7. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Rauch A, Ohrmann P, Bauer J, et al. Cognitive coping styles modulates neural responses to emotional faces in healthy humans: a 3-T FMRI study. Cerebral Cortex. 2007;17:2526–35. doi: 10.1093/cercor/bhl158. [DOI] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46:658–67. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Rotteveel M, de Groot P, Geutskens A, Phaf RH. Stronger suboptimal than optimal affective priming? Emotion. 2001;1:348–64. doi: 10.1037/1528-3542.1.4.348. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–78. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review. 2010;20:236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, Enter D, Hoppenbrouwers SS. High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. Journal of Psychiatry and Neuroscience. 2009;34:60–5. [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1970. [Google Scholar]
- Subic-Wrana C, Thomas W, Huber M, Köhle K. Levels of Emotional Awareness Scale (LEAS) Psychotherapeut. 2001;46:176–81. [Google Scholar]
- Suslow T, Junghanns K, Donges US, Arolt V. Alexithymia and automatic processing of verbal and facial affect stimuli. Current Psychology of Cognition. 2001;20:297–324. [Google Scholar]
- Suslow T, Kugel H, Rauch AV, et al. Attachment avoidance modulates neural response to masked facial emotion. Human Brain Mapping. 2009;30:3553–62. doi: 10.1002/hbm.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Reber H, et al. Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience. 2010;167:111–23. doi: 10.1016/j.neuroscience.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Suslow T, Lindner C, Dannlowski U, et al. Automatic amygdala response to facial expression in schizophrenia: initial hyperresponsivity followed by hyporesponsivity. BMC Neuroscience. 2013;14:140. doi: 10.1186/1471-2202-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Roestel C, Arolt V. Affective priming in schizophrenia with and without affective negative symptoms. European Archives of Psychiatry and Clinical Neuroscience. 2003;253:292–300. doi: 10.1007/s00406-003-0443-4. [DOI] [PubMed] [Google Scholar]
- Tomkins SS. Affect Imagery Consciousness: The Positive Affects. Vol. 1. New York: Springer; 1962. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Social Neuroscience. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1998;54:10–63. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen P, Rauch S, Etcoff N, McInerney S, Lee M, Jenike M. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personality and Social Psychology Bulletin. 2005;31:121–35. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- Wittchen H, Wunderlich U, Gruschwitz S, Zaudig M. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Horgrefe; 1997. [Google Scholar]
- Wong PS, Root JC. Dynamic variations in affective priming. Consciousness and Cognition. 2003;12:147–68. doi: 10.1016/s1053-8100(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Zajonc RB. Feeling and thinking: preferences need no inferences. American Psychologist. 1980;35:151–75. [Google Scholar]
- Zajonc RB. Feeling and thinking: closing the debate over the independence of affect. In: Forgas JP, editor. Feeling and Thinking: The Role of Affect in Social Cognition. Cambridge: Cambridge University Press; 2000. pp. 31–58. [Google Scholar]