Abstract
Background
The value of community-based cancer research has long been recognized. In addition to the National Cancer Institute’s Community Clinical and Minority-Based Oncology Programs established in 1983, and 1991 respectively, the National Cancer Institute established the National Cancer Institute Community Cancer Centers Program in 2007 with an aim of enhancing access to high-quality cancer care and clinical research in the community setting where most cancer patients receive their treatment. This article discusses strategies utilized by the National Cancer Institute Community Cancer Centers Program to build research capacity and create a more entrenched culture of research at the community hospitals participating in the program over a 7-year period.
Methods
To facilitate development of a research culture at the community hospitals, the National Cancer Institute Community Cancer Centers Program required leadership or chief executive officer engagement; utilized a collaborative learning structure where best practices, successes, and challenges could be shared; promoted site-to-site mentoring to foster faster learning within and between sites; required research program assessments that spanned clinical trial portfolio, accrual barriers, and outreach; increased identification and use of metrics; and, finally, encouraged research team engagement across hospital departments (navigation, multidisciplinary care, pathology, and disparities) to replace the traditionally siloed approach to clinical trials.
Limitations
The health-care environment is rapidly changing while complexity in research increases. Successful research efforts are impacted by numerous factors (e.g. institutional review board reviews, physician interest, and trial availability). The National Cancer Institute Community Cancer Centers Program sites, as program participants, had access to the required resources and support to develop and implement the strategies described. Metrics are an important component yet often challenging to identify and collect. The model requires a strong emphasis on outreach that challenges hospitals to improve and expand their reach, particularly into underrepresented populations and catchment areas. These efforts build on trust and a referral pipeline within the community which take time and significant commitment to establish.
Conclusion
The National Cancer Institute Community Cancer Centers Program experience provides a relevant model to broadly address creating a culture of research in community hospitals that are increasingly networked via systems and consortiums. The strategies used align well with the National Cancer Institute—American Society of Clinical Oncology Accrual Symposium recommendations for patient-/community-, physician-/provider-, and site-/organizational-level approaches to clinical trials; they helped sites achieve organizational culture shifts that enhanced their cancer research programs. The National Cancer Institute Community Cancer Centers Program hospitals reported that the strategies were challenging to implement yet proved valuable as they provided useful metrics for programmatic assessment, planning, reporting, and growth. While focused on oncology trials, these concepts may be useful within other disease-focused research as well.
Keywords: Clinical trials, community, culture of research, community hospital research, National Cancer Institute Community Cancer Centers Program
Introduction
Building a strong and sustainable clinical research program involves a comprehensive approach that utilizes strategies to gain leadership support, engage stakeholders, promote awareness, understand infrastructure requirements, and develop useful metrics. The ability to open clinical trials (CTs) and enroll study participants requires a committed multidisciplinary team with expertise and knowledge in clinical, ethical, and regulatory arenas. The conduct of clinical research can seem onerous given the regulatory burden alone.1–3 Yet, it is through this research that novel therapies are developed and patient outcomes are improved. In order to successfully establish a research program, a broad approach should be considered, one that infuses an organization with a deep understanding of the value of research and the acknowledgment that CTs are an important option along the cancer care continuum.
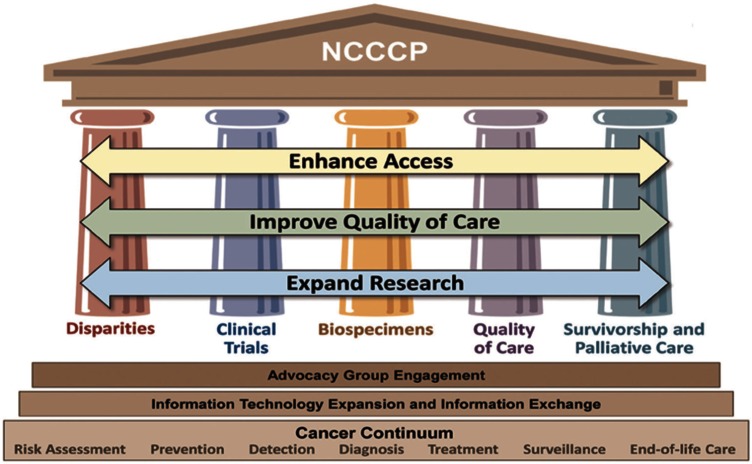
Historically, clinical research trials only took place in academic medical centers. However, in the 1980s, the National Cancer Institute (NCI) Community Clinical Oncology Program (CCOP) and Minority-based CCOP (MB-CCOP) expanded cancer research into the community setting where the majority of cancer patients receive their care.4,5 In 2007, the NCI further expanded its community targeted effort with the NCI Community Cancer Centers Program (NCCCP), a program designed to facilitate patients’ access to quality cancer care and improve the capacity to conduct research at community cancer centers. The overarching goal of the NCCCP initiatives was to address health-care disparities across the full cancer care continuum. Implementation of the program included five “pillars”: Health Disparities, CT, Biospecimen, Quality of Care, and Survivorship and Palliative Care. A graphic representation displays the broad concept of which CT is one pillar (See Figure 1). Over time, numerous “cross-pillar” efforts evolved such as CT and Disparities outreach efforts described in the strategies presented later in the text.6
Figure 1.
NCCCP “pillars.”
NCCCP: National Cancer Institute Community Cancer Centers Program.
Background
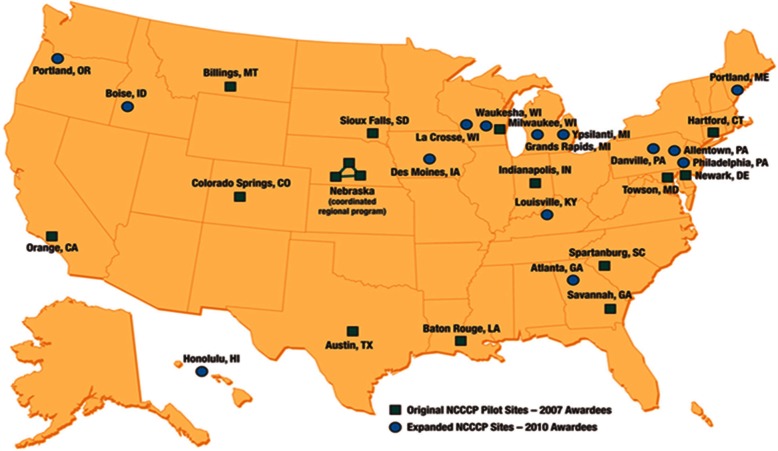
Launched as a pilot program with 16 community hospital–based cancer centers in 2007, the NCCCP added 14 more sites in 2010 (see Figure 2) and had 21 participating centers from 2012 to 2014. For the pilot phase, NCI selected sites with clinical research experience ranging from minimal to extensive, creating a learning collaborative where the more advanced research sites mentored the less experienced programs. The CT pillar initiatives ranged from strategies to assess or overcome accrual barriers, to physician and community engagement tactics, and outcome and metric monitoring in an effort to create real-time interventions directed to improve CT conduct. A subcommittee was formed to operationally support the program’s CT-related objectives, which included development and expansion of research infrastructures and improvement of CT accruals—especially for underserved populations such as racial or ethnic minorities, rurally located patients, and/or the elderly. NCCCP sites were also tasked with broadening trial portfolios to include cancer prevention and control trials as well as potential early phase trials, and documenting CT screening efforts and enrollment barriers.
Figure 2.
NCCCP hospital locations.
NCCCP: National Cancer Institute Community Cancer Centers Program.
The NCCCP sites worked collectively toward achieving program goals, serving as a laboratory for trying new ideas and implementing evidence-based practices. Efforts were also enhanced by knowledge gleaned from the NCI CCOPs and MB-CCOPs and the 2010 NCI and American Society of Clinical Oncology (ASCO) co-sponsored Cancer Trial Accrual Symposium: Science and Solutions.7–9 The symposium examined the state of accrual science by looking at three influential factors related to accrual: site/organizational, physician/provider, and patient/community.10 The NCI advisors to the NCCCP hospitals found this conceptual framework useful in taking a broader view of how to address the program’s CT initiatives related to accrual. Diverging from the traditional (and often failed) tactic of focusing accrual efforts around patient-level recruitment, the NCCCP embraced a more encompassing view that looked across the cancer continuum and at the organizational structure.11,12 This strategy and others described below, and the tools developed within the NCCCP, are shared here in an effort to help other community hospitals learn from this experience.
Four strategies to enhance research culture
Gain executive-level support by engaging senior leadership
The NCCCP was a public–private partnership between NCI and the sites which required chief executive officer (CEO) buy-in demonstrated through a formal letter of support as part of the application process. Also, to promote financial commitment and program sustainability, co-investment through cost sharing was required. Additionally, to foster a greater connection to program goals, CEOs were invited to annual meetings as both attendees and speakers. Sites reported that these strategies not only elevated awareness of the value of a research program to a community hospital but also garnered institutional support for research infrastructure enhancements. Co-investment also promoted financial stewardship and allowed for greater sustainability for research positions and efforts beyond program funding.13
Create a learning collaborative/ mentor sites
The CT Subcommittee implemented five topic-driven workgroups consisting of site members, NCI advisors, and NCI’s program/ project management staff at Leidos Biomedical Research, Inc. to address multiple operational focus areas of CTs in the community setting: (1) CT Best Practices or Infrastructure, (2) Early Phase CTs, (3) CT Portfolio, (4) CT Screening and Accrual Log, and (5) Underserved Accrual. Their efforts and outcomes are described in the “Build infrastructure” section.
A monthly CT Subcommittee call, co-chaired by site members with CT expertise, used a variety of approaches to facilitate continuous and collaborative learning. The five working groups reviewed work progress for the larger membership, thus keeping members informed while avoiding duplication of efforts. Best practice presentations were shared by speakers from the sites, the NCI, and other agencies, such as the Food and Drug Administration, covering an array of topics such as central institutional review boards (IRBs), research department infrastructure, portfolio analysis including trial types and accrual activity, the NCI CT Network (NCTN), and organizational research models.
Quarterly accrual reports were generated and shared using de-identified data, and each site could evaluate their accrual progress in the context of others. Sites also consulted among themselves and those with less developed infrastructures visited stronger research programs to receive more intense mentoring. The sites often identified this learning collaborative environment as a highlight of their progress because it provided them a unique opportunity for accelerated program development and benchmarking.
Build infrastructure
Although sites had varied levels of CT programmatic maturity, enhancing infrastructure was a focus for all sites. The network took a multi-step approach to fostering a research culture that would promote increased patient and provider participation in CTs and expand the types and number of trials each site could open.
Assess the research program
ASCO supported a series of articles addressing minimum standards and exemplary attributes of CT sites.14–16 These articles were used by the CT Subcommittee to conceptually frame and create the “Clinical Trials Assessment of Infrastructure” or “CT AIM” tool to assess site CT infrastructure.† The tool identified nine attributes, each with three assessment levels progressively moving from less to more exemplary CT infrastructure. Examples of attributes in the tool include Physician Engagement, Trial Portfolio Diversity and Management, Education Standards, and Quality Assurance. The process of creating the tool, obtaining feedback on the tool attributes from across the network, and having sites rate themselves over multiple years positively impacted the sites’ research infrastructures.17 The self-assessment raised awareness regarding strengths and opportunities for improvement, served as a communication tool to discuss issues with hospital leadership, and generated strategies to move to the next level. For example, one site’s assessment revealed the lack of a community advisory panel, so they addressed this by establishing a panel to facilitate community input into their hospital research program.
Consider conducting early phase trials
An Early Phase Working Group engaged sites interested in developing infrastructure to activate and conduct early phase trials. Accrual to early phase trials across the network was evaluated, as was the infrastructure to conduct these trials at each of the sites.18 A checklist was created to assist sites in evaluating resources and staff needs for early phase trial implementation.19 Sites with established early phase trial programs were generally those with decades of CT experience and sound infrastructures. These sites mentored other NCCCP sites by presenting their experiences via teleconferences, answering questions, and hosting site visits to facilitate collaborative learning in this arena.
Understand and address barriers to accrual
Collecting and analyzing trial accrual barriers in real time was an essential step toward creating a culture of research. Building upon existing literature and tools, the Screening and Accrual Log working group created the NCCCP CTs Screening and Accrual Log as a mechanism to collect and analyze enrollment barriers across and within sites.20 The information technology platform allowed for a secure, web-based tool that collected the aggregate screening data entered by the sites for selected NCI trials. More than 7000 entries were placed into the Screening Log between 2009 and 2013.21,22 Aggregated data were used to inform research efforts such as the lack of association between race and ethnicity and rates of trial enrollment refusal.21 Also, some barrier data were shared with NCI Cooperative Group investigators to create awareness of enrollment challenges.
Of 21 sites, 20 expanded their data collection to include every cancer trial open at their site, not just the select NCI trials identified for the Log. Sites reported the utility of having screening and barrier information on all trials, including better portfolio awareness and management (see Table 1). The Log Case Report Form (CRF) ultimately became incorporated into several sites’ CT management systems.
Table 1.
Potential site uses for Screening Log Data.
| Adjust trial portfolios | Close slow-accruing trials |
| Open trials to meet patient needs | |
| Consider feasibility of future trials based on past accrual performance | |
| Identify barriers | Transportation |
| Language or translation needs | |
| Primary care providers’ lack of CT knowledge | |
| Report and communicate metrics | Administration |
| Cancer Committee | |
| Steering Committee | |
| Commission on Cancer | |
| Disease-site working groups | |
| Grant writing | |
| Assist with programmatic planning | Assess MD accrual rates |
| Compare staff case loads | |
| Guide funding needs | |
| Track screening versus accrual rates |
CT: clinical trial.
Encourage community and provider CT outreach
In 2010, specific projects were implemented by the Underserved Accrual Working Group to engage physicians and promote a broader understanding of CTs in the community. Particular attention was placed on community providers and racial and ethnic minority and rural populations. Projects included the development of multiple communication and information tools that were shared with the full NCCCP network. Sites customized them based on locally determined needs, for example, choosing education strategies based on the cultural preferences of populations served.
Examples of provider outreach tools included a slide template which was customizable for state cancer rates, pertinent catchment area details (percents of minorities, other demographic data like poverty rate or education level, etc.). This tool also defined trials, types and phase, and randomization and addressed cultural differences that may affect CT participation. Slides were shared at informal “lunch and learns” and were also presented at formal education dinners such as those geared toward primary care physicians. Other outreach strategies included the following: focusing efforts on physicians most likely to refer potential trial patients and highlighting relevant trials; ensuring availability of research nurses to discuss trials and facilitate community physicians’ participation; creating and distributing template educational sheets for complex trials;23 and detailing information about the study’s aim, eligibility requirements, and potential side effects from trial interventions. In addition, sites were encouraged to utilize NCI’s relevant accrual and education related resources available on AccrualNet.24
Through community outreach, the hospitals learned to address barriers to CT enrollment among underserved populations by developing culturally appropriate strategies that fostered trusting relationships with specific communities. For example, one site used a staff liaison to foster relationships with its local Native American community, implemented cancer and CT education activities with the Indian Health Services, and worked with the Tribal IRB. This work led to awareness of competing health needs which were so great that they needed to be addressed before any CT conversation was even appropriate. See Table 2 for outreach examples and proposed metrics; also see the NCCCP Template for Community Outreach25 developed through the Disparities Subcommittee of NCCCP.26
Table 2.
NCCCP outreach efforts and proposed metrics.
| Focus area | Examples of efforts | Potential metrics |
|---|---|---|
| Improve physician/community outreach related to clinical trials (CTs) | Share best practices for how to reach out to local providers
Create templates for CT talks and highlight site trials relevant to practice (e.g. prostate trials for urology outreach) |
Number of referrals from primary care physicians (PCPs)
Referrals from other health-care providers (nurse practitioners (NPs)/physician assistants (PAs), specialists) Track distance traveled by referred patients |
| Explore use of technology to increase accrual of underrepresented populations to CTs | Virtual tumor boards
Virtual phase 1 clinics Skype or telemedicine |
Number of sites actively working toward or implementing technology to address underserved accrual
Number of PCPs involved in cancer conferences Number of underrepresented patients served by virtual technologies Volume of virtual efforts and outcomes |
| Improve navigator–CT team coordination to increase referrals of underrepresented populations to CTs | Navigators attend weekly multidisciplinary conference (MDC) presentations
Navigators educate patients about CTs |
Number of navigator referrals to CT research team
Number of referrals on screening log increase by __X_% Number of patients enrolled from navigator referral (i.e. navigator educated patient regarding availability of CTs and referred to CTs) |
| CT patient mentor program | Mentors with other language as first or native language who are past or current CT participants speak with potential CT patients about their experience | Track number of mentors matched with a patient
Track accrual for these patients |
| CT translation | Translate “short form” for top 2–3 languages at site | Number of consents translated and approved for use
Number of patient interactions related to translation and consent Number of non-English-speaking patients consented |
| Lodging and transportation | Provide daily transport van for rural patients receiving radiation
Provide gas cards Partner with local hotel for reduced patient rate Partner with American Cancer Society (ACS) |
Number of van rides provided
Downstream revenue from patients brought to hospital (e.g. CT scans and treatments) Distance of enrolled trial patients and volume of transportation support given Number of combined partner services with ACS or other community partners |
NCCCP: National Cancer Institute Community Cancer Centers Program.
While obtaining consistent data and establishing systems to track outreach activities remained challenging, over time, sites became aware of the need for early identification of metrics and the complexities and benefits of capturing this type of data for broader use such as for support for an outreach coordinator position or to report to the hospital Cancer Committee. Many sites refined the way they collected this type of information in order to support site-specific priorities and strategic plans: an example involved tracking outreach to and accrual from underrepresented catchment areas to support a van for radiation patient transportation from distant, low-income communities and then monitor downstream revenue related to these patients’ needs (see Table 2 for other metric ideas).
Collaborate beyond CTs to promote a culture of research
Often community hospitals have distinct departments that address pieces of the research process (e.g. community outreach offices, navigation programs, screening, and prevention clinics or staff). The NCCCP’s goal to work and build research capacity across the full continuum of cancer care and its underlying context of “pillars” led to significant collaborative interactions and decreased silos between departments within the participating hospitals as described below (see Figure 1).
Quality of care: multidisciplinary care and navigation
The NCCCP Quality of Care pillar included a focus on the concepts of multidisciplinary care conference teams and navigation. Viewed as an essential component of CT promotion and accrual, the multidisciplinary care conference process can be useful in creating a culture of research as it provides a venue for increasing awareness, highlighting trials, identifying eligible patients, and seeing the gaps where there are no available trials for local populations.27,28 Each site was required to formally assess its structure, function, and operation according to standards prescribed by the NCCCP-developed Multidisciplinary Care Assessment Tool.29 Levels in the CT category ranged from “evolving” (patient not screened for CT eligibility and no CT literature provided to patient) to “achieving excellence” (CT staff reviews all eligible charts and engages care coordinators and treating physicians prior to initial treatment).30
One key facet of multidisciplinary care is an engaged team with strong physician leadership committed to CTs in cancer care and treatment. With this paradigm, CT screening, eligibility, and accrual become a formalized, embedded function of the patient treatment planning process, as opposed to an ad hoc or cursory review of patient eligibility by independent clinicians. Sites encouraged primary care and other outside practitioners to attend multidisciplinary care conferences by giving continuing medical education credits for attendance and promoting “virtual” attendance, meaning practitioners could hear their patient presented without leaving their offices.
Multidisciplinary care experience within the NCCCP also identified navigation as key to promoting the culture of research as the patient moves through the cancer continuum.31 The nurse navigator was encouraged to actively participate in patient education and referrals to appropriate CT team members. A Navigation Assessment Tool32 was developed within the Navigation Working Group that included a component for CTs. At the highest level, this component states, “Navigator engages with research team, assists with specific trial referrals for underserved populations.” Sites reported that this increased collaboration between navigation and the research team positively impacted their CT programs.33 An engaged multidisciplinary care team that includes a patient navigator knowledgeable in CTs can facilitate the patient experience from the initial diagnosis, through treatment and survivorship phases, again supporting a culture of clinical research reaching beyond just the CT team in an institution.
Biospecimens
With the shift toward CTs involving molecularly targeted therapies and biospecimen collection, sites were encouraged to understand the necessary infrastructure requirements to collect high-quality biospecimens. Sites worked to implement the NCI Best Practices for Biospecimen Resources 34 which lists competencies such as (1) biospecimen consenting, annotating, collecting, processing, storing, and distributing; (2) quality assurance and quality control; (3) biosafety; and more. An NCCCP-developed Gap and Fill Assessment Tool tracked sites’ challenges and solutions associated with implementing the NCI Best Practices relevant to locally determined needs. Completing the tool engendered cross-department collaboration between oncology research, information technology, and pathology. This exercise became a roadmap for improving biospecimen collection and storage capabilities that are essential to the NCI research enterprise.35 With this expanded capacity, some NCCCP sites opened an onsite biorepository, and more than one-third met the high selection standards for participation in The Cancer Genome Atlas Project.
Disparities
Given the NCCCP’s overarching goal to reduce cancer health-care disparities, sites placed a particular emphasis on strategies to increase their underrepresented populations’ access to cancer education, screening, and potential accrual to CTs. To do this, the sites completed a Minority Matrix, a tool to assess factors related to accruing their community’s underrepresented population(s) to CTs.36 This was accomplished by using a strengths-weaknesses-opportunities-and-threats analysis of the following areas: information tracking systems, institution infrastructure, research infrastructure, minority navigator and personnel programs, CT education, accrual barriers, strategies to improve trial accrual, internal resources, interpreters and translation service, and community partnerships and patient advocates relevant to their selected population of focus.37 The sites shared their completed matrices to mentor and learn from one another, particularly about strategies for populations they had in common.
Cultural awareness webinars were developed in collaboration with NCI Community Network Program centers to discuss variations among different minority populations in their perceptions of CTs and to offer strategies for addressing myths and enhancing accrual within these populations. Sites expanded the use of consent short forms in different languages to cater to non-English-speaking patients interested in CTs.38 In addition, navigation programs were expanded at many sites to include outreach navigators and continuum of care nurse navigators to address disparate populations. The sites also began to track race and ethnicity information in their CTs database following the Office of Management and Budget guidelines; while challenging to implement, over 3 years, the original 16 sites improved from 16% to 100% compliance tracking.39
Limitations
In the current health-care environment, with its complexities and competing demands, implementing a culture of research is challenging. Extensive communication with each of the NCCCP hospitals’ leadership has revealed that the program’s strategies and tools contributed to more rapid progress and made a positive impact on their research infrastructure, yet the accompanying reality is that the strategies described in this article required resources, support, and time to germinate before changes in outcome were observed. The NCCCP developed over 7 years, providing many sites time to see change. The sites also had federal resource support–both financially and via NCI advisors unique to this program.
The model’s strong emphasis on outreach challenged sites to move beyond their local referral patterns. Hospitals wishing to use these strategies must also consider options to access populations beyond their usual catchment areas. Philosophical shifts to make clinical research a priority in an institution occur slowly. Building trust and rapport with the community, assessing practice patterns, creating referral pipelines, and most appropriately addressing the needs of local underserved populations can often take years to translate into significant changes. That said, an external evaluation of the NCCCP pilot phase (2007–2010), reported improved accrual, expanded CT portfolios, and improved participation of underrepresented populations in CTs over the 3 years.40
How program efforts will be sustained beyond the NCCCP funding is unknown, which may be a limitation of translating this model to other community hospital–based cancer centers. While several sites reported to NCI that some positions will be decreased or eliminated after NCCCP, they also indicated that most of the initiatives are now embedded into the culture of their research programs and will continue. This sustainability is attributed to the broader leadership engagement and buy-in that occurred at the outset of participation in the NCCCP and the program’s required co-investment feature.13
It is also critical to acknowledge that patient accrual is impacted by many factors, including trial availability, staffing, interested physicians, IRB ability to open trials, pharmacy support, and institutional support to name a few, so it is difficult to attribute shifts to a specific intervention. Rather, enhancing accrual requires a persistent and cumulative effort that touches many points across an institution and its staff to create a culture where research is valued and is prioritized. While not easy, one signal that this has been achieved is when discussing or thinking about a patient’s CT options is more the first thought than the last.41–43
Finally, the NCCCP CT experience confirmed the importance as well as the challenges of capturing tailored metrics that measure overall CT impact: quality of care as reflected in numbers and types of multidisciplinary conferences; prospective versus retrospective case reviews; CT/Navigation collaborations and referrals; and physician engagement, community physician outreach, underrepresented community outreach, trial portfolio diversity and management, biospecimen infrastructure, and screening and accrual tracking were all sought after as part of the program metrics and processes. Sites reported that determining, capturing, and reporting these metrics evolved over time. Areas of success provided critical data to evaluate return on investment and that data, despite the challenges, became extremely valuable in the current evidence-driven research and health-care environment.44
Conclusion
As more community hospitals move from single institutions to becoming part of larger health systems and consortiums, the NCCCP experience provides a relevant model for broadly addressing the culture of research across sites that are connected or “networked” in some way. While focused on oncology trials, the authors believe that these concepts may apply to other disease entities as well. CTs are critical to scientific discoveries, medical innovations, and improving the lives and health outcomes of patients.45 Building a robust clinical research program, particularly in the community setting where most cancer patients are treated, is a crucial component of this effort. Sustaining it through creative executive management support and public–private partnerships is also a consideration in the current changing and fiscally challenging health-care environment.13
Strategies used in the NCCCP program align well with the NCI/ASCO Accrual Symposium recommendations for patient/community, physician/provider, and site/organizational level approaches which indeed underpinned many of the initiatives that the sites used to create a research culture at the participating hospitals.10 In addition, while establishing goals at the beginning of a program, it is also important to consider defining metrics upfront that will be used to measure progress (Tables 2 and 3).
Table 3.
NCCCP strategies aligned with NCI/ASCO Symposium recommendations.10
| Site/organizational (system)-level centered | Physician/provider-level centered | Patient/community-level centered |
|---|---|---|
| CEO or leadership buy-in and support of CTs
OMB guided race and ethnicity data capture Clinical Trial Assessment of Infrastructure Matrix Tool use for program assessment Weekly research activities (e.g. multidisciplinary conferences, tumor boards, and research staff meetings which include navigators) Employee education on CTs (e.g. CT 101), disparities, and cultural competence for nonresearch staff Dedicated CT positions for trial implementation with ongoing workforce assessment of trial workload |
Multidisciplinary approach—cancer conferences with CT screening of patients
Clinical research team interface with patient navigation—involvement of navigators in CT education and screening Community physician office visits to discuss open trials and research program Virtual and telemedicine for rural patient screening and follow-up for trials Routine CT portfolio assessment Physician leadership involvement in local, regional, and/or national meetings or societies |
Understanding the underrepresented population (e.g. rural, minority, low SES, elderly, and AYA) in site’s catchment area
Increasing outreach activities and building relationships that include cancer prevention, education, and screening events, as well as accrual promotion Improving CT access (e.g. virtual MDC, transportation voucher and remote telemedicine) Utilizing a CT Screening and Accrual Log to assess enrollment barriers Developing community partnerships (e.g. with faith-based organizations and NCI-sponsored Community Network Partners) |
|
Cross cutting strategies:
Consent translation or interpreter services for relevant populations CT communications/marketing CT education across the cancer care continuum | ||
NCCCP: National Cancer Institute Community Cancer Centers Program; NCI/ASCO: National Cancer Institute/American Society of Clinical Oncology; CT: clinical trial; CEO: chief executive officer; SES: socioeconomic status; OMB: Office of Management and Budget; AYA: adolescent and young adult.
The strategies presented in this article helped NCCCP sites achieve organizational culture shifts that ultimately enhanced their cancer research programs. The sites moved their programs forward faster by garnering executive-level support, sharing best practices, addressing challenges among clinicians and staff—both locally and across the network—assessing their programs to prioritize improvements, and interacting across different hospital departments that traditionally operated in silos. Implementing specific strategies allowed NCCCP sites to create oncology business plans that recognize and support CT infrastructure. Becoming aware of and better defining metrics promoted focus on measuring the overall benefits of a strong clinical research program. The ability to capture, report, and evaluate metrics provided necessary data that help sustain program activities. Additionally, the focused commitment to cancer research became a model for research programs in other disease entities within some of the hospitals. We share the strategies and tools from the NCCCP to stimulate collaborative learning and to give other community cancer centers a framework for creating a culture of research that promotes both the value of research and its sustainability.
Acknowledgments
The authors are grateful to all sites that participated in the NCCCP: Ascension Health sites (Brackenridge Hospital, Columbia St. Mary’s, and St. Vincent), Billings Clinic, Catholic Health Initiatives sites (Good Samaritan, Penrose-St. Francis Health Services, St. Elizabeth Regional Medical Center, St. Francis Medical Center, and St. Joseph Medical Center), Helen F. Graham Cancer Center at Christiana Care, Einstein Healthcare Network, Geisinger Medical Center, Gundersen Health System, Hartford Hospital, Lehigh Valley Health Network, Maine Medical Center, Mercy Medical Center-Des Moines, Northside Hospital, Norton Cancer Institute, Our Lady of the Lake Regional Medical Center, Providence Portland Medical Center, Saint Mary’s Health Care, Sanford USD Medical Center, Spartanburg Regional Healthcare System, St. Joseph Health, St. Joseph Mercy Hospital, St. Joseph’s/Candler, St. Luke’s Regional Medical Center, The Queen’s Medical Center, and Waukesha Memorial Hospital. In addition, the authors wish to thank the NCCCP Program Advisory Committee (NPAC) members for their leadership and the Leidos Biomedical Research team for their tireless dedication and superb project support.
Footnotes
Declaration of conflicting interests: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Funding: This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health (Contract No. HHSN261200800001E).
Work on the tool created and used by the National Cancer Institute Community Cancer Centers Program (NCCCP) sites continued with a formative evaluation. Based on the evaluation, which included input from multiple stakeholders in the community oncology setting, the “CT AIM” tool was recently revised. The process and revisions of the infrastructure assessment tool were presented at the 2014 American Society of Clinical Oncology (ASCO) Quality of Care Symposium, and a publication about the revised tool is near submission.
References
- 1. McMahon AD, Conway DI, Macdonald TM, et al. The unintended consequences of clinical trials regulations. PLoS Med 2009; 3: e1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burman W, Daum R. Grinding to a halt: the effects of the increasing regulatory burden on research and quality improvement efforts. Clin Infect Dis 2009; 49: 328–335. [DOI] [PubMed] [Google Scholar]
- 3. Reith C, Landray M, Devereaux PJ, et al. Randomized clinical trials—removing unnecessary obstacles. N Engl J Med 2013; 369: 1061–1065. [DOI] [PubMed] [Google Scholar]
- 4. Warnecke RB, Johnson TP, Kaluzny AD, et al. The Community Clinical Oncology Program: its effect on clinical practice. Jt Comm J Qual Improv 1995; 21: 336–339. [DOI] [PubMed] [Google Scholar]
- 5. Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer 2010; 116: 4440–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson MR, Clauser SB, Beveridge JM, et al. Translating scientific advances into the community setting: the National Cancer Institute Community Cancer Centers Program pilot. Oncology Issues, May-Jun 2009, pp. 24–28. [Google Scholar]
- 7. Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care 2011; 49: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCaskill-Stevens W, McKinney MM, Whitman CG, et al. Increasing minority participation in cancer clinical trials: the Minority-based Community Clinical Oncology Program experience. J Clin Oncol 2005; 23: 5247–5254. [DOI] [PubMed] [Google Scholar]
- 9. McKinney MM, Morrissey JP, Kaluzny AD. Interorganizational exchanges as performance markers in a community cancer network. Health Serv Res 1993; 28: 459–478. [PMC free article] [PubMed] [Google Scholar]
- 10. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract 2013; 9: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wujcik D, Blakeney N, Michaels M. Making a difference in cancer trials accrual: the integration of pre-screening all patients for eligibility in oncology studies. Applied Clinical Trials Online 2014; 23: 24. [Google Scholar]
- 12. ENACCT. A quality improvement program to improve cancer clinical trial recruitment, accrual, and retention: lessons learned from the National Clinical Trials Pilot Breakthrough Collaborative. Bethesda, MD: Education Network to Advance Cancer Clinical Trials, 2012. [Google Scholar]
- 13. O’Brien DM, Kaluzny AD. The role of a public-private partnership: translating science to improve cancer care in the community. J Healthc Manag 2014; 59: 17–29. [PubMed] [Google Scholar]
- 14. Zon R, Meropol NJ, Catalano RB, et al. American Society of Clinical Oncology Statement on minimum standards and exemplary attributes of clinical trial sites. J Clin Oncol 2008; 26: 2562–2567. [DOI] [PubMed] [Google Scholar]
- 15. Baer AR, Cohen G, Smith DA, et al. Implementing clinical trials: a review of the attributes of exemplary clinical trial sites. J Oncol Pract 2010; 6: 328–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zon R, Cohen G, Smith DA, et al. Part 2: implementing clinical trials: a review of the attributes of exemplary clinical trial sites. J Oncol Pract 2011; 7: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dimond EP, Zon R, St. Germain D, et al. The clinical trial assessment of infrastructure matrix tool (CT AIM) to improve the quality of research conduct in the community. In: ASCO annual meeting, Chicago, IL, 31 May 2014, Abstract 6512 (J Clin Oncol 32: 5s). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaren HA, Nair S, Go RS, et al. Early-phase clinical trials in the community: results from the National Cancer Institute Community Cancer Centers Program early-phase working group baseline assessment. J Oncol Pract 2013; 9: e55–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NCCCP Early Phase Working Group. Early phase trial feasibility checklist, http://ncccp.cancer.gov/files/EarlyPhase_Feasibility_Checklist_20120409.pdf
- 20. St. Germain D, Denicoff AM, Dimond EP, et al. Use of the National Cancer Institute Community Cancer Centers Program screening and accrual log to address cancer clinical trial accrual. J Oncol Pract 2014; 10: e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langford AT, Resnicow K, Dimond EP, et al. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer 2014; 120: 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez MM, Berger M, Brown T, et al. Using an online tool to understand and improve clinical trial accruals. Oncology Issues (Association of Community Cancer Centers), Mar-Apr 2011, pp. 50–55. [Google Scholar]
- 23. St Joseph Hospital trial sheet: The Center for Cancer Prevention and Treatment, http://ncccp.cancer.gov/files/Provider_Outreach_TrialFS_508Comp_20140905.pdf
- 24. NCI. AccrualNet, https://accrualnet.cancer.gov/
- 25. NCCCP. Template for community outreach, http://ncccp.cancer.gov/files/NCCCP-Template-for-Community-Outreach.pdf
- 26. Katurakes N, Hood D, Berger M, et al. How NCCCP outreach efforts help reduce cancer disparities. Oncology Issues (Association of Community Cancer Centers), Jul-Aug 2011, pp. 40–45. [Google Scholar]
- 27. McNair AG, Choh CT, Metcalfe C, et al. Maximizing recruitment into randomised controlled trials: the role of multidisciplinary cancer teams. Eur J Cancer 2008; 44: 2623–2626. [DOI] [PubMed] [Google Scholar]
- 28. Kuroki L, Stuckey A, Hirway P, et al. Addressing clinical trials: can the multidisciplinary tumor board improve participation? A study from an academic women’s cancer program. Gynecol Oncol 2010; 116: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swanson PL, Strusowski P, Asfeldt T, et al. Expanding multidisciplinary care in community cancer centers. Oncology Issues, Jan-Feb 2011, pp. 33–37. [Google Scholar]
- 30. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute Community Cancer Centers Program. J Oncol Pract. Epub ahead of print 21 October 2014. DOI: 10.1200/JOP.2014.001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swanson J, Strusowski P, Mack N, et al. Growing a navigation program: using the NCCCP navigation assessment tool. Oncology Issues, Jul-Aug 2012, pp. 36–45. [Google Scholar]
- 32. NCCCP. NCCCP Navigation Assessment Tool, http://ncccp.cancer.gov/files/NavAssessTool_508Comp_20130318-2.pdf
- 33. St Germain D, Dimond EP, Olesen K, et al. The NCCCP patient navigation project: using patient navigators to enhance clinical trial education and promote accrual. Oncology Issues, May-Jun 2014, pp. 44–53. [Google Scholar]
- 34. NCI Biorepositories and Biospecimen Research Branch. NCI best practices for biospecimen resources, 2011, http://biospecimens.cancer.gov/practices/
- 35. Berger M, Christinson J, Gansauer L, et al. NCCCP biospecimen initiatives: bringing research advances to the community setting. Oncology Issues (Association of Community Cancer Centers), Nov-Dec 2011, pp. 32–44. [Google Scholar]
- 36. NCCCP. Minority Rural Matrix Template, http://ncccp.cancer.gov/files/NCCCP-Minority-Rural-Matrix-Template_508Comp_20140605.pdf
- 37. Gonzalez MM, Berger M, Bryant DM, et al. Using a minority matrix and patient navigation to improve accrual to clinical trials. Oncology Issues (Association of Community Cancer Centers), Mar-Apr 2011, pp. 59–60. [Google Scholar]
- 38. US Department of Health & Human Services. Office for Human Research Protections (OHRP), http://www.hhs.gov/ohrp/policy/ic-non-e.html
- 39. Office of Management and Budget. Standards for the classification of federal data on race and ethnicity, http://www.whitehouse.gov/omb/fedreg_1997standards
- 40. Abernethy AP, Locke S. The relationship between participation in the National Cancer Institute’s Community Cancer Centers Program (NCCCP) and Clinical Trial Activity, 2012, http://ncccp.cancer.gov/files/Clinical-Trials-Analysis-Report.pdf
- 41. Carson W, Thomas J, Hofacker J, et al. A comprehensive program for the enhancement of accrual to therapeutic clinical trials. NCI ASCO cancer trial accrual symposium: science and solutions 2010. Bethesda, MD: ASCO University, 2011. [Google Scholar]
- 42. Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst 2011; 103: 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minasian LM, O’Mara AM. Accrual to clinical trials: let’s look at the physicians. J Natl Cancer Inst 2011; 103: 357–358. [DOI] [PubMed] [Google Scholar]
- 44. Holcombe RF. Cancer clinical research: return on investment in the era of value-based purchasing. J Oncol Pract 2014; 10: 327–328. [DOI] [PubMed] [Google Scholar]
- 45. Institute of Medicine (IOM). A national cancer clinical trials system for the 21st Century: reinvigorating the NCI cooperative group program (ed Nass SJ, Moses HL, Mendelsohn J.). Washington, DC: IOM, 2010. [PubMed] [Google Scholar]