Abstract
Myopathies typically present with proximal or generalised muscle weakness, but it is important for clinicians to recognise they may also have other distributions. This paper describes a case of distal myopathy that was confirmed genetically as ZASP (Z-band alternatively spliced PDZ motif-containing protein) myofibrillar myopathy (MFM). MFMs are particularly topical because the genetic basis of several have recently been established, enabling diagnosis of conditions previously labelled ‘idiopathic myopathy’, and shedding new light on their pathophysiology. This paper describes a purely distal lower limb phenotype of ZASP MFM, the pathophysiology of ZASP and other MFMs, and the differential diagnosis of late-onset distal symmetrical weakness. The case includes several learning points: ZASP MFM is a new diagnosis; it should be included in differential diagnoses for late-onset myopathy, especially if there is a distal pattern or autosomal dominant inheritance; testing for cardiomyopathy is recommended, and a genetic test is now available.
Background
Distal symmetrical weakness is typically considered to be due to a neuropathy, but it is important that clinicians also recognise myopathy as a possible cause. With an ageing population, the prevalence of late-onset distal weakness will be an increasingly common neurological presentation and it is important for clinicians to be familiar with the likely causes, including distal presentations of myopathy.
This paper helps clinicians approach the differential diagnosis of late-onset distal symmetrical weakness in a logical manner. It also informs clinicians about a relatively new diagnosis—ZASP (Z-band alternatively spliced PDZ motif-containing protein) myofibrillar myopathy (MFM)—that presents with distal weakness. The case reminds clinicians to include ZASP MFM in their differential diagnosis of distal late-onset myopathies and that a genetic blood test is available. This particular case of ZASP MFM is unusual in that the weakness remains confined to the distal lower limbs 21 years after symptom onset. Other reports of ZASP MFM in the literature have described distal upper limb weakness and/or proximal leg weakness typically developing within 15 years of distal leg weakness. It may thus be the first case of ZASP MFM with a purely distal lower limb weakness phenotype.
Finally, this case benefits from the patient's meticulous objective measurements of walking speed recorded every month over 9 years, which provide a fascinating insight into the natural history of functional impairment in ZASP MFM; to the best of the authors’ knowledge, this case is unique in this aspect.
Case presentation
A 65-year-old man was referred to the neurology clinic with a 10-year history of progressive leg weakness. A keen hiker, he had first presented 8 years earlier having noticed that he was struggling to keep pace with fellow ramblers. However, since his weakness was mild he declined invasive investigations.
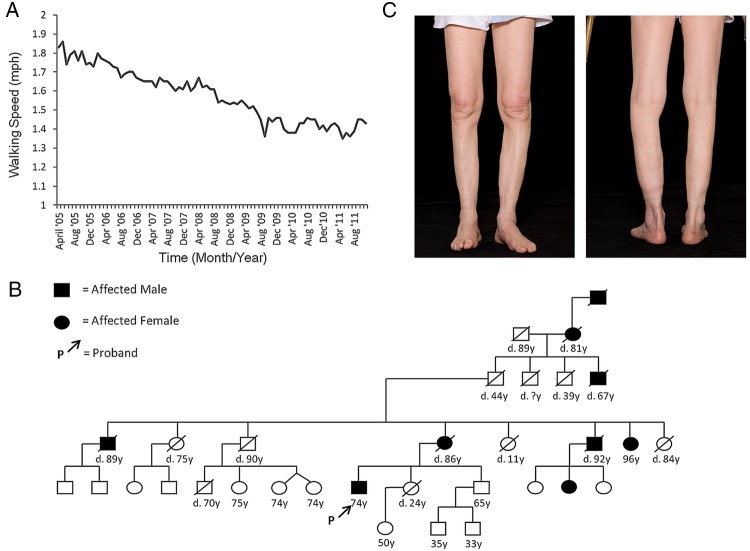
Over the intervening years his symptoms had progressed to the extent that he needed a stick to mobilise, and had difficulty rising from a chair and climbing stairs. Despite this, he continued to take regular walks and had calculated his walking speed over several years, noting a marked decline (figure 1A and see online supplementary file). He had no significant medical history and had not taken regular medications; he was a non-smoker and drank little alcohol. He reported a strong family history of similar problems displaying an autosomal dominant inheritance pattern (figure 1B) with considerable phenotypic variation but presentation consistently in the sixth or seventh decades.
Figure 1.
(A) Graph of patient's walking speed (miles per hour) over 9 years (measured monthly between April 2005 and October 2014). (B) Genogram showing the pattern of inheritance of adult-onset distal weakness in the patient's family. (C) Anterior and posterior views of the patient's lower limbs showing marked muscle wasting of the gastrocnemius and anterior compartment muscles.
Neurological examination of mental status, cranial nerves and upper limbs was normal. In the lower limbs, there was marked wasting of the medial quadriceps, gastrocnemius and anterior compartment muscles bilaterally (figure 1C), but no fasciculations were seen. Tone was normal, power was reduced distally (3/5 for dorsiflexion and 4/5 for plantarflexion), reflexes were absent and plantar responses were flexor. Sensory examination was normal. Gait was high-stepping.
Investigations
Initial laboratory tests included normal renal and liver function, electrolytes and creatine kinase (CK). At second presentation the CK was mildly raised at 481 IU/L. ECG revealed left anterior fascicular block and an echocardiogram was normal. Nerve conduction studies (NCS) were normal. Needle electromyography (EMG) was consistent with myopathy: spontaneous activity at rest with pockets of fibrillation and high-frequency sharp discharge in some muscles, and an excess of brief polyphasic units in all muscles. Interference pattern showed rapid recruitment with low amplitude and a fast firing pattern.
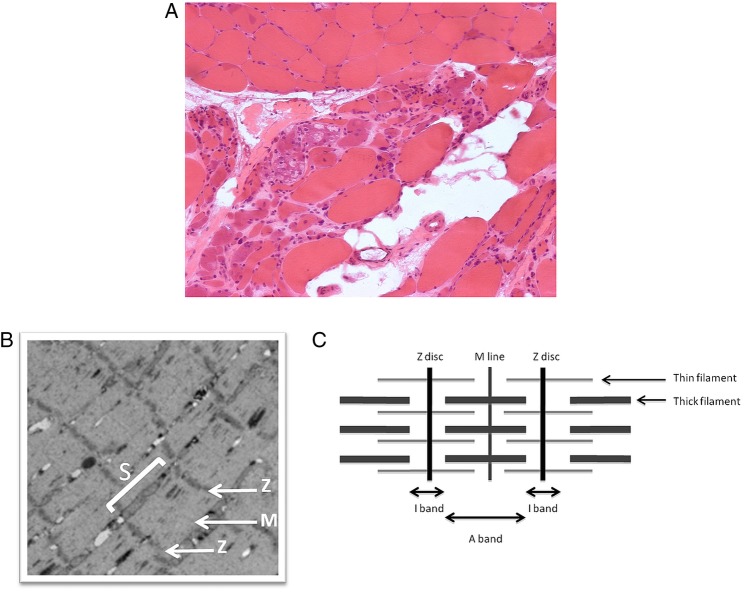
A muscle biopsy, taken from tibialis anterior, demonstrated muscle extensively replaced by collagenous and adipose tissue with marked variation in fibre size, and a few necrotic fibres. Granular basophilic and glassy eosinophilic inclusions, and a few rimmed vacuoles, were present and 5% of fibres were centrally nucleated. Figure 2A shows atrophic and basophilic fibres with vacuoles. Oxidative stains showed whorled fibres, phosphorylase staining was normal and ATPase stains demonstrated a normal chequerboard distribution. The appearances were consistent with end-stage myopathy, but non-specific, and the advanced nature of the pathology made subtyping difficult. MRI of the lower limbs was requested to further delineate the pattern of myopathy, and hence narrow the differential diagnosis, but the request was declined by the radiology department. In light of the strong family history (figure 1B), samples from the patient and a symptomatic maternal aunt were thus sent for genetic testing.
Figure 2.
(A) Muscle biopsy showing atrophic and basophilic fibres with vacuoles in Z-band alternatively spliced PDZ motif-containing protein (ZASP) myofibrillar myopathy (MFM). (B) Electron micrograph of muscle shows the sarcomere (S), Z-discs (Z) and M line. (These images were kindly provided by Professor Bjarne Udd and are not from the patient described in this report.) (C) Illustration of muscle microanatomy.
Differential diagnosis
When considering the differential diagnosis of distal symmetrical weakness, it is useful to think about whether the signs are broadly typical of either upper motor neuron (UMN) pathology or of a neuromuscular disorder.
The UMNs are located in the brain and spinal cord. Pathology at the level of the UMNs produces a ‘pyramidal pattern’ of weakness (ie, flexor muscles are weaker than extensor muscles in the lower limbs) and is associated with increased tone, clonus and brisk reflexes. In this case, there was no evidence of UMN pathology as tone was normal, reflexes were absent and the weakness was not in a pyramidal pattern.
A ‘neuromuscular disorder’ is an umbrella term to describe pathology at either the level of the lower motor neuron (anterior horn cell or nerve), the neuromuscular junction or muscle. There are thus four broad groups of neuromuscular disorders: anterior horn cell disorders, neuropathies, neuromuscular junction disorders and myopathies, and these will each be considered in light of this patient's clinical features. The signs are not suggestive of anterior horn cell disorders (such as motor neuron disease or spinal muscular atrophy) as these are typically associated with fasciculations and more widespread weakness, that is, not only confined to the legs, after so many years of symptoms. Neither is there evidence of fatigable weakness or eye signs that would be typical of a neuromuscular junction disorder such as myasthenia gravis.
Hence the differential diagnosis is narrowed down to myopathy or neuropathy, and both may be associated with the examination findings in this case—muscle wasting, normal tone, distal weakness and arreflexia. Peripheral neuropathies may be motor, sensory or mixed sensorimotor. As the sensory examination was normal in this case, the latter two classifications of neuropathy are unlikely; however, a purely motor neuropathy should remain in the differential. In the case of myopathies, information regarding the pattern of muscular involvement, rate of progression and family history may direct the clinician towards a more specific diagnosis. For instance, weakness of finger and wrist flexion, disproportionate to any other weakness, is typical of a type of myopathy called inclusion body myositis.
Ancillary tests may be required to aid clinical discrimination of motor neuropathy from myopathy. The CK blood level can be helpful as it is typically normal in a neuropathy and may be raised, or normal, in a myopathy. Neurophysiology of the nerve (NCS) and muscle (EMG) are also very helpful investigations to provide objective evidence of a neuropathy and/or myopathy. If a myopathy is confirmed, MRI of the limbs can build on the information gleaned from power testing of the various muscle groups to provide a more detailed topographic map of the muscular disease.
In this case, the CK was mildly raised and the neurophysiology was consistent with a myopathy. These investigation results taken together with the distal pattern of weakness, late age of onset and autosomal dominant inheritance, narrowed the differential diagnosis to: Welander distal myopathy (WDM), tibial muscular dystrophy (TMD), distal myotilinopathy and MFMs due to mutations in ZASP or αB-crystallin.
In selecting gene mutations to test for, it is helpful to consider the typical clinical phenotypes. WDM, typified by hand muscle weakness, was felt to be an unlikely diagnosis in this case. Distal myotilinopathy is a better fit, as this tends to present with ankle weakness, but it is more rapidly progressive, with upper limb weakness, proximal leg weakness and loss of ambulation, all developing within 10 years. In contrast, TMD spares the hand muscles, and usually presents with late adult-onset foot drop and later proximal leg weakness. Likewise, ZASP MFM often presents with foot drop in the fifth decade, and slowly progresses to later involve finger and wrist extensors, followed by proximal leg weakness. Table 1 compares these conditions in further detail. Initial testing for titin mutation (TMD) was negative, but subsequent testing revealed an A165V mutation of the ZASP gene confirming ZASP MFM.
Table 1.
Comparison of late-onset myopathies included in the differential diagnosis
| Myopathy | Pattern of weakness | Age of onset | Progression | Associated features | CK | Biopsy | Muscle MRI | Molecular pathology |
|---|---|---|---|---|---|---|---|---|
| WDM | Finger extensors Wrist flexors then toe and ankle extensors Lower limb first in a minority |
40s–50s | Slow. Remain ambulant Normal life span |
Normal or mildly elevate | Rimmed vacuoles and atrophic fibres | Fatty degeneration in anterior and LL posterior compartments | Missense mutation in TIA1 Rare outside Scandinavia |
|
| TMD | Ankle DF Upper limb spared Asymmetrical |
35–40 years Rare recessive forms in 20s |
Insidious. proximal (hamstring) involvement 65–70 years | Cardiomyopathy | Normal or mildly elevated | Rimmed vacuoles and atrophic fibres Necrosis rare | Selective fatty degeneration LL anterior compartment Hamstring and gluteus minimus later | C-terminus mutation of titin Rare outside Finland |
| ZASP | Ankle weakness then wrist and finger extensors Proximal LL muscles also in 50% Exclusively distal in 10% |
40–50 years | Slow. Loss of ambulance 25–30 years postonset |
Cardiomyopathy Heart block very late |
Mildly elevated | Extensive myofibrillar abnormalities Rimmed and non-rimmed vacuoles |
Early fatty degeneration in gastrocnemius and soleus Later all LL muscles with milder proximal changes | Two European founder mutations (A165V and A147T) in ZASP |
| Distal myotilinopathy | Ankle weakness Later proximal and UL involvement |
45–60 years | Swift UL and proximal weakness. Loss of ambulance <10 years |
Mildly elevated | Similar to ZASP | Similar to ZASP | Mutation in 2nd exon of the myotilin gene |
DF, dorsiflexion; LL, lower limb; TMD, tibial muscular dystrophy; UL, upper limb; WDM, Welander distal myopathy; ZASP, Z-disc alternatively spliced PDZ-domain containing protein.
Treatment
The patient currently receives supportive management from physiotherapy and occupational therapy. They have provided him with exercises to maintain muscle strength and advice on ways of coping with the functional challenges presented by his progressive weakness. He has also been supplied with bilateral ankle-foot orthoses (AFOs).
Outcome and follow-up
The patient remains under follow-up annually in the neurology clinic. At his last clinic review in October 2014, he could walk approximately one mile with two sticks and AFOs.
Discussion
This section is presented in three parts. First, in order to understand the pathophysiology of ZASP MFM, a summary of muscle microanatomy is presented. Second, MFMs in general are discussed and finally ZASP MFM is described in further detail.
Muscle microanatomy
Myofibrils are slender threads within skeletal and cardiac muscle fibres that comprise serial subunits called sarcomeres throughout their length. Each sarcomere contains bundles of thick myosin, thin actin and third filament components (including titin) that act as the contractile units. The striation of muscle fibres is delineated by the organisation of the sarcomere: dark coloured bands (Z-discs) form the borders, light coloured I-bands the thin filaments and semidark A-bands the thick myosin filaments. The Z-discs comprise many different proteins that have structural functions (anchoring the contractile filaments) as well as signalling functions, and damage to these proteins may result in a myopathy.1 The subregions of the sarcomere are illustrated in figure 2B, C.
Myofibrillar myopathies
MFMs share distinct morphological features characterised by a pathological pattern of Z-disc disintegration, followed by accumulation of myofibrillar degradation products and ectopic expression of proteins, resulting in destruction of the myofibrils.2 Several MFM genetic mutations are now known, with alterations in Z-disc-associated proteins desmin, αB-crystallin, myotilin, ZASP, filamin C and Bag3 being reported in recent years. MFMs have heterogeneous clinical phenotypes; most patients present with progressive skeletal muscle weakness but varied distributions are described including distal myopathy, scapuloperoneal syndrome, rigid spine syndrome, generalised myopathy and limb girdle muscular dystrophy. MRI of the limbs may help narrow the differential diagnosis as it can demonstrate the pattern of muscle involvement (anterior vs posterior or mixed). Peripheral neuropathy and cardiomyopathy may occur, especially with desmin and Bag3 mutations, and may antecede the skeletal myopathy.2 EMG studies typically reveal myopathic motor unit potentials and myotonic discharges.
ZASP myofibrillar myopathy
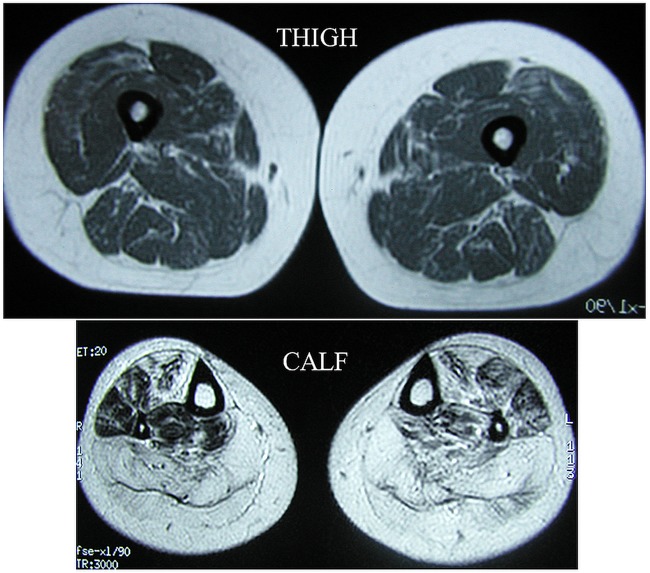
ZASP, encoded on chromosome 10, binds to α-actinin, which cross-links thin filaments of adjacent sarcomeres. ZASP MFM was first described in 2005 by Selcen and Engel in a series of 11 patients: the mean age of onset was in the sixth decade and most patients presented with distal leg muscle weakness and autosomal dominant family history. There is a slow progression of weakness, typically involving ankle dorsiflexion first, followed by finger and wrist extension and, after approximately 15 years, proximal leg muscle weakness. Cardiac involvement, such as cardiomyopathy and heart block, have been reported, although these tend to be late complications.3 CK levels are usually slightly raised and MRI shows fatty replacement of the calf muscles. Figure 3 demonstrates the typical MRI findings in ZASP MFM. In total, only a further 19 cases of ZASP MFM have been reported since Selcen and Engel's original paper. In 2007, Griggs et al4 reported that a family with autosomal dominant late-onset distal myopathy, first described by Markesbery et al,3 in 1974, was subsequently found to carry a ZASP gene mutation. Four more patients with ZASP MFM were described by Claeys et al in 2009 and three by Olivé in 2011.
Figure 3.
MRI demonstrates almost age-normal thigh muscles but extensive fatty degeneration in calf muscles, significant also in tibialis anterior and less in peroneal, long toe extensors and flexors.
Two mutations in ZASP reoccur in families of European descent. The A165V mutation in our patient was also reported in the Markesbery-Griggs family and in the patients described by Selcen and Engel. This mutation was shown to occur on the same haplotype in different families, confirming its founder nature with apparent French-English origin. The other reoccurring mutation is A147T, with a Germanic background. Both mutations are located in the second exon of the gene, so the molecular genetic approach is to start sequencing in this exon.5 Based on the clinical, morphological and muscle imaging phenotype, ZASP MFM cannot be easily separated from myotilinopathy MFM, so the distinction requires genetic investigation.6
Patient's perspective.
I’m a scientist, with degrees in physics, so my response to the changing condition of my legs was to think that I might understand it better if I could find something to measure. The most obvious measurement was the time it took me to cover a route I was already walking regularly for pleasure and exercise.
Walking speeds calculated from this turn out to be a good indicator of how I’m coping more generally, for example, with shopping or stairs, or just getting up from a chair. They also reveal a pattern in which periods of apparent stability end with a fairly rapid deterioration to a new, lower level of ability. This was most marked when a phase of rapid deterioration in walking speed set in 3 months before my 76th birthday.
Close relatives with the disease have included my mother and three of her siblings, and although none took measurements as I have done, their walking seemed to decline slowly until their late 70s, and then much more rapidly. Family members have been relatively long-lived, and anecdotally, have no history of heart trouble.
Learning points.
Distal late-onset weakness may be due to a myopathy.
ZASP (Z-band alternatively spliced PDZ motif-containing protein) myofibrillar myopathy (MFM) is usually dominantly inherited and typically presents as a late-onset distal myopathy, predominantly affecting posterior muscle groups.
Limb MRI may be helpful in distinguishing ZASP MFM from other distal myopathies.
Definitive diagnosis of ZASP MFM relies on genetic testing and a blood test is now available.
Cardiomyopathy and heart block have been reported in advanced ZASP MFM, so ECG and echocardiogram should be performed.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Udd B. Distal muscular dystrophies. In: Griggs R, Amato A, Aminoff M, Boller F, Swaab D, eds. Handbook of clinical neurology. Amsterdam: Elsevier, 2011:239–62. [DOI] [PubMed] [Google Scholar]
- 2.Selcen D. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain 2004;127:439–51. 10.1093/brain/awh052 [DOI] [PubMed] [Google Scholar]
- 3.Markesbery W, Griggs RC, Leach RP et al. . Late onset hereditary distal myopathy. Neurology 1974;24:127–34. 10.1212/WNL.24.2.127 [DOI] [PubMed] [Google Scholar]
- 4.Griggs R, Vihola A, Hackman P et al. . Zaspopathy in a large classic late-onset distal myopathy family. Brain 2007;130:1477–84. 10.1093/brain/awm006 [DOI] [PubMed] [Google Scholar]
- 5.Udd B. Genetics and pathogenesis of distal muscular dystrophies. Adv Exp Med Biol 2009;652:23–38. 10.1007/978-90-481-2813-6_3 [DOI] [PubMed] [Google Scholar]
- 6.Udd B. Distal myopathies. Curr Neurol Neurosci Rep 2014;14:434 10.1007/s11910-013-0434-4 [DOI] [PubMed] [Google Scholar]