Sporadic cerebral amyloid angiopathy (CAA) denotes a common form of microangiopathy in the brain—its pathologic hallmark is vascular amyloid-β protein deposition affecting small vessels in the cerebral cortex and overlying leptomininges.1 Although CAA often does not result in clinically recognizable symptoms and might be diagnosed only at autopsy, it is now considered as a major risk factor for spontaneous lobar intracerebral hemorrhage in elderly people and an important contributor to age-related cognitive decline and dementia.1, 2 In vivo knowledge on CAA has developed largely as a result of progress in neuroimaging, which has allowed the characterization of the spectrum of hemorrhagic and ischemic brain injury associated with CAA as well as different clinical phenotypes.1, 3 The entity of CAA now encompasses not only a specific cerebrovascular pathologic trait and disorder, but also a clinical syndrome (or syndromes) and brain parenchymal lesions seen on neuroimaging (including a set of validated imaging diagnostic criteria—the Boston criteria).4
In this issue of the Journal, Martínez-Lizana et al5 report on a multicenter biomarker and neuroimaging study, which focused on the increasingly recognized imaging feature of acute nontraumatic convexity subarachnoid hemorrhage (cSAH) in the context of CAA. Although cSAH has a number of possible etiologies, previous small case series have suggested that CAA is perhaps the main cause of cSAH in elderly patients (generally older than 60 years).6, 7 While data on mechanims and clinical relevance are limited, the most widely accepted notion is that brittle and fragile leptomeningeal or very superficial cortical CAA-affected vessels might lead to blood leaking episodes into the subarachnoid space (clinically silent or symptomatic depending on its location) and thus cSAH in some patients.
The authors studied 22 patients (over 60 years old) with symptomatic cSAH. The cSAH patients presented with fairly stereotyped, positive and/or negative, transient focal neurologic episodes, largely corresponding to the topography of acute cSAH.5 The neuroimaging, apolipoprotein E (APOE) genotype, and CSF profile of the cSAH cohort was suggestive of underlying CAA and patients actually fulfilled the modified Boston criteria.4 During a median follow-up of 30.7 months, 5 patients had died, 6 survivors showed functional disability, and 12 cognitive impairment.5 Over the same period, six patients had suffered an intracerebral hemorrhage. Compared to healthy controls, cSAH patients had an overrepresentation of the APOE ɛ2 allele (thought to be associated with small vessel fragility in CAA), whereas the non-cSAH CAA comparison group more often had the APOE ɛ4 allele.
These results were obtained in a small ‘convenient' patient sample, thus limiting their generalization. Because of the possibility of selection bias, low statistical power and the lack of systematic longitudinal evaluation of unselected CAA patients with or without cSAH, the poor outcome identified in the study might represent a Proteus phenomenon.8 Moreover, it is hard to disentangle the independent contribution of cSAH in the outcome, or whether the clinical CAA phenotype primarily associated with cSAH conveys poorer prognosis compared with other CAA presentations. Notwithstanding, the study contributes a number of important hypothesis-generating observations on the topic and reinforces the view of cSAH being another neuroimaging hemorrhagic signature of CAA along with lobar microbleeds and cortical superficial siderosis. In fact, recent data indicate that cortical superficial siderosis on blood-sensitive magnetic resonance imaging often results from prior episodes of acute cSAH (Figure 1).9 In the current study, 19/22 patients with acute cSAH also had evidence of (chronic) cortical superficial siderosis, mostly affecting multiple areas. Hence, cSAH and cortical superficial siderosis should be viewed as part of the same pathophysiologic process, providing information about the timing, as well as pattern, of this particular bleeding event in CAA.9 Consisted with this view, cortical superficial siderosis is also characteristically associated with transient focal neurologic episodes (‘amyloid spells'), and a high risk of future intracerebral hemorrhage in CAA cohorts,10 with potential implications for antithrombotic decisions. Furthermore, in a recent cross-sectional analysis APOE ɛ2 was overrepresented in advanced CAA patients with superficial siderosis.11 In the current study, there was a trend worth exploring for APOE ɛ2 being more common in the cSAH CAA patients compared with the non-cSAH CAA group (19% vs. 4%, respectively).
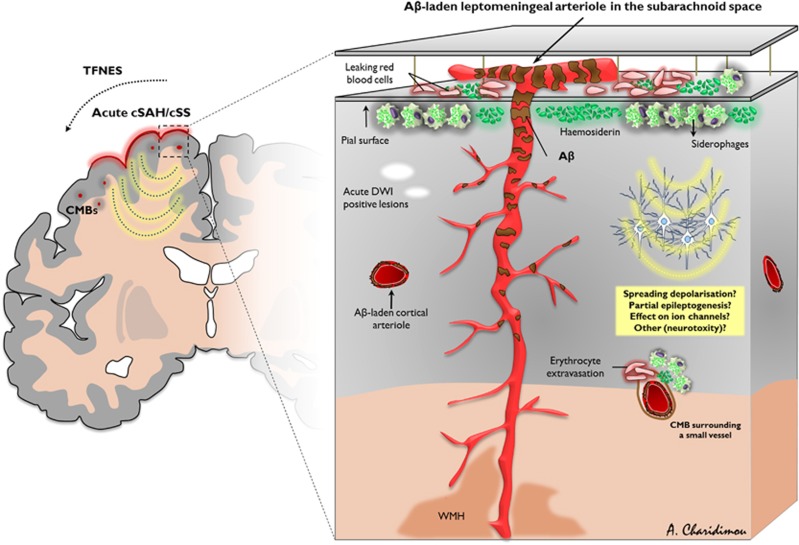
Figure 1.
Schematic diagram of leptomeningeal and superficial parenchymal arterioles showing key features of acute convexity subarachnoid hemorrhage (cSAH) and cortical superficial siderosis (cSS) in sporadic cerebral amyloid angiopathy (CAA). Leptomeningeal and perforating arterioles of a brain section showing amyloid-β (Aβ) deposits (brownish discoloration). Note the reducing vascular Aβ severity moving from the cortical surface into the cerebral white matter. Repeated episodes of hemorrhage (acute cSAH, which can be either clinically silent or symptomatic) from these brittle leptomeningeal or very superficial cortical vessels into the adjacent subarachnoid space are probably an important cause of acute cSAH and cortical superficial siderosis in the chronic phase, which has a characteristic predilection for the cerebral convexities, reflecting linear blood residues, including haemosiderin and hemosiderin-laden macrophages (siderophages) in the subarachnoid space or superficial subpial cortical layers. However, the exact pathophysiologic mechanisms have not yet been proven in detail. Acute cSAH and cSS may induce transient focal neurologic dysfunction (related to spreading depolarization, partial seizure activity focal seizure activity, or other mechanisms), acute microinfarcts on diffusion-weighted (DWI) magnetic resonance imaging (MRI) or be associated with future risk of intracerebral hemorrhage. CMB, cerebral microbleed; TFNEs, transient focal neurological episodes; WMH, white matter hyperintensities.
The paper by Martínez-Lizana et al5 has the potential to identify new pathophysiologic mechanisms within the clinical-imaging spectrum of CAA3, 12 and prompts key questions that should be evaluated in future projects. Can patients with CAA-related isolated cSAH be diagnosed based on the CSF profile and APOE genotype?13 Does cSAH and cortical siderosis denote an otherwise distinct CAA phenotype with more amyloid deposits and vasculopathic changes in leptomeningeal vessels? Do they have a role in the development of CAA-related cognitive decline and symptomatic intracerebral hemorrhage? The intriguing finding of small acute ischemic lesions or chronic cortical ischemia in close proximity to cSAH/cortical superficial siderosis in some cases in the current cohort might indicate a possible cross-talk between hemorrhagic and ischemic brain injury in CAA affecting clinical outcome (including cognition).14 Another recent study of acute cSAH in seven cases with 11C-Pittsburgh compound B positron emission tomography scans also reported small ischemic lesions on diffusion-weighted magnetic resonance imaging as well as future intracerebral hemorrhage occurrence.15 Of note, areas of restricted diffusion in close proximity to cSAH might instead be due to the presence of acute blood products. Furthermore, this association with ischemic lesions might just indicate focally active severe CAA-related disease, not necessarily a causal link. A better understanding of pathways underlying both ischemic and hemorrhagic brain injury and their clinical consequences in CAA is needed. The ongoing SuSPect-CAA trial (Superficial Siderosis in Patients with suspected Cerebral Amyloid Angiopathy) is an important step in further exploring aspects of cSAHs/cortical superficial siderosis and validating them as disease biomarkers. Another key future goal should be the systematic radiologic-pathologic correlation of these imaging markers of small vessel disease in the context of coexisting pathology. Martínez-Lizana et al5 provide inspiration and hypotheses for multiple future avenues of investigation—until then, the saga continuous.
Acknowledgments
Special thanks to Jennifer Linn, Meike W Vernooij, Christian Opherk, Saloua Akoudad, Jean-Claude Baron, Steven M Greenberg, Hans Rolf Jäger and David J Werring for their very useful comments on an earlier version of the illustration included in the manuscript.
The author declares no conflict of interest.
Footnotes
Special thanks to Jennifer Linn, Meike W Vernooij, Christian Opherk, Saloua Akoudad, Jean-Claude Baron, Steven M Greenberg, Hans Rolf Jäger, and David J Werring for their very useful comments on an earlier version of the illustration included in the manuscript.
References
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83:124–37. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–80. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology. 1993;43:2073–9. doi: 10.1212/wnl.43.10.2073. [DOI] [PubMed] [Google Scholar]
- Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–50. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lizana E, Carmona-Iragui M, Alcolea D, Gómez-Choco M, Vilaplana E, Sánchez-Saudinós BM, et al. Cerebral amyloid angiopathy-related atraumatic convexal subarachnoid hemorrhage: an ARIA before the tsunami J Cereb Blood Flow Metab 2015(in this issue). [DOI] [PMC free article] [PubMed]
- Kumar S, Goddeau RP, Selim MH, Thomas A, Schlaug G, Alhazzani A, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010;74:893–9. doi: 10.1212/WNL.0b013e3181d55efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitzke M, Gattringer T, Enzinger C, Wagner G, Niederkorn K, Fazekas F. Clinical presentation, etiology, and long-term prognosis in patients with nontraumatic convexal subarachnoid hemorrhage. Stroke. 2011;42:3055–60. doi: 10.1161/STROKEAHA.111.621847. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Revi Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Linn J, Herms J, Dichgans M, Bruckmann H, Fesl G, Freilinger T, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol. 2008;29:184–6. doi: 10.3174/ajnr.A0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn J, Wollenweber FA, Lummel N, Bochmann K, Pfefferkorn T, Gschwendtner A, et al. Superficial siderosis is a warning sign for future intracranial hemorrhage. J Neurol. 2013;260:176–81. doi: 10.1007/s00415-012-6610-7. [DOI] [PubMed] [Google Scholar]
- Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, Reijmer Y, Falcone GJ, Ayres A, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology. 2014;83:1838–43. doi: 10.1212/WNL.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Mackenzie IR, Feldman HH. Clinical phenotypes of cerebral amyloid angiopathy. J Neurol Sci. 2007;257:23–30. doi: 10.1016/j.jns.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Tamura R, Tomita H, Mizutani K, Miwa T. The importance of amyloid beta protein in cerebrospinal fluid when you recognize convexal subarachnoid hemorrhage. Eur Neurol. 2014;71:283–7. doi: 10.1159/000357426. [DOI] [PubMed] [Google Scholar]
- Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–82. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JV, Singhal S, Rowe CC, Kempster P, Bower S, Phan TG.Convexity subarachnoid hemorrhage with PiB positive PET scans: clinical features and prognosis J Neuroimaging 2014. doi: 10.1111/jon.12188 [DOI] [PubMed]