Abstract
Human cooperation and competition is modulated by oxytocin, a hypothalamic neuropeptide that functions as both hormone and neurotransmitter. Oxytocin’s functions can be captured in two explanatory yet largely contradictory frameworks: the fear-dampening (FD) hypothesis that oxytocin has anxiolytic effects and reduces fear-motivated action; and the social approach/avoidance (SAA) hypothesis that oxytocin increases cooperative approach and facilitates protection against aversive stimuli and threat. We tested derivations from both frameworks in a novel predator–prey contest game. Healthy males given oxytocin or placebo invested as predator to win their prey’s endowment, or as prey to protect their endowment against predation. Neural activity was registered using 3T-MRI. In prey, (fear-motivated) investments were fast and conditioned on the amygdala. Inconsistent with FD, oxytocin did not modulate neural and behavioral responding in prey. In predators, (greed-motivated) investments were slower, and conditioned on the superior frontal gyrus (SFG). Consistent with SAA, oxytocin reduced predator investment, time to decide and activation in SFG. Thus, whereas oxytocin does not incapacitate the impulsive ability to protect and defend oneself, it lowers the greedy and more calculated appetite for coming out ahead.
Keywords: endocrinology, approach motivation, cooperation, aggression, executive control
Human societies function, and their individuals prosper, when and because individuals extend trust and cooperate, control their greedy desires to appropriate other’s wealth and properly defend and protect themselves against predatory exploitation. Cumulating evidence from neurobiology and behavioral sciences suggests that human cooperation and competition is modulated by oxytocin, an evolutionary ancient neuropeptide that is produced in the human hypothalamus and functions as both hormone and neurotransmitter (Ludwig and Leng, 2006; Carter et al., 2008; Donaldson and Young, 2008; Meyer-Lindenberg et al., 2011; Bos et al., 2012). Across mammalian species, oxytocin sustains pair bond formation and maintenance (Carter et al., 2008; Donaldson and Young, 2008). In humans, genetic polymorphism in the oxytocin receptor gene associates with generosity in humans (Israel et al., 2009; Roderigues et al., 2009), and intranasal administration of oxytocin appears to facilitate empathic responding and cooperation (Kirsch et al., 2005; Kosfeld et al., 2005; Baumgartner et al., 2008; Guastella et al., 2008; Petrovic et al., 2008; De Dreu et al., 2010; De Dreu, 2012a; Israel et al., 2013). These and related findings have been captured in two largely competing frameworks, suggesting that oxytocin enables cooperation because (i) it mitigates the fear of being exploited and the concomitant need to protect oneself against predation, or (ii) it tempers the greedy desire to accumulate wealth at the expense of others. Below, we elaborate on the evidence and suspected mechanisms underlying these respective scenarios, and device a novel predator/prey decision-making game to pit the competing hypotheses against each other.
The first perspective on the influence of oxytocin on human cooperation and competition rests on the fact that, on its hypothalamic release, oxytocin targets the regions of the spinal cord that regulate the parasympathetic branch of the autonomic nervous system (Ludwig and Leng, 2006; Donaldson and Young, 2008). Oxytocin interacts with the hypothalamic–pituitary–adrenal axis to attenuate stress responses: it reduces cortisol levels after exposure to stressors, inhibits cardiovascular stress responses and modulates neural circuitries involved in the processing of fear-related information (Carter et al., 2008; Meyer-Lindenberg et al., 2011; Bos et al., 2012). Accordingly, participants receiving intranasal oxytocin (vs placebo) showed reduced activation of the amygdala and attenuated coupling of the amygdala to brainstem centers responsible for autonomic and behavioral components of fear when processing fearful faces (Kirsch et al., 2005; Petrovic et al., 2008; MacDonald and Feifel, 2014), were less fearful of being exploited and more trusting of others (Kosfeld et al., 2005; Baumgartner et al., 2008; De Dreu et al., 2010). Together, these works converge on the fear-dampening (FD) hypothesis: oxytocin dampens activation in the amygdala and its direct and indirect role in fear-responding and thus enables humans to extend trust.
The alternative social approach/avoidance (SAA) perspective on the influence of oxytocin on human cooperation and competition (Guastella et al., 2008; Kemp and Guastella, 2011; Striepens et al., 2012) rests on the well-established notion that human emotion and behavior are grounded in a tendency to approach positively valued and to avoid aversive negatively valued states (Higgins, 1997; Watson et al., 1999; Cardinal et al., 2002; Roskes et al., 2013). It accordingly proposes that oxytocin (i) promotes approach-related exploration rather than exploitation (Kemp and Guastella, 2011, De Dreu et al., 2014), including pro-social behavior such as cooperation, while (ii) allows to adaptively respond to aversive stimuli and threat by, for example, flight, seeking shelter or, if needed, lashing out to neutralize the threat (De Dreu, 2012b; Striepens et al., 2012).
That oxytocin drives pro-social approach fits studies showing that oxytocin increases trust and generosity (Kosfeld et al., 2005; Baumgartner et al., 2008; De Dreu et al., 2010; Chang et al., 2012), and studies showing that oxytocin upregulates neural circuitries involved in empathic concern and reward processing (Skuse and Gallagher, 2005; Carter et al., 2008; Donaldson and Young, 2008; Rilling et al., 2012; Hurlemann et al., 2010). That oxytocin enables adaptive responding to threat contrasts with the FD hypothesis that oxytocin reduces fear and anxiety, yet resonates with work showing that oxytocin blunts attention to negative facial expressions in rhesus macaque (Ebitz et al., 2013; Parr et al., 2013) and, in humans, reduces the tendency to withdraw from angry faces (Hurlemann et al., 2010), potentiates startle reactivity to threat and facilitates protective (neural) responses to aversive stimuli, especially when unpredictable (Striepens et al., 2012; Grillon et al., 2013). It also fits work showing that oxytocin motivates protective competitive behavior when exchange partners are unfamiliar, potentially untrustworthy and belonging to rivaling out-groups (Declerck et al., 2010; De Dreu et al., 2010; Mikolajczak et al., 2010; De Dreu et al., 2011; Ten Velden et al., 2013).
Earlier analyses of oxytocin assumed that pro-social approach may be the result of lowered anxiety and reduced fear (e.g. Bartz et al., 2010), or used oxytocin-reduced amygdala activation in the face of threatening stimuli as evidence for pro-social approach (e.g. Kemp and Guastella, 2011). Here we disentangled fear-responding from pro-social approach conceptually, and to illuminate the precise functions of oxytocin in social exchange problems, we tested derivations of the FD-hypothesis on the one hand, and the SAA-hypothesis on the other. We did so using a newly developed, two-player Predator–Prey Game (PPG). The PPG is grounded in the economic theory of predation and economic growth (Grossman and Kim, 1996; Carter and Anderson, 2001), and models the conflict between survival and preservation of the status quo on the one hand, and the drive toward appropriation and expansion on the other. In the PPG, one player (henceforth predator) has to decide how much to invest in predation (X) out of a given endowment E (with 0 ≤ X ≤ E), while the other player (henceforth prey) simultaneously decides how much to invest in defense (Y) out of an equal endowment E (with 0 ≤ Y ≤ E). If X > Y then the predator obtains all of E–Y; added to the remaining endowment E–X, this leads to a total payoff for the predator of 2E–X–Y, while the prey is left with 0. If X ≤ Y then the predator appropriates nothing, leading to a payoff of E–X for the predator and E–Y for the prey. The PPG is formally equivalent to a contest with as contest success function f = Xm/(Xm + Ym), where f is the probability that the predator wins, m→∞ for X ≠ Y, and f = 0 if Y = X (Tullock, 1980).
In the PPG, the predator’s decision not to invest reflects cooperation because it is collectively beneficial; any amount invested reflects the greedy desire to accumulate personal wealth by subordinating the prey. Vice versa, the prey’s decision not to invest reflects trust and is collectively beneficial; any amount invested reflects fear of being exploited and concomitant defensive aggression (Coombs, 1973; Camerer, 2003). Presumably because detecting opportunities and acquiring gains is less essential and less basic to survival than detecting threat and preventing loss (Delgado et al., 2008; De Martino et al., 2010), animal studies show that predatory greed is relatively slow, goal-directed and controlled by brain circuitries centered on prefrontal cortex. Defensive aggression in prey is faster, more relying on sensorimotor links and conditioned on fear-signaling in amygdala-centered networks (Albert et al., 1993; Siegel et al., 1999; Nelson and Trainor, 2007; Choi and Kim, 2010). Along similar lines, human decision making geared at the inconsiderate accumulation of wealth rests on brain circuitries involved in impulse control, such as the superior frontal gyrus (SFG; Polosan et al., 2011; Chaminade et al., 2012; Fahrenfort et al., 2012; Krutschwitz et al., 2012), whereas decisions geared at protection and defense are strongly amygdala-dependent (Baumgartner et al., 2008; Delgado et al., 2008; De Martino et al., 2010; Rilling et al., 2012).
Whereas we know that oxytocin shapes the neural circuitries and behavioral tendencies in cooperation and competition, precise predictions as to what oxytocin does starkly differ depending on the FD vs SAA hypothesis. SAA predicts that among predators, oxytocin promotes prosocial approach and tempers calculated greed, as reflected in reduced prefrontal activity as well as reduced predator investments. Among prey, oxytocin enables fear-driven defensive aggression, as reflected in maintained or even increased amygdala activity as well as maintained or intensified investments. By contrast, FD predicts that among prey, oxytocin dampens the fear of being exploited and mitigates defensive aggression, as reflected in reduced amygdala activity as well as reduced defensive investments. Among predators, no such effect will be observed.
MATERIALS AND METHODS
Overview
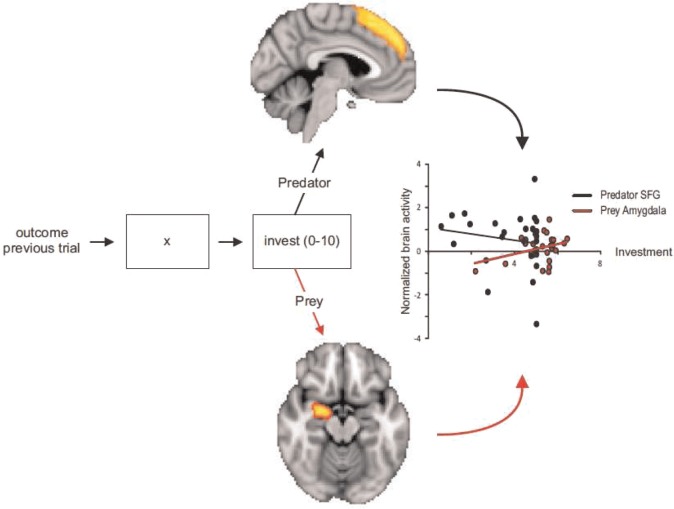
Predictions derived from FD and SAA were tested with the PPG in a double-blind placebo controlled cross-over design. Twenty-seven males without neurological or psychiatric history and not using drugs or prescription-based medication participated in two sessions of 1.5 h, with 1 week in between. They received instructions for the PPG and self-administered intranasally 24 IU oxytocin (three puffs per nostril) or placebo (containing all carrier ingredients except for the neuropeptide; see below). Because oxytocin effects on brain and behavior emerge ∼35 min after administration (e.g. Kosfeld et al., 2005; De Dreu, Greer et al., 2010; also see Kirsch et al., 2005; Baumgartner et al., 2008), we ensured a 30 min gap before participants were placed in the scanner and made six blocks of five investments as predator and six blocks of five investments as prey. Role alternated between blocks, and starting role was randomly determined. Per trial (see Figure 1), a randomly generated prompt (range: 0–10) was given; participants moved up or down by button presses to indicate desired investment. A trial ended by providing feedback about the protagonist’s investment and the resulting payoff. BOLD-MRI was recorded using a 3T Philips Achieva TX MRI scanner.
Fig. 1.
Outline of Decision Trial, ROIs (SFG shown above; Right Amygdala shown below) and their regressions on investment decisions. Fixation was jittered at 6000–9000 ms, and decision making (and trial feedback) was maximized at 6000 ms. Scatterplot shows predator (prey) investments associate negatively (positively) with SFG (Right Amygdala).
Participant recruitment and test medication
The experiment was approved by the Psychology Ethics Committee of the University of Amsterdam, and complied with American Psychological Association guidelines, and the Helsinki Protocols. Male participants (M = 25.31 years) were recruited via an online recruiting system and offered a monetary reward of €60 for participating in a neuroimaging study on the effects of medication on test scores and decision making. Participants were informed about the medication provided only after both test sessions were over and informal debriefing revealed no indication that participants knew they received oxytocin or placebo. Exclusion criteria were significant neurological or psychiatric history, prescription-based medication, smoking more than five cigarettes per day and drug or alcohol abuse. Eligible participants were assigned to a session and instructed to refrain from smoking or drinking (except water) for 2 h before the experiment. They were promised a €60 show-up fee for two separate magnetic resonance imaging (MRI) sessions, each lasting ∼ 1.5 h, and that during each session they could earn up extra money during decision making (i.e. across the two sessions, three predator and three prey decision trials would be randomly selected and paid out, for a maximal total extra earning of €87 ( = 3 × 19 + 3 × 10). For each session, they provided written informed consent.
Participants self-administered a single intranasal dose of 24 IU oxytocin (Syntocinon-Spray, Novartis; 3 puffs per nostril, each with 4 IU oxytocin, with 2 min interval between puffs) or placebo. To avoid any subjective effects (for example, olfactory effects) other than those caused by oxytocin, the placebo contained all the active ingredients except for the neuropeptide. The placebo was manufactured by Stichting Apothekers Haarlemse Ziekenhuizen in coordination with the pharmacy at the Amsterdam Medical Centre, adhering to the European Union guidelines on good manufacturing practice and good clinical practice. The placebo was produced using the exact same recipes and procedures used by Novartis Inc. to produce the carrier of Syntocinon—the synthetic analogue of oxytocin. Placebos were delivered in the same bottles as Syntocinon. In short, participants were ignorant about what treatment they received, and the only difference between the placebo and treatment was the absence vs presence of the active neuropeptide.
Experimental procedures and materials
Experimental sessions were conducted between noon and 4 p.m. and participants were tested individually. On arrival, participants were escorted to a private cubicle where they read and signed an informed consent form. They read the instructions for the PPG, and answered a series of quiz questions to verify whether they accurately understood the decision-making task and consequences. [Because past work on oxytocin revealed no effects on mood or changes therein (e.g. Kosfeld et al., 2005), a mood measure was not included here or in the post-experiment debrief]. Experimenters verified responses and provided the participant with test-medication (double-blind) and instructions. Following self-administration, the experimenter prepared the participant for neuroimaging. The time between neuropeptide administration and entrance into the scanner was fixed at 30 min.
Participants received a booklet with instructions for the PPG (labeled Investment Task), containing several examples of investments and their consequences to both predator (labeled Role A) and prey (labeled Role B), and several questions to probe understanding of the game structure and decision consequences. Neutral labeling was used throughout. We introduced a group-version in which participants represented themselves and two others (further ignored), and an individual version in which participants represented themselves only. In each of the two functional magnetic resonance imaging (fMRI) sessions, participants played 6 × 5 trials as predator and 6 × 5 trials as prey. For each trial, participants received a prompt, randomly generated between 0 (indicating no investment) and 10 (indicating investment of the entire endowment) and used a button-press to adjust the given number up or down to indicate their desired investment. Subsequently, they received feedback about their protagonist’s investment, and were shown the respective payoffs to oneself and to the protagonist [who was randomly chosen on each trial from a pool of 150 predator (prey) investments, see below]. This completed one trial. Data for one participant were discarded because of answers to quiz questions indicated failure to understand the payoff structure.
Trial feedback during the experiment was real and the ‘no deception policy’ was explicitly stated on several occasions during the recruitment and instruction phase. For each trial that participants in the MRI scanner played (henceforth ‘scanner participant’), s/he was paired to a matching trial that was played by a randomly selected subject in an independent behavioral experiment (henceforth ‘non-scanner participant’). This behavioral experiment involved a 2 (Order: Predator Role first/second) × 2 (Role: Predator vs Prey) factorial, with the second factor within participants. Fifteen students served as ‘non-scanner participants’. They were invited to a study on investment decisions and arrived in the laboratory in groups of 2–4, where they were provided informed consent, and were placed in individual soundproof cubicles preventing them from seeing and/or communicating with others. After participants were seated, the experimenter handed them instructions that were identical to those given to the scanner participants. Non-scanner participants made 10 decisions as predator, denoted as Role A (or prey, denoted as Role B), followed by 10 decisions as prey, denoted as Role B (or predator, denoted as Role A). For each investment decision, non-scanner participants were reminded that actual pay was at stake, in that of the 10 trials they played in Role A (and later or earlier in Role B), one trial would be randomly chosen to be matched with a randomly chosen investment decision of their counterpart (i.e. the investment decision made by the matched scanner participant). For each decision, participants entered a number between 0 (no investment) and 10 (their entire endowment), and they indicated how confident they were their investment was sufficient to settle the game in their favor (both 1 = not at all, to 5 = very much; not analyzed).
The 15 non-scanner participants in the behavioral experiment thus produced 15 × 10 trials = 150 prey investments, and 15 × 10 trials = 150 predator investments. In line with the results from the fMRI experiment (see below), average investments across the 10 trials were higher than theoretically predicted and higher among prey than predator (M = 5.953, s.d. = 1.782 vs M = 4.953, s.d. =1.821; F(1, 14) = 6.76, P = 0.021. These 150 predator and 150 prey decisions generated in this behavioral experiment provided the pool of trials from which we randomly chose one on each trial and for each scanner participant to provide actual feedback to the decision made by the scanner participant. Thus, the scanner participant played a trial as predator (prey), and feedback was given based on a randomly chosen investment decision made by a non-scanner participant in the role of prey (predator). We verified that, in the fMRI study, there were no inadvertent effects of Treatment on the investments from the randomly chosen (non-scanner) protagonists, F(1, 26) < 1. This indicates that our procedures provided scanner participants in oxytocin and placebo conditions with similar ‘tough’ protagonists, and rules out the possibility that results from the fMRI experiment were (partially) due to differences in feedback provided.
After both the behavioral experiment and the scanner experiment were completed, we determined actual earnings by non-scanner and scanner participants (range €0–€8, with M = €5 for non-scanner participants, and €0–€33, with M = €19 for scanner participants). Participants received their participation fee and earnings by bank transfer; the acting administrator was unaware of the fact that the money was earned in a decision-making experiment. Accordingly, at any point deception was avoided and participant pay was private and conditioned on their performance.
NeuroImage acquisition
Scanning was performed on a 3T Philips Achieva TX MRI scanner using a 32-channel head coil. Each subject participated in two scanning sessions, one for the placebo and one for the oxytocin condition. Each session consisted of six blocks of the PPG during which functional data were acquired using a gradient-echo, echo-planar pulse sequence (TR = 2000 ms, TE = 27.63 ms, FA = 76.1°, 280 volumes, FOV =192∧2 mm, matrix size = 64∧2, 38 ascending slices, slice thickness =3 mm, slice gap = 0.3 mm) covering the whole brain. We also recorded a 3DT1 recording in one of the sessions (3D T1 TFE, TR = 8.2 ms, TE = 3.8 ms, FA = 8°, FOV = 256∧2 mm, matrix size = 256∧2, 160 slices, slice thickness = 1 mm). During acquisition we also recorded respiration and the pulse oximetry signal and the breath rate of the subject. Stimuli were back-projected onto a screen that was viewed through a mirror attached to the head-coil.
FEAT (fMRI Expert Analysis Tool) version 4.1, part of FSL [Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library; www.fmrib.ox.ac.uk/fsl] was used to analyze the fMRI data (Smith et al., 2004; Woolrich et al., 2009). Preprocessing steps included slice-time correction, motion correction, high-pass filtering in the temporal domain (σ = 100 s), spatially filtered (5 mm) and pre-whitening (Woolrich et al., 2009). Apart from the modeled condition we also included, in the GLM, nuisance variables for movement (six parameters), the pulse oximetry signal (one parameter) and respiration (two parameters). The respiration parameters were modeled using the PhLEM toolbox (Versteynen and Deshpande, 2011).
Structural images were co-registered to the functional images and transformed to MNI standard space (Montreal Neurological Institute) using FLIRT (FMRIB's Linear Image Registration Tool; FSL). The resulting normalization parameters were applied to the functional images. We excluded between one and four blocks of eight subjects and removed two subjects completely because of excessive head movement. Given past work on oxytocin showing differential effects on left and right amygdala (Baumgartner et al., 2008; Rilling et al., 2012), and decision-making research indicating bilateral activation in the SFG (Polosan et al., 2011; Chaminade et al., 2012, Krutschwitz et al., 2012), subcortical regions of interest (ROIs; left and right amygdala) were segmented per individual using FIRST (Patenaude et al., 2011) and the cortical ROI (bilateral SFG) were generated on the basis of the Harvard-Oxford atlas included in FSL (Woolrich et al., 2009). The normalized predictor estimates were extracted from each ROI (weighted for the probabilistic Harvard-Oxford atlas), per subject, per condition, and averaged over voxels. The resulting values were analyzed using SPSS.
ANALYSES AND RESULTS
Making the standard assumption of rational selfish behavior, with an endowment of €10 per trial, the following mixed strategies for predator [with probability of investing X denoted by p(X)] and prey [with probability of investing Y denoted by p(Y)] define a unique Nash equilibrium for PPG: Predator: p(X = 1) = 2/45, p(X) = p(X – 1)[(12 – X)/(10 – X)] for 2 ≤ X ≤ 6, p(X = 0) = 1 – [p(X = 1) + … +p(X = 6)] = 0.4, and p(X) = 0 for X ≥ 7; Prey: p(Y) = 1/(10 – Y) for 0 ≤ Y ≤ 5, p(Y = 6) = 1 – [p(Y = 0) + … + p(Y = 5)] = 0.15, and p(Y) = 0 for Y ≥ 7. In the PPG, it is collectively irrational and wasteful to invest in either predation or defense because the money involved is lost for both predator and prey. Nevertheless, it is individually rational to invest, as indicated by the Nash-equilibrium. On average, prey is expected to invest 3.38 per trial and predator 2.62. This can be broken down into a prediction for ‘aggression frequency’ (expected number of trials per block in which an investment is made; range: 0–5), which equals 3 for predator and 4.5 for prey, and a prediction for ‘force of aggression’ (expected investment across five trials, with no-investment excluded; range: 1–10), which equals 4.36 for predator and 3.75 for prey.
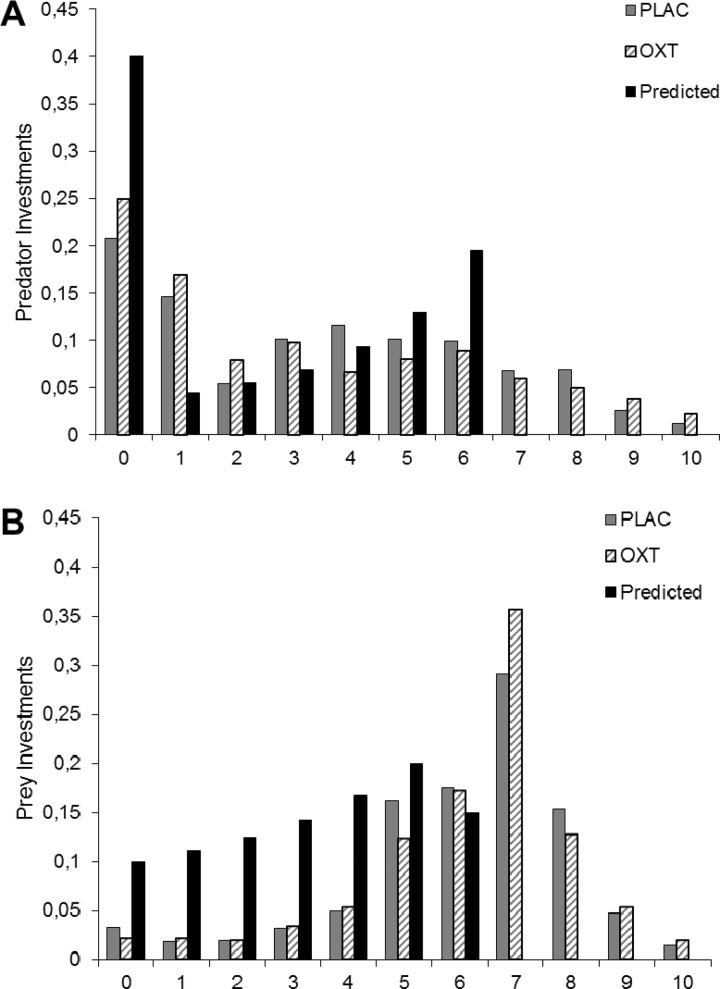
Neither Session (whether placebo was given first or second) nor Trial Block interacted with Treatment and Role (all F < 2.7, all P > 0.18) and we proceeded by collapsing investment decisions across these factors. Figure 2 shows the distribution of investment choices for predator and prey. In line with game-theoretical predictions, we find that participants do invest, that investments are spread and that prey clearly invests more than predator. Unlike these game-theoretical predictions, but fitting regular findings in experimental contests (Abbink, 2012), both predator and prey overinvest: the spread of investment levels frequently exceeds 6, which should never occur theoretically. These results are suggestive for not fully rational but impulsive behavior. Our treatment effect findings, discussed next, are supportive in this respect.
Fig. 2.
Frequency with which investment options were chosen, compared with game-theoretic predictions. (A) Predators aggress more frequently than predicted, and overinvest; (B) Prey aggress more frequently than predicted, and overinvest substantially.
Figure 2 suggests that predators under oxytocin opt for lower investment than predators given placebo, and that prey under oxytocin opt for higher investments than those given placebo. To test this, we first analyzed average investment across trials in a 2 (Role: Predator/Prey) × 2 (Treatment: Oxytocin/Placebo) within-subjects analysis of variance (ANOVA). This revealed no effect for treatment, F(1, 26) = 0.38, P = 0.541, partial η2 = 0.015, a main effect for Role, F(1, 26) = 36.03, P = 0.001, partial η2 = 0.581, and a marginally significant interaction among Role and Treatment, F(1, 26) = 3.48, P = 0.074, partial η2 = 0.118. Means and standard deviations are shown in Table 1. Fitting SAA, oxytocin somewhat reduced overall investment in predators, directional t(26) = 1.50, P = 0.073, partial η2 = 0.080. Oxytocin did not significantly increase investment among prey, t(26) = 0.87, P = 0.392, partial η2 = 0.028.
Table 1.
Means and standard deviations for behavioral responses in predator–prey conflict broken down by role and treatment
| Measure | Predator |
Prey |
||
|---|---|---|---|---|
| Placebo | Oxytocin | Placebo | Oxytocin | |
| Overall investment | 3.504 (2.218) | 3.198 (2.36) | 6.074 (1.397) | 6.21 (0.959) |
| Force of attack | 4.208 (1.752) | 3.924 (1.836) | 6.275 (1.184) | 6.449 (0.853) |
| Frequency of attack | 3.963 (1.407) | 3.753 (1.552) | 4.833 (0.704) | 4.895 (0.304) |
| Decision latency | 4523.52 (1309.68) | 4104.03 (1136.37) | 4215.41 (1511.5) | 4364.04 (1785.9) |
Note: Entries are averages within 5-trial blocks; Standard deviations in brackets (N = 27); Overall Investment ranges between 0 and 10; Force of attack ranges between 1 and 10; Frequency of attack ranges between 0 and 5; Decision Latency ranges between 0 and 6000 ms.
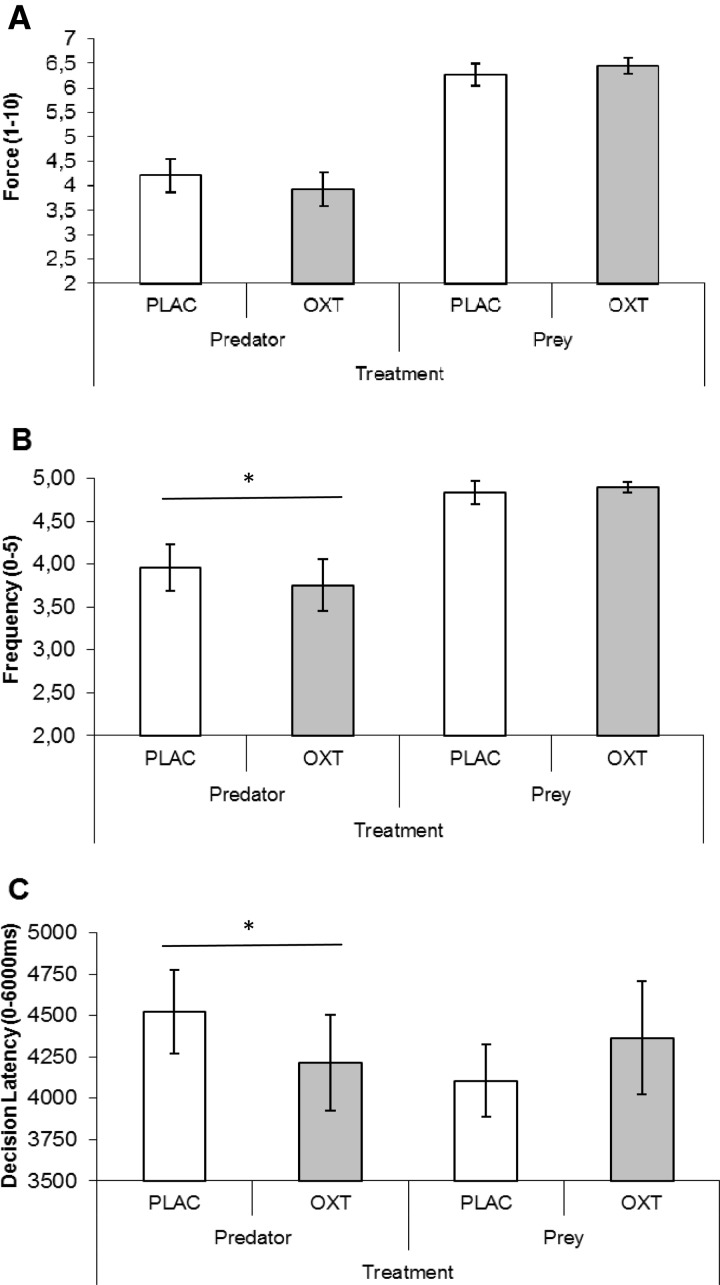
A similar pattern of results was obtained when we analyzed force of aggression (average investment across trials, with no-investment trials excluded; range: 1–10). ANOVA revealed no Treatment effect, F(1, 26) = 0.20, P = 0.650, partial η2 = 0.001, a significant Role effect, F(1, 26) = 37.31, P = 0.001, partial η2 = 0.570, and a marginal Role × Treatment effect, F(1, 26) = 3.91, P = 0.059, partial η2 = 0.11. Mean force patterned as predicted in SAA, and not in FD (see Figure 3A). Compared with placebo, oxytocin reduced force of aggression in predators, directional t(26) = 1.733, P = 0.048, partial η2 = 0.056, and did not significantly increase force of aggression in prey, directional t(26) = 1.243, P = 0.12, partial η2 = 0.056.
Fig. 3.
Aggression in predator and prey as a function of treatment. (A) Force of aggression in predators and prey under oxytocin vs placebo (range: 1–10, displayed ± SE); (B) Compared with placebo, oxytocin reduces aggression frequency of predator but not of prey (range: 0–5, displayed ± SE); (C) Compared with placebo, oxytocin reduces time in milliseconds taken to decide in predators but not in prey (range: 0–6000; displayed ± SE). Contrasts marked with * are significant at P < 0.05 (directional t-tests, with N = 27).
Figure 3B shows aggression frequency (average number of trials within blocks in which investments were made; range: 0–5). A Role (Predator/Prey) × Treatment (Placebo/Oxytocin) within-subjects ANOVA revealed no Treatment effect, F(1, 26) = 1.12, P = 0.300, partial η2 = 0.041, and significant Role, F(1,26) = 10.48, P = 0.003, partial η2 = 0.287, and Role × Treatment effects, F(1, 26) = 4.50, P = 0.044, partial η2 = 0.148. Directional t-tests revealed that compared with placebo, oxytocin reduced aggression frequency among predators, t(26) = 2.035, P = 0.026, partial η2 = 0.137, but did not reduce or increase aggression frequency in prey, t(26) = 0.53, P = 0.478, partial η2 = 0.019. These results fit SAA and were not anticipated by FD.
In addition to investments, we analyzed decision latency. If indeed greed-driven investments among predators are more calculated whereas fear-driven investments among prey are relatively impulsive, the former should take more time (Rand et al., 2012). Furthermore, FD implies that oxytocin slows down decision making among prey because it reduces impulsive fear; SAA, on the other hand, implies that oxytocin accelerates decision making among predators because it promotes pro-social approach and tempers calculated greed. We analyzed decision time averaged across trials in a 2 (Role: Predator/Prey) × 2 (Treatment: Oxytocin/Placebo) ANOVA (observed latencies are shown because log-transformation reduced skewness but did not affect results). This revealed a Treatment × Role interaction, F(1, 26) = 4.26, P = 0.049, partial η2 = 0.141. Directional t-tests show that predators took less time when given oxytocin rather than placebo, t(26) = 1.749, P = 0.046, partial η2 = 0.105 (see Table 1 and Figure 3C); correlational analyses revealed that shorter decision latency tended to associate with frequency of predator attack (r = −0.304, P = 0.12, vs r = −0.254, P = 0.202), but these relationships were not significant. Prey decision latencies were not influenced by treatment, t(26) = 0.16, P = 0.782, partial η2 = 0.003; correlational analyses revealed that decision latency did not associate with reduced frequency of prey defense (r = −0.002, P = 0.991, and r = −0.206, P = 0.31). Results fit with SAA, although they could have been stronger.
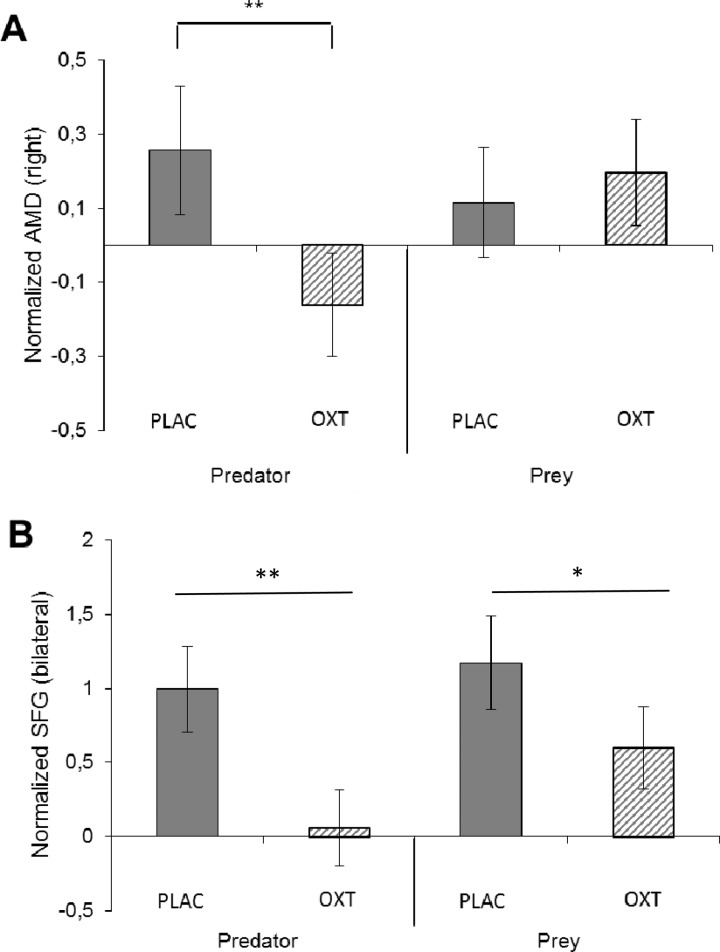
Finally, we considered neural activity in (left and right) amygdala and (bilateral) SFG ROIs during and collapsed across investment decisions. Figure 4A shows right amygdala activation as a function of Treatment × Role, F(1, 26) = 6.35, P = 0.018, partial η2 = 0.196. Oxytocin compared with placebo dampened amygdala in predators, t(26) = 2.48, P = 0.020, partial η2 = 0.202, but not in prey, t(26) = 0.01, P = 0.99, partial η2 = 0.008. This interaction effect was replicated in the left amygdala, F(1, 26) = 5.92, P=0.022, partial η2 = 0.185: oxytocin compared with placebo reduced amygdala activity in predators (M = −0.161 vs M = 0.257; t(26) = 2.57, P = 0.016, partial η2 =0.192), but not in prey (M = 0.197 vs M = 0.116; t(26) = 0.41, P = 0.646, partial η2 = 0.002). These results fail to confirm the FD prediction that oxytocin dampens amygdala among prey; maintained amygdala activation is consistent with SAA. Dampened amygdala among predators given oxytocin was anticipated by neither hypothesis.
Fig. 4.
Activation in amygdala and SFG as a function of treatment and role. (A) Oxytocin dampens right amygdala in predators but not in prey (normalized activity; displayed ± SE); (B) Oxytocin reduces activation in SFG in predators more than in prey (normalized activity; displayed ± SE). Contrasts marked with * (**) are significant at P < 0.05 (P < 0.025) (directional t-tests, with N = 27).
Figure 4B shows activation in the SFG time-locked to investment decisions. Although Role and Role × Treatment effects were not significant, both F(1, 26) < 2.64, Ps > 0.12, the effect for oxytocin was, F(1, 26) = 13.71, P = 0.001, partial η2 = 0.345. As can be seen in Figure 4B, oxytocin compared with placebo reduced activation in the SFG. Figure 4B also shows that this effect of oxytocin was particularly pronounced in predators, t(26) = 4.096, P = 0.001, partial η2 = 0.392 [in prey, t(26) = 2.29, P = 0.030, partial η2 = 0.168]. This effect is consistent with the SAA prediction of tempered calculated greed, and is not anticipated in FD.
Correlational analyses revealed no relationships between neural activity in amygdala or SFG on the one hand, and decision latency on the other (all − 0.11 < r < 0.265, all Ps > 0.190). However, covariance analyses (see also Figure 1) revealed a significant regression of investment decisions in prey on right amygdala, β = 0.412, t = 2.258, P = 0.033; no such relationship emerged for decision making in predators, β = 0.01, t = 0.13, P = 0.989. Oxytocin did not modulate these relations, all Ps > 0.20. Across treatment conditions, predator investments were (marginally) correlated with SFG activation, β = − 0.331, t = −1.756, P = 0.091; no such relationship emerged in prey, β = −0.017, t = −0.284, P = 0.778. These correlations provide some initial evidence that while defensive aggression in prey is conditioned on the (right) amygdala, greed-driven aggression in predators is calculated and conditioned on the SFG.
CONCLUSIONS AND DISCUSSION
Results showed that oxytocin modulates greed-driven predation and leaves unaffected fear-driven prey defense. Compared with placebo, oxytocin reduced investments among predators, reduced the time predators take to decide and dampened activity in both amygdala and SFG. We conclude that predator investment is calculated, and that oxytocin reduces the greedy appetite to accumulate wealth at the expense of others. With regard to prey defense, we observed that oxytocin (compared with placebo) had little effect. Regardless of treatment, prey invested frequently and forcefully, did so relatively fast, and fear-driven prey defense was conditioned upon (right) amygdala. We conclude that prey defense is impulsive rather than calculated, and that oxytocin does not incapacitate such impulsive defense.
From earlier work, we derived two largely competing predictions about the possible effects of oxytocin on cooperative and competitive decision making. The FD hypothesis is grounded in oxytocin’s anxiolytic effects (Kirsch et al., 2005; Baumgartner et al., 2008; Meyer-Lindenberg et al., 2011) and implies effects of oxytocin especially in prey; the SAA hypothesis (Guastella et al., 2008; Kemp and Guastella, 2011; Striepens et al., 2012), in contrast, predicted effects of oxytocin especially in predators. Results fit the latter prediction, and are difficult to reconcile with the FD hypothesis. Furthermore, results fit recent work by Rilling and colleagues (2012) who had participants given oxytocin or placebo making cooperation decisions in prisoner’s dilemma games with partners who cooperated or who did not cooperate. When partners responded with non-cooperation to participant’s cooperation, and thus exploited them, oxytocin did neither increase nor decrease cooperation compared with placebo. As in the current study, oxytocin seems to have no impact on participants seeking to protect themselves against exploitation. Accordingly, we conclude that in social exchange problems oxytocin modulates cooperation and aggressive tendencies because it tempers (calculated) greed more than it mitigates (impulsive) fear of being exploited.
The FD hypothesis derived from oxytocin’s anxiolytic effects (Carter et al., 2008; MacDonald and Feifel, 2014), and especially studies showing oxytocin dampens (amygdala) responding to threatening stimuli and fearful facial expressions (e.g. Kirsch et al., 2005; Petrovic et al., 2008; for a review see Meyer-Lindenberg et al., 2011). The current study allows for the possibility that oxytocin has such anxiolytic effects in these contexts yet highlights that these do not translate into reduced vigilance and readiness to aggress and defend oneself against predation. Accordingly, current results illustrate a broader point, namely that neural responses such as reduced activation in brain regions typically associated with vigilance and threat-detection (e.g. amygdala) not necessarily lead to reduced vigilance and threat-detection, let alone peaceful generosity and defenseless surrender. At best, we would argue, reduced fear-responding at the brain level permits but not necessarily leads to increased pro-social approach.
Although our findings fit the pro-social approach/inhibited avoidance hypothesis better than the FD hypothesis, effect sizes were sometimes small and replication studies would be welcome. New studies could address also whether current findings, obtained with a male sample, extend to female participants as well. Doing so would be important not only because relatively few administration studies include female participants, and recent work suggest oxytocin may have qualitatively different effects on cooperation and related brain circuitries in male compared with female (Rilling et al., 2014). In addition, including females would enable the testing of specific predictions regarding sex differences in aggression (e.g. Archer and Coyne, 2005). An interesting hypothesis, grounded in evolutionary theory, is that female aggression, compared with male aggression, is constrained by the greater centrality of mothers’ to offspring survival, and this resulted in a lower threshold for fear among women (Campbell, 2013). Work in economic decision making indicates that women, compared with men, are more generous and more likely to cooperate (Croson and Gneezy, 2009). In terms of predator–prey contests, this suggests that compared with males observed in the current study, females may be less aggressive in both predator and prey roles.
Some scientists proposed that oxytocin increases social salience, such that what is positive and attractive becomes more positive and attractive under oxytocin while what is negative and aversive becomes more negative and aversive (Shamay-Tsoory et al., 2009; Bartz et al., 2010). Within predator–prey contests, the social-salience hypothesis implies increased fear and defense-related aggression among prey, which we did not find. With regard to predator greed, the social-salience hypotheses is less clear-cut. When we assume that greedy aggression is the default among predators, the social salience hypothesis predicts increased predator aggression under oxytocin, which we clearly did not observe. Because there is no evidence for this assumption, however, the current context neither allows a test of the social-salience hypothesis nor any conclusions about its validity. Future work aimed at discriminating between the SAA hypothesis and the closely related social-salience hypothesis may proceed by identifying individual differences that dictate default tendencies in predators to aggress vs to appease, and among prey to aggress vs to withdraw. Such individual differences should have little effect under the currently supported SAA framework, but should interact with oxytocin under the social-salience prediction.
Two findings regarding oxytocin were not anticipated, and may be specific to the predator–prey conflict examined here. First, we observed reduced activation in the amygdala for predators given oxytocin. Predation is, as argued, relatively instrumental and deliberated, but may not only recruit prefrontal activation related to impulse-control and cost-benefit analyses, but also sub-cortical circuitries involved in threat-detection and emotion-regulation (i.e. for predators the possibility of loss and injury are not at all excluded). Oxytocin in predators lowered decision latency, reduced activation in the SFG and dampened activation in the amygdala. Combined this suggests that oxytocin lowers greedy appetite and therefore the risk of losing out; hence, not only activation in the SFG but also in the amygdala was observed. Second, we observed reduced activation in the SFG for prey given oxytocin; possibly oxytocin reduces impulse-control in both predators and prey, with different consequences—reduced aggression in predators and maintained or even increased aggression in prey. We note, however, that we only obtained correlational evidence supporting the link between SFG and predator aggression.
The PPG enables a decomposition of competitive decision making, and aggression, in terms of its two core motives of greed and fear (Coombs, 1973; Ostrom, 1998; Camerer, 2003). We uncovered that competition is relatively deliberate and calculated when greed-motivated, and relatively impulsive when fear-driven. In addition, we observed that human decision makers switched within a few trials from predator to prey and back, with massive consequences—aggression was up- or downregulated instantly, associated neural circuitries were differentially activated and neurohormonal modulation by oxytocin was flipped between present and absent. The human species operates as both predator and prey, and social contexts may dictate alternating roles; the ability to immerse oneself into either role swiftly and confidently sustains survival as well as prosperity (Sallan et al., 2011). Our data suggest that humans evolved a capacity to compete impulsively as well as deliberately, and that greed rather than fear gives way to the evolutionary ancient oxytocinergic circuitry.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Jolien van Breen and Yara van Someren for collecting data and Jasper Wijnen for computer programming and technical support. Financial support was provided by an internal grant from the Research Priority Area on Brain and Cognition of the University of Amsterdam.
REFERENCES
- Abbink K. The Oxford Handbook of the Economics of Peace and Conflict. Oxford, UK: Oxford University Press; 2012. Laboratory experiments on conflict; pp. 532–53. [Google Scholar]
- Albert DJ, Walsh ML, Jonik RH. Aggression in humans: what is its biological foundation? Neuroscience and Biobehavioral Reviews. 1993;17:405–25. doi: 10.1016/s0149-7634(05)80117-4. [DOI] [PubMed] [Google Scholar]
- Archer J, Coyne SM. An integrated review of indirect, relational, and social aggression. Personality and Social Psychology Review. 2005;9:212–30. doi: 10.1207/s15327957pspr0903_2. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, et al. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21:1426–8. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe R-M, Van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton NJ: Russell Sage; 2003. [Google Scholar]
- Campbell A. The evolutionary psychology of women’s aggression. Philosophical Transactions of the Royal Society Biology. 2013;368:20130078. doi: 10.1098/rstb.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;3:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin, and sociality. Progress in Brain Research. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Carter JR, Anderton CH. An experimental test of a predator-prey model of appropriation. Journal of Economic Behavior and Organization. 2001;45:83–97. [Google Scholar]
- Chaminade T, Marchant JL, Kilner J, Firth CD. An fMRI study of joint action-varying levels of cooperation correlates with activity in control networks. Frontiers in Human Neuroscience. 2012;6:179. doi: 10.3389/fnhum.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macace mulatta) Proceedings of the National Academy of Sciences USA. 2012;109:959–64. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences USA. 2010;107:21773–7. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs CH. A reparametrization of the prisoner's dilemma game. Behavioral Science. 1973;18:424–8. [Google Scholar]
- Croson R, Gneezy U. Gender differences in preferences. Journal of Economic Literature. 2009;47:448–74. [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and Behavior. 2010;57:368–74. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012a;37:871–80. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Hormones and Behavior. 2012b;61:419–28. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Baas M, Roskes M, et al. Oxytonergic circuitry sustains and enables creative cognition in humans. Social Cognitive and Affective Neuroscience. 2014;9:1159–65. doi: 10.1093/scan/nst094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences USA. 2011;108:1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science. 2008;321:1849–52. doi: 10.1126/science.1158860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences USA. 2010;107:3788–99. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–3. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the Rhesus Macaque. Proceedings of the National Academy of Sciences USA. 2013;110:11630–5. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort JJ, van Winden F, Pelloux B, Stallen M, Ridderinkhof KR. Neural correlates of dynamically evolving interpersonal ties predict prosocial behavior. Frontiers in Neuroscience. 2012;6:28. doi: 10.3389/fnins.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HI, Kim M. Predation and accumulation. Journal of Economic Growth. 1996;1:333–50. [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biological Psychiatry. 2008;64:256–8. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Molecular Psychiatry. 2013;18:958–60. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur O, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;12:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PloS ONE. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Weisman O, Ebstein RP, Bornstein G. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology. 2013;37:1341–4. doi: 10.1016/j.psyneuen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: a novel hypothesis. Current Directions in Psychological Science. 2011;20:222–31. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krutschwitz JD, Simmons AN, Flagan T, Paulus MP. Nothing to lose: processing blindness to potential losses drives thrill and adventure seekers. NeuroImage. 2012;59:2850–9. doi: 10.1016/j.neuroimage.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviors. Nature Review Neuroscience. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Research. 2014 doi: 10.1016/j.brainres.2014.01.025. pii: S0006-8993(14)00078-X. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin: social neuropeptides for translational medicine. Nature Neuroscience Review. 2011;12:524–37. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mikolajczak M, Gross JJ, Lane A, Corneille O, de Timary P, Luminet O. Oxytocin makes people trusting, not gullible. Psychological Science. 2010;21:1072–5. doi: 10.1177/0956797610377343. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Review Neuroscience. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Ostrom E. A behavioural approach to the rational choice theory in collective action. American Political Science Review. 1998;92:1–22. [Google Scholar]
- Parr LA, Modi M, Sibert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38:1748–56. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain. NeuroImage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosan M, Baciu M, Cousin E, Perrone M, Pichat C, Bougerol T. An fMRI study of the social competition in healthy subjects. Brain and Cognition. 2011;77:401–11. doi: 10.1016/j.bandc.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Rand DG, Greene JD, Nowak MA. Spontaneous giving and calculated greed. Nature. 2012;489:427–30. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–61. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–48. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences USA. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskes M, Elliot A, Nijstad BA, De Dreu CKW. Avoidance motivation and conservation of energy. Emotion Review. 2013;5:264–8. [Google Scholar]
- Sallan LC, Kammer TW, Ausich WI, Cook LA. Persistent predator-prey dynamics revealed by mass extinction. Proceedings of the National Academy of Sciences USA. 2011;108:8335–8. doi: 10.1073/pnas.1100631108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dyash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biological Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neuroscience and Biobehavioral Reviews. 1999;23:359–98. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Science. 2005;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Striepens N, Scheele D, Kenrick KM, Hurlemann R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings of the National Academy of Sciences USA. 2012;109:18144–9. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Velden FS, Baas M, Shalvi S, Kret ME, De Dreu CKW. Oxytocin differentially modulates competitive approach and withdrawal to antagonists from own versus rivaling other groups. Brain Research. 2013 doi: 10.1016/j.brainres.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Tullock G. Efficient rent seeking. In: Buchanan JM, Tollison RD, Tullock G, editors. Toward a Theory of the Rent-Seeking Society. College Station, TX: Texas AandM University Press; 1980. pp. 97–112. [Google Scholar]
- Verstynen TD, Deshpande V. Using pulse oximetry to account for high and low frequency physiological artifacts in the BOLD signal. NeuroImage. 2011;55:1633–44. doi: 10.1016/j.neuroimage.2010.11.090. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–38. [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]