Abstract
As P-glycoprotein (Pgp) inhibition at the blood–brain barrier (BBB) after administration of a single dose of tariquidar is transient, we performed positron emission tomography (PET) scans with the Pgp substrate (R)-[11C]verapamil in five healthy volunteers during continuous intravenous tariquidar infusion. Total distribution volume (VT) of (R)-[11C]verapamil in whole-brain gray matter increased by 273±78% relative to baseline scans without tariquidar, which was higher than previously reported VT increases. During tariquidar infusion whole-brain VT was comparable to VT in the pituitary gland, a region not protected by the BBB, which suggested that we were approaching complete Pgp inhibition at the human BBB.
Keywords: blood–brain barrier, P-glycoprotein, pituitary gland, positron emission tomography, (R)-[11C]verapamil, tariquidar
Introduction
Adenosine triphosphate-binding cassette transporters, such as P-glycoprotein (Pgp), breast cancer resistance protein and multidrug resistance protein 4 have a pivotal role in controlling brain distribution of drugs.1 These transporters are expressed in the luminal membrane of brain capillary endothelial cells that form the blood–brain barrier (BBB) and are capable of effluxing a multitude of chemically diverse compounds, often in cooperation with transporters expressed at the abluminal membrane, from brain into blood.1 Transporter-mediated drug–drug interactions at the BBB are of great concern as they may lead to altered brain distribution of drugs and hence to severe central nervous system side effects.1 Studies in mice have shown that genetic knockout of Pgp can result in up to 2,600% increases in brain-to-plasma ratios of drugs, which are selective Pgp substrates (e.g., ivermectin).2 However, for drugs that are in addition to Pgp recognized by other efflux transporters at the BBB much smaller increases in brain distribution will be achieved when only Pgp is inhibited, as the other non-inhibited transporter(s) may compensate the function of Pgp.1 To better understand whether results from rodent studies translate to humans it is important to assess the effect of transporter inhibition on drug brain distribution in humans.
Positron emission tomography (PET) imaging with radiolabelled Pgp substrates, such as [11C]verapamil or [11C]N-desmethyl-loperamide, in combination with administration of Pgp inhibitors has now made it possible to directly study Pgp-mediated drug–drug interactions at the human BBB.3 Two Pgp inhibitors have so far been used in clinical PET studies, the immunosuppressant cyclosporine A and the non-marketed third-generation Pgp inhibitor tariquidar.4, 5, 6, 7 Whereas cyclosporine A caused only small increases in brain uptake of [11C]verapamil5 and the use of higher doses is limited by safety concerns, tariquidar is relatively well tolerated at doses required to inhibit Pgp at the BBB. Administration of tariquidar at a dose of 6 mg/kg body weight was shown to result in 129% and 223% increases in brain distribution of (R)-[11C]verapamil and [11C]N-desmethyl-loperamide, respectively, in humans.6, 7 However, as these effects were smaller than those observed in rodent studies it was not clear whether complete inhibition of Pgp at the human BBB had been achieved. Knowing the maximum possible effect of Pgp inhibition on brain distribution of a Pgp substrate in humans is important to better understand the safety risks associated with Pgp-mediated drug–drug interactions at the human BBB and to obtain much needed scaling factors for extrapolation of data from rodents to humans.
In previous studies, PET scans with (R)-[11C]verapamil or [11C]N-desmethyl-loperamide were performed at approximately 1 hour after completion of an intravenous tariquidar infusion.6, 7 As tariquidar plasma concentrations rapidly decline after end of infusion,4 we investigated in the present study the effect of continuous infusion of tariquidar for the duration of the PET scan on (R)-[11C]verapamil brain distribution.
Materials and methods
The study was registered with EUDRACT (number 2012-005796-14), approved by the Ethics Committee of the Medical University of Vienna, and conducted in accordance with the Declaration of Helsinki and its amendments. From all subjects written informed consent was obtained before enrollment into the study. Five healthy men (mean age: 27±3 years, mean weight: 79±14 kg) were included into the study. Subjects were required to be free of any medication for at least 14 days before start of the study.
Subjects underwent two consecutive 60-minute dynamic (R)-[11C]verapamil PET scans (mean injected activity: 400±16 MBq) on a GE Advance scanner (General Electric Medical Systems, Milwaukee, WI, USA) and serial arterial blood sampling via the radial artery as described previously.8 One hour before start of the second PET scan an intravenous infusion of tariquidar was started at an infusion rate of 375 mL/h and maintained until the end of the scan (mean infusion time 123±3 minutes). For formulation of the infusion solution, a stock solution (70 mL) of 7.5 mg/mL of tariquidar free base in 20% ethanol/80% propylene glycol (AzaTrius Pharmaceuticals Pvt Ltd, London, UK) was diluted with aqueous dextrose solution (5%, w/v, 805 mL) to a final concentration of 0.6 mg tariquidar free base per mL. Three venous blood samples (5 mL) were collected at the beginning, in the middle and at the end of the second PET scan to measure plasma concentrations of tariquidar by a previously described liquid chromatography tandem mass spectrometry assay.7
An arterial input function was constructed by correcting total activity counts in arterial plasma for polar [11C]metabolites of (R)-[11C]verapamil as described previously.8 Plasma protein binding of (R)-[11C]verapamil in blood samples obtained immediately before each PET scan was determined as described before.9
All subjects underwent T1-weighted 3 tesla magnetic resonance imaging on an Achieva 3.0 T scanner (Philips Medical Systems, Best, The Netherlands). The PET and magnetic resonance data were processed with Analyze 8.0 (Biomedical Imaging Resource, Mayo Foundation) and SPM5 (Wellcome Department of Imaging Neuroscience, UCL) software and whole-brain gray matter time-activity curves were extracted using the Hammersmith n30r83 three-dimensional maximum probability atlas of the human brain as described previously.8 In addition, the pituitary gland was manually delineated on axial slices of coregistered PET and magnetic resonance data sets with PMOD 3.6 software (PMOD Technologies Ltd, Zürich, Switzerland). A standard 2-tissue 4-rate-constant (2T4K) compartment model was fitted to the time-activity curves from 0 to 60 minutes after radiotracer injection as described previously.4
All data are given as mean±standard deviation (s.d.). Differences in outcome parameters of scan 1 and 2 were tested using the Wilcoxon matched pairs test (Statistica 6.1, StatSoft Inc., Tulsa, OK, USA). P<0.05 was considered as statistically significant. Concentration-effect relationship was analyzed by nonlinear regression based on a sigmoidal model with variable slope using the Prism 5.0 software (GraphPad Software, La Jolla, CA, USA).
Results and Discussion
In a previous study in which we administered tariquidar as a short infusion over 30 minutes during an (R)-[11C]verapamil PET scan we observed that (R)-[11C]verapamil brain concentrations were rising in immediate response to tariquidar infusion and were rapidly falling after the end of infusion in parallel with declining tariquidar plasma concentrations (Supplementary Figure I).4 This suggested that the Pgp-inhibitory effect of tariquidar at the BBB is transient. This is in good agreement with another study, which demonstrated that higher increases in brain uptake of [11C]N-desmethyl-loperamide can be obtained in humans when the PET scan is performed during rather than after tariquidar administration.10
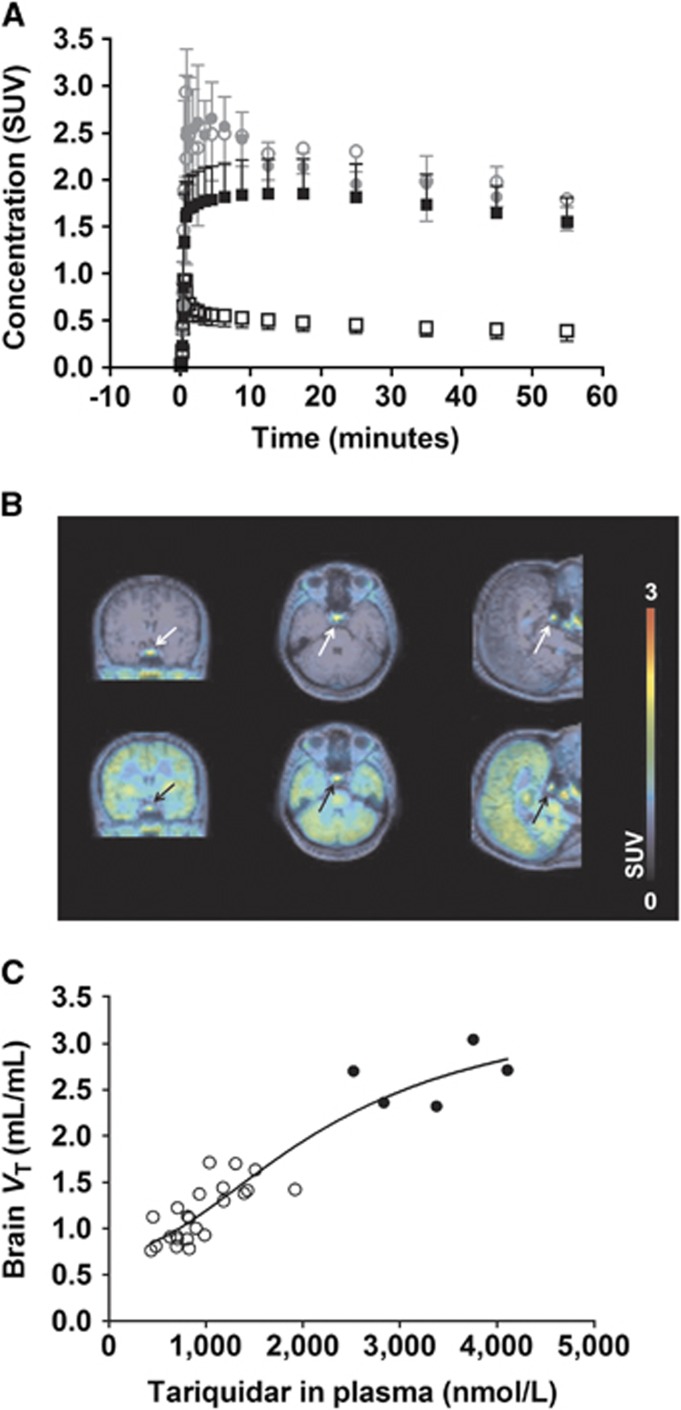
The transient inhibition of Pgp at the BBB by tariquidar prompted us to perform in this study two consecutive (R)-[11C]verapamil PET scans, a first baseline scan and a second scan during continuous infusion of tariquidar, in analogy to a previously described PET protocol using cyclosporine A as Pgp inhibitor.5 We started the tariquidar infusion at 60 minutes before start of the second PET scan and maintained it throughout the PET scan, which resulted in the administration of a mean tariquidar dose of 5.9±1.0 mg/kg body weight. In four out of five subjects previously known, mild adverse reactions that were most likely related to tariquidar occurred (metallic taste in the mouth: N=3 and dizziness: N=1). Mean tariquidar plasma concentration during the PET scan was 2,873±517 nmol/L (Supplementary Table I), which was 2–3 times higher than in our previous study.7 Tariquidar administration had no significant effect on the fraction of non-protein bound (R)-[11C]verapamil in plasma (scan 1: 0.084±0.016, scan 2: 0.103±0.016; P=0.11), which was in good agreement with the previous results.4 Brain time-activity curves were approximately four times higher in scan 2 as compared with scan 1 (Figure 1A).
Figure 1.
(A) Time-activity curves (TACs) (mean standardised uptake value, SUV±s.d., N=5) of (R)-[11C]verapamil in whole-brain gray matter (black) and pituitary gland (gray) before (open symbols) and during (filled symbols) tariquidar infusion. (B) Magnetic resonance (MR)-coregistered PET average images (0 to 60 minutes) of (R)-[11C]verapamil before (upper row) and during (lower row) tariquidar infusion in coronal (left), transaxial (middle) and sagittal (right) views. Radioactivity scale is expressed as SUV. The pituitary gland is indicated by an arrow. (C) Extended concentration-effect curve of tariquidar, in which data from this and two previous studies were pooled.7, 14 Data points from this study are shown as filled circles and from previous studies as open circles. The following parameter estimates (mean±standard error) were obtained: half-maximum effect concentration (EC50): 2,248±987 nmol/L; Hill-slope (n) 2.0±1.1; minimum VT: 0.75±0.26; maximum VT: 3.5±1.2; coefficient of determination (R2): 0.88.
The PET data were analyzed with kinetic modelling using a 2T4K model, which was shown in previous studies to be the model of choice for modelling of [11C]verapamil PET data after Pgp inhibition,4, 5 to obtain VT and the transfer rate constant from plasma into brain K1 as outcome parameters of interest (Table 1). VT and K1 values were significantly increased by 273±78% and 259±74%, respectively, relative to baseline scans (P=0.043). Mean VT and K1 values in scan 2 were 1.7 and 1.6 times higher than mean VT and K1 values in the highest tariquidar dose group (8 mg/kg) of our previous study.7 Interestingly, the increases in brain uptake of (R)-[11C]verapamil observed in the present study were of similar magnitude as the increases observed for [11C]verapamil in another study in non-human primates during administration of a high dose of cyclosporine A, which presumably led to complete Pgp inhibition at the BBB (mean 372% increase in [11C]verapamil K1).11
Table 1. Outcome parameters of kinetic analysis using a 2T4K compartment model.
| Parameter | Whole-brain gray matter without tariquidar | Whole-brain gray matter with tariquidar | Pituitary gland without tariquidar | Pituitary gland with tariquidar |
|---|---|---|---|---|
| K1 (mL/mL/min) | 0.043±0.013 (6) | 0.149±0.021 (5)a | 0.204±0.057 (14) | 0.222±0.061 (32) |
| k2 (1/min) | 0.290±0.318 (23) | 0.310±0.154 (29) | 0.165±0.083 (52) | 0.110±0.054 (209) |
| k3 (1/min) | 0.275±0.339 (26) | 0.646±0.209 (22) | 0.212±0.117 (90) | 0.114±0.049 (1468) |
| k4 (1/min) | 0.074±0.045 (17) | 0.176±0.081 (23)a | 0.597±1.123 (101) | 0.904±1.168 (505) |
| VT (mL/mL) | 0.72±0.14 (3) | 2.63±0.30 (0.7)a | 3.66±0.76 (5) | 3.06±0.72 (7) |
Abbreviations: K1, k2, k3, and k4 are rate constants for transfer of radioactivity between the plasma, the first and the second brain tissue compartments; VT, total distribution volume. Outcome parameters are given as mean±s.d. averaged over five study subjects. Value in parentheses represents precision of parameter estimates (expressed as their coefficient of variation in percentage of the mean), averaged over five study subjects.
Statistically significant difference was observed between scan with and without tariquidar (Wilcoxon matched pairs test).
It has been hypothesized that uptake of [11C]verapamil in the posterior pituitary gland, which has fenestrated capillaries and is therefore not protected by the BBB,12 may provide an estimate of brain uptake of [11C]verapamil in the absence of Pgp function.11, 13 We therefore analyzed distribution of (R)-[11C]verapamil to the pituitary gland (Figures 1A and 1B). While VT in the pituitary gland was 5.3±1.7 times higher than in whole-brain gray matter in scan 1 (P=0.043), VTs in whole-brain gray matter and pituitary gland were comparable and not significantly different in scan 2, which supported the assumption that we were approaching complete inhibition of Pgp at the BBB (Table 1). Moreover, VT in the pituitary gland was similar in scans 1 and 2, which suggested that distribution of (R)-[11C]verapamil to this region is not restricted by Pgp (Table 1). It should be noted, however, that the pituitary gland lies in proximity of two arteries and is smaller (mean volume of interest: 0.11±0.02 cm3) than the spatial resolution of our PET scanner (6 mm) so that quantification of radioactivity in the pituitary gland may be affected by partial volume and/or spill-over effects.
We used the data obtained in this study and data from a previous study14 to extend our previously published concentration-effect curve of tariquidar for enhancement of brain uptake of (R)-[11C]verapamil.7 A sigmoidal curve provided good fits of the combined data (Figure 1C) giving an estimated half-maximum effect concentration (EC50) of 2,248 nmol/L, which was higher than the previously estimated EC50 of tariquidar in humans (868 nmol/L).7 The estimated maximum VT in brain (3.5) was comparable to VT in the pituitary gland in scan 2 (3.06±0.72).
The ratio of nonprotein bound plasma concentration of a transporter inhibitor to its in vitro inhibitory constant (Ki) has been proposed to predict the propensity of an inhibitor to achieve significant transporter inhibition in vivo.1 In our study, tariquidar plasma concentrations ranged from 1,704 to 4,108 nmol/L (Supplementary Table I). The fraction of unbound tariquidar in human plasma has been determined before as 0.0063±0.0021,15 corresponding to unbound tariquidar plasma concentrations in our study ranging from 11 to 26 nmol/L. The Ki of tariquidar for inhibition of transport of [3H]verapamil by human Pgp is 8.5 nmol/L (unpublished data) giving a ratio of the unbound tariquidar plasma concentration to Ki ranging from 1.3 to 3.0.
Since brain distribution of lipophilic Pgp substrates, such as (R)-[11C]verapamil or [11C]N-desmethyl-loperamide, may be dependent on cerebral blood flow (CBF), it has been suggested that extraction fraction (E) given as K1 divided by CBF may be a more suitable parameter of Pgp function than K1 or VT.6, 13 However, since we did not measure CBF in the present study, we were only able to estimate E based on CBF values available from the literature (0.5 mL/g per minute for whole-brain gray matter).16 Normalization of K1 values in scan 2 to CBF provided E values of 0.30±0.04 and 0.44±0.12 in whole-brain gray matter and pituitary gland, respectively, which indicated that for (R)-[11C]verapamil extraction may not reach 100% even in the absence of Pgp activity. It should be noted, however, that CBF in the pituitary gland may differ from CBF in whole-brain gray matter so that our E estimate for the pituitary gland may not be accurate.
In a previous study in rats a 932% increase in (R)-[11C]verapamil brain VT over baseline scans was observed after complete inhibition of Pgp at the BBB by tariquidar.9 According to our concentration-effect curve (Figure 1C), the estimated maximum VT in human brain is 3.5, which allows predicting a maximum VT increase in humans of 367%. This is in good agreement with the prediction made in another study for [11C]verapamil (+400%).13 This indicates that for (R)-[11C]verapamil the scaling factor between rats and humans for VT increase after complete Pgp inhibition is 2.5. It is, however, not known whether this scaling factor will also apply to other substrate-inhibitor combinations than (R)-[11C]verapamil and tariquidar.
In conclusion, our results show that substantial inhibition of Pgp can be safely achieved at the human BBB using an experimental (nonmarketed) Pgp inhibitor, which results in pronounced increases in brain delivery of a radiolabelled Pgp substrate. This approach may be of interest to improve brain penetration of therapeutic drugs, which are cleared from brain by Pgp. Moreover, we experimentally verified the hypothesis that PET analysis of baseline uptake (i.e., without administration of a Pgp inhibitor) of a radiolabelled Pgp substrate in the pituitary gland gives an estimate of brain uptake when Pgp is fully inhibited, thus providing the possibility to predict the maximum possible effect of Pgp-mediated drug–drug interactions at the BBB.11, 13 Finally, our data provide much needed scaling factors for extrapolation of rodent data to humans in pharmacokinetic modelling.
Acknowledgments
The research leading to these results has received funding from the Austrian Science Fund (FWF) (grant F 3513-B20). This study would not have been possible without the excellent support of research nurse Maria Weber (Department of Clinical Pharmacology). The authors wish to thank the staff of the PET center at the Medical University of Vienna for continued support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Kalvass JC, Polli JW, Bourdet DL, Feng B, Huang SM, Liu X, et al. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin Pharmacol Ther. 2013;94:80–94. doi: 10.1038/clpt.2013.34. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Wulkersdorfer B, Wanek T, Bauer M, Zeitlinger M, Müller M, Langer O, et al. Using positron emission tomography to study transporter-mediated drug-drug interactions in tissues. Clin Pharmacol Ther. 2014;96:206–213. doi: 10.1038/clpt.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CC, Bauer M, Karch R, Feurstein T, Kopp S, Chiba P, et al. A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R-11C-verapamil and PET. J Nucl Med. 2009;50:1954–1961. doi: 10.2967/jnumed.109.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzi M, Mankoff DA, Link JM, Shoner S, Collier AC, Sasongko L, et al. Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J Nucl Med. 2009;50:1267–1275. doi: 10.2967/jnumed.108.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Liow JS, Kimura N, Seneca N, Zoghbi SS, Morse CL, et al. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med. 2010;51:559–566. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Zeitlinger M, Karch R, Matzneller P, Stanek J, Jäger W, et al. Pgp-mediated interaction between (R-[11C]verapamil and tariquidar at the human blood-brain barrier: a comparison with rat data. Clin Pharmacol Ther. 2012;91:227–233. doi: 10.1038/clpt.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, et al. Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[11C]verapamil. Epilepsia. 2007;48:1774–1784. doi: 10.1111/j.1528-1167.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- Kuntner C, Bankstahl JP, Bankstahl M, Stanek J, Wanek T, Stundner G, et al. Dose-response assessment of tariquidar and elacridar and regional quantification of P-glycoprotein inhibition at the rat blood-brain barrier using (R-[11C]verapamil PET. Eur J Nucl Med Mol Imaging. 2010;37:942–953. doi: 10.1007/s00259-009-1332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Bhatia R, Morse CL, Woock AE, Zoghbi SS, Shetty HU, et al. Increased permeability-glycoprotein inhibition at the human blood-brain barrier can be safely achieved by performing PET during peak plasma concentrations of tariquidar. J Nucl Med. 2015;56:82–87. doi: 10.2967/jnumed.114.146894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke AB, Eyal S, Chung FS, Link JM, Mankoff DA, Muzi M, et al. Modeling cyclosporine A inhibition of the distribution of a p-glycoprotein PET ligand, 11C-verapamil, into the maternal brain and fetal liver of the pregnant nonhuman primate: impact of tissue blood flow and site of inhibition. J Nucl Med. 2013;54:437–446. doi: 10.2967/jnumed.112.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama S, Pearl GS, Takei Y. Ultrastructural study of the human neurohypophysis. III. Vascular and perivascular structures. Cell Tissue Res. 1980;206:291–302. doi: 10.1007/BF00232773. [DOI] [PubMed] [Google Scholar]
- Eyal S, Ke B, Muzi M, Link JM, Mankoff DA, Collier AC, et al. Regional P-glycoprotein activity and inhibition at the human blood-brain barrier as imaged by positron emission tomography. Clin Pharmacol Ther. 2010;87:579–585. doi: 10.1038/clpt.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Karch R, Zeitlinger M, Stanek J, Philippe C, Wadsak W, et al. Interaction of 11C-tariquidar and 11C-elacridar with P-glycoprotein and breast cancer resistance protein at the human blood-brain barrier. J Nucl Med. 2013;54:1181–1187. doi: 10.2967/jnumed.112.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Hirabayashi H, Amano N, Moriwaki T. Retrospective analysis of P-glycoprotein-mediated drug-drug interactions at the blood-brain barrier in humans. Drug Metabol Dispos. 2013;41:683–688. doi: 10.1124/dmd.112.049577. [DOI] [PubMed] [Google Scholar]
- Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, et al. Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;34:1373–1380. doi: 10.1038/jcbfm.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.