Abstract
Hyperlipidemia is a risk factor for abnormal cerebrovascular events. Rafts are cholesterol-enriched membrane microdomains that influence signal transduction. We previously showed that Rho-kinase-mediated Ca2+ sensitization of vascular smooth muscle (VSM) induced by sphingosylphosphorylcholine (SPC) has a pivotal role in cerebral vasospasm. The goals of the study were to show SPC-Rho-kinase-mediated VSM contraction in vivo and to link this effect to cholesterol and rafts. The SPC-induced VSM contraction measured using a cranial window model was reversed by Y-27632, a Rho-kinase inhibitor, in rats fed a control diet. The extent of SPC-induced contraction correlated with serum total cholesterol. Total cholesterol levels in the internal carotid artery (ICA) were significantly higher in rats fed a cholesterol diet compared with a control diet or a β-cyclodextrin diet, which depletes VSM cholesterol. Western blotting and real-time PCR revealed increases in flotillin-1, a raft marker, and flotillin-1 mRNA in the ICA in rats fed a cholesterol diet, but not in rats fed the β-cyclodextrin diet. Depletion of cholesterol decreased rafts in VSM cells, and prevention of an increase in cholesterol by β-cyclodextrin inhibited SPC-induced contraction in a cranial window model. These results indicate that cholesterol potentiates SPC-Rho-kinase-mediated contractions of importance in cerebral vasospasm and are compatible with a role for rafts in this process.
Keywords: Hyperlipidemia, lipid rafts, Rho-kinase, sphingosylphosphorylcholine, vasospasm
Introduction
Cerebral vasospasm is a sustained abnormal constriction of cerebral arteries that is a major lethal complication for patients with subarachnoid hemorrhage (SAH).1 The mechanism underlying this pathologic condition is not fully understood and this has limited the efficacy of potential therapies. Constriction of the cerebral arteries occurs through contraction of smooth muscle in the arterial walls and the primary determinant of this contraction is phosphorylation of the 20-kDa myosin light chain (MLC).2 Myosin light chain kinase (MLCK) is an elongated and potentially flexible molecule. There is no crystal structure of the full-length kinase, but several structures are available that are representative of various domains. The extended length of MLCK is sufficient to span between the thick and thin filaments in smooth muscle, and the length could be longer if the proline-rich repeat segment is modeled as an extensible linker.3 The primary determinant of smooth muscle contraction is phosphorylation of the 20-kDa MLC. This process is regulated by the calcium/calmodulin-dependent MLCK-mediated pathway and a calcium-independent mechanism, i.e., Ca2+ sensitization.4
Most phenotypes attributed to the monomeric G protein RhoA and mediated by its effector, Rho kinase (ROK), reflect Ca2+ sensitization: inhibition of myosin II dephosphorylation in the presence of basal (calcium dependent or independent) or increased MLCK activity.4 In a variety of normal and pathologic cell types, ROK I and II (ROCK I/II) have pivotal roles in organization of the nonmuscle and smooth muscle cytoskeleton and adhesion plaques, as well as in regulation of transcription factors. Thus, ROCK I/II activity regulates cellular contraction, motility, morphology, polarity, cell division, and expression. Emerging evidence suggests that dysregulation of the Rho-ROCK pathway at different stages is linked to cardiovascular, metabolic, and neurodegenerative diseases, as well as to cancer.5 In addition to RhoA/ROK,6, 7, 8 multiple second messengers and signaling pathways such as the protein kinase C9, 10 and arachidonic acid pathways11 have been linked to the Ca2+-sensitization mechanism. Inhibition of MLC phosphatase, directly by ROK or by phosphorylation of phosphatase inhibitor CPI-17, also increases phosphorylation of the myosin II regulatory light chain and thus the activity of smooth muscle and nonmuscle actomyosin ATPase and motility.4 Rho kinase may also be activated during SAH-induced cerebral vasospasm, suggesting the involvement of a ROK-mediated Ca2+-sensitization mechanism.12, 13 In addition, HA1077 (fasudil), an inhibitor of ROK, prevents development of cerebral vasospasm in vitro14 and in humans.15 Taken together, these observations suggest that a ROK pathway has an important role in the pathogenesis of cerebral vasospasm.
We previously showed that eicosapentaenoic acid inhibits sphingosylphosphorylcholine (SPC)-induced contraction in vitro in porcine coronary arterial strips and in an in vivo study, in which intracisternal injection of eicosapentaenoic acid inhibited SPC-induced vasoconstriction.16, 17 The upstream mediator of the ROK pathway remains to be determined, but we showed that SPC induces Ca2+ sensitization of coronary and cerebral vascular smooth muscle (VSM) through sequential activation Src-PTKs (Src family protein tyrosine kinases) and ROK in vitro, and that SPC-induced activation of Src-PTKs was blocked by eicosapentaenoic acid.16 In a preliminary experiment, docosahexaenoic acid, another polyunsaturated fatty acid (the n-3 series), had the same effect of eicosapentaenoic acid, but this result requires further clarification. Sphingosylphosphorylcholine is a sphingolipid that is generated by N-deacylation of sphingomyelin (SM), one of the most abundant lipids in the cell membrane, and has a critical role in apoptosis, cell proliferation, and endothelial nitric oxide production.18, 19, 20 It may be a novel messenger for vasospasm21 and we have shown that the SPC-ROK pathway may initiate abnormal vascular contractions associated with cholesterol and cholesterol-enriched lipid rafts in vitro.22
Hyperlipidemia is a major risk factor in abnormal cerebrovascular events.23 Lipid rafts are sphingolipid- and cholesterol-rich domains of the plasma membrane that also contain a variety of signaling and transport proteins.24 Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, are neuroprotective through improvement of cerebral vasomotor reactivity and reduction of cytokine responses to cerebral ischemia,25 but some clinical reports showing that acute statin treatment reduces cerebral vasospasm suggest a more direct link between cholesterol and cerebral vasospasm.26, 27 The purposes of this study were (1) to investigate whether SPC can induce ROK-mediated contraction of the rat basilar artery in vivo, (2) if this occurs, to determine the relationship between cholesterol and SPC-induced vasoconstriction in vivo, and (3) to examine the effect of cholesterol and lipid raft depletion in VSM on SPC-induced vasoconstriction in vivo.
Materials and methods
All experimental procedures were performed according to the guidelines of the animal care and ethical committee at Yamaguchi University of the Ube, Yamaguchi, Japan. The guidelines were approved by the Institute of Life Science and Medicine, Yamaguchi University School of Medicine.
General Preparation
Male Sprague-Dawley rats weighing approximately 150 g were divided into three groups that received control diet (n=24), 1% cholesterol diet (n=16), or 1% cholesterol+5% β-cyclodextrin (β-CD) diet (n=5) over 8 weeks. The control diet was a laboratory commercial chow pellet that did not contain fish products (F1, Funabashi Farm, Funabashi, Japan). F1 supplemented with 1% cholesterol and 1% cholic acid (Funabashi Farm) was used as the 1% cholesterol diet.28 The dose of β-CD was determined from a study of the cholesterol-lowering action of β-CD in rats.29 Animals were kept in a temperature-controlled environment under a 12-hour light/dark cycle. All experiments were started at 1000 h. After induction of anesthesia with a mixture of 66% nitrous oxide, 30% oxygen, and 4% halothane, the animals were ventilated mechanically via a tracheostomy and anesthesia was maintained using 0.5% to 1% halothane. The femoral artery was cannulated for continuous monitoring of heart rate and arterial blood pressure, and for obtaining blood samples for determination of arterial blood gas tensions and pH. Serum was separated by centrifugation at 1,500 × g for 15 minutes at 4°C and stored at −80°C. Serum T-Cho was measured using an L-type Wako CHO·H kit (Hitachi-High-Technologies Corporation, Tokyo, Japan).
Basilar Artery Cranial Window
A closed cranial window was used for observation of the basilar artery diameter.30 Rats were anesthetized, placed in a head holder in the supine position, and the clivus was exposed by blunt dissection between the carotid sheath and trachea. After division of the superficial transverse vein and the hyoid bone, the trachea and esophagus were retracted laterally. Compression of the carotid arteries and the descending vagus nerves was avoided. The muscle covering the basioccipital bone was removed by electrocautery. A hole of 3 × 4-mm diameter was then made at the base of the skull between the tympanic bullae, the dura was opened, and the basilar artery was exposed carefully. A polypropylene ring was placed over the hole and secured with dental acrylic resin. After the space inside the cranial window was filled with artificial cerebrospinal fluid (aCSF), the arachnoid membrane around the basilar artery was opened and a glass coverslip was fitted over the window and secured with dental acrylic resin. Vessel diameter, heart rate, blood pressure, and arterial blood gas tensions stabilized within 30 minutes after aCSF suffusion and agents were then delivered through the catheter.
The images obtained in these experiments were stored on a hard disc for later playback and analysis. The basilar artery diameter was measured using a DP-BSW-V3.1 videomicrometer (Olympus, Hiroshima, Japan) on a liquid crystal display attached to a DP71 microscope (Olympus). Intracranial pressure was monitored with a catheter inserted through the cranial window. Artificial cerebrospinal fluid (0.2 mL/min) was delivered through another catheter. The composition of the aCSF was as follows: Na+, 151 mEq/L; K+, 4 mEq/L; Ca2+, 3 mEq/L; Cl−, 110 mEq/L; and glucose, 100 mg/dL; pH adjusted to 7.48.30 This composition of aCSF was used in a previous contractile study,30 and thus should be suitable for a study of basilar artery contraction. The solution was freshly prepared each day and bubbled with 5% CO2 in air at 37.5°C. Ca2+-free CSF included 2 mmol/L EGTA and was prepared without Ca2+. The mean intracranial pressure of the cranial window was 10.0±1.0 cm H2O, respectively (n=15 in each group). Vessel diameter was recorded at the time of the plateau response to each agent, as the mean of 10 consecutive measurements taken at approximately 10-second intervals. Vascular smooth muscle contraction is shown as the % reduction in diameter of the basilar artery relative to the pretreatment artery.
Quantification of Cholesterol and Phosphatidylcholine in Vascular Smooth Muscle
Rats were anesthetized and placed in a head holder in the supine position. The carotid sheath was exposed and the internal carotid artery (ICA) was excised by blunt dissection. Under a microscope, the clot, endothelium, and adventitia were carefully removed with minimal stretching of the smooth muscle, and smooth muscle strips (1.5 mg) were prepared. The strips were quickly frozen in liquid nitrogen and stored at −80°C for later biochemical analyses.
The strips were extracted by the Bligh-Dyer method. Immediately after evaporation of solvent to dryness, the lipid extract was incubated with BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide). The solvent was evaporated under nitrogen and the dried residue was reconstituted in 100 μL of BSTFA containing 1% chlorotrimethylsilane. The T-Cho level was analyzed by gas chromatography (GC-2010; Shimazu, Kyoto, Japan). The sample (1 μL) was injected onto a SPB-1 fused silica capillary column (30 m × 0.32 mm I.D., film thickness 0.25 μm, Sigma-Aldrich Tokyo, Japan) using helium as the carrier gas. The column temperature was initially 50°C (1 minute hold) and was programmed to rise at 8°C/min to 250°C. The detector temperature was 310°C and the injection temperature was 250°C.
Phosphatidylcholine (PC) levels in the sample were measured using thin layer chromatography (TLC) on silica gel 60 HPTLC plates (10 × 20 cm, Merck, Darmstadt, Germany). Phosphatidylcholine, SM, phospatidylethanolamine, phosphatidylserine, phosphatidylinositol, phosphatidic acid, and cardiolipin were separated by a single migration with a chloroform/methanol/petroleum ether/acetone/glacial acetic acid/water mixture (24:15:10:5:1.3:1, v/v). Lipids separated on TLC plates were developed by spraying with a 10% CuSO4·5H2O solution made up in 8% H3PO4 and heating at 150°C until the appearance of brown spots.
Western Blotting Analysis
Rats carotid artery was excised and smooth muscle strips were prepared as described above. Total proteins were extracted from the strips by homogenization in 2 × SDS-PAGE sample buffer containing protease inhibitors (Roche Diagnostics). The concentration of extracted proteins was determined by Bradford assay using bovine serum albumin (BSA) as a standard. The proteins were electrophoresed in 10% polyacrylamide gels and then transferred onto an Immobilon-PSQ PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 0.1% Tween-20 in Tris-buffered saline containing 5% skim milk and 1% BSA, the membranes were reacted with primary antibodies against Flotillin-1 at a final concentration of 0.2 μg/mL. After washing, the membranes were reacted with HRP-conjugated goat anti-rabbit Ig (GE Healthcare, Buckinghamshire, UK) for 1 hour at room temperature, and then signals were detected by ECL-Western Blotting Detection System (GE Healthcare).
Quantitative Real-Time PCR
The rat carotid artery was excised and smooth muscle strips were prepared as described above. The strips were quickly frozen in liquid nitrogen and stored at −80°C for later quantitative PCR analysis. RNA extraction and DNase treatment was performed with a RNeasy Micro kit (Qiagen, KJ Venio, The Netherlands). RNA quantity and purity were determined with a DU640 spectrophotometer (Beckman Coulter, Brea, CA, USA). Total RNA (4 μg) was reverse transcribed using anchored-oligo(dT)18 primers (Transcriptor High Fidelity cDNA Synthesis Kit; Roche, Basel, Switzerland). Quantitative real-time PCR was performed in an Applied Biosystems StepOnePlus real-time PCR system (Life Technologies, Austin, TX, USA) using TaqMan probes. Rat-specific probes were used for glyceraldehyde 3-phosphate dehydrogenase (Rn01775763_g1), flotillin-1 (Rn00575535_m1), and ROCK-2 (Rn00564633_ml). Quantification was performed by normalizing Ct (cycle threshold) values to glyceraldehyde 3-phosphate dehydrogenase and analyzed by the comparative Ct method with Applied Biosystems StepOnePlus real-time PCR system software v2.0 (Life Technologies).
Cholesterol Visualization by Fluorescent Microscopy
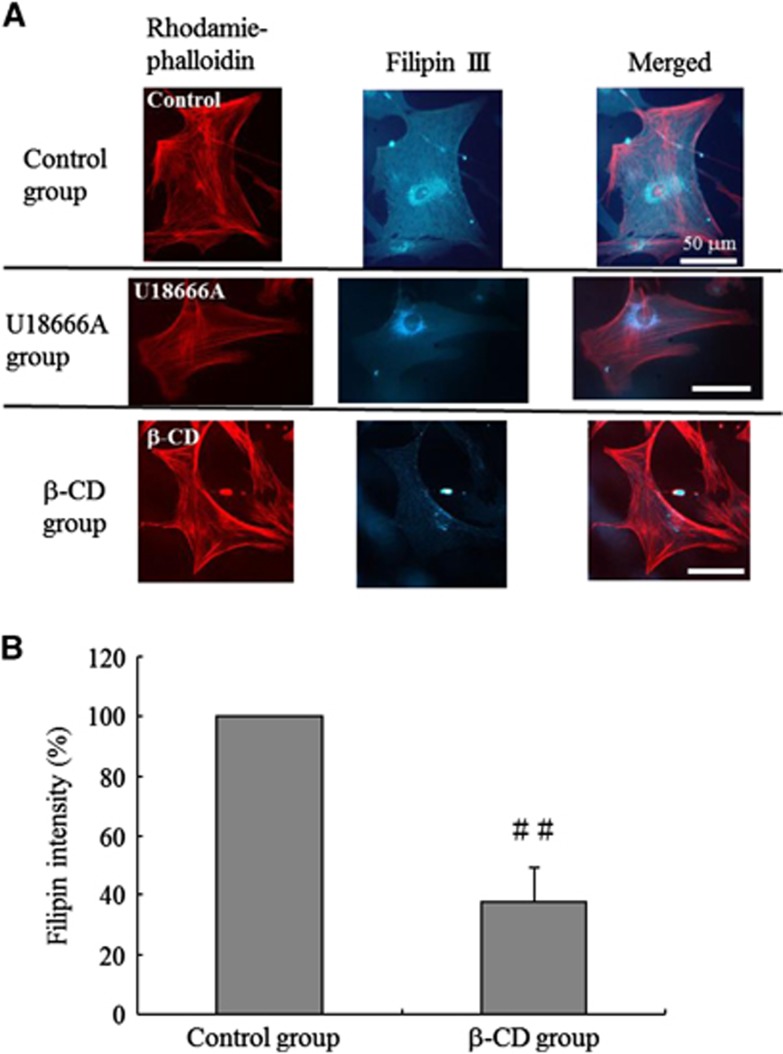
Next, we confirmed the known effect of β-CD on cholesterol histologically.31 Human brain VSM cells (HBVSMCs) (ScienCell Research Laboratories, San Diego, CA, USA) were stained with filipin III to visualize cholesterol (Cholesterol Cell-Based Detection Assay Kit; Cayman Chemical Company, Ann Arbor, MI, USA) and counterstained with rhodamine phalloidin to visualize F-actin (F-Actin Visualization Biochem Kit; Cytoskeleton, Denver, CO, USA). Human brain VSM cells were treated with 5 mmol/L β-CD for 2 hours at 37°C to deplete membrane cholesterol. Control dishes were treated with culture medium alone. Fluorescent cytochemical staining of cholesterol and F-actin was viewed with a fluorescent microscope (Eclipse TS100; Nikon, Minato-ku, Japan) using excitation at 340 to 380 nm and emission at 385 to 470 nm for filipin III, and excitation at 535 nm and emission at 585 nm for rhodamine phalloidin. To measure filipin III intensity, regions of interest were drawn around each cell and the total pixel intensity for each cell was recorded using ImageJ. F-actin staining was used as a reference in the region of interest analysis. Thirty-five cells were analyzed per experiment. The filipin III intensity of β-CD-treated cells was used to calculate the percentage reduction in filipin III intensity compared with the average filipin intensity of untreated cells.
There is strong evidence that phenotypic switching of smooth muscle cells, which we define as any change in the normal structure or function of the differentiated smooth muscle, has a major role in major diseases in humans, including atherosclerosis, cancer, and hypertension.32 Little is known about regulation of smooth muscle cell differentiation in vivo, but studies in cultured smooth muscle cells imply roles of factors including mechanical forces, contractile agonists, extracellular matrix components, and endothelin-smooth muscle cell interactions.32 This background indicates the value of comparison of the in vivo and in vitro models in this study.
Visualization of Lipid Rafts by ELectron microscopy
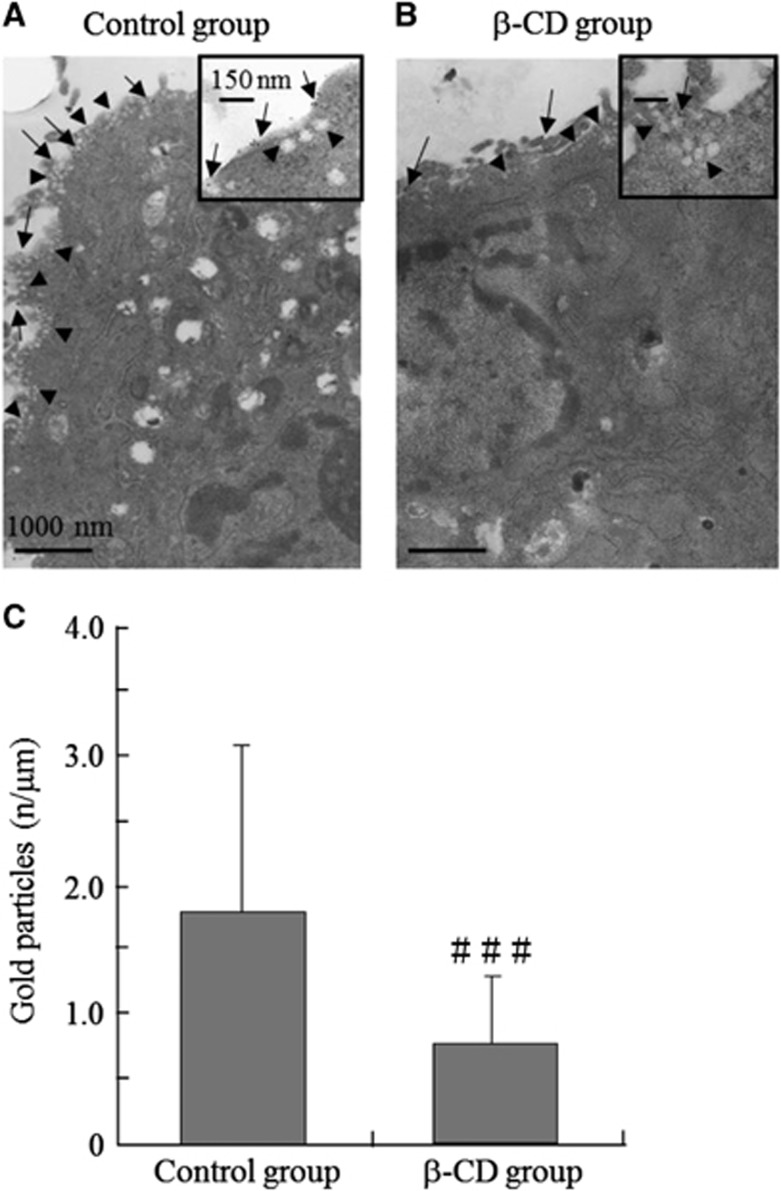
Human brain VSM cells in culture medium were fixed 1:1 (v/v) with 4% paraformaldehyde+0.4% glutaraldehyde (pH 7.4) in 0.2 mol/L PHEM buffer (60 mmol/L PIPES, 25 mmol/L HEPES, 2 mmol/L MgCl2, 10 mmol/L EGTA) for 30 minutes at room temperature. Cells were washed in PHEM and treated with 40 mmol/L glycine. Lipid rafts (raft marker ganglioside GM1) were detected by incubation for 1 hour at room temperature with cholera toxin (CTx) B subunit conjugated to biotin (Molecular Probes, Inc., Eugene, OR, USA) diluted 1:10 in blocking solution (PHEM-2% BSA). Cells were washed with phosphate-buffered saline (PBS) and incubated for 1 hour at room temperature with 6-nm gold particles conjugated to streptavidin (Electron Microscopy Sciences, Hatfield, PA, USA) diluted 1:40 in PBS. Cells were then fixed overnight in 2% glutaraldehyde, washed, post-fixed in 1% osmium tetroxide, dehydrated, and infiltrated by Epon resin. Ultrathin sections were cut using a microtome (EM UC6, Leica, Wetzlar, Germany) and observed using an electron microscope (Quanta3D, FEI, Hillsboro, OR, USA).
Materials
Sphingosylphosphorylcholine (Biomol International, LP, Plymouth Meeting, PA, USA) was dissolved in 100% ethanol and the resulting solution was mixed with PBS (pH 7.5) containing 2 mg/mL BSA to obtain a stock solution (2 mmol/L SPC).21 This stock solution was stored at −80°C and thawed at room temperature before use. Rabbit polyclonal antibody against rat flottilin-1 and mouse monoclonal antibody against rat β-actin were obtained from Santa Cruz (Dallas, TX, USA). Filipin III and U18666A were obtained from Cayman Chemical. Rhodamine phalloidin was from Cytoskeleton. Y-27632 and β-CD were obtained from Calbiochem (San Diego, CA, USA). Bovine serum albumin, EGTA, and PBS were obtained from Sigma (Tokyo, Japan).
Statistical Analysis
Data are expressed as the mean±s.d. Differences among groups (quantification of cholesterol, expression levels of flotillin-1, flotillin-1 mRNA, and ROCK-2 mRNA in VSM) were tested by one-way analysis of variance (ANOVA) followed by Fisher's Protected Least Significant Difference for multiple comparisons. A paired t test was used for analysis of data obtained in the in vivo model to determine the significance of changes of the SPC-treated groups between treatment with and without Y-27632. The association between VSM contractions and serum T-Cho was examined by Pearson correlation analysis. Other results were analyzed by Mann–Whitney U-test. Statistical significance was defined as a probability value of <0.05.
We calculated the statistical power to confirm that sufficient measurements were made to safely make the conclusions. If the calculation was among three groups, then the lowest value was used. The statistical powers are 95.8% in Figure 1B, 83.7% in Figure 2A, and 100% in Figures 3A, 3B, 4B, and 4C. Parametric analysis was used to show a normal distribution.
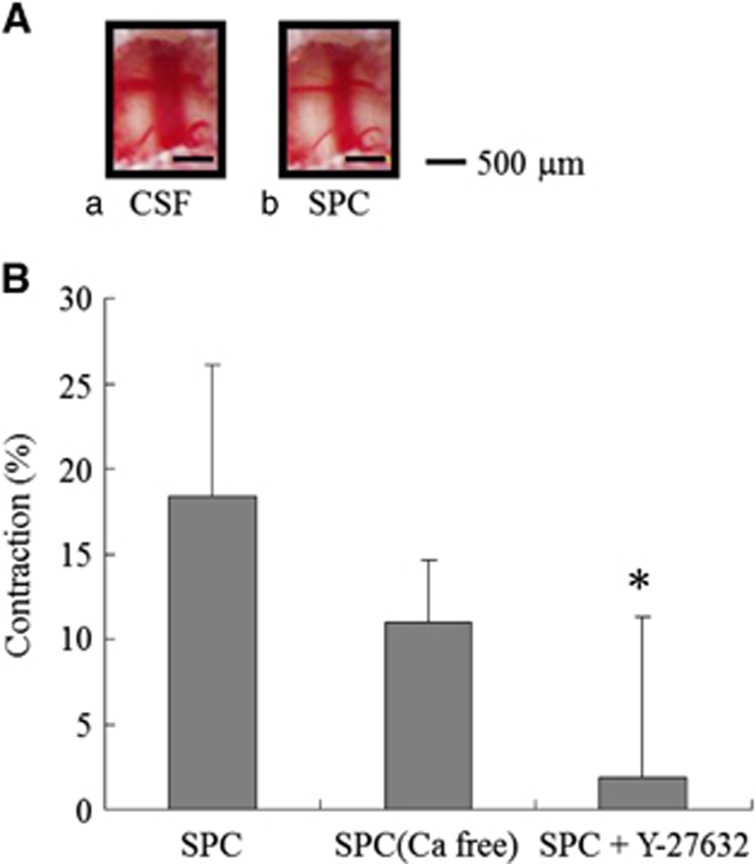
Figure 1.
Changes in the rat basilar artery diameter induced by sphingosylphosphorylcholine (SPC). (A) A control rat basilar artery treated with artificial cerebrospinal fluid (CSF) (a) and vasoconstriction with 100 μmol/L SPC (b). Bars, 500 μm. (B) Percent reduction in basilar artery diameter after treatment with 100 μmol/L SPC, 100 μmol/L SPC and Ca2+-free CSF (n=5), and 10 μmol/L Y-27632 (n=9). Values are shown as means+s.d. *P<0.001 versus 100 μmol/L SPC.
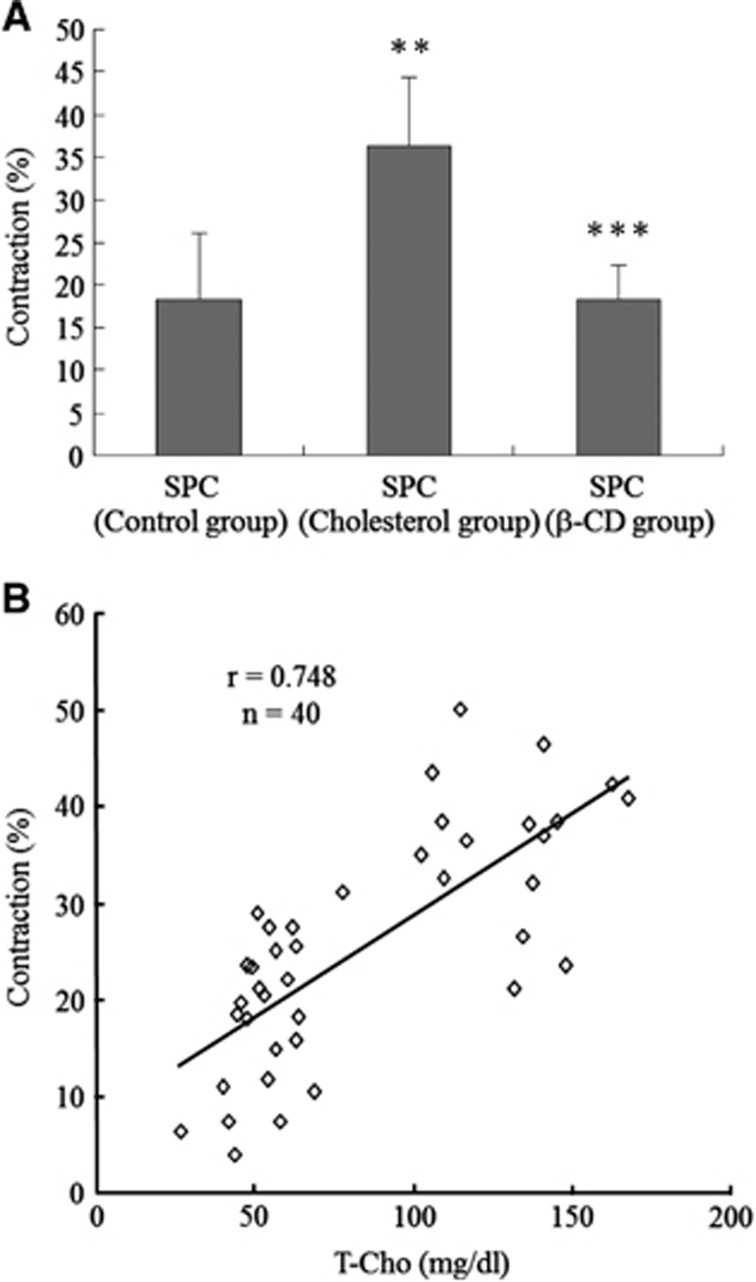
Figure 2.
Link between cholesterol and sphingosylphosphorylcholine (SPC)-induced vascular smooth muscle (VSM) contraction. (A) SPC (100 μmol/L)-induced contraction in rats in the control group (n=24) was increased in those fed a 1% cholesterol diet (cholesterol group, n=16). SPC (100 μmol/L)-induced contraction in animals fed a 1% cholesterol diet was reduced in those fed a 1% cholesterol+5% β-cyclodextrin (β-CD) diet (β-CD group, n=5). (B) The extent of contraction correlated (r=0.75, n=40) with serum T-Cho levels. Values are shown as means+s.d. **P<0.001 versus control group. ***P<0.01 versus cholesterol group.
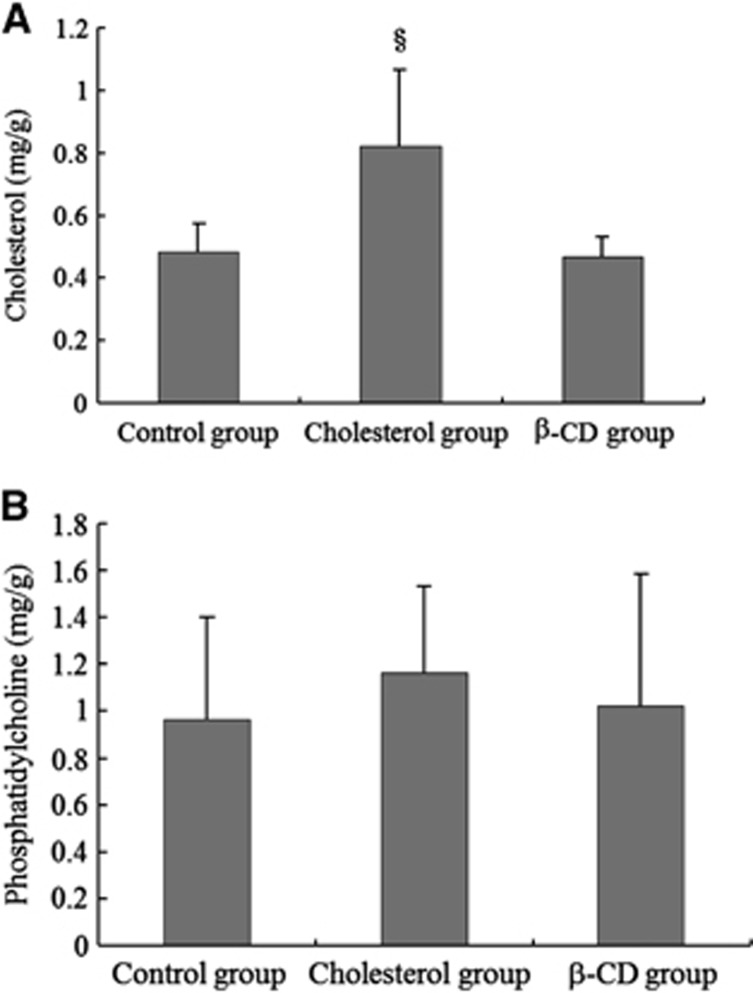
Figure 3.
Quantification of cholesterol and phosphatidylcholine in vascular smooth muscle (VSM). (A) T-Cho in the internal carotid artery (ICA) of rats fed a 1% cholesterol diet (cholesterol group, n=5) was significantly higher than in those fed a control diet (control group, n=5). T-Cho in the ICA of rats fed a 1% cholesterol+5% β-cyclodextrin (β-CD) diet (β-CD group, n=5) was lower than that in rats fed a 1% cholesterol diet (cholesterol group, n=5). (B) There were no significant differences in the PC content in the ICA among the three groups. Values are shown as means+s.d. §P<0.05.
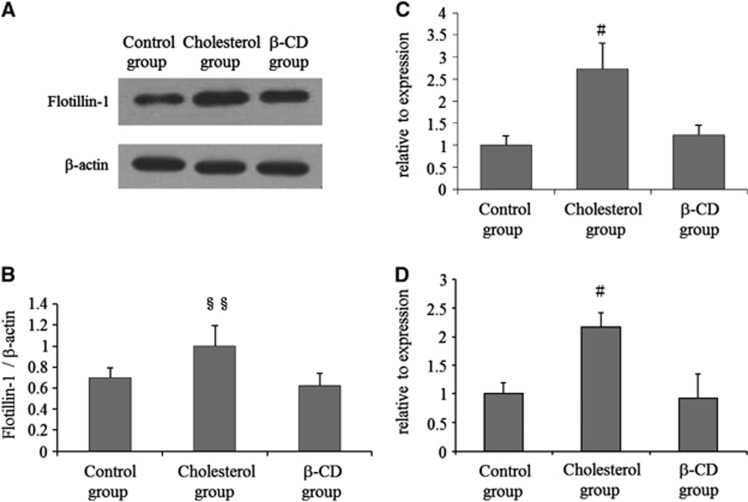
Figure 4.
Altered expression of flotillin-1, flotillin-1 mRNA, and ROCK-2 mRNA in smooth muscle cells of rats fed a 1% cholesterol diet. (A, B) Western blot analysis showing increased protein expression of flotillin-1 in the internal carotid artery (ICA) from rats fed a cholesterol diet. β-Actin was used as a loading control. Three independent experiments were performed and representative data are shown. Values are shown as means+s.d. §§P<0.01. (C, D) Real-time PCR revealed increased flotillin-1 and ROCK-2 mRNA expression in the ICA from rats fed a cholesterol diet, but not in rats fed the β-cyclodextrin (β-CD) diet. All data are normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression level. Results are shown as mean (s.d.) values from at least three independent experiments, with PCR analysis in each experiment performed in triplicate. #P<0.05.
Results
Sphingosylphosphorylcholine-Induced Vascular Contraction in the Control Diet Group
In measurement of the basilar artery diameter in the control diet group, SPC-induced contraction was concentration dependent between 3 and 300 μmol/L with an EC50 of 30 μmol/L (data not shown). At final aCSF concentrations of 100 and 300 μmol/L, SPC induced slowly developing but severe vasoconstriction, reaching maximum vasoconstriction (18% to 20%) within 30 minutes after injection (Figure 1A). The contraction induced by SPC (100 μmol/L in aCSF) was 18.3±7.8% and that induced by SPC (300 μmol/L in aCSF) was 20.2±7.9%. Such vasoconstriction persisted for at least 90 minutes after injection. To test whether SPC induces ROK-mediated Ca2+-independent contraction in vivo, the basilar arteries were treated with 100 μmol/L SPC-aCSF followed by 100 μmol/L SPC-Ca2+-free aCSF or 100 μmol/L SPC-aCSF containing 10 μmol/L Y-27632. The contraction induced by SPC (100 μmol/L in aCSF) was reduced from 18.3±7.8% (n=24) to 1.94±9.3% by Y-27632 (10 μmol/L) (n=9; P<0.001; Figure 1B), indicating complete inhibition of SPC-induced contraction by Y-27632. In contrast, Ca2+-free solution (100 μmol/L SPC-Ca2+-free CSF) had little apparent effect on SPC-induced contraction (11.0±3.6%, n=5; Figure 1B). In all experiments, the respiratory rate and volume were adjusted to maintain expiratory PCO2 between 35 and 40 mm Hg. Body temperature was monitored rectally and maintained at 37°C with a heating pad. Other physiologic parameters were unchanged throughout the experiments (data not shown).
Link Between Cholesterol and Sphingosylphosphorylcholine-Induced Vascular Smooth Muscle Contraction
Serum T-Cho in rats (n=16) fed a 1% cholesterol diet was significantly higher than that in rats (n=24) fed a control diet (131.8±20.0 versus 53.6±10.7 mg/dL, P<0.001). Sphingosylphosphorylcholine (100 μmol/L)-induced contraction increased in rats fed a 1% cholesterol diet (36.4±7.9% versus 18.3±7.8%, P<0.001; Figure 2A) and the extent of contraction was correlated (r=0.75, n=40; Figure 2B) with serum T-Cho levels. The SPC-induced contraction potentiated by cholesterol was reduced in rats (n=5) fed a 1% cholesterol+5% β-CD diet (18.4±4.0%, P<0.01 versus 1% cholesterol diet; Figure 2A). KCl-induced vasocontractions were 47.4±14.5% (Control group, n=11), 47.0±11.6% (Cholesterol group, n=6), and 46.9±12.4% (β-CD group, n=7), and did not differ significantly among the three groups. In the cranial window model, SPC-induced contraction continued for at least 2 hours, as found in our earlier in vitro study.21 These sustained and long-lasting contractions were maintained in the three groups (data not shown).
Quantification of Cholesterol and Phosphatidylcholine in Vascular Smooth Muscle
T-Cho in the ICA measured using gas chromatography was significantly higher in rats (n=5) fed a 1% cholesterol diet compared with that in rats (n=5) fed a control diet (0.82±0.24 versus 0.49±0.09 mg/g, Figure 3A). T-Cho in the ICA was reduced in rats (n=5) fed a 1% cholesterol+5% β-CD diet compared with that in rats fed a 1% cholesterol diet (0.47±0.06 mg/g, Figure 3A). The level of PC, a major component of non-raft membranes, in the ICA measured using TLC did not differ significantly among the three groups (Figure 3B).
Expression Levels of Flotillin-1, Flotillin-1 mRNA, and ROCK-2 mRNA in Vascular Smooth Muscle
Expression levels of flotillin-1 in smooth muscle cells of rats fed a control diet (Control group), 1% cholesterol diet (Cholesterol group), and a 1% cholesterol+5% β-CD diet (β-CD group) are shown in Figures 4A and 4B. Western blot analysis showed increased flotillin-1 in the ICA from rats fed a cholesterol diet, but no change in flotillin-1 in the ICA in rats fed a β-CD diet (Figure 4B). β-Actin was used as a loading control. Quantitative PCR data for the indicated genes in smooth muscle cells of rats fed a control diet, 1% cholesterol diet, and a 1% cholesterol+5% β-CD diet are shown in Figures 4C and 4D, with expression levels in the control group set to 1. These data show upregulation of Flotillin-1 mRNA and ROCK-2 mRNA in smooth muscle cells of rats fed a 1% cholesterol diet.
Evaluation of Cholesterol Depletion in Vascular Smooth Muscle Cells by β-Cyclodextrin
To test the applicability of filipin III staining of cholesterol in HBVSMCs, the cells were first treated with the hydrophobic polyamine U18666A (1.25 μmol/L, 48 hours), a strong inhibitor of lysosomal cholesterol trafficking,33 as a positive control. Filipin III staining of the U18666A-treated cells indicated acute intracellular accumulation of cholesterol in perinuclear vacuoles (Figure 5A). We then determined how β-CD influenced the cholesterol distribution in HBVSMCs. Cells treated with β-CD showed a marked decrease in filipin III staining compared with nontreated control cells, indicating cholesterol depletion (Figure 5A). The % filipin III intensity of HBVSMCs treated with β-CD showed a marked decrease (37.7±12.0%, n=35, P<0.001 versus control cells; Figure 5B).
Figure 5.
Evaluation of cholesterol depletion in vascular smooth muscle (VSM) cells by β-cyclodextrin (β-CD). (A) Human brain VSM cells (HBVSMCs) treated with U18666A (U18666A group) showed intracellular accumulation of cholesterol in perinuclear vacuoles, based on filipin III staining. HBVSMCs treated with β-CD (β-CD group) showed a marked decrease in filipin III staining compared with nontreated cells (Control group). All cells stained with filipin III were counterstained with rhodamine phalloidin to visualize F-actin. Bars, 50 μm. (B) The mean % filipin III intensity of HBVSMCs treated with β-CD showed a marked decrease (n=35). Values are shown as means+s.d. ##P<0.001 versus control group.
Distribution of Lipid Rafts on the Surface of Vascular Smooth Muscle Cells in Electron Microscopy
To examine the effect of β-CD on lipid rafts, transmission electron microscopy was performed in HBVSMCs labeled with the raft marker ganglioside GM1 and the CTx B subunit (6-nm gold particles) (Figures 6A and 6B). Cells treated with β-CD showed a marked decrease in the GM1 count compared with nontreated control cells, indicating raft decrease. At the ultrastructural level, the general morphology of β-CD-treated cells was similar to that of control cells. The GM1 count (gold particles) per unit membrane length (n/μm) was higher in control cells compared with β-CD-treated cells (1.80 versus 0.76 GM1/μm, P=0.037, Figure 6C), based on 20 random images for each sample and three independent counts. Membrane length was measured using ImageJ.
Figure 6.
Effect of β-cyclodextrin (β-CD) on formation of raft clusters in vascular smooth muscle (VSM) cells. (A) Labeling of human brain VSM cells (HBVSMCs) with the raft marker ganglioside GM1 (Control group). Bar, 1,000 nm. (B) GM1 labeling of HBVSMCs treated with β-CD (β-CD group). Bar, 1,000 nm. HBVSMCs treated with β-CD showed a marked decrease in the GM1 count (gold particles, arrows) compared with the control group. Arrowheads revealed caveolar structures, defined as uncoated 50- to 100-nm surface invaginations with clear connections to the plasma membrane. The insets in (A) and (B) show higher magnification images (Bars, 150 nm). (C) The GM1 count (gold particles) per unit membrane length (n/μm) was higher in nontreated cells compared with β-CD-treated cells. Values are shown as means+s.d. ###P<0.05 versus control group.
Discussion
The new findings in this study are that (1) hyperlipidemia increases VSM cholesterol (but not PC, a major component of nonraft membranes) and SPC-induced contraction in vivo, (2) the lowering effect of VSM cholesterol (but not PC) by β-CD inhibits SPC-induced contraction in vivo, (3) hyperlipidemia increases flotillin-1 and flotillin-1 mRNA in the VSM, (4) preventing the increase of VSM cholesterol by β-CD inhibits increases of flotillin-1 and flotillin-1 mRNA in the VSM, and (5) the GM1 count (a raft marker) per unit membrane length of HBVSMCs is decreased by β-CD.
Sphingosylphosphorylcholine is a sphingolipid generated by N-deacylation of sphingomyelin, an abundant lipid in the cell membrane, and is a novel messenger for vasospasm.21 Sphingosylphosphorylcholine, like other membrane lipids, may be released into the perivascular subarachnoid space during degradation of red and white blood cells and platelets after SAH. 16 We have shown that the SPC concentration in CSF is markedly elevated after SAH. The SPC-induced contraction (Ca2+ sensitization) is mediated by ROK activation and the SPC-ROK pathway has been associated with cholesterol in vitro.21, 22 In the present study, a Ca2+-free solution relaxed SPC-induced constriction slightly and Y-27632 induced complete relaxation in vivo. The dose of SPC was determined from a concentration-response curve of the SPC-induced contraction (data not shown) and has been used in a previous study of VSM contraction.16 The dose of Y-27632 used in the study has previously been used for selective inhibition of ROK.8, 16 Furthermore, we previously examined the effect of a dominant-negative ROK, GST-RB/PH (TT), on SPC-induced contraction in the middle cerebral artery permeabilized with β-escin, which allows for large molecules (up to 150 kDa) to enter the cytosol of VSM to specifically inhibit ROK. The SPC-induced contraction was abolished by GST-RB/PH (TT), thus providing direct evidence that SPC-induced contraction is mediated by ROK.21 Therefore, these results suggest that SPC may also be a trigger for the ROK-mediated Ca2+-sensitization mechanism in vivo. The extent of SPC-induced contraction correlated with serum T-Cho levels, which suggests that SPC-ROK-mediated VSM contraction may be regulated by the serum T-Cho level in vivo. To test whether normal and physiologic vascular contractions induced by membrane depolarization could be reproduced in the basilar artery cranial window model, basilar arteries (control diet group) were treated with 118 mmol/L K+-aCSF followed by 118 mmol/L Ca2+-free K+-aCSF. High K+-aCSF induced severe vasoconstriction and the Ca2+-free solution relaxed the KCl-constricted vessels completely, indicating complete inhibition of the Ca2+-dependent contraction by Ca2+-free solution in this model (data not shown).
In addition to the effect of serum T-Cho levels on SPC-induced contraction, we found an effect of the vascular tissue cholesterol level on contraction. The cholesterol level was increased in the VSM and the ICA of rats fed a cholesterol diet and SPC-induced contraction also increased in these rats. This increase in VSM cholesterol level was inhibited in rats treated with a diet containing 5% β-CD, with this dose based on a previous study of the cholesterol-lowering action of β-CD in rats.29 Preventing an increase of VSM cholesterol with β-CD inhibited the cholesterol-dependent SPC-ROK-mediated VSM contractions. These results suggest that SPC-ROK-mediated VSM contraction may be regulated by the cholesterol level in vascular tissue. The concentration of β-CD in cultured cells and that in the cranial window model could not be compared accurately, but the cholesterol depletion effect of β-CD in smooth muscle cells was confirmed histologically by staining of HBVSMCs with filipin III. To test whether filipin III staining of cholesterol in VSM cells was reliable, we first treated HBVSMCs with the hydrophobic polyamine U18666A, one of several hydrophobic amines that block cholesterol release from late endosomes or lysosomes and induce accumulation of low density lipoprotein cholesterol in lysosomes34 U18666A treatment induced acute intracellular accumulation of cholesterol in perinuclear vacuoles based on filipin staining. On the basis of this result, we examined the cholesterol depletion effect of β-CD. Human brain VSM cells treated with β-CD showed a marked decrease in filipin III staining compared with nontreated cells, indicating strong cholesterol depletion.
Next, we examined the mechanisms underlying cholesterol-dependent SPC-ROK-mediated VSM contraction. Western blot analysis and real-time RT-PCR revealed an increase in flotillin-1, flotillin-1 mRNA, and ROCK-2 mRNA expression in the ICA in rats fed a cholesterol diet, whereas these levels were not altered in the ICA in rats fed a β-CD diet. Transmission electron microscopy showed that β-CD decreased the level of the GM1 ganglioside raft marker. These results suggest selective destruction of cholesterol-enriched membrane microdomains (i.e., lipid rafts) by β-CD. Quantification of cholesterol and PCs in the rat ICA showed that neither increasing VSM cholesterol, which potentiates SPC-induced contraction, nor depleting VSM cholesterol, which inhibits cholesterol-dependent SPC-induced contraction, affected the level of PC in VSM.
Lipid rafts are plasma membrane microdomains that contain more cholesterol and higher levels of signaling molecules compared with nonraft membranes, of which PC is a major component, in smooth muscle.24 Lipid rafts are of increasing interest as cellular organelles contributing to the pathogenesis of human diseases, including cancer, atherosclerosis, vasculoproliferative disease, heart failure, and ischemic preconditioning of the heart.24, 34 The results of this study suggest that cholesterol and cholesterol-enriched lipid rafts potentiate VSM contraction mediated by the SPC/ROK pathway. Hyperlipidemia may induce raft formation and SPC-ROK-mediated abnormal vascular contraction, which may have an important role in cerebral vasospasm in patients with hyperlipidemia. We found that KCl-induced VSM contraction was independent of serum cholesterol levels (data not shown), suggesting that the SPC-induced cholesterol-dependent pathway did not affect normal and physiologic vascular contractions induced by membrane depolarization. Previously, we showed that Src-TK mediates SPC-induced contraction and activation of ROK and that SPC induces translocation of Fyn, a member of the Src-TK family that is localized to lipid rafts.16, 35 We have also shown SPC-induced translocation of cytosolic ROK to the cell membrane.16, 21 Furthermore, we previously reported that β-CD blocks translocation of ROK to cell membranes.22 The molecular mechanisms through which cholesterol potentiates VSM contraction are not completely clarified, but these findings are compatible with a crucial role of lipid rafts, in which ROK is translocated to lipid rafts in the SPC/Src-TK/ROK pathway of VSM contraction.
Hyperlipidemia is a major risk factor in abnormal cerebrovascular events.22 Several associated factors, including plaque disruption and thrombosis, are implicated in the pathogenesis of the hyperlipidemia-associated increase in the risk of cardiovascular and cerebrovascular events.36, 37, 38 ROK-mediated Ca2+ sensitization of VSM has a pivotal role in these events in hypertension and vasospasm,8, 13 but the causal link between Ca2+ sensitization of VSM and hyperlipidemia has not been established. Statins are widely used clinically for lipid reduction through inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and recent clinical reports show that acute pravastatin treatment after SAH reduces cerebral vasospasm.26, 27 Statins are neuroprotective through improvement of cerebral vasomotor reactivity and reduction of cytokine responses to cerebral ischemia,25 but the linkage between statin treatment and SPC-ROK-mediated abnormal vascular contraction regulated by cholesterol requires further study. Further work is also required to examine (1) how cholesterol affects SPC levels in the VSM, (2) how cholesterol affects the levels of steroid hormones, which could also influence contractility, and (3) how the detailed molecular mechanism of ROK translocation to lipid rafts of VSM strips depends on SPC-induced Ca2+ sensitization, using sucrose density gradient fractionation analysis.
Acknowledgments
The authors thank H Ohata and H Iida (Department of Anesthesiology and Critical Care Medicine, Gifu University School of Medicine, Gifu, Japan) for support with the cranial window model.
The authors declare no conflict of interest.
Footnotes
This work was supported (in part) by a Grant-in-Aid for Specially Promoted Research (Project No. 20001008) granted in 2008 to Kyushu Institute of Technology, Yamaguchi University, and Shizuoka University by the Japan Ministry of Education, Culture, Sports, Science, and Technology; a Grant-in-Aid for Scientific Research (Project No.22791346) granted by MEXT of Japan; the Kanae Foundation for the Promotion of Medical Science; and the Mitsubishi Pharma Research Foundation.
References
- Findlay JM, Macdonald RL, Weir BK. Current concepts of pathophysiology and management of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cerebrovasc Brain Metab Rev. 1991;3:336–361. [PubMed] [Google Scholar]
- Hartshorne DJ.Biochemistry of the contractile process in smooth muscleIn: Johnson LR ed. Physiology of the Gastrointestinal Tract Raven Press: New York; 1987423–482. [Google Scholar]
- Hong F, Haldeman BD, Jackson D, Carter M, Baker JE, Cremo CR. Biochemistry of smooth muscle myosin light chain kinase. Arch Biochem Biophys. 2011;510:135–146. doi: 10.1016/j.abb.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinase, and Myosin Phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Amin E, Dubey BN, Zang SC, Gremer L, Dvorsky R, Moll JM, et al. Rho-Kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Gong MC, Somlyo AV, Somlyo AP. Separate upstream and convergent downstream pathways of G-protein- and phorbol ester-mediated Ca2+ sensitization of myosin light chain phosphorylation in smooth muscle. Biochem J. 1996;318:469–475. doi: 10.1042/bj3180469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Andrea JE, Allen BG, Clement-Chomienne O, Collins EM, Morgan KG. Smooth muscle protein kinase C. Can J Physiol Pharmacol. 1994;72:1392–1399. doi: 10.1139/y94-201. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fuglsang A, Alessi D, Kobayashi S, Cohen P, Somlyo AV, et al. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992;267:21492–21498. [PubMed] [Google Scholar]
- Miyagi Y, Carpenter RC, Meguro T, Parent AD, Zhang JH. Upregulation of rho A and rho kinase messenger RNAs in the basilar artery of a rat model of subarachnoid hemorrhage. J Neurosurg. 2000;93:471–476. doi: 10.3171/jns.2000.93.3.0471. [DOI] [PubMed] [Google Scholar]
- Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- Satoh S, Suzuki Y, Harada T, Ikegaki I, Asano T, Shibuya M, et al. Possible prophylactic potential of HA1077, a Ca2+ channel antagonist and vasodilator, on chronic cerebral vasospasm. Eur J Pharmacol. 1992;220:243–248. doi: 10.1016/0014-2999(92)90754-r. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, et al. Involvement of Src family protein tyrosine kinase in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- Shirao S, Fujisawa H, Kudo A, Kurokawa T, Yoneda H, Kunitsugu I, et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc Dis. 2008;26:30–37. doi: 10.1159/000135650. [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf D, van Koppen CJ, Windorfer B, Himmel HM, Jakobs KH. Calcium signalling by G protein-coupled sphingolipid receptors in bovine aortic endothelial cells. Naunyn-Schmiedeberg's Arch Pharmacol. 1996;354:397–403. doi: 10.1007/BF00168428. [DOI] [PubMed] [Google Scholar]
- Mogami K, Mizukami Y, Todoroki-Ikeda N, Ohmura M, Yoshida K, Miwa S, et al. Sphingosylphosphorylcholine induces cytosolic Ca2+ elevation in endothelial cells in situ and causes endothelium-dependent relaxation through nitric oxide production in bovine coronary artery. FEBS Lett. 1999;457:375–380. doi: 10.1016/s0014-5793(99)01076-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstein S. Sphingolipid metabolites: members of a new class of lipid second messengers. J Membr Biol. 1995;146:225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T, et al. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- Morikage N, Kishi H, Sato M, Guo F, Shirao S, Yano T, et al. Cholesterol primes vascular smooth muscle to induce Ca2+ sensitization mediated by a sphingosylphosphorylcholine-Rho-kinase pathway: possible role for membrane raft. Circ Res. 2006;99:299–306. doi: 10.1161/01.RES.0000235877.33682.e9. [DOI] [PubMed] [Google Scholar]
- Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41:1636–1640. doi: 10.1161/STROKEAHA.110.584672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Gherghiceanu M, Lekli I, Mukherjee S, Popescu LM, Das DK. Essential role of lipid raft in ischemic preconditioning. Cell Physiol Biochem. 2008;21:325–334. doi: 10.1159/000129391. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Meintzschel F, Rösler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001;32:2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- Tseng MY, Hutchinson PJ, Turner CL, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Biological effects of acute pravastatin treatment in patients after aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled trial. J Neurosurg. 2007;107:1092–1100. doi: 10.3171/JNS-07/12/1092. [DOI] [PubMed] [Google Scholar]
- Mizuguchi K, Yano T, Kojima M, Tanaka Y, Ishibashi M, Masada A, et al. Hypolipidemic effect of ethyl all-cis-5,8,11,14,17-eicosapentaenoate (EPA-E) in rats. Jpn J Pharmacol. 1992;59:307–312. doi: 10.1254/jjp.59.307. [DOI] [PubMed] [Google Scholar]
- Kaewprasert S, Okada M, Aoyama Y. Nutritional effects of cyclodextrins on liver and serum lipids and cecal organic acids in rats. J Nutr Sci Vitaminol (Tokyo) 2001;47:335–339. doi: 10.3177/jnsv.47.335. [DOI] [PubMed] [Google Scholar]
- Iida M, Iida H, Dohi S, Takenaka M, Fujiwara H. Mechanisms underlying cerebrovascular effects of cigarette smoking in rats in vivo. Stroke. 1998;29:1656–1665. doi: 10.1161/01.str.29.8.1656. [DOI] [PubMed] [Google Scholar]
- Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Pietrini SD, D'Souza-Schorey C. ARF6-mediated endosome recycling reverses lipid accumulation defects in Niemann-Pick Type C disease. PLoS One. 2009;4:e5193. doi: 10.1371/journal.pone.0005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Brown BG, Zhao XQ, Sacco DE, Albers JJ.Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease Circulation 1993871781–1791.Circulation 1995;92:3172–3177. [DOI] [PubMed] [Google Scholar]
- Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol. 2009;8:569–580. doi: 10.1016/S1474-4422(09)70092-4. [DOI] [PubMed] [Google Scholar]