Abstract
Strong evidence links the 5-HTTLPR genotype to the modulation of amygdala reactivity during fear conditioning, which is considered to convey the increased vulnerability for anxiety disorders in s-allele carriers. In addition to amygdala reactivity, the 5-HTTLPR has been shown to be related to alterations in structural and effective connectivity. The aim of this study was to investigate the effects of 5-HTTLPR genotype on amygdala reactivity and effective connectivity during fear conditioning, as well as structural connectivity [as measured by diffusion tensor imaging (DTI)]. To integrate different classification strategies, we used the bi-allelic (s-allele vs l/l-allele group) as well as the tri-allelic (low-functioning vs high-functioning) classification approach. S-allele carriers showed exaggerated amygdala reactivity and elevated amygdala-insula coupling during fear conditioning (CS + > CS−) compared with the l/l-allele group. In addition, DTI analysis showed increased fractional anisotropy values in s-allele carriers within the uncinate fasciculus. Using the tri-allelic classification approach, increased amygdala reactivity and amygdala insula coupling were observed in the low-functioning compared with the high-functioning group. No significant differences between the two groups were found in structural connectivity. The present results add to the current debate on the influence of the 5-HTTLPR on brain functioning. These differences between s-allele and l/l-allele carriers may contribute to altered vulnerability for psychiatric disorders.
Keywords: classical conditioning, fear, 5-HTTLPR genotype, amygdala, emotion
INTRODUCTION
Fear conditioning is an established model for the development and maintenance of anxiety disorders (Hamm and Weike, 2005; Delgado et al., 2006). In fear conditioning paradigms, a neutral stimulus (CS+) is paired with an aversive stimulus (unconditioned stimulus, UCS) while a second stimulus (CS−) predicts the absence of the UCS. After a few trials, the CS+ elicits conditioned responses (CRs) like increased skin conductance responses (SCRs), changes in preference ratings and altered neural activity (Delgado et al., 2006; Olsson and Phelps, 2007; Tabbert et al., 2011). Regarding the neural correlates of fear conditioning, studies have identified a network including the amygdala, the anterior cingulate cortex (ACC), the insula, the orbitofrontal cortex (OFC) and the occipital cortex (LeDoux, 2000; Olsson and Phelps, 2007). The amygdala is essentially involved in the formation of the conditioned stimulus (CS)/UCS association (LaBar and LeDoux, 1996; Büchel and Dolan, 2000). Conditioned blood oxygen level–dependent signal change (BOLD) responses within the OFC, the insula and the occipital cortex are often interpreted as neural correlates of conscious evaluation processes of bodily arousal, increased attention and the evaluation of the CS value (O'Doherty, 2007; Ochsner et al., 2008; Craig, 2011; Klucken et al., 2012).
Substantial effort has been made to investigate the association between specific genetic variations and fear conditioning because fear conditioning is considered to be a central mechanism in the development of psychiatric disorders (Mineka and Oehlberg, 2008; Lonsdorf et al., 2009, 2015; Caspi et al., 2010; Schweckendiek et al., 2011). The functional genetic variation within the promoter region of the serotonin transporter gene (SLC6A4; serotonergic transporter-linked polymorphic region; 5-HTTLPR) is of special interest (Lesch et al., 1996; Hariri et al., 2002; Munafò et al., 2008; Caspi et al., 2010). A 43 base pair insertion/deletion located in the promoter region of the serotonin transporter gene results in two allelic variations: a long (l-) and a short (s-) allele. Initial in vitro studies point to a (‘bi-allelic’) dominant effect of the s-allele, with reduced 5-HTT availability and 5-HTT functioning in s-allele carriers compared with homozygote l-allele carriers (l/l-allele group) (Lesch et al., 1996; Stoltenberg et al., 2002 but see Heinz et al., 2000). However, this classification strategy is currently under debate (Jonassen and Landro, 2014). More recent studies assume an alternative ‘tri-allelic’ classification owing to the observation that a single nucleotide polymorphism [A/G polymorphism (rs25531)] renders the LG-allele functionally similar to the s-allele in terms of the reduced 5-HTT availability (‘low-functioning group’: S, LG vs ‘high-functioning group’: LA) (Nakamura et al., 2000; Hu et al., 2006; Praschak-Rieder et al., 2007). S-allele carriers and subjects with the low-functioning allele are characterized by an increased vulnerability for psychiatric disorders, which could be explained by exaggerated fear processing (Hariri et al., 2002; Caspi et al., 2003; Heinz et al., 2004; Munafò et al., 2008, 2009a, b; Karg et al., 2011; Jiang et al., 2013; Northoff, 2013).
Although a prominent twin study has suggested that genetic factors explain a substantial proportion of the variance in CRs (Hettema et al., 2003), so far only few studies have investigated the association between the 5-HTTLPR and fear conditioning. Thereby, the majority of studies reported increased CRs in s-allele carriers as compared with the l/l-allele group, as measured by startle responses and hemodynamic activity. However, no group differences were found in conditioned SCRs (Lonsdorf et al., 2009; Agren et al., 2012; Klucken et al., 2013a, b; for an exception see Garpenstrand et al., 2001). Regarding neural activity, increased BOLD responses to the CS+ were observed in the low-functioning group as compared with the high-functioning group, respectively, in s-allele carriers as compared with l/l-allele carriers in different brain structures like the amygdala, the insula, the ACC and the occipital cortex (Hermann et al., 2012; Klucken et al., 2013a). increased amygdala, ACC and occipital responses were often associated with increased attention to salient stimuli and/or an exaggerated stress response (Bradley et al., 2003; Delgado et al., 2006; Alexander et al., 2009; Homberg and Lesch, 2011; Klucken et al., 2013b). The enhanced insula activity to the CS+ in s-allele carriers was interpreted to mirror an increase of anticipatory anxiety and/or an increased sensitivity to bodily cues (Crişan et al., 2009; Hermann et al., 2012).
In addition to enhanced BOLD responses, alterations of effective connectivity and structural connectivity (e.g. white matter microstructure integrity) have been hypothesized to contribute to the development of psychiatric disorders (Cremers et al., 2010; Meyer-Lindenberg, 2010; Ayling et al., 2012). In detail, amygdala-insula and amygdala-ventromedial prefrontal cortex (vmPFC) connectivity have been related to (dysfunctional) emotion regulation as well as state and trait anxiety (e.g. Baur et al., 2013; Hilbert et al., 2013). Regarding structural connectivity, the delineation of the fiber architecture of tissue using diffusion tensor imaging (DTI) has recently gained increased attention (White et al., 2008; Ayling et al., 2012; Jones et al., 2013). Although a number of different tracts have recently been associated with psychiatric disorders (White et al., 2008; Ayling et al., 2012; Baur et al., 2013), we focused on the uncinate fasciculus (UF) due to the following reasons: first, the UF connects the limbic system with the prefrontal cortex and has repeatedly been linked to (dysfunctional) emotion processing, psychiatric disorders and subclinical (e.g. trait) anxiety (White et al., 2008; Thomason and Thompson, 2011; Ayling et al., 2012; Montag et al., 2012; Baur et al., 2013). Second, the 5-HTTLPR has been associated with altered white matter microstructure in the UF (Pacheco et al., 2009; Jonassen et al., 2012). However, DTI results are not always consistent. For instance, Montag and colleagues (2012) found a positive correlation between the UF and trait anxiety in males but not in females (and also further positive correlations with other tracts), while other studies showed negative correlations with trait anxiety and/or anxiety disorders (Kim and Whalen, 2009; Phan et al., 2009). Nevertheless, it has been speculated that serotonergic transmission and hence related polymorphisms like the 5-HTTLPR might be critical for neuroplasticity, which could influence the development and strength of connectivity of the PFC and other areas (like the amygdala) involved in the serotonergic circuitry (e.g. Homberg et al., 2011; Jonassen and Landro, 2014 for a comprehensive review). For instance, studies suggest that postnatal serotonergic transmission has long-term effects on the neuroplasticity within and around the amygdala (Homberg et al., 2011) and thus may alter its connectivity. In addition, serotonin is involved in early differentiation and maturation of nerve cells not only in the amygdala but also in many other cortical and subcortical brain areas (Jonassen and Landro, 2014).
To date, associations of the 5-HTTLPR genotype with fear conditioning as well as with structural and functional connectivity alterations have been investigated only separately. The integration of these measurements constitutes an essential step toward a more detailed understanding of the impact of 5-HTTLPR genotype on brain mechanisms. Therefore, the first aim of the study was to analyze the association between 5-HTTLPR genotype and fear conditioning. The second aim was to examine effective as well as structural connectivity of the amygdala. We expected increased amygdala responses to the CS+ during fear conditioning as well as increased connectivity in s-allele carriers because fear conditioning may provoke (state) anxiety and probably emotion regulation (e.g. by trying to down-regulate) when confronted with the CS+ (e.g. Delgado et al., 2008; Hermann et al., 2014). We analyzed the data with respect to the bi-allelic and the tri-allelic classification approach to add to the ongoing classification debate.
METHODS
Participants
For the present study, 107 participants (mean age: 24.3; s.d.: 4.4, 58 males) were recruited. To avoid potential confounds due to stratification strategy, we included only Caucasian participants with European background, who were native German speakers. Current or past mental, sexual or chronic health problems as well as consumption of psychotropic drugs were defined as exclusion criteria. All participants were right-handed, had normal or corrected-to-normal vision and received 40 Euro for their participation. Participants signed an informed consent. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee. Seven participants (five males) were excluded owing to neurological problems (n = 1; arachniodal cyst) or owing to excessive (>6 mm) head motion during scanning (n = 6), leaving 100 participants in the final sample. The genotype frequencies were as follows: 9 s/s (4 males; mean age: 23.3; s.d.: 2.6), 42 s/l (24 males; mean age: 23.2; s.d.: 3.0) and 49 l/l (25 males; mean age: 24.7; s.d.: 5.5). There was no significant deviation from Hardy–Weinberg Equilibrium (x2(1) < .1; P > 0.9).
Genotyping
DNA was extracted from buccal cells using a standard commercial extraction kit (High Pure PCR Template Preparation Kit; Roche, Mannheim, Germany) in a MagNA Pure1 LC System (Roche). Participants were genotyped for the 5-HTTLPR genotype (and rs25531) by means of polymerase chain reaction and gel electrophoresis. A detailed protocol is provided elsewhere (Alexander et al., 2009). The bi-allelic results (s carrier vs l/l homozygote) are presented in the result section. As a supplement, additional results are provided using a tri-allelic dichotomized model (‘SS, SLG, LGLG, SLA, LALG’ vs ‘LALA’). We decided to use this tri-allelic dichotomized model because no group differences occurred between the two ‘low-functioning’ groups (‘SS, SLG, LGLG’ and ‘SLA, LALG’).
Conditioning procedure
A differential fear conditioning procedure (each CS: 16 trials) was conducted using colored squares as reinforced conditioned (CS+) or as non-reinforced stimuli (CS−). Electrical stimulation was used as UCS (50% reinforcement). Each CS was presented for 8 s. The UCS duration was 100 ms. The UCS was delivered 7.9 s after the CS+ onset and co-terminated with the CS+ offset. The intertrial interval ranged from 4.5 to 7 s. Electrodes were fixed to the middle of the left shin and stimulus intensity was set individually using a gradually increasing procedure to achieve an ‘unpleasant but not painful’ level of sensation. A custom-made impulse generator (833 Hz) provided transcutaneous electrical stimulation (UCS) for 100 ms through two Ag/AgCl electrodes (1 mm2 surface). Two different colored CS+ were used (Wittmann et al., 2007). The two CS+ differed significantly neither in valence, arousal and UCS-expectancy ratings nor in SCRs (all P > 0.700) or hemodynamic responses, and are summarized in the analyses. The stimuli were projected onto a screen at the end of the scanner (visual field = 18°) using a liquid-crystal display (LCD) projector and were viewed through a mirror mounted on the head coil. A magnetic resonance imaging (MRI)-compatible video camera was used to check whether participants watched the stimuli. Throughout the experiment, SCRs were sampled simultaneously. Immediately after the conditioning procedure, preference and expectancy ratings of the CS were collected. An extinction phase was further assessed but will not be reported in the present manuscript.
Subjective ratings
Valence and arousal ratings of the CS were collected using nine-point Likert scales that ranged from 1, very pleasant/not arousing at all, to 9, very unpleasant/very arousing. In addition, UCS-expectancy was rated from 0 (no shock) to 100 (certain shock) using a Likert scale. For the CS ratings (arousal, valence, UCS-expectancy), statistical analyses were performed via analysis of variance (ANOVA) in a 2 (stimulus: CS+ vs CS−) × 2 (genotype: s-allele vs l/l-allele group, respective low-functioning vs high-functioning group) design followed by post hoc tests in SPSS 21 (IBM Corporation, Armonk, USA) for each rating. Appropriate Bonferroni-corrected post hoc t-tests were conducted to further analyze significant effects.
Skin conductance measures
SCRs were sampled simultaneously with MR scans using Ag/AgCl electrodes filled with isotonic (0.05 M NaCl) electrolyte medium placed hypothenar at the non-dominant (left) hand. SCRs were defined in two analysis windows: the maximum response within the time window 1–5 s after the CS (CS+ or CS−) onset was counted as the first interval response (FIR) and within the time windows 5.1–9 s as the second interval responses (SIR). Statistical analyses were performed via ANOVAs in a 2 (stimulus: CS+ vs CS−) × 2 (group: s-allele vs l/l-allele group; or low-functioning vs high-functioning group) design followed by Bonferroni-corrected post hoc tests in SPSS 21.
Magnetic resonance imaging
Hemodynamic activity
All images were acquired with a 1.5 Tesla whole-body tomograph (Siemens Symphony with a quantum gradient system) with a CP head coil. Structural image acquisition consisted of 160 T1-weighted sagittal images (MPRage, 1 mm slice thickness; TR = 1.9 s; TE = 4.16 ms; field of view 250 mm × 250 mm). For functional images, 292 images were registered using a T2*-weighted gradient echo-planar imaging (EPI) sequence with 25 slices covering the whole brain (slice thickness = 5 mm; 1 mm gap; descending slice order; time of repetition (TR) = 2.5 s; echo time (TE) = 55 ms; flip angle = 90°; field of view 192 mm × 192 mm; matrix size = 64 × 64). The first two volumes were discarded owing to the incomplete state of magnetization. The orientation of the axial slices was paralleled to the OFC tissue–bone transition. Data were analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London UK; 2008) implemented in MATLAB 7.5 (Mathworks Inc., Sherbourn, MA). Before all statistical analyses, data were preprocessed as described before (Klucken et al., 2012). The experimental conditions were the CS+, the CS−, the UCS and the non-UCS modelled as events. Regressors were convolved with the hemodynamic response function. The six movement parameters of the rigid body transformation obtained by the realignment procedure were introduced as covariates in the model. The voxel-based time series were filtered with a high pass filter (time constant = 128 s). On the first level of analysis, the contrast CS+ > CS− was analyzed for each participant and introduced as dependent variable into the group analyses to investigate the potential effects of 5-HTTLPR genotype.
On the second level, a full-factorial model was used to avoid potentially biased type I errors due to the use of pooled errors (Boik, 1981; Barcikowski and Robey, 1984). The full-factorial model included the group factor 5-HTTLPR genotype implemented in SPM8 and was analyzed for main effects of task (CS+ > CS− and CS− > CS+). Whole brain analyses were conducted with P < 0.05 [family-wise error (FWE) corrected] and k > 10 voxels. Region of interest (ROI) analyses were performed using the small volume correction in SPM8 P < 0.05 (FWE-corrected; k > 5 voxels). ACC, amygdala, insula, OFC and thalamus masks were taken from the ‘Harvard-Oxford cortical and subcortical structural atlases’ provided by the Harvard Center for Morphometric Analysis. The occipital cortex mask was created with MARINA (Walter et al., 2003). Hermann and colleagues (2012) kindly provided the vmPFC mask.
Effective Connectivity Analyses
To assess connectivity, we conducted a psychophysiologic interaction (PPI) analysis, which explores the effective connectivity between a seed region and other brain areas in interaction with an experimental task (Friston et al., 1997; Gitelman et al., 2003; O'Reilly et al., 2012). We used the amygdala as seed region (volume of interest; VOI) and extracted the first eigenvariate as implemented in SPM8. Then, the interaction term was created by multiplying the extracted signal with the contrast of interest (CS+ > CS−) for each participant. Three vectors were created containing (i) the psychological variable (main effect of the contrasts of interest), (ii) the physiological variable (VOI time-course) and (iii) the interaction term. First-level analysis was conducted for each participant and included the three regressors (psychological variable, physiological variable, interaction term) in the design matrix. At the second level, we analyzed genotype-dependent differences in the effective connectivity in the insula and the vmPFC.
Diffusion Tensor Imaging
Diffusion-weighted images were acquired using a single shot, pulsed gradient, EPI protocol (slice thickness = 3 mm; interleaved slice procedure; TR = 9.8 s; TE = 111 ms; field of view 192 mm × 192 mm; matrix size = 128 × 128, 12 directions, b-values = 0 and 1000 s/mm2, 3 averages). Skeletonization was carried out using the Tract-Based Spatial Statistics module implemented in FSL. First, data were preprocessed (eddy current and head motion correction, brain mask for the DTI data, tensor calculation). Anisotropy was expressed as fractional anisotropy (FA). The fiber tract of interest (UF) was taken from the John Hopkins University (JHU) DTI white matter atlas provided by the FMRIB software library (FSL) software package (Wakana et al., 2007; Hua et al., 2008). We were interested in group differences in (the frontal part of) the UF tract, which is provided in the JHU DTI white matter atlas because Jonassen et al. (2012) found differences in the frontal but not in the temporal part. To analyze further if potential differences occur exclusively in the hypothesized UF-tract or are found generally in white matter microstructure integrity, we analyzed the posterior corona radiata as a ‘control tract’. This tract is assumed to be uninfluenced by the 5-HTTLPR. In the first-level analyses, FA-values were computed for each participant and introduced as dependent variable for the group analyses. In the group analyses, main effects as well as differences between the genotype groups were calculated using the permutation program ‘randomise’ (FSL software package). In addition, we analyzed both groups separately to test whether group means are significantly increased against chance. A threshold of P < 0.05 (FWE-corrected) was used.
RESULTS
Subjective ratings
ANOVAs showed strong main effects of stimulus-type for valence [F(1,98) = 275.93, P < 0.001], arousal [F(1,98) = 271.63, P < 0.001] and UCS-expectancy ratings [F(1,98) = 469.46, P < 0.001], but no interaction effects with 5-HTTLPR genotype as compared with previous studies. Post hoc tests showed that the CS+ was rated as significantly more unpleasant, more arousing and with a higher UCS-expectancy rating as compared with the CS− (all P < 0.001; see Table 1).
Table 1.
Mean subjective ratings (s.d.) and SCRs (FIR and SIR) for the CS+ and the CS−
| Stimulus | Valence | Arousal | UCS expectancy | FIR | SIR |
|---|---|---|---|---|---|
| CS+ | 6.6 (1.3) | 6.7 (1.2) | 6.5 (1.5) | 0.14 (0.13) | 0.16 (0.16) |
| CS− | 2.3 (1.8) | 2.4 (1.9) | 1.6 (1.4) | 0.09 (0.09) | 0.10 (0.12) |
Skin conductance responses
ANOVAs revealed a significant main effect of stimulus-type regarding the FIR [F(1,98) = 43.54, P < 0.001] and the SIR [F(1,98) = 37.10, P < 0.001], showing increased SCRs to the CS+ as compared with the CS− (all P < 0.05). Neither a main effect of 5-HTTLPR genotype nor a stimulus × genotype interaction effect was found for SCRs (all P > 0.1). We also analyzed group differences regarding the first half (trial 1–10) and the second half (trial 11–20) of the experiment because time might be an important variable in fear conditioning. Again, the results remain stable: No group differences were found, neither in the first nor in the second half (all P > 0.3) of the experiment.
MRI results
Fear conditioning
Main effect of stimulus (CS+ > CS−)
Whole brain results showed significant differences between the CS+ and the CS− within many different brain structures. We found increased BOLD responses to the CS+ in the occipital cortex (x/y/z = 15/ − 79/10, zmax = 7.82, P < 0.0001), the left OFC (x/y/z = − 3/35/ − 14, zmax = 6.280, P < 0.0001), the supplemental motor area (x/y/z = − 6/11/43, zmax = 5.73, P < 0.0001), the middle temporal gyrus (x/y/z = 54/ − 7/52, zmax = 5.45, P = 0.0001), the rolandic operculum (x/y/z = − 51/2/13, zmax = 5.55, P < 0.001), the supramarginal gyrus (x/y/z = − 51/ − 28/28, zmax = 5.17, P < 0.001) and the postcental gyrus (x/y/z = 18/ − 43/61, zmax = 5.34, P < 0.001). Further, ROI analyses revealed significant results in the contrast CS+ > CS− (see Table 2 for detailed P-values and localization). Finally, no main effect of stimulus was found in the contrast CS− > CS+.
Table 2.
Significant ROI activations (localization and statistics of the peak voxels) for the contrast CS+ > CS− and for group differences (s-allele group vs l/l-allele group)
| Group analysis | Contrast | Structure | Side | k | x | y | z | zmax | p corr | Effect size |
|---|---|---|---|---|---|---|---|---|---|---|
| Main effect of stimulus | CS+ > CS− | ACC | L | 420 | −3 | 35 | −8 | 5.27 | <0.001 | 0.50 |
| Amygdala | L | 10 | −12 | −7 | −17 | 3.23 | 0.022 | 0.32 | ||
| Amygdala | R | 23 | 24 | −1 | −29 | 4.33 | 0.001 | 0.42 | ||
| Insula | L | 172 | −33 | 20 | 7 | 3.99 | 0.012 | 0.37 | ||
| Insula | R | 97 | −30 | 23 | 7 | 3.76 | 0.015 | 0.37 | ||
| Occipital cortex | L | 3257 | −6 | −82 | 10 | 7.80 | <0.001 | 0.68 | ||
| Occipital cortex | R | 2920 | 15 | −79 | 10 | 7.82 | <0.001 | 0.68 | ||
| OFC | L | 96 | −21 | 32 | −17 | 5.02 | <0.001 | 0.48 | ||
| OFC | R | 23 | 15 | 14 | −14 | 3.60 | 0.025 | 0.35 | ||
| Thalamus | L | 208 | −12 | −16 | 7 | 3.49 | 0.029 | 0.34 | ||
| Thalamus | R | 165 | 21 | −28 | 4 | 3.52 | 0.026 | 0.34 | ||
| vmPFC | L | 70 | −3 | 35 | −14 | 6.28 | <0.001 | 0.58 | ||
| vmPFC | R | 189 | 3 | 38 | −14 | 5.66 | <0.001 | 0.53 | ||
| s-allele group > l/l-allele group | CS+ > CS− | Amygdala | R | 11 | 24 | −1 | −29 | 3.19 | 0.026 | 0.32 |
| Insula | L | 35 | −36 | −19 | 19 | 3.55 | 0.029 | 0.35 | ||
| Occipital cortex | L | 54 | −27 | −1 | −44 | 4.21 | 0.030 | 0.41 |
The threshold was P < 0.05 (FWE-corrected; small volume correction according to SPM8). L: left hemisphere, R: right hemisphere. k: cluster size. Effect sizes are given in point biserial correlation of the respective peak voxels. All coordinates are given in MNI space.
Group differences in the contrast CS+ > CS−
Whole brain results showed increased BOLD responses in the contrast CS+ > CS− in s-allele carriers as compared with the l/l-allele group in the left insula (x/y/z = − 36/ − 19/22, zmax = 4.47, P < 0.001). ROI analyses further displayed higher amygdala reactivity in s-allele carriers as compared with the l/l-allele group for the contrast CS+ > CS− (x/y/z = 24/ − 1/ − 29, zmax = 3.19, P < 0.05; see Figure 1a). In addition, we found increased insula (x/y/z = − 36/ − 19/19, zmax = 3.55, P < 0.05) and occipital (x/y/z = − 27/ − 1/ − 44, zmax = 4.21, P < 0.05) activity in s-allele carriers as compared with the l/l-allele group (see Table 2). In addition, no group differences occurred for the contrast CS− > CS+. Finally, whole brain and ROI analyses showed no significantly elevated activity in l/l-allele carriers as compared with the s-allele group.
Fig. 1.
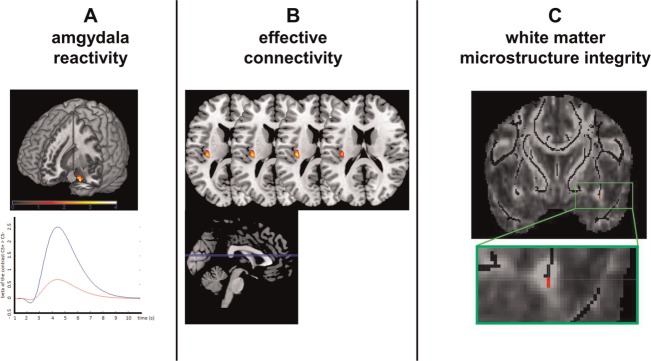
(A) Neural activations for the main effect of 5-HTTLPR genotype (s-allele carriers vs l/l-allele group) in the right amygdala (above) and a standard modeled canonical hemodynamic response function (HRF) depicting the neural activation from the s-allele (blue line) and the l/l-allele group (red line) for the peak voxel within the amygdala for contrast CS+ > CS− (below). (B) Neural activations for the main effect of 5-HTTLPR genotype in the left insula (above) for the effective connectivity results. (C) Coronar view of the higher FA values in s-allele carriers as compared with the l/l-allele group observed in the limbic part of the UF (red; x/y/z = 34/3 − 12). The displaying threshold was P < 0.05. Results are displayed on the mean FA skeleton overlaid on the mean FA image.
Effective connectivity
In addition to amygdala reactivity, we used PPI to explore effective connectivity between the amygdala and cortical brain structures. Increased effective connectivity in s-allele carriers as compared with the l/l-allele group was found between the amygdala and the left insula (x/y/z = − 36/ − 22/16; z = 3.48; P < 0.05; effect size = 0.33; see Figure 1b), but not between the amygdala and the right insula or between amygdala and the vmPFC. In addition, no significant results occurred in further areas in the whole brain analysis.
White matter microstructure integrity
Next, we analyzed the DTI data to investigate white matter microstructure integrity. An effect of genotype was found in the UF but not in the control tract. S-allele carriers showed significantly higher FA-values (max. peak: x/y/z = 24/ − 1/ − 29; z = 3.19; P = 0.027) as compared with the l/l-allele group (see Figure 1c). Notably, the peak FA-values differentiating s-allele carriers from the l/l-allele group were located in the limbic part of the UF. As an additional finding, we also analyzed the s-allele group separately and found increased FA-values bilaterally in both UF-tracts. Peak voxels were located in the limbic part of the UF-tract (see Figure 1c).
Finally, to investigate if age and gender influenced the effects of the 5-HTTLPR, we entered age and gender as (co)variates into an AN(C)OVA (cf. Jonassen et al., 2012). We did not find any interaction effects of age and gender on FA-values or on other measurements. One reason might be that participants were mostly students, which may have lead to restricted variance at least in age. Jonassen and colleagues (2012) investigated a sample with a broader age range.
DISCUSSION
The aim of the present study was to investigate the relationship between 5-HTTLPR genotype and fear conditioning, effective coupling and white matter microstructure integrity. As the main result, s-allele carriers exhibited enhanced amygdala reactivity, increased amygdala-insula connectivity during fear learning and increased white matter integrity in the UF tract.
Fear conditioning
In detail, we found elevated amygdala responses in s-allele carriers as compared with the l/l-allele group during fear learning. Enhanced BOLD responses in response to fear stimuli in s-allele carriers is a frequently observed finding, which has been reported by many studies using various stimuli and designs (Hariri et al., 2002, 2006; Munafò et al., 2008; Alexander et al., 2012; Klucken et al., 2013a). The substantial influence of the serotonergic system on amygdala functioning and fear learning is also mirrored in pharmacological studies showing alterations by direct manipulation of serotonergic neurotransmission (Inoue et al., 2004; Burghardt et al., 2007; Almada et al., 2009; Homberg, 2012). The increased activity might constitute an important process for the stabilization of the fear learning signal, the production of conditioned fear responses and the maintenance of anxiety disorders (Delgado et al., 2006; Schweckendiek et al., 2011).
Importantly, the increased amygdala reactivity during fear conditioning in the s-allele group offers an explanation for the observed dissociation of the 5-HTTLPR on different response levels: As mentioned in the introduction, in contrast to startle responses (Lonsdorf et al., 2009) and hemodynamic activity (Klucken et al., 2013a), most studies have not found a genotype-dependent effect on SCRs. While amygdala reactivity is crucially involved in conditioned startle responses, conditioned SCRs have been reported to be (mostly) dissociated from amygdala responses (Davis and Whalen, 2001; Hamm and Weike, 2005; Weike et al., 2005; Tabbert et al., 2006; Klucken et al., 2009). We assume that the 5-HTTLPR may predominantly alter amygdala-dependent CRs (like startle response) rather than CRs (e.g. SCRs) modulated by other brain areas (Critchley et al., 2002).
Effective connectivity
We observed increased amygdala-insula coupling dependent on 5-HTTLPR genotype. Altered amygdala-insula coupling during (dysfunctional) emotion processing is a robust finding (Stein et al., 2007) and has been reported to be modulated by 5-HTTLPR genotype in the context of emotion regulation (Schardt et al., 2010; Lemogne et al., 2011). In detail, s-allele carriers showed increased (anterior) insula responses and altered insula coupling processes as compared with l/l-allele carriers (Schardt et al., 2010; Lemogne et al., 2011). The enhanced coupling in s-allele carriers possibly reflects the increased effort necessary for emotion regulation during the anticipation of the unconditioned fear stimulus (Hermann et al., 2014). However, because in the present study subjects were not explicitly instructed to (actively) regulate their emotions, this interpretation should be treated with caution. Alternatively, because amygdala and insula responses are involved in interoceptive processing, the enhanced connectivity could be caused by increased interoceptive processing in s-allele carriers (Paulus and Stein, 2006; Domschke et al., 2010; Drabant, 2012; Klucken et al., 2013b). However, this is a post hoc explanation and future studies have to examine this in more detail.
Structural connectivity
In addition to effective connectivity, the DTI data provides evidence that the 5-HTTLPR genotype is linked to alterations of structural connectivity within the UF. The UF constitutes the white matter tract that connects structures of the limbic system with prefrontal cortex areas (Ebeling and Cramon, 1992; Pacheco et al., 2009; Ayling et al., 2012). Alterations of UF white matter microstructure integrity have been speculated to influence bottom-up processing of fear-relevant signals from the limbic system to cortical structures (Montag et al., 2012), which are responsible for CS evaluation (Milad and Rauch, 2007; O'Doherty, 2007). This view is supported by recent reports of positive correlations between anxiety-related traits and white matter microstructure integrity in the UF (Montag et al., 2012; Modi et al., 2013). Notably, previous studies have also reported negative associations between the UF-tract and anxiety-related traits. Thus, the functionality of white matter microstructure integrity is to date unclear (Phan et al., 2009; Baur et al., 2011, 2013).
The present results allow speculations about larger scale network functioning: The observed increased amygdala reactivity could be a correlate of a facilitated acquisition process, which renders formerly neutral stimuli into salient stimuli more easily in s-allele carriers (Klucken et al., 2013b). Moreover, the fear signal could be further augmented by increased bottom-up processing enabled by altered white matter microstructure integrity of the UF (Montag et al., 2012). Consequently, s-allele carriers may be prone to encounter a larger number of fear-provoking stimuli (Lonsdorf et al., 2009), which may lead to the experience of negative affect more often in s-allele carriers. This in turn might require enhanced emotion regulation efforts, which could be reflected in increased coupling processes (Volman et al., 2013). It is then conceivable that additional stressors such as stressful life events hamper successful emotion regulation and therefore increase the risk for psychiatric disorders (Karg et al., 2011; Alexander et al., 2012; Miller et al., 2013).
Limitations and overall conclusion
When evaluating the findings of the present study, some limitations have to be considered. First, although we showed that the 5-HTTLPR genotype is associated with white matter microstructure integrity, the biological mechanisms that link altered serotonergic transmission to structural connectivity are undetermined so far. The understanding of the functional consequences of altered white matter microstructure integrity is to date far from comprehensive and thus the current results should be interpreted with caution until replication is available. It is possible that other white matter tracts, which were not investigated in this study, are also associated with the 5-HTTLPR. Only two other studies investigated the 5-HTTLPR genotype and white matter microstructure integrity and observed enhanced white matter microstructure integrity in high-functioning allele carriers in the UF or no clear relationship, which is in contrast to our findings (Pacheco et al., 2009; Jonassen et al., 2012). These findings could have been caused by small sample sizes (37 participants in both studies) and the investigation of female subjects only. Further, the present study only included individuals of European decent. Results based on other (e.g. Asian) backgrounds may not be comparable with the present findings. In addition, we investigated potential variance in the group differences with respect to classification strategies (bi-allelic vs tri-allelic). The low-functioning group displayed increased amygdala activation and amygdala-insula coupling, but no group differences regarding the UF-tract were found. These discrepancies might be explained by the increase of error variance in the tri-allelic dichotomized model (see Jonassen and Landro, 2014, for a comprehensive overview), which decreases the power of the analysis. Finally, in contrast to previous studies, we found increased activations in the vmPFC in the contrast CS+ > CS. Previous results have linked vmPFC activity to fear inhibition (e.g. during fear extinction) and/or to emotion regulation (Milad et al. 2007; Goldin et al., 2008; Hermann et al., 2009; Merz et al., 2012; Lissek et al., 2014; Klucken et al., 2013c). The vmPFC is a brain area that has been associated with many different functions. Thus, the effects observed in this study could be caused by functionally heterogeneous subdivisions. However, this is a post hoc interpretation. Further studies should investigate the vmPFC during fear acquisition to clarify its role.
In conclusion, in search of the determinants of the present results, it has been suggested that the association between the 5-HTTLPR and altered emotional processing are determined by early neural developmental rather than differences in 5-HT transmission in adulthood (Jonassen and Landro, 2014). If such early differences in neural plasticity occur, it seems plausible that s-allele carriers show dysfunctional connectivity in the serotonergic circuitry, which is associated with increased stress and fear (e.g. Alexander et al., 2009, 2012; Belsky et al., 2009; Kuepper et al., 2012).
Supplementary Material
Acknowledgments
This work was supported by a research grant from the DFG German Research Foundation (KL 2500-1). We thank Luise Blochberger, Paul R. Bretschneider, and Aisha J. Munk for data acquisition.
REFERENCES
- Agren T, Furmark T, Eriksson E, Fredrikson M. Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes. Translational Psychiatry. 2012;2:e76. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N, Klucken T, Koppe G, et al. Interaction of the serotonin transporter-linked polymorphic region and environmental adversity: increased amygdala-hypothalamus connectivity as a potential mechanism linking neural and endocrine hyperreactivity. Biological Psychiatry. 2012;72:49–56. doi: 10.1016/j.biopsych.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Almada RC, Borelli KG, Albrechet-Souza L, Brandão ML. Serotonergic mechanisms of the median raphe nucleus-dorsal hippocampus in conditioned fear: output circuit involves the prefrontal cortex and amygdala. Behavioural Brain Research. 2009;203:279–87. doi: 10.1016/j.bbr.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Ayling E, Aghajani M, Fouche JP, van der Wee N. Diffusion tensor imaging in anxiety disorders. Current Psychiatry Reports. 2012;14:197–202. doi: 10.1007/s11920-012-0273-z. [DOI] [PubMed] [Google Scholar]
- Barcikowski RS, Robey RR. Decisions in single group repeated measures analysis: statistical tests and three computer packages. The American Statistician. 1984;38:148–50. [Google Scholar]
- Baur V, Brühl AB, Herwig U, et al. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Human Brain Mapping. 2013;34:437–46. doi: 10.1002/hbm.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Rufer M, et al. White matter alterations in social anxiety disorder. Journal of Psychiatric Research. 2011;45:1366–72. doi: 10.1016/j.jpsychires.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–54. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boik RJ. A priori tests in repeated measures designs: effects of nonsphericity. Psychometrika. 1981:241–55. [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology. 2000;10:219–23. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DEA, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biological Psychiatry. 2007;62:1111–18. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–89. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Craig ADB. Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Crişan LG, Pana S, Vulturar R, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Social Cognitive and Affective Neuroscience. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans. Neuron. 2002;33:653–63. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nature Neuroscience. 2008;11:880–81. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Drabant EM. Neural Mechanisms Underlying 5-HTTLPR-Related Sensitivity to Acute Stress. American Journal of Psychiatry. 2012;169:397. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochirurgica. 1992;115:143–48. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behavioral Neuroscience. 2001;115:358–64. [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006;59:888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2004;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biological Psychiatry. 2000;47:643–9. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hermann A, Keck T, Stark R. Dispositional cognitive reappraisal modulates the neural correlates of fear acquisition and extinction. Neurobiology of Learning and Memory. 2014;113:115–24. doi: 10.1016/j.nlm.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Hermann A, Küpper Y, Schmitz A, et al. Functional gene polymorphisms in the serotonin system and traumatic life events modulate the neural basis of fear acquisition and extinction. PloS One. 2012;7:e44352. doi: 10.1371/journal.pone.0044352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Archives of General Psychiatry. 2003;60(7):702–8. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: a systematic review. Journal of affective disorders. 2014;158:114–26. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Homberg JR. Serotonergic modulation of conditioned fear. Scientifica. 2012;2012:1–16. doi: 10.6064/2012/821549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Lesch K. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69(6):513–19. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JDA, Blom T, et al. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One. 2011;6(1):e16646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Molecular Psychiatry. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Li XB, Abekawa T, et al. Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. European Journal of Pharmacology. 2004;497:311–16. doi: 10.1016/j.ejphar.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Qiao F, Xu XF, Yang Y, Bai Y, Jiang LL. Meta-analysis confirms a functional polymorphism (5-HTTLPR) in the serotonin transporter gene conferring risk of bipolar disorder in European populations. Neuroscience Letters. 2013;549:191–6. doi: 10.1016/j.neulet.2013.05.065. [DOI] [PubMed] [Google Scholar]
- Jonassen R, Endestad T, Neumeister A, Foss Haug KB, Berg JP, Landrø NI. The effects of the serotonin transporter polymorphism and age on frontal white matter integrity in healthy adult women. Frontiers in Human Neuroscience. 2012;6:19. doi: 10.3389/fnhum.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen R, Landrø NI. Serotonin transporter polymorphisms (5-HTTLPR) in emotion processing: implications from current neurobiology. Progress in Neurobiology. 2014;117:41–53. doi: 10.1016/j.pneurobio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29(37):11614–18. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, et al. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Social Cognitive and Affective Neuroscience. 2013a;8:318–25. doi: 10.1093/scan/nss005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Kagerer S, Schweckendiek J, Tabbert K, Vaitl D, Stark R. Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture-picture conditioning paradigm. Neuroscience. 2009;158:721–31. doi: 10.1016/j.neuroscience.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, et al. Neural correlates of disgust- and fear-conditioned responses. Neuroscience. 2012;201:209–18. doi: 10.1016/j.neuroscience.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Vaitl D, Stark R. Dissociation of neuronal, electrodermal, and evaluative responses in disgust extinction. Behavioral Neuroscience. 2013c;127:380–6. doi: 10.1037/a0032331. [DOI] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, et al. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Human Brain Mapping. 2013b;34:2549–60. doi: 10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper Y, Wielpuetz C, Alexander N, Mueller E, Grant P, Hennig J. 5-HTTLPR S-allele: a genetic plasticity factor regarding the effects of life events on personality? Genes, Brain, and Behavior. 2012;11(6):643–50. doi: 10.1111/j.1601-183X.2012.00783.x. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE. Partial disruption of fear conditioning in rats with unilateral amygdala damage: correspondence with unilateral temporal Lobectomy in humans. Behavioral Neuroscience. 1996;110:991–7. doi: 10.1037//0735-7044.110.5.991. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Boni C, Pessiglione M, Lehéricy S, Fossati P. Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Human Brain Mapping. 2011;32:1856–67. doi: 10.1002/hbm.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, et al. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Social cognitive and affective neuroscience. 2014;9:1134–42. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindström KM, Haaker J, Ohman A, Schalling M, et al. BDNFval66met affects neural activation pattern during fear conditioning and 24 h delayed fear recall. Social cognitive and affective neuroscience. 2015;10(5):664–71. doi: 10.1093/scan/nsu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive and Affective Neuroscience. 2012;7:819–30. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Behavioural neuroscience: genes and the anxious brain. Nature. 2010;466:827–8. doi: 10.1038/466827a. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007;Vol. 1121:546–61. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Neurocircuitry and neuroplasticity abnormalities in mood and anxiety disorders. Biological Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: a meta-analysis. Molecular Psychiatry. 2013;18:1018–24. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–80. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Modi S, Trivedi R, Singh K, et al. Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: preliminary evidence from a DTI based tractography study. Behavioural Brain Research. 2013;238:188–92. doi: 10.1016/j.bbr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M, Weber B, Markett S, Schoene-Bake J-C. Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience. 2012;217:77–83. doi: 10.1016/j.neuroscience.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Morris J, DeGelder B, Weiskrantz L, Dolan R. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–52. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009a;65:211–19. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, et al. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. American Journal of Medical Genetics. 2009b;150B:271–81. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry. 2000;5:32–8. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Northoff G. Gene, brains, and environment—genetic neuroimaging of depression. Current Opinion in Neurobiology. 2013;23:133–42. doi: 10.1016/j.conb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Social Cognitive and Affective Neuroscience. 2008;3:144–60. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Annals of the New York Academy of Sciences. 2007;1121:254–72. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience. 2007;10:1095–102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7:604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. The Journal of Neuroscience. 2009;29:6229–33. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, et al. Preliminary evidence of white matter abnormality in the Uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry. 2009;66:691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biological Psychiatry. 2007;62:327–31. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Schardt DM, Erk S, Nüsser C, et al. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2010;53:943–51. doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Schweckendiek J, Klucken T, Merz CJ, et al. Weaving the (neuronal) web: fear learning in spider phobia. Neuroimage. 2011;54:681–8. doi: 10.1016/j.neuroimage.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Twitchell GR, Hanna GL, et al. Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. American Journal of Medical Genetics. 2002;114:230–4. doi: 10.1002/ajmg.10187. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, et al. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Social Cognitive and Affective Neuroscience. 2011;6:495–506. doi: 10.1093/scan/nsq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage. 2006;32(2):761–70. doi: 10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Volman I, Verhagen L, den Ouden HEM, et al. Reduced serotonin transporter availability decreases prefrontal control of the amygdala. The Journal of Neuroscience. 2013;33:8974–9. doi: 10.1523/JNEUROSCI.5518-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, et al. MARINA: an easy to use tool for the creation of MAsks for Region of INterest Analyses. Neuroimage 19, 2 (supplement), p. 1899. . 2003 Available on: www.bion.de. [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HWS, Kessler C. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. The Journal of Neuroscience. 2005;25:11117–24. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in Magnetic Resonance Imaging:TMRI. 2008;19:97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Düzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


