Abstract
Recent human imaging studies indicate that reduced blood flow through pial collateral vessels (‘collateral failure') is associated with late infarct expansion despite stable arterial occlusion. The cause for ‘collateral failure' is unknown. We recently showed that intracranial pressure (ICP) rises dramatically but transiently 24 hours after even minor experimental stroke. We hypothesized that ICP elevation would reduce collateral blood flow. First, we investigated the regulation of flow through collateral vessels and the penetrating arterioles arising from them during stroke reperfusion. Wistar rats were subjected to intraluminal middle cerebral artery (MCA) occlusion (MCAo). Individual pial collateral and associated penetrating arteriole blood flow was quantified using fluorescent microspheres. Baseline bidirectional flow changed to MCA-directed flow and increased by >450% immediately after MCAo. Collateral diameter changed minimally. Second, we determined the effect of ICP elevation on collateral and watershed penetrating arteriole flow. Intracranial pressure was artificially raised in stepwise increments during MCAo. The ICP increase was strongly correlated with collateral and penetrating arteriole flow reductions. Changes in collateral flow post-stroke appear to be primarily driven by the pressure drop across the collateral vessel, not vessel diameter. The ICP elevation reduces cerebral perfusion pressure and collateral flow, and is the possible explanation for ‘collateral failure' in stroke-in-progression.
Keywords: collaterals, collateral failure, experimental stroke, infarct expansion, intracranial pressure, ischemic stroke, stroke-in-progression
Introduction
The term stroke-in-progression has been used to describe patients who present with mild or rapidly improving symptoms and then show a decline in neurologic function within 24 to 72 hours after stroke onset.1 It occurs in approximately 10% to 40% of patients2 and has a poor prognosis, with over 50% of patients requiring assistance with daily tasks at 3 months.3 Until recently, the assumed pathophysiology of stroke-in-progression was that patients experience clinical improvement then subsequent deterioration due to spontaneous reperfusion, then rethrombosis of the initially occluded artery.4 This is the basis for the frequent use of anticoagulants such as heparin in these patients, despite lack of evidence of clinical benefit.5 Recent use of sequential advanced imaging has permitted identification of patients with early infarct expansion as the cause for their neurologic deterioration, and for these patients to be distinguished from those with a 2nd stroke (in a separate arterial territory) and those with deterioration from other factors such as brain swelling. Such studies have shown fluctuating symptoms that occur in patients with infarct expansion despite stable arterial occlusion.6 Infarct expansion typically occurs within the first day post admission, and is associated with reduced flow through pial collateral vessels.7, 8
Pial collaterals provide anastomoses between distal branches of adjacent arterial territories, and permit retrograde residual perfusion of the ischemic penumbra after stroke.9 They also provide perfusion to penetrating arterioles at the ‘watershed' between different arterial territories under normal circumstances.10, 11 Good collateral supply is strongly associated with better clinical outcome post-stroke.12 Adequacy of preformed collaterals is strongly influenced by genetics.13 However, failure of flow through adequate preformed collaterals has been recognized as occurring in some patients.7 Proposed mechanisms include collateral vessel thrombosis,14 venous steal,15 reversed Robin Hood syndrome,16 and blood pressure fluctuations secondary to autonomic dysfunction.17 However, evidence has not been strong for any of these mechanisms. Recent data obtained by our group indicate that there is a transient but dramatic elevation of intracranial pressure (ICP) 24 hours after minor experimental stroke.18 Perhaps surprisingly, we can find no published data regarding ICP in patients with small strokes. We hypothesize that a similar transient elevation in ICP 24 hours after minor stroke may occur in patients and cause ‘collateral failure' leading to neurologic deterioration in stroke-in-progression. The aims of this study were to (1) investigate the key regulators of collateral and associated penetrating arteriole blood flow during stroke and reperfusion; (2) investigate changes in anterior cerebral artery-middle cerebral artery (ACA–MCA) ‘watershed' perfusion during stroke and reperfusion; (3) determine the effect of ICP elevation on collateral and ‘watershed' penetrating arteriole blood flow during stroke.
Materials and methods
Ethics Statement
All animal experiments were performed on male outbred Wistar rats (aged 7 to 12 weeks, body weight 300 g to 500 g; ASU breeding facility University of Newcastle, Australia). Experiments were approved by the Animal Care and Ethics Committee of the University of Newcastle (Protocols # A-2011-131 and # A-2011-112) and were in accordance with the requirements of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The studies were conducted and the manuscript prepared in accordance with the ARRIVE guidelines.19
In Vitro Microsphere Flow Validation
The fluorescent microsphere method for blood flow calculation was validated in vitro by comparison with the weighed volume of blood through the same system over a 10-minute time interval. Fluorescent microspheres (1 μm in diameter) (Molecular Probes, Eugene, OR, USA) diluted in heparinized rat blood (0.03% w/v) were infused via an automated syringe driver (Pump 11 Elite, Harvard Apparatus, Holliston, MA, USA) through polyurethane tubing (internal diameter 127 μm). Multiple flow rates (0.25 μL/min to 10 μL/min) were tested. At each rate, the end of the tubing was positioned inside an Eppendorf tube. Eppendorf tubes were weighed before the experiment and reweighed after exactly 10 minutes infusion to calculate the volume of fluid in the tube, i.e., ‘Measured Blood flow' (Qm), calculated in μL/min. Microsphere flow (Qmicro) was imaged at the midpoint of the 10-minute collection interval. Microspheres passing through the tube were recorded at 300 frames per second with a digital camera (Genie HM640, Teledyne Dalsa, Waterloo, ON, Canada) connected to a × 10 objective fluorescent microscope (BX60, Olympus, Tokyo, Japan). Microsphere blood flow was calculated using the following equation: Blood Flow=πr2 × v, where v is microsphere velocity in μm/s and r is the radius (half the diameter) of the vessel (tubing) in μm.
Experimental Protocols
Study I
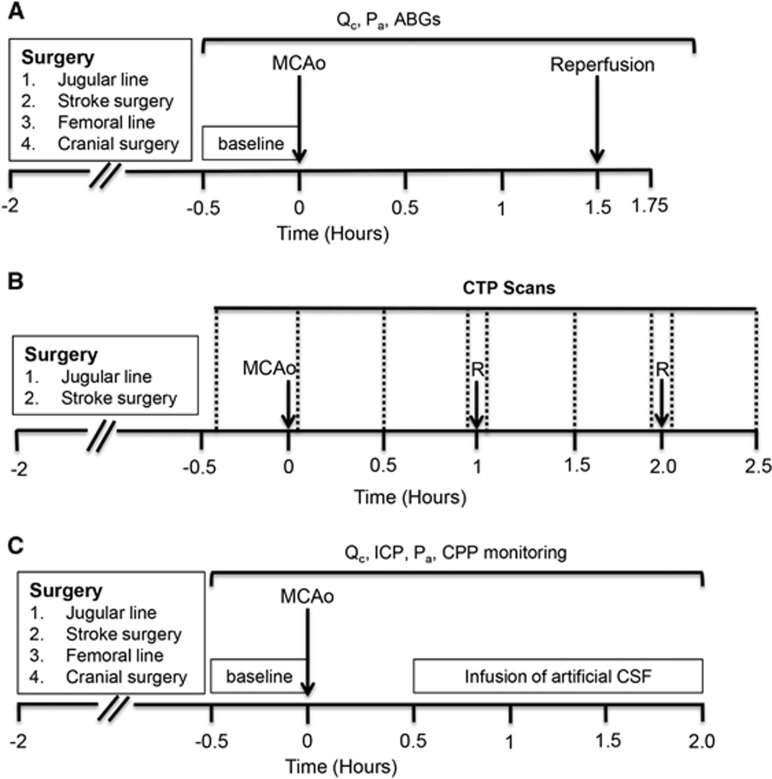
In Study I, we investigated the relative importance of changes in blood flow velocity and collateral vessel diameter in determining collateral blood flow during MCA occlusion (MCAo) and reperfusion. Animals underwent baseline recordings of ACA–MCA pial collateral and ‘watershed' penetrating arteriole blood flow before induction of experimental stroke (90 minutes MCAo, n=6). Collateral and ‘watershed' penetrating arteriole blood flow recordings were then taken every 10 minutes throughout occlusion and every 5 minutes for 15 minutes after reperfusion (Figure 1A).
Figure 1.
Experimental timelines. (A) Study I. Collateral and ‘watershed' penetrating arteriole blood flow was measured during 90 minutes MCAo and 15 minutes reperfusion. (B) Study II. CTP imaging (vertical dotted lines) was performed to measure whole brain perfusion changes during both permanent and temporary (1 hours or 2 hours) MCAo. After baseline CTP scans, scans were taken every 30 minutes during MCAo. Further scans were taken immediately after reperfusion (R) and at 30 minutes after reperfusion. Images obtained from these scans were then used to assess changes in ‘watershed' perfusion during occlusion and reperfusion. (C) Study III. ICP was artificially elevated by fluid infusion into the lateral ventricle and effects on collateral blood flow were determined. ABG, arterial blood gas; CPP, cerebral perfusion pressure; CTP, computed tomography perfusion; ICP, intracranial pressure; MCAo, middle cerebral artery occlusion; Pa, arterial pressure; Qc, collateral blood flow.
Study II
The aim of study II was to measure tissue perfusion in the ‘watershed' region supplied by the penetrating arterioles arising from ACA–MCA collaterals, using computed tomography perfusion (CTP) imaging. Animals were subjected to either permanent MCAo (n=6), or 1 hours (n=8) or 2 hours (n=7) temporary MCAo. CTP scans were taken at baseline and immediately after MCAo. For temporary strokes, CTP scans were taken every 30 minutes during occlusion, immediately after reperfusion, and at 30 minutes after reperfusion. For permanent strokes, CTP scans were taken every 30 minutes for the first 2 hours after occlusion (Figure 1B).
Study III
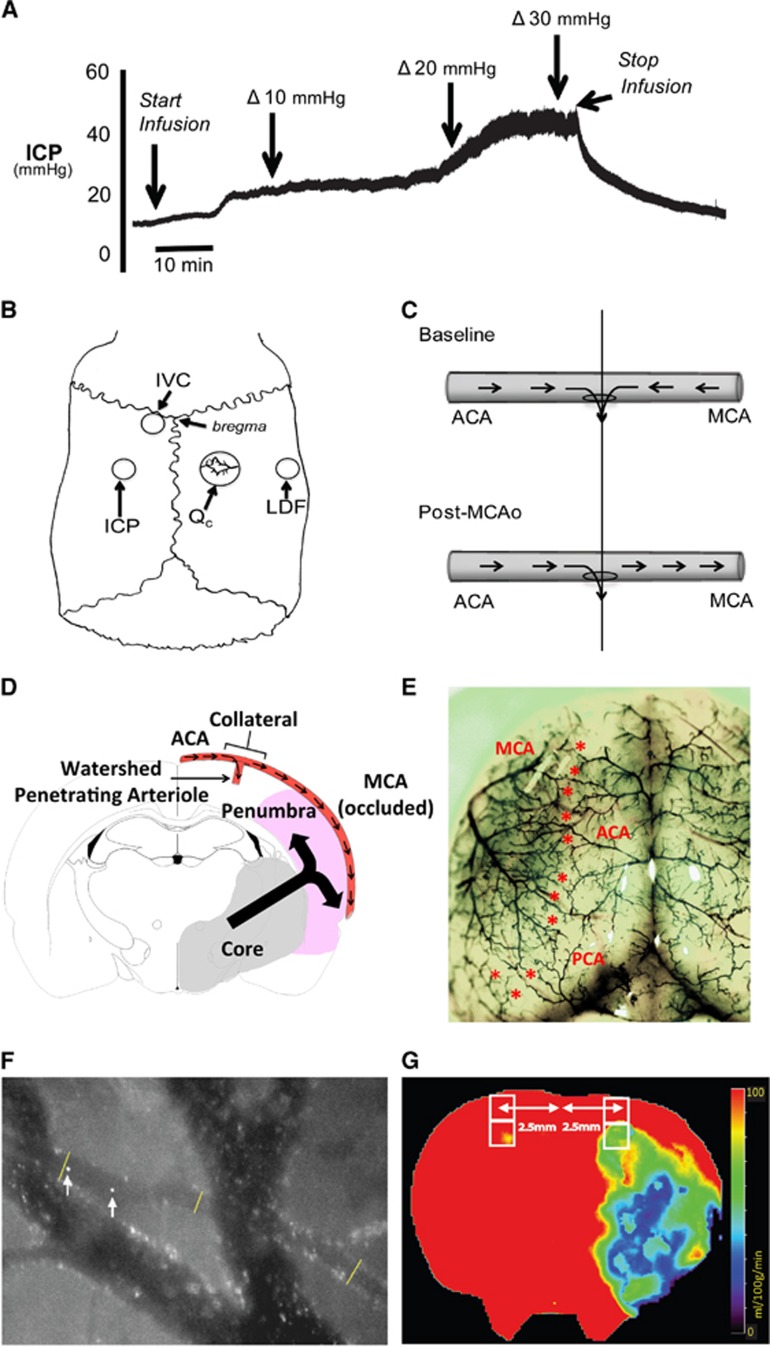
In study III, our aim was to determine the effect of ICP elevation on collateral and ‘watershed' penetrating arteriole flow during MCAo. After pre-MCAo baseline measurements (collateral and 'watershed' penetrating arteriole flow, ICP, and mean arterial pressure (MAP)), rats were subjected to permanent MCAo (n=6). A post-occlusion baseline measurement of collateral and 'watershed' penetrating arteriole flow was conducted 30 minutes after MCAo. Then, artificial ICP elevation was started by infusing artificial cerebrospinal fluid (aCSF) (SDR Scientific, Sydney, Australia) into the lateral ventricle, starting at a rate of 4 μL/min. The rate of aCSF infusion was gradually increased to raise ICP in 5 mm Hg increments (infusion rates needed for each 5 mm Hg increase are presented in Supplementary Table 1). At each 5 mm Hg ICP increment, a recording of collateral and 'watershed' penetrating arteriole flow was made. These incremental increases in ICP were continued until ICP reached 30 mm Hg above baseline (Figures 1C and 2A), equivalent to the values that occur naturally 24 hours after stroke in this model.18
Figure 2.
Surgical and experimental procedures. (A) Representative intracranial pressure (ICP) trace. ICP was increased by infusion of artificial cerebrospinal fluid (aCSF) into the left lateral ventricle of a rat post-stroke. The infusion rate was increased stepwise from 4 μL/min to 40 μL/min. This resulted in an increase in ICP with no significant change in arterial blood pressure, so cerebral perfusion pressure (CPP) (not shown) mirrored ICP changes. (B) Schematic of skull surgery and monitoring: cranial window used to measure collateral flow (Qc), laser Doppler flow (LDF) probe to measure middle cerebral artery (MCA) perfusion, intraventricular catheter (IVC) for infusion of aCSF and ICP probe location. (C) Schematic of collateral blood flow direction before and after MCA occlusion (MCAo). The ‘watershed' penetrating arteriole at the confluence of the bidirectional collateral vessel blood flow (top panel) was used to demarcate the anterior (ACA) and middle (MCA) cerebral artery portions of the vessel (vertical line). This same landmark was also used after MCAo (bottom panel). Blood flow was measured in both ACA and MCA portions of the vessel at each imaging time point. (D) Schematic showing the relationship of the collateral and 'watershed' penetrating arteriole vessels in relation to infarct core and penumbra in the MCA territory at 2.3 mm behind bregma (corresponding to the center of the cranial window). Arrows within the blood vessel indicate direction of blood flow during MCAo. The large black arrow indicates direction of infarct expansion into the penumbra toward the collateral vessel. Area of infarction is based of infarct probability maps 24 hours after permanent MCAo in our model.23 (E) Rat cortical vasculature showing the location of pial collateral vessels (colored latex perfusion). Collaterals between the ACA and the MCA, and posterior cerebral artery and MCA are marked with asterisks. (F) Calculation of collateral vessel flow. Image shows two merged frames, taken 3.3 ms apart. A collateral vessel and larger bridging veins are seen. Arrows show the location of a microsphere at two time points—distance travelled between frames is used to calculate velocity; lines show locations of vessel diameter measurements. (G) Representative computed tomography perfusion (CTP) cerebral blood flow (CBF) map at bregma. Two regions of interest (ROIs) were fitted (white boxes) 2.5 mm lateral to midline in each hemisphere, corresponding to the location of the penetrating arterioles that are supplied by the collaterals imaged in study I. Perfusion was measured by taking the average CBF within these ROIs.
Anesthesia and Monitoring
The anesthetic and monitoring protocols were as previously reported.20 Rats were anesthetized with 5% isoflurane in O2/N2 (1:3) and maintained with 1% to 2% isoflurane. Core temperature was maintained at 37°C by a thermocouple rectal probe (RET-2, Physitemp Instruments Inc., Clifton, NJ, USA) and warming plate (HP-1M, Physitemp). Incision sites were shaved, cleaned, and injected subcutaneously with 2 mg/kg 0.05% Bupivacaine (Pfizer, Sydney, Australia). Blood gases were monitored intermittently throughout study I and at baseline in study III. Blood samples (0.1 mL) were taken from a right femoral arterial line. This line was also used for continuous arterial pressure monitoring. Heart rate and respiratory rate were calculated from the clearly discernible cardiac and respiratory waveforms on the arterial pressure tracing.21
Surgical Procedures
Middle cerebral artery occlusion
Middle cerebral artery occlusion was induced using the silicone-tipped intraluminal thread occlusion method, using 4-0 monofilament occluding threads with 3.5 mm length × 0.35 mm diameter silicone tips, as previously reported.22, 23 For CT studies, final thread advancement to produce MCAo was performed on the CT scanning table, as previously reported.20
Collateral and ‘watershed' penetrating arteriole blood flow quantification method
A jugular venous line was placed for the constant infusion of microspheres, used to quantify collateral and associated penetrating arteriole blood flow.23, 24 A portion of the right parietal bone overlying the MCA–ACA ‘watershed' territory was removed using a high speed dental drill (Forte 100, Saeshen, Daegu, Korea) to create a cranial window extending 5 mm caudal × 5 mm lateral from a point at bregma and 1 mm lateral to midline (Figure 2B). Care was taken to leave the dura intact. The hole was filled with aCSF and sealed with a glass coverslip. A laser Doppler probe (LDF) (Moore Instruments, Sussex, UK) was placed just lateral to the cranial window to monitor the tissue perfusion of the MCA territory (Figure 2B). Middle cerebral artery occlusion was confirmed by >50% decrease in LDF signal. Prespecified exclusion criteria were lack of LDF drop, suboptimal cranial window, or subarachnoid hemorrhage on postmortem.
Placement of intracranial pressure monitor and intraventricular catheter
Two hollow PEEK (poly-ethyl ether ketone) screws (Solid Spot LLC, Santa Clara, CA, USA) of 2 mm diameter × 5 mm length were inserted for monitoring of epidural ICP and for insertion of an intraventricular catheter for artificial elevation of ICP by aCSF infusion (Figure 2B), according to our published method.18, 21 Intracranial pressure was measured using a fiber optic pressure transducer (420-LP, SAMBA Sensors, Gothenburg, Sweden)18 sealed into a saline-filled screw with biocompatible caulking material (Silagum, DMG Dental, Hamburg, Germany). Recent studies indicate negligible differences between ICP measurements taken epidurally or intraparenchymally in rats, and the epidural method avoids brain trauma and the risk of creating a CSF leak.25, 26 Probe location was validated by ensuring clear cardiac and respiratory waveforms and responsiveness of signal to abdominal compression.21 Cerebral perfusion pressure (CPP) was calculated using the formula CPP=MAP−mean ICP. In the more rostral screw, an intraventricular catheter was inserted 8 mm, so that the tip was in the lateral ventricle (Figure 2B). The catheter was also sealed into the screw with caulking material before aCSF infusion.
Collateral and ‘Watershed' Penetrating Arteriole Blood Flow Measurements
Fluorescently labelled microspheres (1 μm diameter, 0.2% w/v) (Molecular Probes) were continuously infused through the jugular line (4 mL/h) and could be seen transiting vessels within the cranial window. Pial collateral arterioles were identified as vessels between the ACA and MCA territories with microspheres entering from both ends, and exiting at the midpoint of the vessel (bidirectional flow). Microspheres exit the vessel at the confluence of ACA and MCA origin flow down a ‘watershed' penetrating arteriole, typically with a diameter of ~30 μm (illustrated in Figure 2C, Supplementary Figure 1A, shown in Supplementary Video 1). In each experiment, one collateral vessel and one ‘watershed' penetrating arteriole were visualized. The collateral vessel selected was the first vessel in which fluorescent microspheres could be visualized flowing in the characteristic bidirectional pattern previously reported.10, 11 The ‘watershed' penetrating arteriole located at the confluence of the bidirectional baseline collateral vessel flow was used to demarcate the ACA and MCA portions of the vessel for the duration of the study. The exact location of each vessel relative to bregma varied, since, as in much of the cerebral vasculature in rats and humans, there is significant interindividual anatomic variability. The available human imaging and animal experimental evidence indicates that in the setting of complete MCAo, the collateral vessels are the major source of residual perfusion to the entire ischemic penumbra. Moreover, infarcts expand toward these vessels from the more centrally located infarct core (Figure 2D).27 Proximal branches of the MCA receive collateral flow from multiple collaterals via distal branches (Figure 2E). Therefore, both pressure and vasodilation effects are anticipated to be equal in all of the ACA–MCA collateral vessels. Therefore, we did not focus on the exact location of the individual vessel studied, since the above data indicate that all ACA–MCA collateral vessels are likely to respond and contribute similarly.
Tracking of microspheres and calculation of blood flow were performed as per the in vitro microsphere flow validation (Figure 2F). Blood flow was calculated in both the ACA and MCA portions of the collateral vessel at each time point. To calculate ‘watershed' penetrating arteriole flow, the following equations were used, dependent on the type of flow:
During bidirectional flow, penetrating arteriole flow=ACA flow+MCA flow;
During unidirectional flow, penetrating arteriole flow=proximal flow—distal flow (proximal and distal flow were defined relative to the direction of flow, Figure 2C).
Computed Tomography Perfusion ‘Watershed' Image Acquisition, Processing, and Analysis
Detailed methods and CTP data have previously been reported for this cohort of animals;20, 23, 24 however, the ‘watershed' territory perfusion was not previously analyzed. Imaging was performed using a Siemens 64 slice CT scanner (Siemens, Erlangen, Germany) with a 512 × 512 matrix, 50 mm field of view with twelve 2.4 mm slices. Radio-opaque contrast for perfusion imaging was injected through a jugular venous catheter.20
Post-processing of perfusion data was performed using the MIStar imaging software (Apollo Medical Imaging Technology, Melbourne, Australia), as previously described with some minor modifications.20 In brief, cerebral blood flow (CBF) maps were generated from CTP images. The 2.4mm CTP slice at 0 mm bregma was analyzed for each animal. Two 1 mm × 1 mm regions of interest (ROIs; 1 superficial, 1 deep relative to skull) were generated and placed 2.5 mm lateral to the sagittal sinus (corresponding to the location of the ‘watershed' penetrating arterioles that are supplied by the collaterals imaged in study I) on the ipsilateral (right) and contralateral (left) cortex (Figure 2G). This allowed assessment of perfusion changes in the ‘watershed' territory between the ACA and the MCA. The CBF from the superficial and deep ROIs was then averaged.
Testing Cerebral Vasoreactivity
A control animal was subjected to isoflurane+endogenous CO2 challenge to ensure collateral vessel reactivity was preserved under experimental conditions. This animal underwent the same surgical procedures as the animals included in our analysis but without MCAo. Serial arterial blood gas analyses were undertaken while PaCO2 was increased in a stepwise manner by incremental increases in inhaled isoflurane concentration and accompanying reduction in respiratory rate. A recording of the diameter of a collateral vessel was made at each PaCO2.
Statistics
Statistical tests were performed using Graphpad Prism 5.04 (La Jolla, CA, USA) unless otherwise stated. Only data meeting criteria for normality, including visual examination of histograms as calculated skewness and kurtosis between +1 and −1, was analyzed. Linear regression analysis was performed to assess the correlation between microsphere blood flow and measured blood flow in vitro. Accuracy was calculated using the standard error of the estimate divided by the mean. Changes in collateral blood flow, collateral blood flow velocity, collateral vessel diameter, ‘watershed' penetrating arteriole blood flow, CTP-determined ‘watershed' perfusion, and physiologic variables during MCAo reperfusion or during ICP elevation were assessed using repeated measures one-way ANOVA with post hoc Dunnett's test. Repeated-measure observations during MCAo reperfusion or ICP elevation were analyzed by repeated-measures linear regression, using Stata 13 (StataCorp, College Station, TX, USA). We planned study III with six animals. We anticipated ICP elevation would reduce collateral flow by 35% with a standard deviation of 0.25. We were able to reject the null hypothesis that ICP does not reduce collateral flow with probability (power) 0.80. The type I error probability associated with the test of this null hypothesis (alpha) was 0.05. Data are presented as mean±standard deviation (s.d.). Statistical significance was accepted at P<0.05.
Results
In Vitro Microsphere Blood Flow Validation
Regression analysis indicated that the relationship between measured blood flow (Qm) and microsphere blood flow (Qmicro) was significant and linear (r2=0.99, P<0.0001). There were no significant differences between the regression line and the line of identity with respect to the intercept or slope. The Qmicro calculation was accurate to 9.1% (Supplementary Figure 2).
Study I—Key Determinants of Collateral Blood Flow During Middle Cerebral Artery Occlusion and Reperfusion
Seven animals were excluded. Reasons for exclusion were subarachnoid hemorrhage (n=1) or suboptimal cranial window (n=6). Physiologic variables for all studies are listed in Table 1. During MCAo and reperfusion, mean heart rate was higher than baseline (389±22 BPM baseline, 451±74 BPM during MCAo and 471±64 BPM reperfusion).
Table 1. Physiologic parameters.
| Baseline | MCAo | Reperfusion | |
|---|---|---|---|
| Study I | |||
| RR (BPM) | 53±7 | 60±13 | 60 ±10 |
| HR (BPM) | 389±22 | 451±74** | 471±64* |
| MAP (mm Hg) | 80±15 | 92±15 | 79±9 |
| pO2 (mm Hg) | 124±49 | 148±59 | 171±71 |
| pCO2 (mm Hg) | 44±12 | 42±7 | 45±5 |
| pH | 7.38±0.07 | 7.39±0.01 | 7.41±0.03 |
| Study II (CTP 1-hour MCAo) | |||
| RR (BPM) | 63±15 | 66±6 | 69±15 |
| HR (BPM) | 378±40 | 395±33 | 360±46 |
| Study II (CTP 2-hour MCAo) | |||
| RR (BPM) | 59±5 | 60±7 | 67±17 |
| HR (BPM) | 381±44 | 387±34 | 379±35 |
| Study II (CTP pMCAo) | |||
| RR (BPM) | 58±10 | 66±11 | |
| HR (BPM) | 381±46 | 371±41 | |
| Pre-MCAo baseline | Post-MCAo baseline | Peak ICP elevation | |
|---|---|---|---|
| Study III | |||
| MAP (mm Hg) | 84±15 | 92±16 | 80±10 |
| ICP (mm Hg) | 12±9 | 14±10 | 37±13###,*** |
| CPP (mm Hg) | 72±21 | 79±24 | 45±21###,*** |
| RR (BPM) | 58±7 | 70±10 | 54±6# |
| HR (BPM) | 368±22 | 434±65* | 402±41 |
| pO2 (mm Hg) | 184±42 | — | — |
| pCO2 (mm Hg) | 45±14 | — | — |
| pH | 7.40±0.04 | — | — |
Abbreviations: CPP, cerebral perfusion pressure; HR, heart rate; ICP, intracranial pressure; MAP, mean arterial pressure; MCAo, middle cerebral artery occlusion; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; RR, respiratory rate. Study I: **P<0.001, versus baseline. Study III: *P<0.05, ***P<0.001 versus pre-MCAo baseline; #P<0.05, ###P<0.001, versus post-MCAo baseline (P-values for illustrative purposes, uncorrected for multiple comparisons).
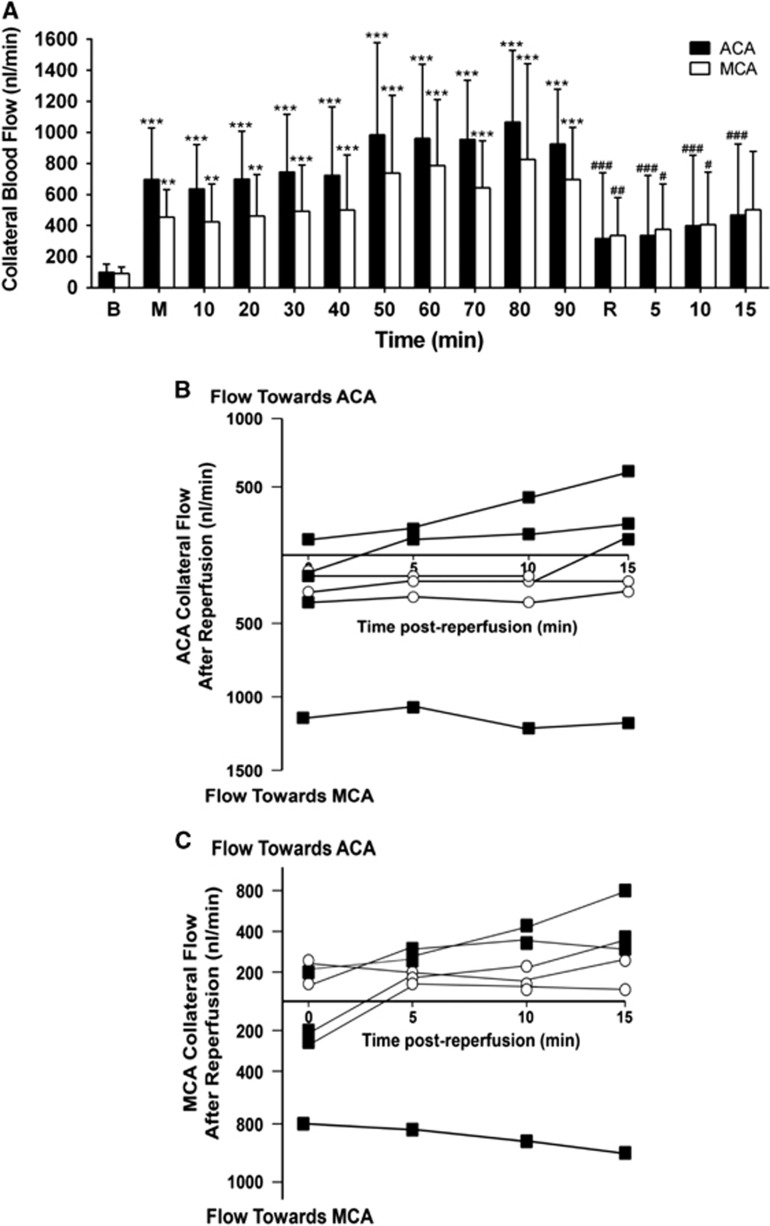
At baseline, slow bidirectional flow was observed in all collateral vessels (99±53 nL/min and 99±32 nL/min in ACA and MCA segments, respectively; Figure 3A). Flow was toward the center of the collateral from both directions, meeting at the central ‘watershed' penetrating arteriole, which microspheres from both ACA and MCA segments were seen to enter (illustrated in Figure 2C and Supplementary Figure 1A, shown in Supplementary Video 1). After MCAo, flow switched from bidirectional flow to unidirectional. Flow from the ACA segment remained anterograde; flow in the MCA segment became retrograde (illustrated in Figure 2C and Supplementary Figure 1B, shown in Supplementary Video 2). Flow increased dramatically in both segments, to 696±331 nL/min and 453±179 nL/min on the ACA and MCA sides of the vessel, respectively (699% and 458% of baseline, both P<0.01, Figure 3A). Further gradual increase in flow occurred, peaking 80 minutes after MCAo (1,066±469 nL/min and 825±617 nL/min; 1071% and 834% of baseline, respectively; both P<0.001, Figure 3A).
Figure 3.
Collateral flow increases during ischemia and decreases during reperfusion. (A) Collateral blood flow: absolute blood flow in the anterior cerebral artery (ACA) segment of the collateral vessel (solid columns) and blood flow in the middle cerebral artery (MCA) segment of the collateral vessel (hollow columns) during MCA occlusion (M) and reperfusion (R); **P<0.01, ***P<0.001 for post-RM-ANOVA Dunnett's test compared with baseline (B); #P<0.05, ##P<0.01, ###P<0.001 for post-RM-ANOVA Dunnett's test compared with 90 minutes MCAo (n=6). Average blood flow is represented irrespective of direction. Flow direction varied after reperfusion, therefore averaging both + and − flows would leave the impression that there was little to no flow through these vessels, which was not the case. Panels (B and C) illustrate the importance of directionality of flow post-reperfusion in the ACA and MCA segments of the collateral vessel. (B) Individual animal analysis of direction and magnitude of blood flow through the ACA segment of collateral vessels during reperfusion (nL/min). Squares=unidirectional flow, Circles=bidirectional flow. (C) Individual animal analysis of direction and magnitude of blood flow through the MCA segment of collateral vessels during reperfusion (nL/min). Squares, unidirectional flow; circles, bidirectional flow.
At reperfusion, three different blood flow patterns were observed within the ACA and MCA segments of the collateral vessel: return to bidirectional flow (anterograde on both sides, toward the center of the collateral), a complete reversal of flow so that flow was from MCA to ACA side (illustrated in Supplementary Figure 1C, shown in Supplementary Video 3) or persistent ACA to MCA flow (Figures 3B and 3C). Responses varied between animals, and within animals at different time points post-reperfusion. Regardless of the direction of flow, there was an immediate reduction in mean blood flow upon reperfusion, (from 924±353 nL/min pre-reperfusion to 316±424 nL/min post-reperfusion on the ACA side of collateral vessels and from 697±334 nL/min to 337±243 nL/min on the MCA sides, both P<0.01; Figure 3A).
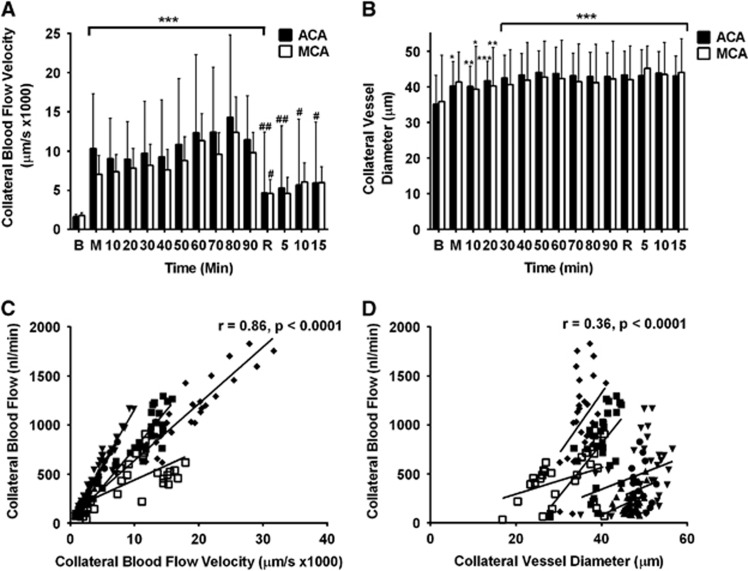
Collateral blood flow was calculated from blood flow velocity and vessel diameter. The contributions of each were examined to explore the major determinants of the dramatic changes in flow that were observed at the times of vessel occlusion and reperfusion. The velocity of collateral blood flow increased significantly immediately post-MCAo to 10,336±6,965 μm/s and 7,041±5,443 μm/s on the ACA and MCA sides of the collateral vessel, respectively (600% and 395% of baseline, respectively, both P<0.001). Velocity remained significantly above baseline throughout MCAo and peaked 80 minutes after MCAo (Figure 4A). The diameter of collateral vessels increased only gradually after MCAo, to 44.1±6 μm and 45.2±6 μm on the ACA and MCA side of the vessel, respectively (125% and 126% of baseline, respectively, P<0.05 versus baseline at all time points) (Figure 4B). Collateral blood flow velocity was strongly correlated, and collateral vessel diameter weakly correlated with collateral blood flow (within subject r=0.86 and 0.36, respectively, both P<0.0001; Figures 4C and 4D).
Figure 4.
Collateral flow change is strongly correlated with blood flow velocity changes and only weakly correlated with vessel diameter. (A) Collateral blood flow velocity and (B) Collateral vessel diameter, in the anterior cerebral artery (ACA, solid columns) and middle cerebral artery (MCA, hollow columns) segments of the collateral vessels during MCA occlusion (MCAo) and reperfusion. *P<0.05, **P<0.01, ***P<0.001 for post-RM-ANOVA Dunnett's test compared with baseline; #P<0.05, ##P<0.01 for post-RM-ANOVA Dunnett's test compared with 90 minutes MCAo. Repeated measures linear regression analysis of collateral blood flow versus blood flow velocity (C) and vessel diameter (D). Data points include measurements from both the ACA and MCA sides of each collateral vessel, recorded at each time point. Separate data symbols and regression lines are shown for each animal (n=6). B, baseline; M, MCAo; R, reperfusion.
In the control experiments to determine the effects of isoflurane and PaCO2 on collateral vasomotor reactivity, the collateral vessel exhibited a stepwise increase in diameter in response to incremental increases in endogenous CO2 concentrations. Diameter increased from 32.42 μm at a PaCO2 of 38 mm Hg (low normal range) to 38.60 μm at 44 mm Hg (high normal range) and 46.04 μm at 60 mm Hg (hypercapnia) (Supplementary Figure 3).
‘Watershed' Penetrating Arteriole Flow and Tissue Perfusion During Middle Cerebral Artery Occlusion and Reperfusion
‘Watershed' penetrating arteriole flow—microsphere technique
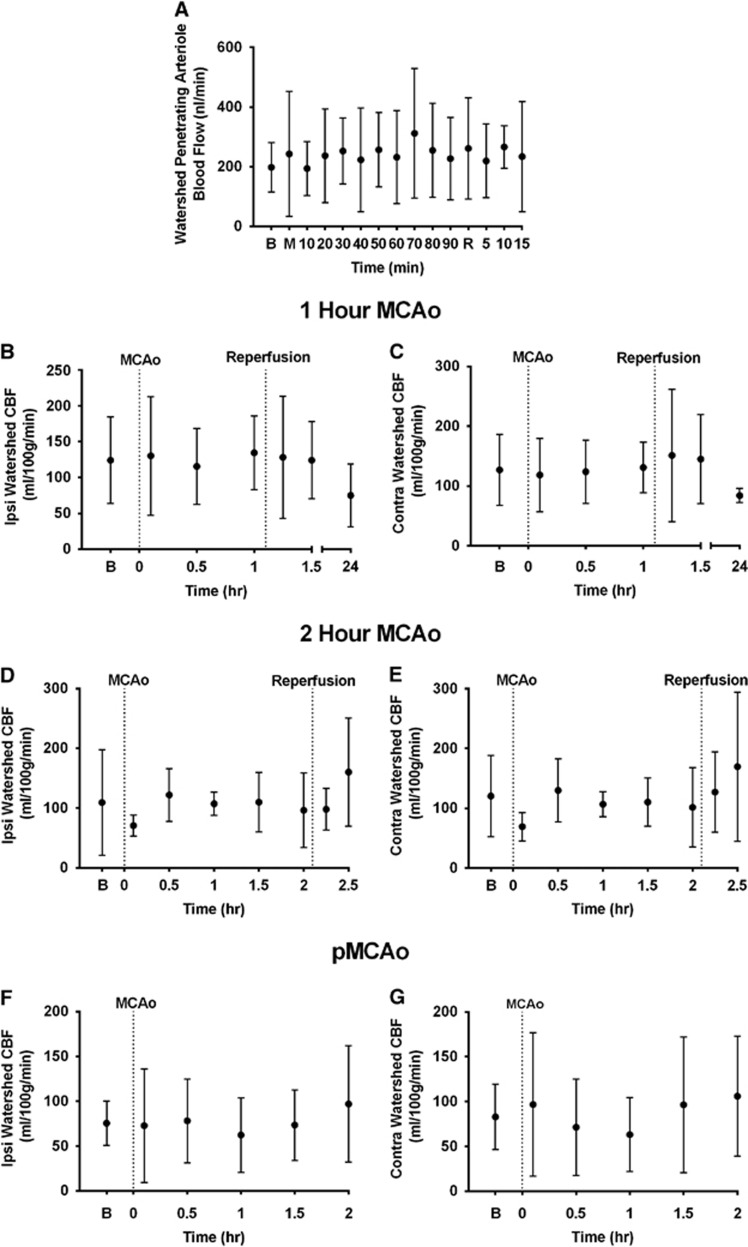
Mean ‘watershed' penetrating arteriole blood flow remained relatively constant throughout MCAo and reperfusion. It was 198±83 nL/min at baseline, and ranged from 186±170 nL/min to 312±217 nL/min during MCAo, and from 219±123 nL/min to 266±71 nL/min during reperfusion, with no clear trend of change over time (Figure 5A).
Figure 5.
‘Watershed' penetrating arteriole blood flow and ‘watershed'-region tissue perfusion remain constant throughout middle cerebral artery (MCA) occlusion (MCAo) and reperfusion. (A) Blood flow in the ‘watershed' penetrating arterioles arising from collateral vessels calculated using fluorescent microspheres. (B–G) Ipsilateral and contralateral 'watershed' tissue perfusion assessed using serial computed tomography perfusion (CTP) cerebral blood flow (CBF) during MCA occlusion±reperfusion, during 1-hour MCAo (B and C), 2-hour MCAo (D and E) and permanent MCAo (pMCAo) (F and G). B, baseline; M, MCAo; R, reperfusion.
‘Watershed' tissue perfusion—computed tomography perfusion technique
Mean ‘watershed' CBF in the ipsilateral and contralateral cortex pre-MCAo were 105±63 mL/100 g per minute and 112±57 mL/100 g per minute, respectively. These values showed little variability immediately post-MCAo (95±67 mL/100 g per minute and 97±60 mL/100 g per minute, respectively). In the individual experimental groups, CBF in the ‘watershed' also varied very little after reperfusion after either 1-hour or 2-hour MCAo (Figures 5B–E). In the permanent occlusion group, values also varied little within 2 hours of occlusion (Figures 5F and 5G) There was a nonsignificant trend to reduction in perfusion to both the ipsilateral and contralateral ‘watersheds' at 24 hours in the 1-hour MCAo group (75±44 mL/100 g per minute and 84±12 mL/100 g per minute, respectively; Figures 5B and 5C). (Supplementary Figure 4 for representative CTP maps).
Study III—Effect of Intracranial Pressure Elevation on Collateral and ‘Watershed' Penetrating Arteriole Blood Flow
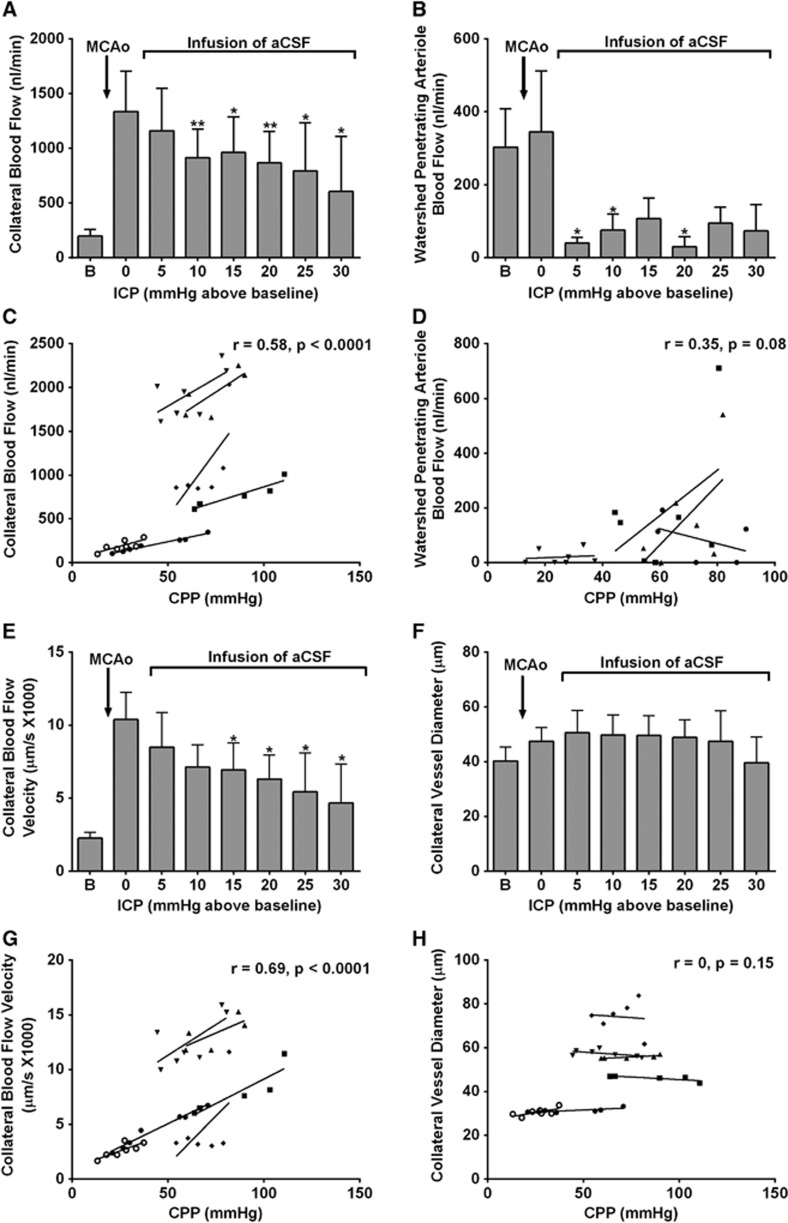
Two animals were excluded because of suboptimal cranial window. Heart rate was higher at post-MCAo baseline compared with pre-MCAo baseline (434±65 BPM versus 368±22 BPM; Table 1). Both blood pressure and heart rate were not significantly different between post-MCAo baseline and at peak ICP. Progressive elevation of ICP above baseline resulted in progressive reduction of CPP and significantly reduced collateral blood flow (1,337±900 nL/min at post-MCAo baseline versus 915±640 nL/min at 10 mm Hg and 605±872 nL/min at 30 mm Hg above baseline, P<0.05; Figure 6A. ‘Watershed' penetrating arteriole flow decreased dramatically even with a small increase of ICP to 5 mm Hg above baseline (Figure 6B). Flow reductions were statistically significant at ICPs of 5, 10, and 20 mm Hg above baseline (40±31, 75±88, and 30±56 nL/min, respectively, versus 303±210 nL/min at post-MCAo baseline, P<0.05). There was a significant correlation between CPP and collateral blood flow (within subject r=0.58, P<0.0001, Figure 6C) but not ‘watershed' penetrating arteriole blood flow (r=0.35, P>0.05; Figure 6D).
Figure 6.
Artificial elevation of intracranial pressure (ICP) reduces both collateral and ‘watershed' penetrating arteriole flow. The reduction in collateral flow is primarily driven by a significant reduction in blood flow velocity in the collateral vessel, not changes in vessel diameter. (A) Collateral blood flow and (B) ‘Watershed' penetrating arteriole flow, at baseline and at each incremental increase of ICP. *P<0.05, **P<0.01 for post-RM-ANOVA Dunnett's test compared with post-MCAo baseline. Linear regression analysis of cerebral perfusion pressure (CPP) versus: (C) collateral blood flow, and (D) ‘Watershed' penetrating arteriole blood flow, during ICP elevation; (E) collateral blood flow velocity and (F) collateral vessel diameter, at baseline and at each incremental increase of ICP. *P<0.05, for post-RM-ANOVA Dunnett's test compared with post-MCAo baseline. Linear regression analysis of CPP versus: (G) collateral blood flow velocity and (H) collateral vessel diameter, during ICP elevation (data points are measurements recorded at each time point, n=6 animals for collateral flow, blood flow velocity, and vessel diameter; n=4 animals for penetrating arteriole flow, individual regression lines are shown). aCSF, artificial cerebrospinal fluid; MCAo, middle cerebral artery occlusion.
Elevation of ICP to between 15 and 30 mm Hg above baseline significantly reduced collateral blood flow velocity (10,393±4,533 μm/s at post-MCAo baseline versus 6,939±4,508 μm/s at 15 mm Hg, and 4,668±4611 μm/s at 30 mm Hg above baseline, P<0.05; Figure 6E), but did not significantly alter collateral vessel diameter (47±13 μm at post-MCAo baseline versus 50±18 μm at 15 mm Hg, and 40±16 μm at 30 mm Hg; both P>0.05; Figure 6F). There was a significant correlation between CPP and collateral blood flow velocity (within subject r=0.69, P<0.0001; Figure 6G) but no significant correlation with diameter (within subject r=0, P>0.05; Figure 6H).
Discussion
Several findings from this study shed new light on the regulators of collateral flow and advance our understanding of the potential cause of ‘collateral failure' associated with infarct expansion in stroke patients. Changes in collateral blood flow after stroke were associated with only modest changes in vessel diameter, but large changes in blood flow velocity. From our knowledge of hydrodynamics, we know that the latter is a surrogate for changes in the pressure drop across the collateral vessel in response to MCAo. This appears to be the key factor affecting flow during MCAo and reperfusion, rather than collateral diameter changes. Blood flow through ‘watershed' penetrating arterioles supplied by collaterals was maintained during the early phases of ischemia when ICP is known not to be elevated. We were also able to confirm that tissue perfusion to the ‘watershed' territory supplied by these vessels was also stable during ischemia and reperfusion. Intracranial pressure elevation, to levels equivalent to those we have recently shown to occur 24 hours after stroke in this model,18 caused a progressive reduction in collateral blood flow and a dramatic and immediate reduction in penetrating arteriole blood flow.
Collateral vessels exhibited slow, bidirectional flow at baseline. Bidirectional flow has previously been observed in the collateral circulation of mice.10, 11 We show here that this also occurs in rats. At odds with these findings are the results from studies using laser speckle imaging that reported no flow under basal conditions.28, 29 Those studies were interpreted as indicating that these vessels do not ‘open up' until major vessel occlusion occurs. However in light of our current findings and the previous mouse data, a likely alternative explanation is that laser speckle imaging is unsuited to detection of the slow and bidirectional baseline flow seen in collateral vessels. Similarly, we saw that collateral blood flow continued after reperfusion, although at much lower velocities, and with variation in flow direction both between animals and within individuals over time. Again these findings contrast to those obtained with the laser speckle technique,28 likely for similar reasons. Our data indicate that collaterals have an important physiologic role under basal conditions, providing tissue perfusion to the ‘watershed' region located between the two vascular territories.
Immediately after MCAo, collateral flow became unidirectional, toward the ischemic MCA territory. Flow increased dramatically, however counter to our expectations, vessel diameter changes were only modest. Collateral vessels have larger diameters relative to vessels with similar flow velocities elsewhere in the cerebral circulation.30 The importance of this larger diameter becomes apparent during experimental stroke, when there are many-fold increases in velocity and absolute blood flow, which becomes unidirectional toward the occluded territory.10 This aspect of the structure of collateral vessels appears to be optimized to maintain stable baseline perfusion, while allowing for rapid increases of flow into the occluded arterial territory at onset of ischemia. The indication that vessel diameter changes had a relatively modest impact on the flow increase post-MCAo suggests that the CPP-driven pressure differential across the collateral vessel is the major driver of collateral blood flow during stroke.31 This is quite different to flow regulation of vascular beds under physiologic conditions, where vessel diameter is the key regulator of flow. The distinction is important conceptually, in optimizing our efforts to manipulate collateral flow therapeutically.
Flow through the ‘watershed' penetrating vessels arising from collaterals, and perfusion of the ipsilateral and contralateral ‘watershed' remained relatively constant throughout stroke. Consistent with our results, Toriumi et al11 showed that red cell velocity in penetrating arterioles associated with collateral vessels did not significantly change from baseline after MCAo. However in contrast to these findings, Shih et al32 reported a reduction in red blood cell flux in penetrating arterioles (driven by a reduction in red blood cell velocity) during MCAo. The likely explanation for these apparent discrepancies is that Shih et al32 did not specify whether the penetrating arterioles measured in their study arose directly from the collateral vessels, or from within the ischemic MCA territory. In the latter case, a reduction in flow would be anticipated. Sustained flow through the collateral-supplied ‘watershed' penetrating arterioles most likely gives rise to an area of oligemic tissue that borders the penumbra in stroke patients.33
Intracranial pressure elevation caused a significant linear reduction in collateral blood flow. The idea that alterations in CPP can alter perfusion of the ischemic penumbra is long established.34–36 Most of the residual perfusion to the penumbra travels via collateral vessels.12 A number of recent studies in animal models of stroke have sucessfully enhanced penumbral perfusion by increasing dilation of hypoxic arterioles using inhaled nitric oxide.34, 35 Others have increased flow within the terminal branches of the ACA or the pial collaterals between ACA and MCA by using pressor therapy or partial aortic occlusion, respectively.37, 38 A number of small clinical studies have attempted to enhance perfusion to the penumbra using pressor therapy. However, the efficacy of these approaches is yet to shown in large randomized trials.39 The effects of ICP on collateral perfusion are less often considered. We provide the first empirical evidence that elevation of ICP to levels equivalent to those occurring naturally after experimental stroke causes a significant stepwise reduction in CPP and collateral blood flow.18 We were unable to directly measure the pressure gradient between the adjacent territories. However, our data strongly suggest that elevation of ICP, by reducing CPP, reduced the driving pressure across the collateral vessel. This is the explanation for the observed stepwise reduction in blood flow velocity and flow.
As soon as ICP was elevated to as little as 5 mm Hg above baseline, ‘watershed' penetrating arteriole flow decreased dramatically and remained low thereafter. This result may be explained by the differing responses to ischemia of the pial MCA arterial branches (downstream of the retrograde collateral flow during MCAo) and the ‘watershed' penetrating arterioles. Penetrating arterioles are known as the ‘bottleneck' of perfusion to the cortex. They have been shown to maintain basal tone even after 2 hours temporary MCA occlusion reperfusion, whereas the pial MCA branches lose tone after even short durations of ischemia.40 Therefore, small reductions in CPP will have a much greater effect on ‘watershed' penetrating arterioles, since a greater driving pressure (CPP) is required to maintain flow across high resistance vessels. The lack of further flow reductions in the ‘watershed' penetrating arterioles with further ICP elevation may in part represent the counteracting effects of ‘watershed' penetrating arteriolar vasodilation (over a many minutes timescale) and concurrent progressive experimental elevation of ICP. Conversely, the maximally dilated pial MCA vasculature downstream of the collateral vessel is a low resistance circuit.41 Therefore, incremental changes in CPP would be expected to result in the observed linear reduction in collateral blood flow. The apparent selective sensitivity of the ‘watershed' penetrating arterioles arising from collateral vessels to changes in perfusion pressure is also a potential contributor to the selective sensitivity of this region to infarction in the setting of vessel stenosis, in which local fluctuations in perfusion pressure are also implicated.
Recent serial advanced imaging studies have revealed that patients with late infarct expansion have persistent arterial occlusion and good collateral status at baseline. The subsequent infarct expansion is associated with reduced collateral status or ‘collateral failure'.6, 7 Intracranial pressure is known to increase in patients with large malignant infarctions (>80% of the MCA territory),42 however because ICP monitoring is invasive it is not performed in patients with small strokes. We do not know whether these patients experience a transient ICP rise similar to that seen in rats with experimental stroke.18 Clinical data obtained for the CATCH study strongly highlighted the similarities in timing between the ICP elevation seen in our stroke model and clinical deterioration in stroke-in-progression, i.e., 53% of patients with stroke-in-progression deteriorated on day 1 (first full day in hospital).8 The findings of the current study suggest that ICP elevation is a very plausible mechanism for the ‘collateral failure' and subsequent late infarct expansion in ischemic stroke patients.
Our method for collateral blood flow quantification permits a degree of real-time quantification not available with other currently available techniques, and this provided us with tremendous insights regarding the regulation of collateral flow during stroke reperfusion and the response to ICP elevation. However, measurements were limited to a single collateral vessel in each animal. To date, there is no method to quantify total collateral blood flow through all collateral vessels in real time. However, we used CT perfusion imaging of tissue perfusion as a surrogate for this and to confirm our findings that stable ‘watershed' penetrating arteriole flow during MCAo reperfusion is accompanied by stable tissue perfusion of the ‘watershed' territories. The use of isoflurane anesthetic allowed optimal control of anesthetic depth and blood gases in freely breathing animals. However, isoflurane is known to dilate cerebral vessels. Our validation study supported the experimental data indicating that under the conditions the experiments were conducted, significant collateral vasodilatory potential remained. Using these data we have estimated potential effects of greater vasodilatory reserve to determine whether this would be likely to influence our primary findings. Even if vasodilation was assumed to be more than twice that seen under the influence of isoflurane, our estimates indicate that the effects of greater vasodilation on collateral blood flow would still be an order of magnitude less than the approximately 500% increase in flow that was seen immediately after MCAo.
In conclusion, we have shown that collateral vessels provide retrograde perfusion of the occluded arterial territory during stroke, while maintaining stable perfusion to the ‘watershed' territory. Change in collateral vessel diameter had little effect on changes in flow, which appeared to be primarily driven by changes in the pressure drop across the collateral vessel after arterial occlusion. Supporting the key role of this pressure differential, incremental elevation of ICP resulted in decreasing CPP, and caused a stepwise reduction in collateral blood flow. Coupled with our recent findings showing a dramatic but transient ICP elevation after minor experimental stroke, and human imaging studies indicating that ‘collateral failure' rather than thrombus extension is the likely mechanism for most stroke-in-progression, these findings have important potential clinical implications. They suggest that ICP elevation is a likely explanation for ‘collateral failure' and may be the mechanism responsible for late infarct expansion in ischemic stroke patients.
Acknowledgments
Acknowledgments: The authors thank the Faculty of Health Stores Workshop of the University of Newcastle for manufacture of bespoke surgical and anesthetic equipment. The authors also thank Associate Professor Patrick McElduff for his help with the statistical analysis in this project.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported in part by a NHMRC Project Grant, APP1033461, the Hunter Medical Research Institute from funds donated by the Greater Building Society, and by the National Stroke Foundation (Australia). Both D Beard and L Murtha were supported by an Australian Postgraduate Award. N Spratt was supported by a NHMRC career development fellowship, APP1035465.
Supplementary Material
References
- Davalos A, Castillo J.Progressing strokeFisher M, Bogousslavsky J. Current Review of Cerebrovascular Disease Philadelphia: Springer; 2001pp 169–181. [Google Scholar]
- Ali LK, Saver JL. The ischemic stroke patient who worsens: new assessment and management approaches. Rev Neurol Dis. 2007;4:85–91. [PubMed] [Google Scholar]
- Coutts SB, Modi J, Patel SK, Aram H, Demchuk AM, Goyal M, et al. What causes disability after transient ischemic attack and minor stroke?: Results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke. 2012;43:3018–3022. doi: 10.1161/STROKEAHA.112.665141. [DOI] [PubMed] [Google Scholar]
- Caplan LR. Treatment of "progressive" stroke. Stroke. 1991;22:694–695. doi: 10.1161/01.str.22.5.694. [DOI] [PubMed] [Google Scholar]
- Roden-Jullig A, Britton M. Effectiveness of heparin treatment for progressing ischaemic stroke: before and after study. J Intern Med. 2000;248:287–291. doi: 10.1046/j.1365-2796.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- Asdaghi N, Hameed B, Saini M, Jeerakathil T, Emery D, Butcher K. Acute perfusion and diffusion abnormalities predict early new MRI lesions 1 week after minor stroke and transient ischemic attack. Stroke. 2011;42:2191–2195. doi: 10.1161/STROKEAHA.110.611376. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke. 2012;43:1013–1017. doi: 10.1161/STROKEAHA.111.637421. [DOI] [PubMed] [Google Scholar]
- Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriumi H, Tatarishvili J, Tomita M, Tomita Y, Unekawa M, Suzuki N. Dually supplied T-junctions in arteriolo-arteriolar anastomosis in mice: key to local hemodynamic homeostasis in normal and ischemic states. Stroke. 2009;40:3378–3383. doi: 10.1161/STROKEAHA.109.558577. [DOI] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Wiltshire T, Sealock R, Faber JE. Genetic dissection of the Canq1 locus governing variation in extent of the collateral circulation. PLoS ONE. 2012;7:e31910. doi: 10.1371/journal.pone.0031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- Pranevicius O, Pranevicius M, Pranevicius H, Liebeskind DS. Transition to collateral flow after arterial occlusion predisposes to cerebral venous steal. Stroke. 2012;43:575–579. doi: 10.1161/STROKEAHA.111.635037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov AV, Sharma VK, Lao AY, Tsivgoulis G, Malkoff MD, Alexandrov AW. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke. 2007;38:3045–3048. doi: 10.1161/STROKEAHA.107.482810. [DOI] [PubMed] [Google Scholar]
- Palamarchuk I, Kimpinski K, Lippert C, Hachinski V. Nocturnal deterioration after ischemic stroke and autonomic dysfunction: hypothesis and implications. Cerebrovasc Dis. 2013;36:454–461. doi: 10.1159/000356093. [DOI] [PubMed] [Google Scholar]
- Murtha LA, McLeod DD, McCann SK, Pepperall D, Chung S, Levi CR, et al. Short-duration hypothermia after ischemic stroke prevents delayed intracranial pressure rise. Int J Stroke. 2014;9:553–559. doi: 10.1111/ijs.12181. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments—the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DD, Parsons MW, Levi CR, Beautement S, Buxton D, Roworth B, et al. Establishing a rodent stroke perfusion computed tomography model. Int J Stroke. 2011;6:284–289. doi: 10.1111/j.1747-4949.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- Murtha L, McLeod D, Spratt N. Epidural intracranial pressure measurement in rats using a fiber-optic pressure transducer. J Vis Exp. 2012;62:e3689. doi: 10.3791/3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- McLeod DD, Beard DJ, Parsons MW, Levi CR, Calford MB, Spratt NJ. Inadvertent occlusion of the anterior choroidal artery explains infarct variability in the middle cerebral artery thread occlusion stroke model. PLoS ONE. 2013;8:e75779. doi: 10.1371/journal.pone.0075779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DD, Parsons MW, Hood R, Hiles B, Allen J, McCann SK, et al. Perfusion computed tomography thresholds defining ischemic penumbra and infarct core: studies in a rat stroke model. Int J Stroke. 2013;6:284–289. doi: 10.1111/ijs.12147. [DOI] [PubMed] [Google Scholar]
- Hiploylee C, Colbourne F. Intracranial pressure measured in freely moving rats for days after intracerebral hemorrhage. Exp Neurol. 2014;255:49–55. doi: 10.1016/j.expneurol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Uldall M, Juhler M, Skjolding AD, Kruuse C, Jansen-Olesen I, Jensen R. A novel method for long-term monitoring of intracranial pressure in rats. J Neurosci Methods. 2014;227:1–9. doi: 10.1016/j.jneumeth.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Wright P, Chambers BR, Sachinidis JI, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34:2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- Armitage GA, Todd KG, Shuaib A, Winship IR. Laser speckle contrast imaging of collateral blood flow during acute ischemic stroke. J Cereb Blood Flow Metab. 2010;30:1432–1436. doi: 10.1038/jcbfm.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Luo W, Zhou F, Li P, Luo Q. Dynamic change of collateral flow varying with distribution of regional blood flow in acute ischemic rat cortex. J Biomed Opt. 2012;17:125001. doi: 10.1117/1.JBO.17.12.125001. [DOI] [PubMed] [Google Scholar]
- Rovainen CM, Woolsey TA, Blocher NC, Wang DB, Robinson OF. Blood flow in single surface arterioles and venules on the mouse somatosensory cortex measured with videomicroscopy, fluorescent dextrans, nonoccluding fluorescent beads, and computer-assisted image analysis. J Cereb Blood Flow Metab. 1993;13:359–371. doi: 10.1038/jcbfm.1993.49. [DOI] [PubMed] [Google Scholar]
- Alexandrov AW. Hyperacute ischemic stroke management: reperfusion and evolving therapies. Crit Care Nurs Clin North Am. 2009;21:451–470. doi: 10.1016/j.ccell.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Shih AY, Friedman B, Drew PJ, Tsai PS, Lyden PD, Kleinfeld D. Active dilation of penetrating arterioles restores red blood cell flux to penumbral neocortex after focal stroke. J Cereb Blood Flow Metab. 2009;29:738–751. doi: 10.1038/jcbfm.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Bonnin P, Gharib A, Leger PL, Villapol S, Pocard M, et al. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke. 2012;43:3078–3084. doi: 10.1161/STROKEAHA.112.664243. [DOI] [PubMed] [Google Scholar]
- Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110:727–738. doi: 10.1161/CIRCRESAHA.111.253419. [DOI] [PubMed] [Google Scholar]
- Urrutia VC, Wityk RJ.Blood pressure management in acute stroke Neurol Clin 200826565–583.x-xi. [DOI] [PubMed] [Google Scholar]
- Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Armitage GA, Ramakrishnan G, Dong B, Todd KG, Shuaib A. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab. 2014;34:61–71. doi: 10.1038/jcbfm.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke: systematic review. Stroke. 2006;37:1565–1571. doi: 10.1161/01.STR.0000222002.57530.05. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–1492. doi: 10.1038/jcbfm.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.