Highlights
-
•
CARD16 is the most abundant CARD-only protein in hematopoietic cells.
-
•
Unlike CARD17, CARD16 oligomerizes to form a filament-like structure.
-
•
The filament-like structure formed by CARD16 promotes caspase 1 (CASP1) assembly.
-
•
CASP1-dependent IL-1β processing is enhanced by CARD16.
-
•
CARD16 colocalizes in ASC-speck by interacting with ASC.
Abbreviations: ANOVA, analysis of variance; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; Bcl10, B-cell lymphoma/leukemia 10; BS3, bis(sulfosccinimidyl)suberate; CARD, caspase recruitment domain; CARMA1, CARD-membrane-associated guanylate kinase 1; COPs, CARD-only proteins; CASP1, caspase-1; ELISA, enzyme-linked immunosorbent assay; FCS, fetal calf serum; IL, interleukin; LPS, lipopolysaccharide; LRRs, leucine-rich repeats; NLRs, nucleotide-binding domain leucine-rich repeat containing receptors; NF-κB, nuclear factor kappa beta; PYD, pyrin domain
Keywords: Caspase, Cytokine, Inflammation, Interleukin
Abstract
Increasing evidence indicates that caspase recruitment domain (CARD)-mediated caspase-1 (CASP1) assembly is an essential process for its activation and subsequent interleukin (IL)-1β release, leading to the initiation of inflammation. Both CARD16 and CARD17 were previously reported as inhibitory homologs of CASP1; however, their molecular function remains unclear. Here, we identified that oligomerization activity allows CARD16 to function as a CASP1 activator. We investigated the molecular characteristics of CARD16 and CARD17 in transiently transfected HeLa cells. Although both CARD16 and CARD17 interacted with CASP1CARD, only CARD16 formed a homo-oligomer. Oligomerized CARD16 formed a filament-like structure with CASP1CARD and a speck with apoptosis-associated speck-like protein containing a CARD. A filament-like structure formed by CARD16 promoted CASP1 filament assembly and IL-1β release. In contrast, CARD17 did not form a homo-oligomer or filaments and inhibited CASP1-dependent IL-1β release. Mutated CARD16D27G, mimicking the CARD17 amino acid sequence, formed a homo-oligomer but failed to form a filament-like structure. Consequently, CARD16D27G weakly promoted CASP1 filament assembly and subsequent IL-1β release. These results suggest that oligomerized CARD16 promotes CARD-mediated molecular assembly and CASP1 activation.
1. Introduction
Activation of caspase-1 (CASP1) and subsequent processing of interleukin (IL)-1β are essential for initiation of the inflammatory response. In this process, large molecular complexes, known as the inflammasomes, serve as platforms for CASP1 activation [1,2]. Several types of inflammasomes have been reported and typically contain one of the NLR family proteins, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and cysteine protease CASP1. Among the inflammasomes, the NLRP3 inflammasome is the most extensively studied; it recognizes danger signals and induces the sterile inflammatory response in various diseases. NLRP3 is composed of C-terminal leucine-rich repeats (LRRs), a central nucleotide domain termed the NACHT domain, and an N-terminal effector domain [pyrin domain (PYD)] [2]. ASC contains an N-terminal PYD and a C-terminal caspase recruitment domain (CARD) [3]. CASP1 consists of a CARD and catalytic domains (p10 and p20) [4,5]. Notably, each inflammasome component possesses oligomerization activity. NLRP3 homo-oligomerizes via its NACHT domain when stimulated by danger signals and the NLRP3 PYD homotypically interacts with that of ASC after which CARD of ASC recruits and binds to CASP1. These interactions finally assemble the NLRP3 inflammasome, leading to the formation of the active CASP1 tetramer, which processes pro-IL-1β into its biologically active mature form. Interestingly, both PYD and CARD of ASC were originally identified as the domains that oligomerize to form filament-like aggregates [3]. Although their physiological relevance remains unclear, a recent investigation revealed that both PYD and CARD of ASC and CASP1 form filaments and that the endogenous NLRP3 inflammasome forms a large filamentous complex [6].
NLRP3 inflammasomes are activated by various endogenous danger signals such as extracellular adenosine triphosphate (ATP), monosodium urate, and cholesterol crystals and induce IL-1β release and subsequent inflammatory responses that contribute to disease development [7–9]. We previously demonstrated the importance of the NLRP3 inflammasome in the pathophysiology of myocardial ischemia–reperfusion, vascular injury, abdominal aneurysm, and chronic kidney disease [10–13]. Furthermore, recent clinical studies have demonstrated the therapeutic effects of blocking IL-1 in several types of sterile inflammatory diseases [14,15].
Several CARD-only proteins (COPs) are CASP1 CARD homologs. Of these, previous investigations suggested that CARD16 (also known as pseudo-ICE), CARD17 (also known as INCA), and CARD18 (also known as Iceberg) can function as negative regulators of CASP1 activity by binding the CARD of CASP1 [16–19]. All of these COPs are located on chromosome 11q22.3 and share highly conserved amino acid sequences with CASP1 [20]. CARD16 consists of 97 amino acids and shows 92% amino acid identity with the CARD of CASP1. CARD17 also shares 81% sequence identity with the CARD of CASP1. In contrast, CARD18 is less similar to CASP1 than CARD16 and has 53% identity with the CARD of CASP1. These COPs are not present in the rodent genome but are present in the human genome, suggestive of the complexity of CASP1 regulation in humans. Although the CARD interaction is essential for the inflammasome assembly, the functional difference among these COPs remains unclear. In the present study, we investigated the molecular characteristics of CARD16 and CARD17 with respect to the recruitment and activation of CASP1.
2. Materials and methods
2.1. Plasmids
The polymerase chain reaction (PCR)-generated cDNAs encoding human CARD16, CARD17, CASP1, CASP1CARD (1–102), ASC, ASCPYD (1–100), and ASCCARD (101–195) were subcloned into the N-terminal 3 × Flag-tagged pCDNA3.1 vector or N-terminal Myc-tagged pMG2 vector. Enzymatically inactive CASP1C285A, mutated CARD16D27G, and mutated CARD16R45C were generated using the PrimeSTAR Mutagenesis Basal kit (Takara Bio, Shiga, Japan).
2.2. In silico analysis of promoter region
The promoter region of human CASP1 (2000 base pairs upstream of the initiation codon) was compared with that of CARD16, CARD17, and CARD18 using a BLAST algorithm (http://blast.ncbi.nlm.nih.gov).
2.3. Isolation of human PBMCs and cell culture
HEK-293T and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Wako, Osaka, Japan) supplemented with 10% fetal calf serum (FCS) and antibiotics. THP-1 cells, HL60 cells, and human PBMCs were cultured in RPMI1640 (Sigma, St Louis, MO, USA) supplemented with 10% FCS and antibiotics. PBMCs were isolated from five healthy male volunteers (25–40 years old) with Ficoll-Paque PLUS (GE Healthcare, Little Chalfont, UK). This study protocol was approved by the ethical committee of Jichi Medical University and all subjects provided written informed consent. THP-1 macrophages were differentiated with 200 nM PMA for 24 h. Transfection was performed using Lipofectamine 2000 (Life Technologies, Grand Island, NY, USA) according to manufacturer’s protocol or PEI MAX (Polysciences, Warrington, PA, USA) as described previously [21].
2.4. Reverse transcription and real-time PCR
Total RNA was prepared using the ISOGEN system (Nippon Gene Co., Tokyo, Japan) according to the manufacturer’s instructions. Total RNA was reverse transcribed using a Super Script VILO cDNA Synthesis kit (Life Technologies). Real-time PCR were performed using SYBR Premix Ex Taq II (Takara Bio). The primers used in the assay were as follows: CASP1 (forward, 5′-GAAGCTCAAAGGATATGGAAACAAA-3′; reverse, 5′-AAGACGTGTGCGGCTTGACT-3′), CARD16 (forward, 5′-TGCTCCCCTTGCATAAAGGA-3′; reverse, 5′-CCAGTTTGCAACTCTTTACCTAAACC-3′), CARD17 (forward, 5′ CTTCCTTCCTAGGTTCAACTTTCATT-3′, Reverse; 5′-GTGCTGGGCATCTGTGCTT-3′), CARD18 (forward, 5′–AAGATGGGTTTGCACTAAGAGAGAA-3′; reverse, 5′- TGGAAGAAGCTCTGGGAAGTCT-3′), and ACTB (forward, 5′- GGCACTCTTCCAGCCTTCCTTC-3′; reverse, 5′-GCGGATGTCCACGTCACACTTCA-3′). A dilution series of the pCR2.1 plasmid encoding the target sequence was used as the standard for absolute quantification.
2.5. IL-1β measurement
The IL-1β level was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (R&D Systems, Minneapolis, MN, USA). The supernatants were precipitated with ice-cold acetone and resolved in 1 × Laemmli buffer for western blot analysis.
2.6. In vitro protein interaction assays
HeLa cells in 12-well culture plates were transfected with 1.6 μg of the indicated plasmids for the co-immunoprecipitation assay. After 24 h, they were lysed in Nonidet P (NP)-40 buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 1% NP-40) supplemented with a protease inhibitor cocktail (Sigma). After centrifugation, the supernatants were subjected to immunoprecipitation, and the precipitated proteins were analyzed as the insoluble fraction. The supernatants were immunoprecipitated using specific antibodies in combination with protein G or protein A-Sepharose (GE Healthcare). HeLa cells were lysed in cross-linking buffer (20 mM phosphate buffer, pH 8.0, 150 mM NaCl, and 1% NP-40) for the cross-linking assay. After centrifugation, the supernatants were placed on ice with 2 mM bis(sulfosccinimidyl)suberate (BS3) for 2 h, and the crosslinking reaction was terminated by adding an excess of glycine.
2.7. Western blot analysis
Samples were separated by SDS–PAGE and transferred to PVDF membranes. After blocking with Tris-buffered saline containing 2% casein, the membranes were incubated with the following primary antibodies: anti-β actin monoclonal antibody (Ab) (clone AC-15; Sigma), anti-Flag monoclonal Ab (clone M2; Sigma), anti-IL-1β polyclonal Ab (H153; Santa Cruz Biotechnology, Dallas, TX, USA), anti-Myc polyclonal Ab (MBL, Nagoya, Japan).
2.8. Immunocyotochemistry
Cells were cultured in 8-well slide glass chambers and fixed with 10% neutral buffered formalin for 10 min at room temperature, and then permeabilized with PBS containing 0.1% Triton X-100. After blocking with PBS containing 3% bovine serum albumin, the slides were incubated with primary antibodies for 1 h. The following antibodies were used: anti-human ASC polyclonal Ab (Enzo Life Sciences), anti-Flag M2 monoclonal Ab (Sigma), and anti-Myc polyclonal Ab (MBL). Unbound antibodies were washed with PBS and the slides were incubated with the following secondary antibodies: Alexa Fluor 488 donkey anti-rabbit IgG, Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 594 donkey anti-rabbit IgG, and Alexa Fluor 594 goat anti-mouse IgG (Life Technologies). Nuclei were co-stained with Hoechst33342 and fluorescence was detected using confocal laser scanning microscopy (Fv10i, Olympus).
2.9. Statistical analysis
Data are expressed as mean ± standard error. Differences between multiple group means were determined by one-way analysis of variance (ANOVA) combined with the Turkey–Kramer test. All analyses were performed using the Stat Plus, ver. 2009 (Analyst Soft). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. CARD16 and CARD17 expression in human hematopoietic cells
We first compared the CASP1 and COPs promoter regions and found that the CASP1 promoter regions are conserved within CARD16 and CARD17 but not within CARD18. In silico analyses showed that CASP1 and CARD16 or CARD17 promoter regions share 83.8% or 80.2% sequences homology in the 1314 bp or 1334 bp upstream of the initiation codon, respectively. The CASP1 and COP expression profiles were analyzed to investigate the most abundantly expressed COPs in human hematopoietic cells. Although both CARD16 and CARD17 possessed similar promoter regions, only CARD16 exhibited comparable expression levels to CASP1 in PBMCs (Fig. 1). The CARD17 and CARD18 expression levels were considerably lower than that of CARD16 in other human hematopoietic cell lines such as HL-60 and THP-1. In particular, CARD18 expression was not detectable in HL-60 cells.
Fig. 1.
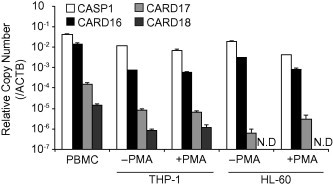
Expression of CARD16 and CARD17 in human hematopoietic cells. Absolute quantification of COP expression in PBMCs, THP-1 monocytes, PMA-differentiated THP-1 macrophages, HL-60 cells, and PMA-differentiated HL-60 cells. The relative copy number of each gene was calculated using a plasmid encoding their target sequence. Data are mean ± standard error. N.D. indicates that no gene expression was not detected.
3.2. CARD16 forms high-molecular weight complexes with CASP1CARD
Homo-oligomerization of CARD is required for CASP1 activation. Thus, we next compared the molecular characteristics of CARD16 and CARD17. We examined their oligomerization activity in HeLa cells, which have lower expression levels of these genes than hematopoietic cells. We transfected plasmids containing the CARD domains of CASP1 (CASP1CARD), CARD16, and CARD17 into HeLa cells, and analyzed their activity using a cross-linking assay. As expected, the formation of the CASP1CARD oligomer was clearly detected (Fig. 2A). Similarly, CARD16 oligomerization was apparently detected, whereas CARD17 did not form any oligomers. We subsequently transfected Flag-tagged and Myc-tagged CASP1CARD, CARD16 or CARD17 to confirm whether a homomeric interaction was responsible for the formation of the oligomer and conducted a co-immunoprecipitation assay. Both CASP1CARD and CARD16 were co-immunoprecipitated by their homo-interactions, but Myc-tagged CARD17 was not co-immunoprecipitated by Flag-tagged CARD17 (Fig. 2B). We assessed intracellular localization to address how these homomeric complexes are formed in cells. Interestingly, both CASP1CARD and CARD16 formed filament-like structures (Fig. 2C). Besides the homomeric interaction, the interaction between FlagCASP1CARD and MycCARD16 or MycCARD17 was assessed by co-immunoprecipitation assay. Substantial quantities of MycCASP1CARD and MycCARD16 were co-immunoprecipitated with FlagCASP1CARD, whereas MycCARD17 was weakly co-immunoprecipitated with FlagCASP1CARD (Fig. 2D). In contrast, a similar quantity of FlagCASP1CARD was detected in each co-immunoprecipitated fraction when the immunoprecipitation was performed using anti-Myc antibody (Fig. 2E). The quantities of immunoprecipitated proteins likely reflected the degree of polymerization; that is, polymerized CARD16 was co-immunoprecipitated with CASP1CARD, but homo-oligomerization defective CARD17 was less precipitated. In accordance with the co-immunoprecipitation results, CARD16 formed a filament-like structure that co-localized with filaments of CASP1CARD (Fig. 2F). In contrast, CARD17 abrogated CASP1CARD filament formation suggesting that the inhibitory effect of CARD17 on CASP1 activation is mediated by inhibiting its homo-oligomerization.
Fig. 2.
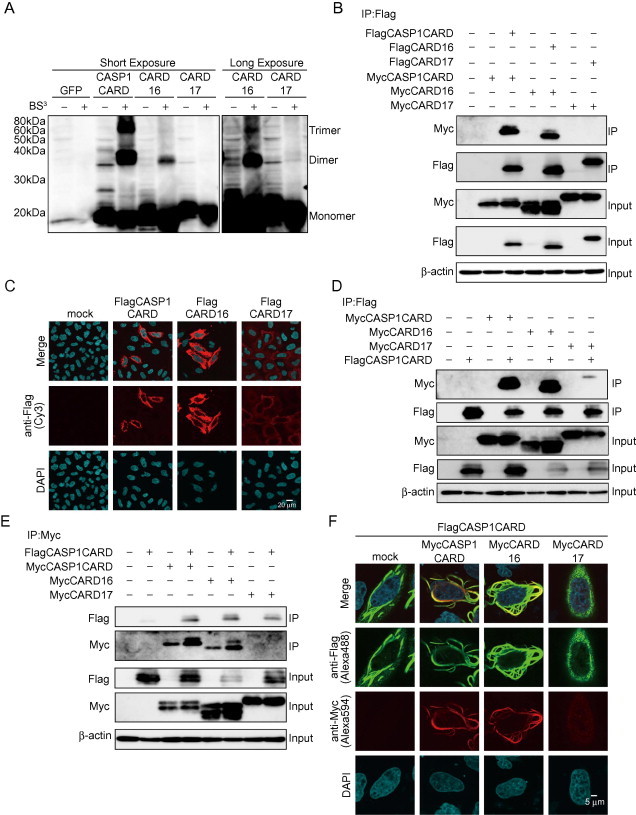
CARD16 forms high-molecular weight complexes with CASP1CARD. HeLa cells were transiently transfected with plasmids encoding FlagCASP1CARD, FlagCARD16, and FlagCARD17. (A) Cell lysates were cross-linked with BS3, then separated by SDS–PAGE and visualized by Western blot. (B) HeLa cells were transiently transfected with plasmids encoding FlagCASP1CARD together with MycCASP1CARD, FlagCARD16 together with MycCARD16, or FlagCARD17 together with MycCARD17. Cell lysates were immunoprecipitated with anti-Flag antibody and detected by Western blot analysis. (C) HeLa cells were transiently transfected with plasmids encoding FlagCASP1CARD, FlagCARD16, or FlagCARD17. Cells were immunostained with anti-Flag antibody. (D and E) The interactions between CASP1CARD and CARD-containing proteins were detected by co-immunoprecipitation. HeLa cells were transiently transfected with FlagCASP1CARD together with MycCASP1CARD, MycCARD16, or MycCARD17. Cell lysates were immunoprecipitated with (D) anti-Flag antibody or (E) anti Myc antibody and detected by Western blot analysis. (F) Co-localization of CASP1CARD and CARD-containing proteins was analyzed by immunocytochemistry. Transfected Hela cells were immunostained with anti-Flag and anti-Myc antibodies.
3.3. CARD16 interacts with ASC and localizes in ASC speck aggregates
Since ASC also contains CARD, we assessed the interaction between CARD16 and ASCCARD. As reported previously [22], ASC forms insoluble high-molecular weight complexes through polymerization, even in the presence of detergents when expressed transiently (Fig. 3A). In addition, CASP1CARD and CARD16 were also detected in the insoluble fraction probably because of its polymerization activity. Therefore, we constructed a plasmid encoding only PYD of ASC (ASCPYD) or CARD of ASC (ASCCARD) to avoid the formation of insoluble complexes. A co-immunoprecipitation assay in transfected HeLa cells revealed that ASCCARD interacted with CARD16 but not with CARD17 (Fig. 3B and C). In contrast, ASCPYD did not interact with CARD16 or CARD17. Immunocytochemistry showed co-localization of CARD16 in ASC-speck (Fig. 3D). CARD16 formed a filament-like structure that co-localized with ASCARD filaments but not with that of ASCPYD (Fig. 3E and F). Therefore, it was likely that the localization of CARD16 in ASC-speck was mediated through co-polymerizing with ASCCARD. In support of these results, Western blot analysis showed that both CARD16 and ASC were detected in the insoluble fraction (Fig. 3A). Conversely, CARD17 failed to co-localize with ASC-speck (Fig. 3D).
Fig. 3.
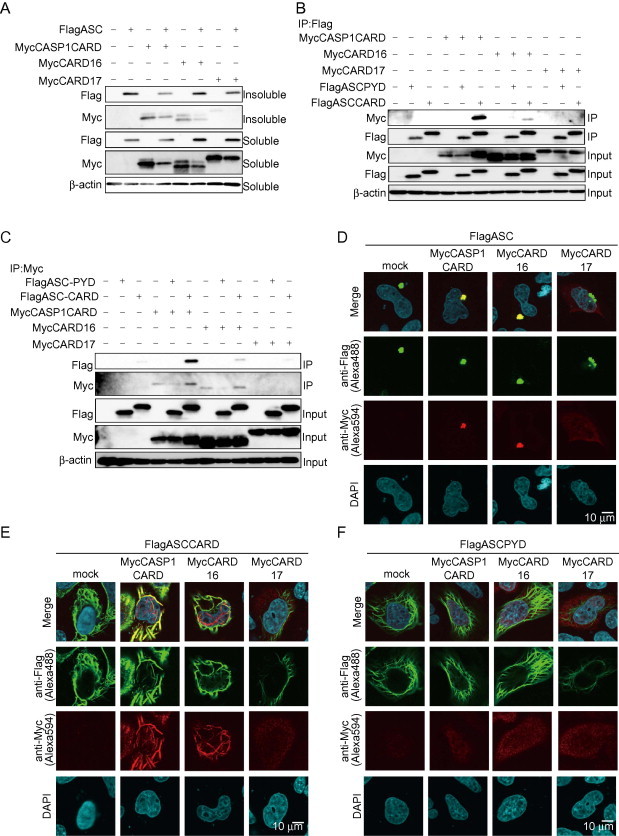
CARD16 localizes in ASC-Speck. (A) HeLa cells were transiently transfected with plasmids encoding FlagASC together with MycCASP1CARD, MycCARD16, or MycCARD17. Cell lysates and the insoluble fraction were analyzed by Western blotting. (B, C) HeLa cells were transiently transfected with plasmids encoding MycCASP1CARD, MycCARD16, or MycCARD17 together with FlagASCCARD or FlagASCPYD. Cell lysates were immunoprecipitated with (B) anti-Flag antibody or (C) anti-Myc antibody and detected by Western blot analysis. (D–F) Co-localization of CARD-containing proteins was analyzed by immunocytochemistry. HeLa cells were transiently transfected with (D) FlagASC, or (E) FlagASCCARD, or (F) Flag ASCPYD together with MycCASP1CARD, MycCARD16, or MycCARD17. Cells were immunostained with anti-Flag and anti-Myc antibodies.
3.4. CARD16 promotes assembly of CASP1 filaments
CARD16 interacts with CASP1CARD and forms a filament-like structure. Thus, we hypothesized that CARD16 promotes CASP1 assembly. To test this hypothesis, CASP1C285A, a mutant deficient in protease activity, was used to avoid the induction of auto-catalytic activation. We analyzed CASP1C285A assembly by immunocytochemistry in transiently transfected HeLa cells. CASP1C285A was dispersed throughout the cells when it was expressed alone (Fig. 4A). Notably, CASP1C285A formed a filament-like structure that co-localized with CARD16 but not with CARD17 when it was co-transfected with CARD16 or CARD17 (Fig. 4A). Supporting this result, Western blot analysis revealed that both CASP1C285A and CARD16 were detected in the insoluble fraction (Fig. 4B). These results suggest that CARD16 promotes CASP1 filament assembly. CASP1, CARD16, and IL-1β were co-expressed in HeLa cells to assess whether the assembled CASP1 and CARD16 complex is functional. Indeed, co-expression of CARD16 enhanced CASP-1-mediated IL-1β release, whereas CARD17 reduced it (Fig. 4C). Notably, a similar change in CASP1-dependent mature IL-1β release was detected in supernatants by Western blot analysis (Fig. 4D, 1.6-fold change in CARD16 co-expressing cells and 0.76-fold change in CARD17 co-expressing cells). Furthermore, we investigated whether CARD16 or CARD17 affects the interaction between CASP1 and ASC. As expected, CASP1C285A co-localized with ASC-speck when CASP1C285A was co-expressed with ASC (Fig. 4E). The co-expression of CARD16 retained CASP1C285A in ASC-speck. In contrast, the co-expression of CARD17 reduced CASP1C285A localized in the ASC-speck and increased dispersed CASP1C285A. In fact, CARD17 reduced the formation of insoluble CASP1C285A when it was co-expressed with ASC (Fig. 4F). These results suggest that CARD16 could participate in the formation of ASC-speck with CASP1C285A, whereas CARD17 prevents CASP1 co-localization with ASC speck.
Fig. 4.
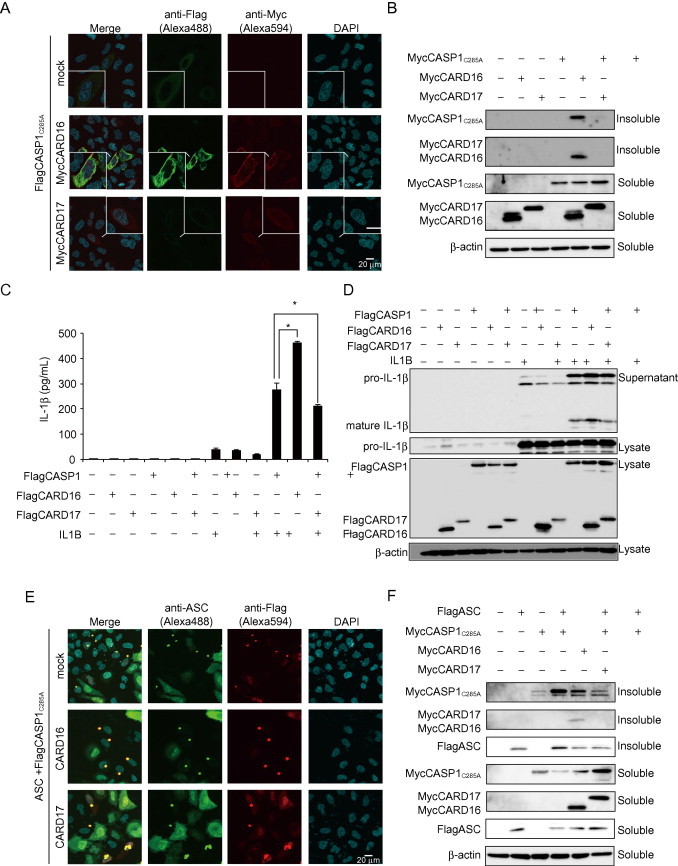
CARD16 recruits CASP1 to its filament-like structure. (A) Assembly of CASP1C285A was analyzed by immunocytochemistry. HeLa cells were transiently transfected with FlagCASP1C285A together with MycCARD16 or MycCARD17. After 24 h, the cells were immunostained with anti-Flag and anti-Myc antibodies. (B) Formation of the insoluble fraction was analyzed by Western blot analysis. HeLa cells were transiently transfected with MycCASP1C285A together with MycCARD16 and MycCARD17. Cells were lysed with 1%NP lysis buffer, and the insoluble fraction was analyzed. (C and D) IL-1β secretion from transiently expressing Hela cells. HeLa cells were transiently transfected with IL1B, FlagCASP1, Flag CARD16, and FlagCARD17. IL-1β levels in the supernatant were determined by ELISA (C) and Western blot analysis (D). (E) Co-localization of CASP1 with ASC in the presence of CARD16 or CARD17 was analyzed by immunocytochemistry. HeLa cells were transiently transfected with FlagCASP1C285A together with ASC and MycCARD16 or MycCARD17. Cells were immunostained with anti-Flag and anti-ASC antibodies. (F) Formation of the insoluble fraction by CASP1 and ASC in the presence of CARD16 or CARD17. HeLa cells were transiently transfected with MycCASP1C285A together with FlagASC, MycCARD16, or MycCARD17. The insoluble fraction was analyzed by Western blot analysis. Data are mean ± standard error. *P < 0.05.
3.5. Filament formation is required for CARD16-induced CASP1 filament assembly
We attempted to generate a CARD16 mutant that did not form a filament to investigate whether the formation of the CARD16 filament is required for CARD16-induced CASP1 assembly. We generated two CARD16 mutants mimicking CARD17 using alignment analysis (Fig. 5A). Amino acids Asp-27 and Arg-45 are critical for the interaction between CASP1 and other CARD-containing proteins [23]. Immunocytochemistry revealed that no filaments formed in CARD16D27G-expressing HeLa cells, whereas they were formed weakly in CARD16R45C-expressing cells (Fig. 5B). However, CARD16D27G preserved its homo-oligomerization activity (Fig. 5C). CARD16D27G was not co-localized with the CASP1CARD filament-like structure (Fig. 6A), although CARD16D27G interacted with CASP1CARD (Fig. 6B). We transfected CARD16, CARD16D27G, and CARD16R45C into HeLa cells and examined their localization by immunocytochemistry to determine whether CARD16D27G lacks the ability to assemble CASP1. As expected, CARD16D27G failed to induce filamentous CASP1 assembly (Fig. 6C). In support of this result, the formation of insoluble CASP1C285A was prevented when it was co-expressed with CARD16D27G (Fig. 6D). Finally, we examined whether CARD16 mutants enhance CASP1-dependent IL-1β release and confirmed that both CARD16D27G and CARD16R45C weakly promoted IL-1β release compared to CARD16 (Fig. 6E and F).
Fig. 5.
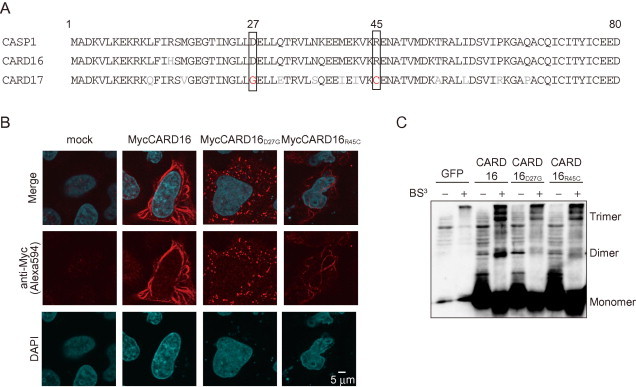
Generation of a non-filament forming CARD16 mutant. (A) Alignment of CASP1CARD, CARD16, and CARD17. CARD16 point mutations were made to mimic CARD17. (B) HeLa cells were transiently transfected with MycCARD16, MycCARD16D27G, or MycCARD16R45C. After 24 h, the cells were immunostained with anti-Myc antibody. (C) HeLa cells were transiently transfected with FlagCARD16, FlagCARD16D27G, or FlagCARD16R45C. Cell lysates were cross-linked with BS3 and analyzed by Western blot analysis.
Fig. 6.
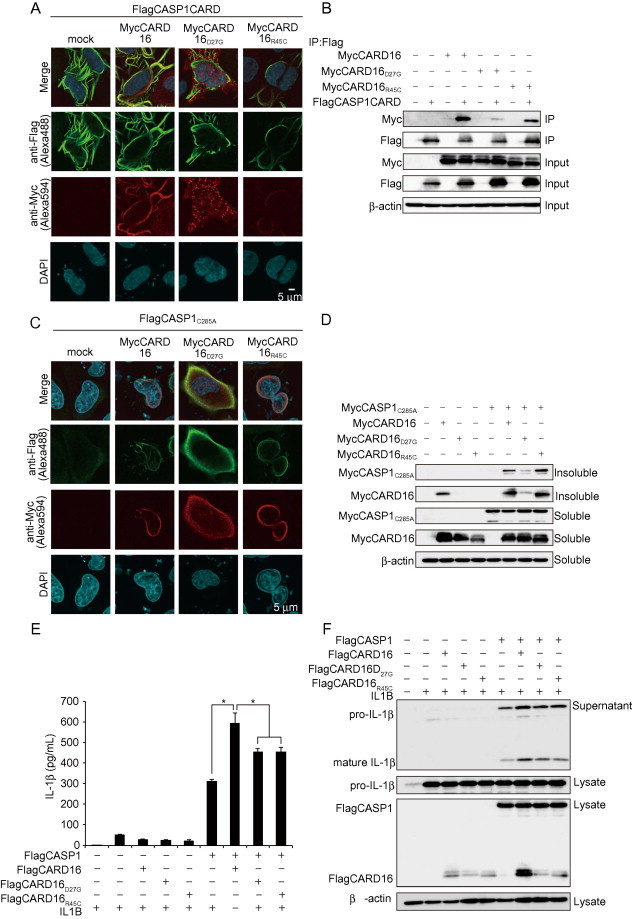
Non-filament forming CARD16 mutant disrupts CASP1 filament assembly. (A) Localization of CARD16 mutants and CASP1CARD. HeLa cells were transiently transfected with FlagCASP1CARD together with MycCARD16, MycCARD16D27G, or MycCARD16R45C. After 24 h, cells were immunostained with anti-Flag and anti-Myc antibodies. (B) Interaction between CARD16 mutants and CASP1CARD. HeLa cells were transiently transfected with plasmids encoding MycCARD16, Myc CARD16D27G, or CARD16R45C together with FlagCASP1CARD. Cell lysates were immunoprecipitated with anti-Flag antibody. (C) The ability of CASP1C285A to form filaments was analyzed by immunocyotochemistry. HeLa cells were transiently transfected with FlagCASP1C285A together with MycCARD16, MycCARD16D27G, or MycCARD16R45C. After 24 h, the cells were co-stained with anti-Flag and anti-Myc antibodies. (D) Formation of the insoluble fraction was analyzed by Western blot analysis. HeLa cells were transiently transfected with MycCASP1C285A together with MycCARD16, MycCARD16D27G, or MycCARD16R45C. Cells were lysed with 1%NP lysis buffer and the insoluble fraction was analyzed by Western blot analysis. (E and F) IL-1β secretion from transiently expressed Hela cells. HeLa cells were transiently transfected with IL1B, FlagCASP1, Flag CARD16, FlagCARD16D27G, or FlagCARD16R45C. IL-1β levels in the supernatant were determined by ELISA (E) and Western blot analysis (F). Data are mean ± standard error. *P < 0.05.
4. Discussion
In the present study, we found that CARD16 and CARD17, but not CARD18, have a conserved promoter region with CASP1. The CARD16 expression level was comparable to that of CASP1. In contrast, the CARD17 and CARD18 expression levels were considerably lower than that of CASP1. Molecular characteristics analyses revealed that CARD16 demonstrated potent oligomerization activity, whereas CARD17 failed to form a homo-oligomer. CARD16 clearly formed a large filament-like structure, indicating a highly polymerized molecular complex. CARD16 promoted a filamentous assembly of CASP1C285A, which lacks autocatalytic activity. Moreover CARD16 enhanced CASP1-dependent IL-1β release. Furthermore, the CARD16 mutants with attenuated oligomerization activity weakly promoted IL-1β release. These results indicate that CARD16 and CARD17 have different roles in CARD-mediated molecular assembly and CASP1 activation and provide new insights into the molecular mechanism underlying the regulation of the inflammasomes.
Although COPs are reportedly CASP1 inhibitors, the most abundant COPs in human hematopoietic cells have not been determined. We determined that CARD16 and CARD17 shared a similar promoter region with CASP1, but no homology was detected in the CARD18 promoter region. In addition, a comparison of their expression levels revealed that CARD16 was the most abundant COP in hematopoietic cells. The CARD17 and CARD18 expression levels were considerably lower than that of CASP1. Therefore, we assumed that CARD16 may contribute to regulating CASP1 in hematopoietic cells.
We clarified two functional differences between CARD16 and CARD17: an interaction with ASC and oligomerization activity. Previous studies reported the interaction between CARD16 or CARD17 and CASP1. However, their interaction with ASC remains to be determined. We clearly showed that CARD16 interacts with ASC via CARD–CARD interactions and co-localizes with ASC-speck. Although the precise role of CARD16 in ASC-speck was not elucidated in this study, CARD16 could not prevent CASP1 co-localization with ASC-speck. Another remarkable difference between CARD16 and CARD17 is their oligomerization activity. Cross-linking and immunocytochemical analyses revealed that CARD16 not only formed an oligomer but also formed large filament-like structures with CASP1. One study demonstrated that both PYD and CARD in the inflammasomes can form filaments [6]. Activated NLRP3 nucleates the PYD filaments of ASC, which cluster ASCCARD. The clustered ASCCARD causes the filamentous assembly of CASP1. By analogy, we postulated that CARD16-mediated filament formation is critical for CASP1 filament assembly. In support of this finding, the CARD16D27G mutant, which lacks the activity to form filaments, assembled CASP1 filaments less effectively compared with that of CARD16. The significance of CARD-mediated filament formation has been investigated in other CARD-containing proteins. For example, Qiao et al. [24] recently reported that the filament formation by B-cell lymphoma/leukemia 10 (Bcl10), CARD-membrane-associated guanylate kinase 1(CARMA1), and Malt1 is necessary to form the CARMA1 signalosome, which induces nuclear factor kappa beta (NF-κB) activation [24,25]. Since CARD16 interacts with Bcl10 [18], it may be possible that the filament formed by CARD16 is also involved in other inflammatory processes. Unlike CARD16, CARD17 inhibited both filamentous assembly and ASC-mediated assembly of CASP1. The reason for the distinct functions of CARD16 and CARD17 may depend on the lower homology between CASP1 and CARD17. The defective homo-oligomerization activity may permit CARD17 to inhibit CASP1. Although there is no direct evidence, it is plausible that CARD17 abrogates the filamentous assembly of CASP1 by terminating polymerization.
Previous investigations suggested that COPs such as CARD16, CARD17, and CARD18 act as endogenous inhibitors of CASP1 activation [16–19]. However, we found that CARD16 clearly promoted CASP1 filament assembly and subsequent IL-1β release. Although the reason for the discrepancy between previous studies and our study is currently unclear, it could be based on differences in experimental conditions. In previous reports, lipopolysaccharide (LPS) was used to induce IL-1β release, and the IL-1β levels were measured only by ELISA, which detects both pro-IL-1β and mature IL-1β [17–19]. Therefore, the effects of COPs on IL-1β processing were not determined. As LPS up-regulates IL-1β at the transcriptional level, it cannot be ruled out that CARD16 may be involved in transcriptional regulation of IL-1β. In addition, CARD16 is also involved in RIP2-mediated NF-κB induction [17–19]. Further investigations are needed to clarify the role of CARD16 in transcriptional and post-transcriptional regulation of IL-1β.
In conclusion, functional differences between CARD16 and CARD17 may be explained by their oligomerization activity; CARD16 assists with CASP1 assembly, resulting in CASP1 activation and subsequent IL-1β release. However, several limitations should be noted. First, because CARD16 and CARD17 are present in humans, but not in rodents, loss-of-function studies are limited. Second, because the molecular characteristics of COPs were mainly analyzed in HeLa cells, the exact role of endogenous CARD16 in hematopoietic cells remains unclear. Further studies are needed to clarify the precise roles of CARD16 and CARD17 in physiological and/or pathological inflammatory processes.
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Acknowledgments
We are grateful to Masako Sakurai and Yumi Ohde for their technical assistance and Dr. Tadashi Kasahara for invaluable suggestions. This study received Grants from the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for Next Generation World-Leading Researchers (NEXT Program),” initiated by the Council for Science and Technology Policy (CSTP) (M.T.), the MEXT-supported program for the Strategic Foundation at Private Universities (M.T.), a Grant-in-Aid for Research Activity Start up, (T.K.), the Japan Heart Foundation Young Investigator’s Research Grant (T.K.), and Banyu Life Science Foundation International (T.K.). T.K. and M.T. designed the experiments; T.K., A.K., K.S., Y.I., T.K., M.K., and Y.M. performed the experiments; T.K. and A.K. contributed reagents and other essential material; F.U., H.K., K.S., and J.S. analyzed data; T.K. wrote the manuscript; and M.T. critically revised the manuscript.
Contributor Information
Tadayoshi Karasawa, Email: tdys.karasawa@jichi.ac.jp.
Masafumi Takahashi, Email: masafumi2@jichi.ac.jp.
References
- 1.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Masumoto J., Taniguchi S., Sagara J. Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem. Biophys. Res. Commun. 2001;280:652–655. doi: 10.1006/bbrc.2000.4190. [DOI] [PubMed] [Google Scholar]
- 4.Cerretti D.P. Molecular cloning of the interleukin-1β converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry N.A. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 6.Lu A. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariathasan S. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 9.Duewell P. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yajima N. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation. 2008;117:3079–3087. doi: 10.1161/CIRCULATIONAHA.107.746453. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi M. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 12.Usui F. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015;35:127–136. doi: 10.1161/ATVBAHA.114.303763. [DOI] [PubMed] [Google Scholar]
- 13.Komada T. ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am. J. Pathol. 2014;184:1287–1298. doi: 10.1016/j.ajpath.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Larsen C.M., Faulenbach M., Vaag A., Volund A., Ehses J.A., Seifert B., Mandrup-Poulsen T., Donath M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 15.So A. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–3076. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 16.Humke E.W., Shriver S.K., Starovasnik M.A., Fairbrother W.J., Dixit V.M. ICEBERG: a novel inhibitor of interleukin-1β generation. Cell. 2000;103:99–111. doi: 10.1016/s0092-8674(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 17.Druilhe A., Srinivasula S.M., Razmara M., Ahmad M., Alnemri E.S. Regulation of IL-1β generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 2001;8:649–657. doi: 10.1038/sj.cdd.4400881. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.H., Stehlik C., Reed J.C. Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J. Biol. Chem. 2001;276:34495–34500. doi: 10.1074/jbc.M101415200. [DOI] [PubMed] [Google Scholar]
- 19.Lamkanfi M., Denecker G., Kalai M., D’Hondt K., Meeus A., Declercq W., Saelens X., Vandenabeele P. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1β generation. J. Biol. Chem. 2004;279:51729–51738. doi: 10.1074/jbc.M407891200. [DOI] [PubMed] [Google Scholar]
- 20.Stehlik C., Dorfleutner A. COPs and POPs: modulators of inflammasome activity. J. Immunol. 2007;179:7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed S.E., Staley E.M., Mayginnes J.P., Pintel D.J., Tullis G.E. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J. Virol. Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Masumoto J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 23.Kersse K., Lamkanfi M., Bertrand M.J., Vanden Berghe T., Vandenabeele P. Interaction patches of procaspase-1 caspase recruitment domains (CARDs) are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor kappaB signaling. J. Biol. Chem. 2011;286:35874–35882. doi: 10.1074/jbc.M111.242321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao Q. Structural architecture of the CARMA1/Bcl10/MALT1 signalosome: nucleation-induced filamentous assembly. Mol. Cell. 2013;51:766–779. doi: 10.1016/j.molcel.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guiet C., Vito P. Caspase recruitment domain (CARD)-dependent cytoplasmic filaments mediate bcl10-induced NF-kappaB activation. J. Cell Biol. 2000;148:1131–1140. doi: 10.1083/jcb.148.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]


