Abstract
In vivo studies have shown that blood–brain barrier (BBB) dysfunction is involved in the course of Parkinson's disease (PD). However, these have lacked either anatomic definition or the ability to recognize minute changes in BBB integrity. Here, using histologic markers of serum protein, iron, and erythrocyte extravasation, we have shown significantly increased permeability of the BBB in the postcommissural putamen of PD patients. The dense innervation of the striatum by PD-affected regions allows for exploitation of this permeability for therapeutic goals. These results are also discussed in the context of the retrograde trans-synaptic hypothesis of PD spread.
Keywords: α-synuclein, blood–brain barrier, Parkinson's disease, striatum
Introduction
Parkinson's disease (PD) is characterized by aggregation of filamentous or oligomerized α-synuclein and degeneration of specific neuronal regions. According to the dual-hit hypothesis of PD pathogenesis, a microbial or toxic pathogen within the gut lumen and the olfactory mucosa represents the pathogenic agent, with subsequent prion-like spread of pathology, first to the dorsal motor nucleus of the vagus and central olfactory areas, respectively, then to higher centers. The connectional anatomy linking the dorsal motor nucleus and olfactory bulb with higher centers such as the substantia nigra is unproven.
We have previously proposed that disease could be spread hematogenously.1 This is conceivable within the paradigm of retrograde (axon terminal to soma) Lewy pathology spread.2, 3 Almost all regions affected in Braak stage 1 have axon terminals outside the blood–brain barrier (BBB). That same BBB, however, protects the higher-order regions, whose axon terminals reside within the central nervous system, from blood-borne substances. Cell-to-cell spread is a potential mechanism to circumvent this protection but the connectional neuroanatomy is not consistent with the temporal order of Lewy pathology development as it is currently understood.
Nigrostriatal dopaminergic neuronal cell bodies develop significant Lewy pathology, but recent studies strongly suggest that α-synuclein aggregation in these cells begins at presynaptic axon terminal in the striatum and spreads retrogradely to nigral cell bodies.3, 4 In this context, the absence of an intrinsic striatal source of aggregated αsyn before αsyn aggregation in nigrostriatal axon terminals would seem to preclude cell-to-cell spread as a mechanism by which such aggregation is initiated in nigrostriatal neurons. Instead, we postulate that, as in the peripheral nervous system, synuclein aggregation in nigrostriatal neurons, as well as in other higher-order neurons in the brain, is initiated by a soluble, pathogenic, aggregation-inducing agent present in the systemic circulation, which requires disruption of the BBB in striatum to achieve access to nigrostriatal axon terminals. In this context, we sought to evaluate the integrity of the BBB in PD striatum.
Although immunohistochemical methods in the past often proved inadequate to distinguish premortem BBB permeability from postmortem leakage, modern techniques and reagents have proven that this is no longer the case. Leakiness of BBB has been confirmed using these techniques in many neurologic disease.5, 6 There is evidence for BBB hyperpermeability in PD by cerebrospinal fluid analysis and positron-emission tomography imaging but immunohistochemical proof remains elusive.7, 8
In the present study, we have used classic histologic staining and immunohistochemical techniques to examine BBB integrity in a postmortem study of the striata of a well-characterized cohort of PD patients and controls. The results are discussed in the context of an alternative model for the pathogenesis of PD.
Materials and methods
Patient Tissues
Formalin-fixed, paraffin-embedded tissue from postcommissural putamen was obtained from the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona. Ethics approval was received from the Ottawa Hospital Research Ethics Board (#20140364-01H). Relevant patient information can be found in Supplementary Table 1. There were no statistically significant differences in age (P=0.16) or postmortem interval (P=0.87) between groups. Vascular risk factors including hypertension, coronary artery disease, and diabetes mellitus were present in 8/11 healthy controls and 8/12 subjects with PD. In all, 12/12 of PD subjects had been treated with L-DOPA with various dosages for various time periods, with 1/12 having a history of dyskinesia. Previous studies have found similar sized sample groups to be adequate for determination of BBB integrity.5, 6
Staining Procedures
Hematoxylin & eosin, Perls' Prussian Blue, and immunofluorescent staining procedures are described in Supplementary Information.
Image Analysis
For evaluation of erythrocyte extravasation and perivascular hemosiderin deposits, three stained sections per patient were scanned using a ScanScopeCS (Aperio, Vista, CA, USA) and analyzed using the Imagescope software (Aperio). To perform quantification, 10 areas of 1 mm2 were randomly selected on each section and the number of extravasated erythrocytes or perivascular Prussian Blue staining was manually quantified. Error bars represent the standard error of the mean (s.e.m.).
For quantification of extravasated serum proteins, five images of 0.15 mm2 were randomly selected on each of three sections per patient. Images captured in Axiovision were then transferred to ImageJ (NIH, Bethesda, MD, USA) and quantification was performed as previously described.6
Analysis of all images was performed blinded to the disease status of the sample.
Results
Extravasated Erythrocytes Are More Abundant in Parkinson's Disease than in Control Striatum
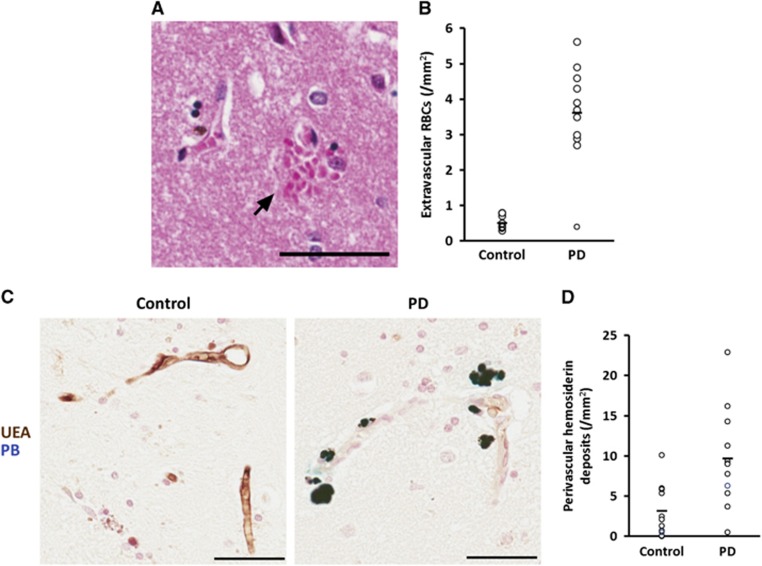
To characterize the integrity of the BBB in PD striatum, we examined hematoxylin & eosin stained sections for evidence of erythrocytes lying within the striatal gray matter but outside vascular lumina (Figure 1A). We found PD striatum to have sevenfold more extravascular erythrocytes per mm2 (P<2 × 10−7), compared with the minimal extravasation seen in controls (Figure 1B).
Figure 1.
Extravasated erythrocytes and perivascular hemosiderin deposits are more abundant in Parkinson's disease (PD) striatum. (A) Multiple erythrocytes (arrow) lying within the striatal parenchyma adjacent to the wall of a striatal vessel. Scale bar=50 μm. (B) Quantification of extravascular erythrocytes in the striata of control and PD subjects. P<2 × 10−7. Line represents mean. n=11 controls, 12 PD. (C) Representative micrographs of Prussian Blue staining demonstrating perivascular hemosiderin (dark blue) adjacent to ulex europaeus agglutinin-stained vessels (brown) in PD striatum (right) and lack thereof in control tissue (left). Scale bars=50 μm. (D) Quantification of perivascular hemosiderin in the striata of control and PD subjects. P<0.005. Line represents mean. n=11 controls, 12 PD.
Perivascular Hemosiderin Deposits
To further quantify BBB permeability, we used UEA to stain blood vessels and the Perls' Prussian blue reaction to show ferric iron in hemosiderin. Brown UEA-staining vessels were readily observed throughout striatum in all cases with no obvious differences in endothelial continuity or integrity between PD and control. Hemosiderin was visible in all cases, and was easily distinguishable from UEA staining, appearing as a turquoise to midnight blue reaction product (Figure 1C). Quantification (Figure 1D) showed threefold more deposits in PD brain (P<0.005) when compared with controls and these often appeared as discretely grouped around capillaries.
Serum Protein Leakage from Parkinson's Disease Striatal Vessels
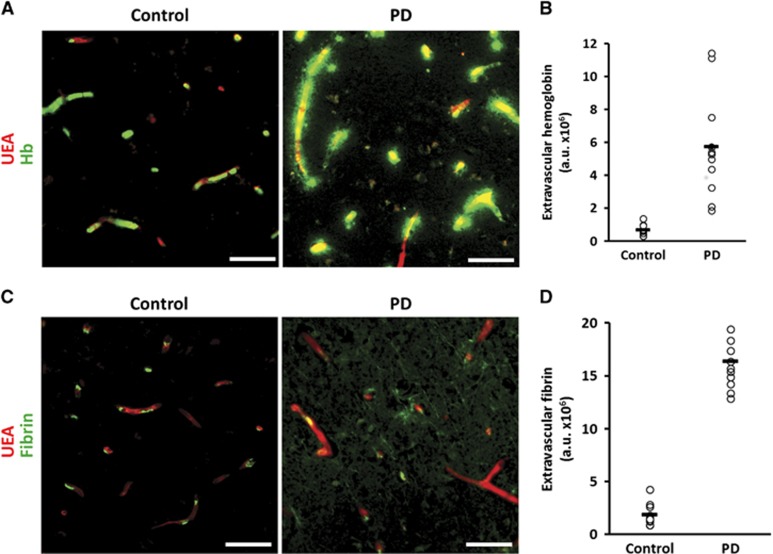
Erythrocyte diapedesis requires significant gaps in the integrity of the BBB. Serum proteins leak faster and further into brain parenchyma than erythrocytes.9 To assess indices of more subtle leakage, we studied the pattern of extravasation of serum proteins by immunofluorescent microscopy. Significant perivascular deposits of hemoglobin (Figure 2A, right) and fibrin (Figure 2C, right) were noted in PD striatum. In contrast, the same proteins in control brain were almost entirely contained within vessel lumina (Figures 2A and 2C, left). Quantification analysis specifically of extravascular protein revealed an 8.6-fold increase in extravasated hemoglobin in PD (P<2 × 10−5; Figure 2B). Fibrin(ogen) showed a similar 9.4-fold extravascular increase in PD (P<2 × 10−5; Figure 2D).
Figure 2.
Leakage of serum proteins is increased in Parkinson's disease (PD) striatum. (A) Hemoglobin (Hb; green) and ulex europaeus agglutinin-positive vessel (red) staining in control (left) and PD (right) striatum. Scale bars=50 μm. (B) Quantification of the extravascular portion of Hb staining P<2 × 10−5. Line represents mean. n=11 controls, 12 PD. (C) Fibrin(ogen) (green) and UEA-positive vessel (red) staining in control (left) and PD (right) striatum. Scale bars=50 μm. (D) Quantification of the extravascular portion of fibrin(ogen) staining P<3 × 10−5. Line represents mean. n=11 controls, 12 PD.
Discussion
The BBB is a key to the vascular system in the central nervous system, where it prevents nonspecific export of large and/or polar molecules. This postmortem study in a well-characterized population shows compromised BBB integrity in the striatum of PD patients by various methods including erythrocyte extravasation, perivascular hemosiderin, and leakage of various serum proteins outside UEA-staining vessel walls. Intraparenchymal erythrocytes were generally found immediately abutting the abluminal vessel wall (Figure 1) and Hb extravasation (Figures 2A and 2B) displayed a markedly more restricted circumferential perivascular migration than that of fibrin (Figures 2C and 2D). This is consistent with the previous reports of BBB disruption that describe a central Hb/erythrocyte core lying within a circumferentially larger serum protein deposit.6, 9
This is the first report of BBB dysfunction in the striatum of PD patients but these findings are supported by the previous animal work. The MPTP and 6-hydroxydopamine PD mouse models show impaired striatal BBB integrity with disruption of tight junctions and gliosis in the former, and increased angiogenesis in the latter.10, 11 MPTP also causes extravasation of serum albumin into brain parenchyma.10 It must be kept in mind that these models result from acute toxic injection and therefore differ from the natural insidious course of PD.
In humans, PD BBB integrity has previously been assessed in the midbrain. Extravascular CD4+ and CD8+ lymphocytes can be showed histologically in PD SNpc.10 By positron-emission tomography, the BBB efflux pump P-glycoprotein shows reduced function in the PD midbrain with increased uptake of the tracer 11C-verapamil.8 Polymorphisms in the P-gp gene have been associated with PD. Biochemically, serum albumin can be found in cerebrospinal fluid from PD patients.7
In addition, BBB dysfunction has been seen in striatum of PD patients experiencing L-DOPA-induced dyskinesia.12 Although all of the PD subjects in this study were treated with L-DOPA, only one experienced dyskinesia. As the BBB disruption in L-DOPA dyskinesia has been attributed to vascular endothelial growth factor-induced angiogenesis, we confirmed that there was no significant difference in UEA-positive vascular area between PD and control striata in this study (P=0.96; Supplementary Figure 1).
The clinical diagnosis of PD requires nigral dysfunction and therefore nigral Lewy pathology. If loss of BBB integrity occurs in the striatum before the onset of higher-order Lewy pathology, then presynaptic axon terminals of striatal afferents would become exposed to blood-borne substances. There is circumstantial anatomical evidence to support this possibility. Striatal afferent fibers are derived from several brain regions affected in Lewy body disease, such as the SNpc and limbic cortex. For example, the cingulate cortex, which develops significant Lewy pathology, provides a dense innervation of the striatum. Within the striatum, striosomes reportedly have a leakier BBB than matrix.13 Both the nigral neurons of the nigrosomes and the limbic cortical neurons of layers V and VI (which develop strong, earlier lewy bodies) project exclusively to striosomes.14, 15
The BBB of the striatum is especially fragile, whether to ischemic, osmotic, or other stressors. After unilateral ischemic stress, increased BBB permeability appears in the striatum before any other brain region, both ipsi- and contralaterally.16 It is important to note that loss of BBB integrity is normal in the aging brain. Subjects experiencing prodromal symptoms of PD (e.g., constipation and hyposmia) from diseased peripheral-projecting neurons (i.e., olfactory bulb and enteric nervous system), may, in the course of BBB breakdown, develop lesions in the striatum as a result of those same soluble agents now having access to central striatopetal axon terminals.
It remains unclear whether striatal BBB permeability changes precede or are a result of inflammation in established central nervous system Lewy body disease. This is an unavoidable limitation of any study involving postmortem PD brain tissue. Given the known role for inflammation after neuronal injury, it is plausible that inflammation follows axonal dying back from a striatal afferent (e.g., SNpc).
The present study shows a loss of integrity of the BBB in the striata of patients with PD. This is consistent with evidence for BBB dysfunction in an increasing number of neurodegenerative disorders, including Alzheimer's disease, amyotrophic lateral sclerosis, and chronic traumatic encephalopathy. The postmortem nature of this study renders interpretation of the temporal aspects of this dysfunction difficult. However, given increasing evidence that α-synuclein aggregation is initiated at the level of axon terminals,3, 4 it is tempting to speculate that altered BBB permeability in the striatum precedes the development of nigral/cortical Lewy body disease. We believe that this would allow blood-borne substances to reach the axon terminals of striatopetal neurons and initiate aggregation of α-synuclein. Further studies in model organisms such as mice or nonhuman primates will allow assessment of this hypothesis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
The authors are appreciative of insights provided by Drs Doug Gray & David Munoz. The authors are grateful to the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human brain tissue.
Funding for research in the Woulfe lab is funded by the Michael J Fox Foundation for Parkinson's Research, Parkinson Society Ottawa, and the Parkinson's Research Consortium Ottawa. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J Fox Foundation for Parkinson's Research.
Supplementary Material
References
- Woulfe JM, Gray MT, Gray DA, Munoz DG, Middeldorp JM. Hypothesis: a role for EBV-induced molecular mimicry in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:685–694. doi: 10.1016/j.parkreldis.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Mori F, Mimura J, Itoh K, Kakita A, Takahashi H, et al. Proteinase K-resistant alpha-synuclein is deposited in presynapses in human Lewy body disease and A53T alpha-synuclein transgenic mice. Acta Neuropathol. 2010;120:145–154. doi: 10.1007/s00401-010-0676-z. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2012;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani V, Stefani A, Pierantozzi M, Natoli S, Stanzione P, Franciotta D, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson's disease. J Neuroinflammation. 2012;9:188. doi: 10.1186/1742-2094-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, et al. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS ONE. 2011;6:e26612. doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, et al. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Ohlin KE, Francardo V, Lindgren HS, Sillivan SE, O'Sullivan SS, Luksik AS, et al. Vascular endothelial growth factor is upregulated by L-dopa in the parkinsonian brain: implications for the development of dyskinesia. Brain. 2011;134:2339–2357. doi: 10.1093/brain/awr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer O, Lawhorn C, Miller T, Smith DM, Brown LL. Functional architecture of the mammalian striatum: mouse vascular and striosome organization and their anatomic relationships. Neurosci Lett. 2005;385:198–203. doi: 10.1016/j.neulet.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195:153–159. doi: 10.1016/s0022-510x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci. 1987;7:3935–3944. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Friedman B, Cheng Q, Tsai P, Schim E, Kleinfeld D, et al. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke. 2009;40:e666–e674. doi: 10.1161/STROKEAHA.109.551341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.