Abstract
The effects of partial acclimatization to high altitude (HA; 5,050 m) on cerebral metabolism and cerebrovascular function have not been characterized. We hypothesized (1) increased cerebrovascular reactivity (CVR) at HA; and (2) that CO2 would affect cerebral metabolism more than hypoxia. PaO2 and PaCO2 were manipulated at sea level (SL) to simulate HA exposure, and at HA, SL blood gases were simulated; CVR was assessed at both altitudes. Arterial–jugular venous differences were measured to calculate cerebral metabolic rates and cerebral blood flow (CBF). We observed that (1) partial acclimatization yields a steeper CO2-H+ relation in both arterial and jugular venous blood; yet (2) CVR did not change, despite (3) mean arterial pressure (MAP)-CO2 reactivity being doubled at HA, thus indicating effective cerebral autoregulation. (4) At SL hypoxia increased CBF, and restoration of oxygen at HA reduced CBF, but neither had any effect on cerebral metabolism. Acclimatization resets the cerebrovasculature to chronic hypocapnia.
Keywords: cerebral blood flow measurement, energy metabolism, environment, high altitude
Introduction
Acclimatization to high altitude (HA) is a multifaceted process involving cardiorespiratory and renal compensatory changes that are most dynamic during the first few weeks of exposure. Hypoxic stimulation of the peripheral chemoreceptors leads to hyperventilation, resulting in hypocapnia and respiratory alkalosis for which renal bicarbonate excretion slowly compensates. Thus, despite the partial pressure of arterial carbon dioxide (PaCO2) normally being reduced to ~25 mm Hg at altitudes of ~5,000 m, blood pH returns to near sea level (SL) values within 2 to 3 weeks. Arterial blood gases (ABG) and pH are major determinates of blood flow to the brain (cerebral blood flow, CBF).1 The sustained alterations and compensations in ABG and pH with ascent to HA thus represent a complex stimulus to the cerebrovasculature, the nature of which is not well understood.
Metabolic compensation for respiratory alkalosis results in marked decreases in plasma bicarbonate ion concentration that while helping to return pH to normal levels also reduces buffering capacity. Cerebrovascular reactivity (CVR) to changes in PaCO2 after ascent to HA have been reported to increase, decrease, or remain unchanged with ascent to HA.2 Between-study differences in altitude, degree of acclimatization, and method of reactivity assessement likely underscore many of these reported differences. Moreover, most of these data come from transcranial Doppler ultrasound estimations of CBF that are confounded by constriction or dilation of the insonated vessel in response to both changes in blood gases and ascent to HA.3 In addition, ventilatory acclimatization elicits slightly different ramifications to arterial than cerebral extracellular fluid; internal jugular venous blood provides a better estimation of brain extracellular fluid parameters than does arterial blood4 but has not been measured at HA.
A number of studies have measured CBF over the course of acclimatization to HA and have found CBF to return to SL values, or remain slightly elevated, after 2 weeks (see ref. 3). Severinghaus et al5 reported in four subjects that acute correction of hypoxia (via administration of 30% O2) after 5 days at 3,810 m returned CBF to SL values when PaCO2 was maintained at HA values (~31 mm Hg). Similarly, at 4,300 m CBF was reduced in Andean natives by 18% after 60 minutes of oxygen breathing.6 Interestingly, oxygen delivery appears to be maintained during ascent and acclimatization to HA, with the initial increase in CBF compensating for reductions in arterial oxygen content (CaO2) until oxygen carrying capacity of blood improves after a few days at altitude. Hypoxic cerebral vasodilation—which is mediated via PaO2 rather than SaO2—thereby affects the initial increase in CBF with ascent to HA, which prevails in the face of hypocapnia, but the mechanisms facilitating the diminished influence of CO2 have not been studied.
The majority of studies have reported unchanging cerebral metabolic rate of oxygen (CMRO2) with severe (PaO2~35 mm Hg) or moderate acute hypoxia at SL;7 and after 3 weeks acclimatization to HA (5,260 m; PaO2~51 mm Hg) in lowlanders8 or in HA residents exposed to acute hypoxia at 3,800 m (PaO2~41 mm Hg9). Only one study has reported a 5% increase in CMRO2 (using magnetic resonance imaging) during acute hypoxia roughly equivalent to 4,000 m HA (SaO2 was not reported but was likely ~85%)10—this discrepancy likely reflects different methodologies and their respective assumptions. Consistent with a stable CMRO2, cerebral carbohydrate utilization has been reported to be relatively consistent in acute7 and chronic hypoxia;9 however, this is not a universal finding as other studies have reported increased cerebral carbohydrate metabolism during acute hypoxia.11 Conversely, older studies using positron emission tomography indicate that cerebral glucose metabolism might decrease at altitude in populations evolutionarily less adapted to HA12 than the Sherpa in whom it is similar to SL dwellers at SL.13 Studies in animals indicate decreased cerebral carbohydrate metabolism,14 and in humans small decreases (~13%) in CMRO2 with hypercapnia were reported by Xu et al,15 although this is not a universal finding,16 and has not been assessed using cerebral arteriovenous differences in humans at SL or at HA. It thus remains unclear whether the prevailing CBF after partial acclimatization is regulated by oxygen delivery, sustained hypocapnia, altered acid-base buffering, changes in cerebral metabolism or the balance between them all.
We aimed to characterize these effects of acclimatization (compensated acid-base balance and buffering to respiratory alkalosis) on both cerebrovascular function and cerebral metabolism. The importance of blood gases and cerebral metabolism on CBF was assessed by measuring arterial–jugular venous differences across the brain during acute changes in blood gases at SL and after partial acclimatization to HA. To achieve this, at SL we induced changes to PaO2 and PaCO2 to approximate those that would be experienced at HA; and after 6 to 10 days at 5,050 m blood gases were altered to acutely simulate those at SL. We were thus able to assess the effect of partial acclimatization and altered acid-base balance on prevailing brain blood flow and metabolism. How cerebrovascular reactivity is altered at HA was addressed by inducing steady-state iso-oxic changes in PaCO2 at both SL and 5,050 m. We hypothesized that metabolic compensation for respiratory alkalosis with ascent to HA, and the resultant leftward shift in the CO2-pH relation would yield steeper cerebrovascular reactivity and ventilatory sensitivity to changes in PaCO2, but not when expressed as [H+]. Because of this greater acidic response and hence chemoreflex drive, we also reasoned there might be a greater pressor response17 leading to an additional elevation in CBF for which cerebral autoregulation would not compensate.1 Finally, we hypothesized that acute correction of hypoxia at HA would not affect cerebral metabolism.
Materials and methods
Participants
Eleven healthy young subjects (1 female; age 30.4±7; body mass index 24.9±3.7) gave written informed consent before study participation. The University of British Columbia Clinical Review Ethical Board granted ethical approval, and the study conformed to standards set by the Declaration of Helsinki. None of the volunteers were smokers, had history of cardiovascular diseases, were taking any medications, or had been to altitudes greater than 3,000 m in the 6 months before testing. Each subject was prescreened via a 12-lead electrocardiogram, pulmonary function test, full polysomnography (to rule out any sleep-disordered breathing), and transthoracic echocardiogram. This study was one component of a large expedition to 5,050 m. Sea level data were collected during April 2012 in Kelowna, British Columbia (altitude 344 m) and HA experiments were completed over 3 weeks during May 2012 at the Ev-K2-CRN Pyramid laboratory, Khumbu region, Nepal (altitude 5,050 m). Some studies from this expedition have been reported elsewhere. Expedition members participating as subjects had a minimum of 48 hours between studies involving pharmaceutical interventions or exercise to mitigate contamination.
Experimental Design
Subjects were first familiarized with the experimental protocol, as detailed below. Testing was completed on 2 days, once at SL and once at 5,050 m, separated by ~2 months. On both days subjects abstained from any caffeine containing beverages or alcohol at least 24 hours before testing. The HA protocol was completed between 6 and 10 days after arrival to 5,050 m during which time no subjects displayed any signs or symptoms of acute mountain sickness. At both altitudes, the subject's preparedness and physical status was reassessed, after which two catheters were placed under local anesthesia (1% lidocaine) and by ultrasound guidance by an experienced anesthesiologist. A 20G arterial catheter (Arrow, Markham, Ontario, Canada) was inserted into the left radial artery, and a jugular bulb catheter (Edwards PediaSat Oximetry catheter, Irvine, CA, USA) was placed by the Seldinger technique into the right internal jugular vein (IJV) and advanced to the jugular bulb. Both arterial and IJV catheters were attached to pressure transducers and isolated sampling reservoirs for sampling of arterial and jugular venous blood (VAMP system (VMP160), Edwards Lifesciences, Mississauga, ON, Canada). The radial arterial transducer was calibrated at the level of the right atrium for the measurement of beat-to-beat blood pressure. After arterial and jugular cannulation, subjects rested supine for at least 30 minutes breathing ambient air while they were equipped with measurement apparatuses as detailed below. End-tidal partial pressures of CO2 (PETCO2) and O2 (PETO2) were controlled to target specific PaCO2 and PaO2 values using a portable end-tidal forcing system (AirForce, GE Foster, Kelowna, BC, Canada). The system prospectively targets inspired gases by a feedback control and error reduction algorithm to achieve desired end-tidal values.18 Discrete PETO2 values were targeted instead of SaO2 values because (1) it is the PaO2 not SaO2 that stimulates vasomotion at the arteriolar bed and (2) a given SaO2 may entail differing PaO2 after changes in acid-base balance and hematocrit after acclimatization. At both SL and HA, four permutations of PETO2 and PETCO2 were targeted: 100 mm Hg PETO2/40 mm Hg PETCO2 (resting SL values); 100 mm Hg PETO2/25 mm Hg PETCO2 (SL hypocapnia, HA hyperoxia); 47 mm Hg PETO2/40 mm Hg PETCO2 (SL hypoxia, HA hypercapnia); and 47 mm Hg PETO2/25 mm Hg PETCO2 (resting HA values). These values were targeted with varying precision and are described in detail next (see Supplementary information for protocol diagram, Supplementary Figure S1).
End-Tidal Forcing at Sea Level
Steady-state ABG were maintained for 3 to 5 minutes with each stage separated by 10 minutes breathing ambient air. Clamp 1: First, subjects hyperventilated while PETO2 was clamped at 100 mm Hg, reducing PETCO2 which was clamped to 25 mm Hg (equivalent to normal baseline PaCO2 at 5,050 m); PETCO2 was then increased in 5 mm Hg increments for 3 to 5 minutes at each level until a PETCO2 of 15 mm Hg above eupnic PETCO2 was reached. Clamp 2: PETO2 was lowered to 47 mm Hg and PETCO2 to 25 mm Hg with subjects instructed to hyperventilate further if the hypoxic ventilatory response was insufficient to reduce PaCO2 to the desired level (to bring both PaO2 and PaCO2 to normal baseline values at 5,050 m as measured during our earlier studies.19 Finally, in Clamp 3, PETO2 was reduced to 47 mm Hg (normal PaO2 at 5,050 m) while PETCO2 was maintained at SL baseline values for each subject (~40 mm Hg; Supplementary Figure S1).
End-Tidal Forcing at 5,050 m
Per the SL protocol clamping was maintained for 3 to 5 minutes at each end-tidal target. The study was originally designed to target resting SL PaCO2 (~40 mm Hg), but individuals were generally unable to cope with such relative hypercapnia. Consequently, hypercapnic stages at HA reached PETCO2 of ~35 mm Hg. Subjects first hyperventilated with maintenance of euoxia (40 mm Hg PaO2) to decrease PETCO2 to 10 mm Hg below eupnic levels. PETCO2 was then increased in a stepwise manner in 5 mm Hg increments to 35 mm Hg to mimic SL normocapnic hypoxia (Clamp 1), followed by 10 minutes rest breathing ambient air. Next, PETO2 was increased to 100 mm Hg (Clamp 2) with maintenance of isocapnia (at 25 mm Hg) for 3 minutes, after which PETCO2 was increased directly to 35 mm Hg (Clamp 3) for >3 minutes, to replicate SL hypocapnia and baseline blood gases, respectively. Clamps 2 and 3 were completed back-to-back due to a limited supply of compressed gases (Supplementary Figure S1). At both SL and HA, PaO2 was targeted rather than SaO2 as the former is the primary stimulus to the cerebrovascular bed.
Measurements
Data acquisition
All data except internal carotid (ICA) and vertebral (VA) artery flows and arterial blood gases were collected continuously at 200 kHz via an analog to digital data acquisition system (Powerlab/16SP ML795; AD Instruments, Colorado Springs, CO, USA). Beat-to-beat blood pressure was measured from the radial artery and the mean arterial pressure (MAP) calibrated offline by manual sphygmomanometry. Heart rate (HR) was measured from the electrocardiogram. The left middle cerebral artery blood velocity (MCAv) and right posterior cerebral artery blood velocity (PCAv) were measured by transcranial Doppler ultrasound (Spencer Technologies, Seattle, WA, USA) using a 2-MHz pulsed probe. The MCA and the PCA are fed from the ICA and VA, respectively; thus, left MCAv and right PCAv were measured for symmetrical consistency with neck measures of CBF (see Quantification of CBF, below). Standard transcranial Doppler ultrasound search techniques that optimize signal quality and reproducibility were utilized as we have previously detailed.17
Blood gases
Arterial and IJV blood samples drawn into preheparinized syringes were analyzed either immediately, or were kept on ice if there was a delay greater than 5 minutes. All samples were analyzed within 30 minutes of collection for pH; PO2; PCO2; percent saturation of hemoglobin (SO2); total hemoglobin (tHb); and, plasma glucose, lactate and electrolyte concentrations (ABL-90, Radiometer, Copenhagen, Denmark).
Quantification of Cerebral Blood Flow
Duplex vascular ultrasound (10 MHz multifrequency linear array probe, Terason 3000, Teratech, Burlington, MA, USA) was used to measure continuous diameters and velocities in the ICA and VA as detailed previously.3, 17 Briefly, the left ICA was measured at least 2 cm from the bifurcation ensuring there was no turbulent or retrograde flow at the site of velocity measurement. The right VA was measured between C6 and C4. Flow in each vessel was calculated and CBF determined as twice their sum. Although both vertebral arteries typically arise from the subclavian arteries, the left VA is usually ~10% to 20% larger in diameter (and flow) than the right VA. Although we measured only flow in the right VA, these bilateral differences would only results in a 6% to 8% systematic bias that would not alter our findings. Moreover, our absolute measures of CBF are consistent with the previous literature, and the changes in ICA or VA flow are largely based on a stimulus response to a manipulation in ABG (which would be expected to be the same between vessels). Continuous screen capture was saved for subsequent offline analysis using proprietary edge-tracking software. Use of this software reduces observer error and bias over manual methods of analysis, possessing an intraobserver coefficient of variation of 6.7%.3, 17 Accurate quantification of flow through a vessel requires extremely good image quality, and the repeated measures design of the present study necessitated that the exact same point of a vessel was insonated with an identical angle of insonation during each measure. We were unable to capture images in every subject at each blood gas target that met these criteria, and in two subjects no usable ICA or VA images could be collected at either altitude. We consequently calculated the CMRO2 at each altitude from ICA and VA flows, and used these resultant mean CMRO2 values (for SL and 5,050 m) and the cerebral arteriovenous difference for oxygen to calculate CBF by the Fick equation, as detailed in the Supplementary information.
Calculation of reactivity
Cerebrovascular reactivity to CO2 was assessed separately for hypo- and hypercapnia as the slope of the linear regression between %ΔCBF and PaCO2, arterial [H+], PIJVCO2, and IJV [H+]. Although at SL a +15 mm Hg PCO2 step was conducted, this was not used in the calculation of SL hypercapnic CVR in order that hypercapnic CVR considered the same ΔPCO2 at both altitudes (subjects were unable to tolerate +15 mm Hg PaCO2 above eupnia at 5,050 m). The R2 of every individual regression line for the calculation of reactivity was greater than 0.6 indicating linearity. Ventilatory and MAP reactivity was calculated in the same manner as CVR, but the former in the hypercapnic range only (because volitional increase in ventilation is required to attain hypocapnia).
Statistical Analysis
That data were normally distributed which was confirmed by the Shapiro-Wilk test. Repeated-measures ANOVA was used to compare differences across blood gas targets, and selected bonferroni corrected post-hoc comparisons (one of the simplest and most conservative comparisons) of a priori interest were made between blood gas targets at both SL and 5,050 m. Comparisons were made between SL and 5,050 m for a given blood gas target, and between targets within elevation. Cerebrovascular reactivity comparisons between hypo- and hypercapnia, or between altitudes were made with t-tests. Alpha was 0.05, and values are shown as mean±s.d.
Results
Subjects
All 11 subjects completed all ABG tests at SL. Two subjects were airlifted from 5,050 m before data collection due to one developing acute appendicitis; both of these subjects were removed from the data set yielding an n=9, all male, aged 31.9±6.7 years, with a body mass index of 25.5±3.8 kg/m2.
Acid-Base Balance at 5,050 m
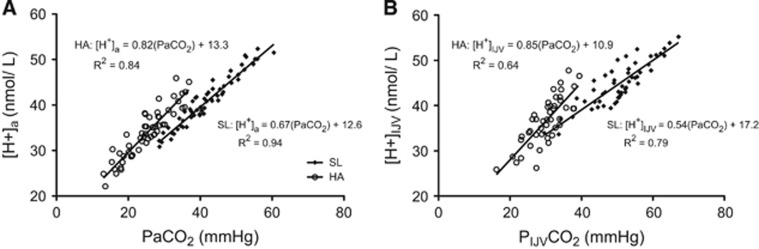
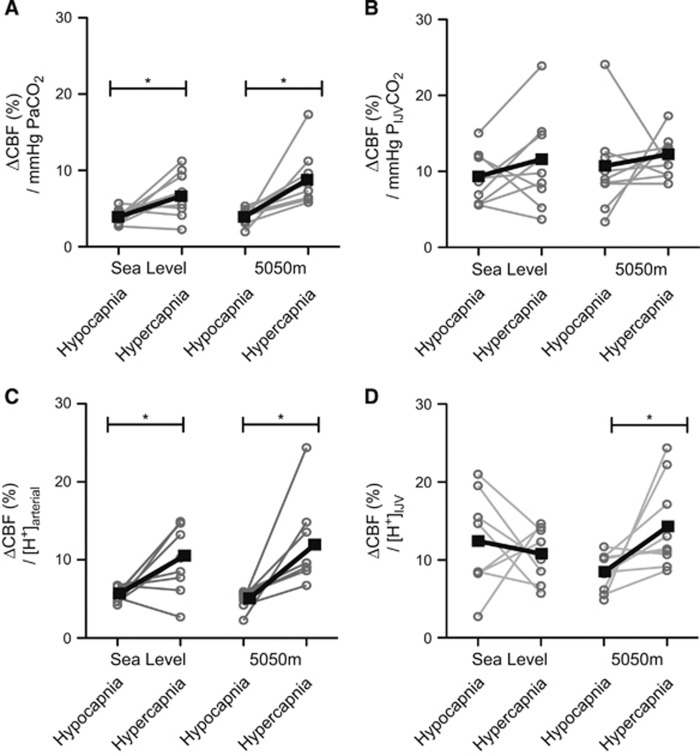
Exposure to 5,050 m resulted in partial metabolic compensation (i.e., decrease in arterial base excess and HCO3−) for the respiratory alkalosis (arterial pH at 5,050 m was 7.47±0.04 versus 7.40±0.01 at SL). The plasma concentration of potassium ion was decreased, and chloride increased at 5,050 m, but neither were influenced by ABG clamping. Acute hypo- and hypercapnia changed arterial and jugular venous pH at both altitudes. Arterial and internal jugular venous HCO3− was decreased at HA relative to SL. Arterial HCO3− was increased during hypercapnia, and decreased during hypocapnia at both altitudes. Conversely, jugular HCO3− was not significantly affected by any acute change to ABG at SL, and at HA was altered only at +10 mm Hg PaCO2. Figure 7 shows that a given iso-oxic change in PCO2 elicits a greater change in proton concentration at HA and that this difference is augmented in internal jugular venous blood. See Supplementary information for all tabular values.
Figure 7.
Proton concentration in arterial (A) and internal jugular venous blood (B) at sea level (SL) and high altitude (HA) during acute euoxic changes in PETCO2. Slopes of the regression were significantly less at SL than at HA for both arterial and internal jugular venous blood (P<0.05). P<0.0001 for the linear fit of all regression equations.
Cardiovascular Effects of High Altitude and Blood Gas Clamping
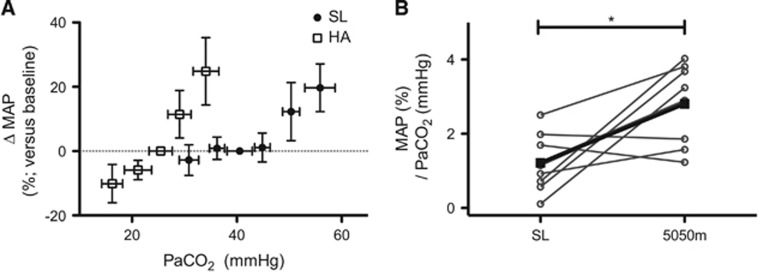
Mean arterial pressure at SL was not significantly altered relative to baseline with any of the PaO2 manipulations. Conversely, hypoxia at SL elevated HR irrespective of PaCO2, whereas at HA clamping elicited no significant change in HR. At rest, MAP was elevated after 6 to 10 days at 5,050 m and was further increased under conditions of euoxic and hyperoxic hypercapnia (+28.4±11% MAP and +13.7±10, respectively). Conversely, hypocapnia at HA elicited a 10.0±6.0% decrease in MAP whereas it was unaltered at SL during this condition (Figure 1). The mean slope of the percent increase in MAP versus PaCO2 in the hypercapnic range was significantly greater at HA (SL: 1.2±0.7 Δ%/mm Hg versus HA 2.8±1.0 Δ%/mm Hg; Figure 1). If the difference between SL and HA was accounted for solely by the change in PCO2-H+ relationship at HA, then MAP reactivity as a function of [H+] should be similar between altitudes; however, MAP reactivity as a function of [H+] was actually greater than that at SL (SL, 1.9±1.1 Δ%/nmol/L; HA, 3.6±1.3 Δ%/nmol/L). Hyperoxic hypercapnia at HA produced a similar increase in MAP as euoxic hypercapnia at SL (HA ΔMAP 13.7±10% SL ΔMAP 12.3±9.0%), suggesting background hypoxia caused the greater hypercapnic increase in MAP with hypercapnia at HA.
Figure 1.
Percent difference in mean arterial pressure (MAP) from baseline (A) during euoxic changes in PaCO2 at sea level (SL) (solid squares) and high altitude (HA) (hollow squares), and the individual slopes of this relationship in the hypercapnic range (B). Mean slopes (±s.d.) for this relationship were SL hypercapnic range 1.2±0.7%/mm Hg; HA, hypocapnic range 1.1±0.7%/mm Hg (not-shown); HA, hypercapnic range 2.8±1.0%/mm Hg. *P<0.05.
Effects of O2
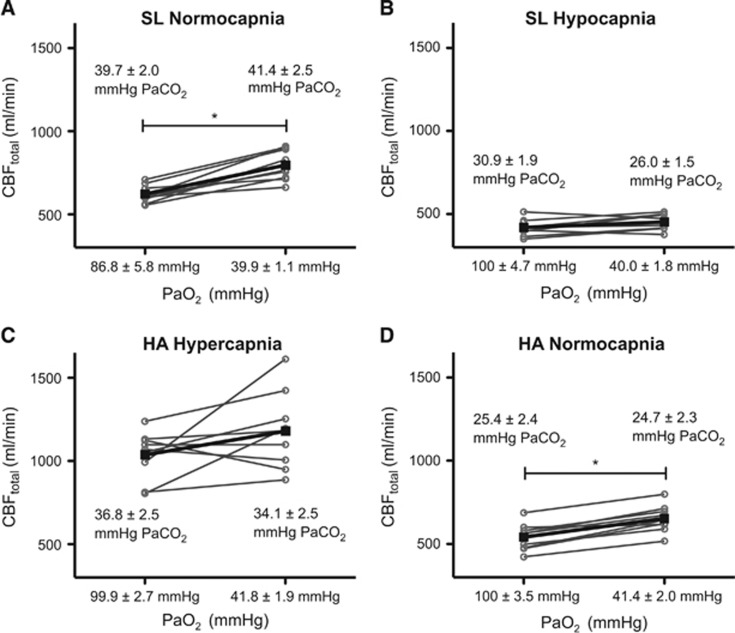
At SL, the isocapnic reduction in PaO2 to 39.9±1.1 mm Hg (78.8± 1.4% SaO2) elicited a 45±16% increase in CBF (Figure 2A), reduced cerebral a-vPaO2 from 53±5.2 mm Hg to 12.9±0.9 mm Hg and maintained O2 extraction fraction (O2EF). At 5,050 m normalization of PaO2 to SL values decreased CBF in all subjects (Figure 2D). As expected, normalization of O2 resulted in a corresponding increase in a-vPaO2 from 14.9±2.2 mm Hg to 65.0±4.9 mm Hg. Compared with SL hypocapnic normoxia, O2 normalization at HA yielded greater cerebral oxygen delivery (CDO2; Equation 10, Supplementary information) and correspondingly lower O2EF (Equation 7, Supplementary information).
Figure 2.
Individual cerebral blood flow (CBFtotal) values during isocapnic changes in PaO2 at sea level (SL) and high altitude (HA). The top plots (A and B) show data from SL; the bottom plots (C and D) show data from 5,050 m. The left side of each plot depicts CBF while PaO2 was ~100 mm Hg (except at SL—plot A—where ambient PO2 yielded a smaller PaO2), whereas hypoxia is shown on the right side of each plot. The left hand plots (A and C) show isocapnia clamped at ~40 mm Hg at SL and ~35 mm Hg at HA (the lower PaCO2 values at HA were due to inability of subjects to tolerate higher values; see Results and Discussion). Right side plots (B and D) show isocapnic clamp at ~25 mm Hg. Please see Materials and methods for further details on gas permutations. *P<0.05.
Effects of CO2
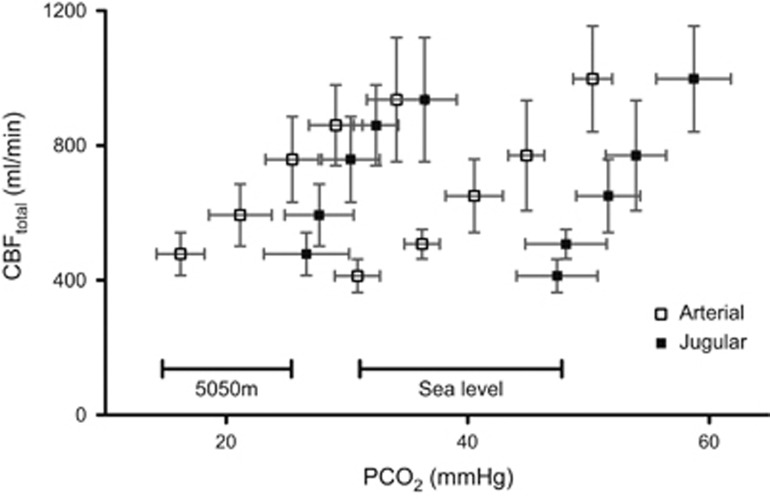
Iso-oxic hypercapnia and hypocapnia resulted in significant increases and decreases, respectively, in CBF at both SL and HA (Figure 3). Hypercapnic reactivity was greater than hypocapnic reactivity at both elevations. In the hypocapnic range, the decrease in CBF was similar between SL and HA, decreasing 25.1±7.0% (−5 mm Hg PaCO2) and 40.0±18% (−10 mm Hg PaCO2) at SL, and 19.7±9.0% (−5 mm Hg PaCO2) and 36.0±6.8% (−10 mm Hg PaCO2) at 5,050 m. The hypocapnic CVR was therefore not different between SL and HA (4.0±1.0%/mm Hg at both SL and HA). In the hypercapnic range at SL, CBF increased 23.9±15% (+5 mm Hg PaCO2) and 67.2±29% (+10 mm Hg PaCO2), whereas at HA CBF increased 38.2±24% (+5 mm Hg PaCO2) and 77.0±27% (+10 mm Hg PaCO2) giving a hypercapnic reactivity at SL of 6.8±2.9%/mm Hg versus 9.0±3.6%/mm Hg at HA (P=0.27; Figure 4A). Consideration of the altered acid-base buffering capacity at HA by determination of the hypercapnic CVR as ΔCBF versus [H+] did not change these relationships (Figure 4C).
Figure 3.
Total cerebral blood flow (CBFtotal) during steady-state euxoic changes in PCO2 at sea level (SL) and high altitude (HA). Hollow squares represent CBF plotted against arterial PCO2 and solid squares against internal jugular vein PCO2. CBF was significantly altered from baseline at all levels of PCO2 at both SL and 5,050 m (P<0.05). Data are mean±s.d.
Figure 4.
Individual (circles, gray) and mean (squares, black) cerebrovascular reactivity (CVR) to euoxic changes in arterial (left) and jugular (right) PCO2 (Top) and [H+] (bottom). CVR in the hypocapnic range was lower than in the hypercapnic at both elevations when determined as a function of PaCO2 or arterial [H+] (A and C; P<0.05). PIJVCO2 CVR did not differ between hypo- and hypercapnia nor between altitudes (B), whereas [H+]IJV was greater in the hypercapnic range at high altitude (HA) (D); P<0.05. The very high arterial CVR in one individual at HA (11.2%/mm Hg) was more than two standard deviations above the mean (6.8±5.4%/mm Hg); however, removal of this individual did not affect statistical significance. IJV, internal jugular vein. *P<0.05.
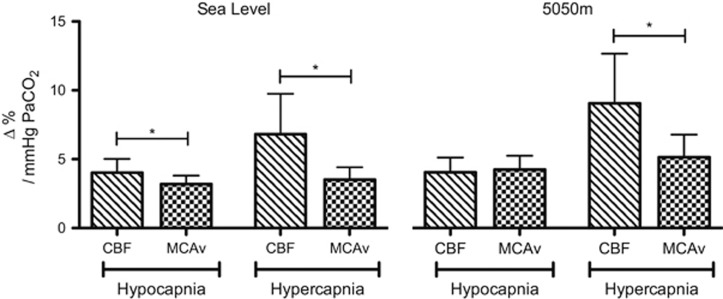
Figure 5 depicts the substantial underestimation of CO2 reactivity when based on MCAv. In the hypercapnic range, MCAv-CVR was approximately half of actual CVR at both SL and HA. In the hypocapnic range, MCAv underestimated reactivity at SL but not at 5,050 m.
Figure 5.
Comparison of cerebral blood flow (CBF) versus middle cerebral artery (MCA) velocity (MCAv) reactivities to change in PaCO2 at sea level (SL) and high altitude (HA). MCAv cerebrovascular reactivity (CVR) was lower than CBF CVR in the hypercapnic range at both altitudes, likely due to dilation of the MCA during increases in CO2, slowing of MCA blood velocity and consequent underestimation of reactivity to increases in PaCO2. Data are mean±s.d. *P<0.05.
The effect of returning PaCO2 to SL values at HA (relative hypercapnia) was anecdotally more unpleasant and difficult at HA for the majority of the volunteers, likely because at HA hypercapnia elicited very high ventilation in most subjects (e.g., +10 mm Hg hypercapnia at HA yielded 83.8±23 L/min; whereas at SL hypercapnia elicited 40.6±17.4 L/min). Ventilatory sensitivity to CO2 increased from SL values of 2.3±1.3 mL/min per mm Hg PaCO2 (3.7±2.8 mL/min per nmol/L H+) to 5.9±1.6 ml/min per mm Hg PaCO2 (7.7±2.2 mL/min per nmol/L H+) at HA. Ventilatory sensitivity as a function of jugular venous blood was similar to arterial values, except for IJV PCO2 sensitivity that was greater than PaCO2 sensitivity at HA (see Supplementary information, Supplementary Figure S2). The O2EF varied inversely with CBF (and PaCO2) at both SL and HA, ranging at SL from 47±4.2% to 12.9±3.1%, and at HA from 51.5±4.8% to 21.0±2.9% (−10 mm Hg PETCO2 to +10 mm Hg PETCO2, respectively).
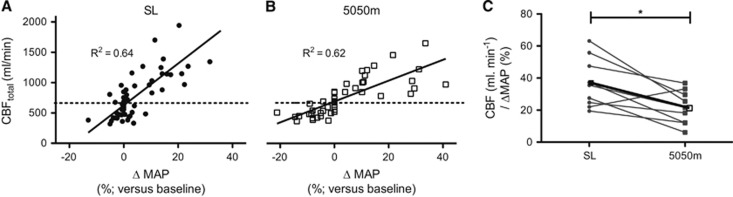
Despite the greater MAP-PaCO2 reactivity at HA, the CBF for a given change in MAP was smaller at HA than at SL in every individual but one (Figure 6C). There was a significant relationship between the change in hypercapnic MAP-CO2 reactivity from SL to HA and the change from SL to HA in hypercapnic CBF-CO2 reactivity (R2=0.47).
Figure 6.
Relationships between cerebral blood flow (CBF) and ΔMAP at sea level (SL) and high altitude (HA). Plots A and B depict CBF versus the percent change in MAP from baseline at SL (A) and HA (B). Each individual's change in CBF per percent ΔMAP within the hypercapnic range is given in plot C. This CBF-MAP reactivity was observed to decrease in every subject but one, despite a greater MAP-PCO2 reactivity at HA that elicited greater hypertension than at SL. P<0.0001 for the linear fit of all regression equations. MAP, mean arterial pressure. *P<0.05.
Combined Effects of O2 and CO2
At SL the CBF increase observed during normocapnic hypoxia was neutralized with concomitant hypocapnia (i.e, CBF was not significantly different between normoxic or hypoxic hypocapnia; Figure 2B). Whereas at HA, increasing PaO2 to SL values during concomitant hypercapnia caused CBF to decrease in five of nine individuals (Figure 2C); changes in PaCO2 elicited significant changes in CBF and MAP regardless of prevailing PaO2. Thus, although hyperoxia decreased CBF at HA by 17±6.7%, CBF decreased by 18±5.1% with hypocapnia despite this eliciting a 31.5±6.0% decrease in CDO2. These findings indicate that oxygen is not as potent a stimulant to cerebral vasomotion as CO2 and that CBF is reset to a lower prevailing PaCO2 at HA.
Cerebral Metabolism
No metric of cerebral carbohydrate metabolism was significantly altered during any change in PaO2 at SL or at HA (i.e., CMRlactate, CMRglucose, oxygen glucose index; oxygen carbohydrate index, or arteriovenous differences for glucose or lactate). At SL, but not at HA, CMRglucose and CMRlactate were reduced with hypercapnia (+10 mm Hg). Lactate arteriovenous differences increased significantly only with hypercapnia at SL.
Discussion
The principal findings of this study were (1) partial acclimatization to HA yields a steeper CO2-H+ relation in both arterial and jugular venous blood; yet (2) whereas ventilatory sensitivity to euoxic ΔPCO2 increased at HA, contrary to our hypothesis, cerebrovascular reactivity did not change, despite (3) MAP-CO2 reactivity being augmented at HA, thus indicating effective cerebral autoregulation. (4) Acute hypoxia at SL increased CBF, and acute restoration of oxygen at HA reduced CBF, but neither had any effect on cerebral metabolism.
Metabolic Compensation and Cerebral Blood Flow
Partial metabolic compensation for respiratory alkalosis (i.e., bicarbonate excretion) after 6 to 10 days at 5,050 m steepened the relationship between PCO2 and pH (proton concentration)—i.e., a given change in PCO2 elicited a greater change in arterial and internal jugular venous [H+] than at SL. We initially hypothesized that changes in buffering status would be responsible for any changes to reactivity (cerebrovascular, MAP, ventilatory), yet the increase in MAP and ventilatory reactivity at HA was similar when reactivity was assessed as a function of proton concentration. Reduced buffering capacity is therefore not the principal mechanism that mediates elevations in MAP and ventilatory reactivity. We did not administer the entire PCO2 range under conditions of hypoxia at SL or hyperoxia at HA. However, that the increase in MAP and ventilation during hyperoxic hypercapnia at HA increased similarly as during normoxic hypercapnia at SL suggests that background hypoxia is responsible for the observed augmentation in MAP and ventilatory reactivity at HA likely via activation of the peripheral chemoreflex and/or increased sensitivity of the carotid body.2
Arterial, Tissue, and Venous PCO2
That the mammalian cerebral vasculature is highly sensitive to changes in PaCO2 has been known for 150 years. At SL there is an ~4% to 5% increase in CBF per mm Hg increase in PaCO2 above eupnic PaCO2, and 3% to 4% decrease in CBF below eupnic PaCO2, affected principally by dilation and constriction (respectively) of the pial arterioles on the brain's surface.1 How CO2 precisely exerts its effects on the cerebrovasculature is not definitively known. Altered arterial pH without changes in PaCO2 does not effect CBF,20 but extravascular application of acidic or basic solutions alters vessel tone.21 The sensitivity to CO2 thus appears to rely on diffusion of molecular CO2 into the vascular wall where the resultant shift in extracellular pH drives changes in smooth muscle tone.22 That CBF is a function of extravascular pH rather than arterial pH is further supported by the present data where CBF at HA was equivalent to SL despite chronic alkalosis, indicating CBF CO2 sensitivity is reset over time at HA. Similar conclusions were made by Fencl et al23 who studied cerebral and ventilatory reactivity to CO2 in four subjects after induced metabolic acidosis and alkalosis through ingestion for 5 days of NH4Cl and NaHCO3, respectively. Under these circumstances, CBF was greater at a given PaCO2 when bicarbonate concentration was low (metabolic acidosis) than during metabolic alkalosis when bicarbonate concentration was high suggesting a further role for bicarbonate concentration, in addition to pH and PCO2. Further studies of these relationships in humans are lacking.
Both ventilatory sensitivity and MAP sensitivity increased at HA. Figure 7 shows the reduction in buffering capacity at HA was particularly manifest in jugular venous blood. This perhaps reflects differential buffering on the tissue/venous side of the cerebral circulation and is likely related to the lack of significant change in IJV bicarbonate ion concentration during acutely altered PCO2. The ventilatory response to CO2 is principally a function of the central chemoreceptor environment,24 for which jugular venous blood provides a much closer approximation as its pH, PCO2, and PO2 are equivalent to that of cerebral spinal fluid. Thus, in addition to the known augmentation of carotid body sensitivity with acclimatization, the significant increase in the slope of the CO2-H+ relationship in jugular venous blood adjunctively contributes to the augmented ventilatory sensitivity at HA.
Cerebral Blood Flow and Arterial Blood Gases
Hypoxia below a PaO2 of ~50 mm Hg is a potent vasodilator of the cerebral circulation; however, the present study clearly shows the comparatively greater importance of CO2 in regulating CBF. For example, at SL hypocapnia reduced CBF equally in normoxia and hypoxia and during normocapnia at HA returning PaO2 to SL values decreased CBF and under hypercapnic conditions normalization of PaO2 caused no uniform change in CBF as CBF was already elevated by ~80%. Indeed, oxygen delivery is well maintained during even severe hypoxia (PaO2 36±4.3 mm Hg), whereas it becomes significantly attenuated during hypocapnia at both SL and HA (present data and ref. 17). Yet despite the decreased delivery, O2EF increases proportionally to maintain cerebral O2 delivery. To our knowledge, this is the first direct evidence that informs previous observations of ‘graying' of vision, carpal-pedal spasm, and profound sleepiness during severe euoxic hypocapnia (PaCO2 16.6±1.7 mm Hg)17, suggesting that these symptoms are due to local vasoconstriction in brain regions associated with these symptoms but not due to global reductions in O2 delivery.
Relationship Between Enhanced Mean Arterial Pressure Reactivity and Cerebral Blood Flow
We observed a greater increase in MAP for a given increase in PaCO2 or [H+] at HA, reflecting the additive chemoreceptor stress of hypercapnia in background hypoxia; removal of the hypoxic stimulus during hypercapnia at HA significantly attenuated the increase in MAP (euxoxic hypercapnia: +28.4±11%, versus hyperoxic hypercapnia: +13.7±10%). In fact, hyperoxic hypercapnia at HA elevated MAP to a similar extent as did euoxic hypercapnia at SL (HA ΔMAP 13.7±10% SL ΔMAP 12.3±9.0%).
Despite greater MAP reactivity at HA, CBF was altered less for a given change in MAP at HA relative to SL. This suggests that the intrinsic cerebrovascular mechanisms serving to buffer changes in perfusion pressure—conventionally termed cerebral autoregulation—were not impaired but were in fact enhanced. Many studies have concluded that autoregulation is impaired at HA (e.g., ref. 25), although this is not a universal finding and surely relates to lack of a consensual metric for the quantification of CA.26 A number of studies have found an important function of the sympathetic nervous system in attenuating surges in CBF (reviewed in ref. 1). Muscle sympathetic activity is increased at HA27 and, if extrapolated to the cerebral circulation, we speculate that this is responsible for the augmented cerebral autoregulation to increased MAP we observed during rising PaCO2 at HA. There did, however, appear to be a within-individual relationship between MAP and CBF reactivity increase at HA because ΔMAP reactivity (from SL to HA) was significantly related to the Δ CBF reactivity (R2=0.47), likely reflecting the influence of perfusion pressure on CBF.
Velocity Versus Flow Cerebrovascular Reactivity
The estimation of CBF by transcranial Doppler ultrasound remains a popular tool. Its use assumes a constant diameter of the insonated vessel, an assumption we have previously found appropriate within a relatively narrow range of ABG.17 During extreme changes in ABG,28 exposure to HA3 or after glycerol trinitrate administration29 the middle cerebral artery dilates. Herein, we show that hypercapnic CVR estimated by transcranial Doppler ultrasound of the middle cerebral artery is approximately half of that based on CBF, consistent with dilation of the vessel, and indicating that previous measurements of hypercapnic CVR at HA were grossly underestimated.19, 30, 31
Comparison to Other Studies
To our knowledge, this is the first study to collect ABG (and IJV blood gases) during a series of steady-state blood gas permutations at both SL and HA. Collection of arterial blood allows for precise quantification of reactivity to changes in blood gases compared with commonly used estimation by end-tidal PCO2 and PO2. We observed an increased PaCO2–PETCO2 gradient that increased from −2.1±1.5 mm Hg at SL baseline to −4.0±2.2 mm Hg at HA baseline, ranging at HA from −2.7±1.3mmH during −10 mm Hg hypocapnia to −5.1±2.1 mm Hg during +10 mm Hg hypercapnia (data not presented). This finding has important implications for interpretation of previous studies where ABG were unknown during the CO2 manipulation.19, 30, 32 Contrary to the present data, some studies have reported a lower hyper- than hypocapnic CVR at HA.19, 33 Overestimation of PaCO2 in the hypercapnic range may provide an explanation for this finding, as it would produce an artificially low hypercapnic CVR. Figure 5 shows how this error is further exacerbated by MCAv-based underestimations of CBF and likely explains why in the present study MCAv hypercapnic CVR was not different from hypocapnic CVR at HA.
Cerebral Metabolism During Changes in Blood Gases
Our finding of reduced CMR for glucose and lactate during hypercapnia at SL is the first reported in conscious humans. Cohen et al34 similarly found a reduced rate of glucose metabolism with hypercapnia during halothane-induced anesthesia in healthy men. Studies in animals later suggested this is due to inhibition of phosphofructokinase by CO2 per se and consequent inhibition of glycolysis.35 In the present study during hypercapnia at SL seven out of nine individuals showed a small efflux of glucose from the brain. However, post hoc power calculations showed that we were likely underpowered and large sample size would be needed to show a significant effect (n=18). Cognizant of this limitation, we also acknowledge that our methodology cannot elucidate the mechanisms responsible; however, it is known that hypercapnia increases the capillary permeability to glucose and amino-acid transport in humans.36 Impaired glycolysis during steady-state hypercapnia increases the intracellular concentration of glucose in rats14 and in conjunction with increased transport of alternative metabolic substrates (e.g., amino acids) it is theoretically possible the gradient for cerebral glucose metabolism was inverted. Given we observed no negative glucose arteriovenous differences at HA we speculate a higher degree of absolute hypercapnia is necessitated to elicit such profound changes to cerebral metabolism. But, studies on human cerebral metabolism during hypercapnia have focused exclusively on CMRO2.15, 16, 37 The study of Hertz and Paulson36 is the only other study, to our knowledge, assessing cerebral substrate metabolism during acute hypercapnia in conscious humans (who required carotid angiography). They reported a decrease in mean a-vGlucose but there was no mention of a negative a-vGlucose difference in any subjects despite a similar magnitude of hypercapnia. Moreover, acute and chronic hypercapnia actually increased CMRglucose in sheep38 and had no effect on CMR in pigs.39 These results are difficult to reconcile but likely reflect known between-species anatomical and physiological variability, as well as differing methodologies. Regardless, a definitive study in humans utilizing advanced imaging and tracer substrate modalities during suitably elevated PaCO2 has not been conducted.
Technical Limitations
Our intention was to measure regional CBF in the ICA and VA using Duplex ultrasound, a technique we have previously found to reliably quantify CBF during experimentally altered ABG17 and during ascent to 5,050 m on this same expedition.3 We impose strict requirements for image quality to ensure all factors are kept constant for repeated measures within subjects (e.g., section of vessel and steering angle) and carefully discard images that do not meet these standards. Such standards are difficult—if not impossible—to attain when very high ventilation recruits accessory neck muscles such as during severe hypo- or hypercapnia (especially at HA). With the numerous blood gas permutations, the repeated measures design in the field at 5,050 m, and the loss of one sonographer and two subjects early in the study at 5,050 m, we were unable to produce enough measurements that passed our quality control to report repeatedly measured regional CBF changes within individuals for all blood gas permutations at both altitudes. We consequently chose to use those accurate measures of CBF we did collect to estimate CMRO2 to quantify CBF by the Fick principle in all subjects for every blood gas permutation.
Previous studies have often used a fixed CMRO2 (typically 3.2 mL/100 g per minute)40 to estimate CBF from arterial and cerebral venous samples based on evidence that CMRO2 remains constant during profound changes in ABG.40 In the subjects in whom we successfully measured CBF by Duplex ultrasound we observed a 12% increase in CMRO2 at 5,050 m that did not reach significance, and in four subjects CMRO2 varied inversely with changes in PaCO2 at both elevations. We pooled CMRO2 measures at SL and HA (N=7 with different subjects contributing a different number of values to the mean) and calculated CBF from these SL and HA mean CMRO2 values. If CMRO2 does, in fact, vary inversely with PaCO2 then it is possible we underestimated and overestimated CBF values during hypocapnia and hypercapnia, respectively; indeed, there is some evidence for small (<10%) increase and decrease in CMRO2 with moderate hypo- and hypercapnia, respectively.16, 37 However, our CVR values at SL are highly consistent with previous reports17 and we do not think that subtle underestimated and overestimated CBF values during hypocapnia and hypercapnia detract from our main conclusions.
Finally, due to the invasive nature and extensiveness of this field-based study, our sample size was relatively small. Nevertheless, with the exception of a-v glucose and CMRglu, clear significant differences were evident indicating that additional numbers would be unlikely to alter our findings in the majority of our outcome variables. Moreover, we clearly highlight the variability in all of our major outcome variables by displaying individual data and related responses.
Conclusion
Despite some loss of buffering capacity and left shift of the pH–H+ relationship that produced a significantly greater change in pH for a given change in CO2 at HA, we observed no alteration to cerebrovascular responses to CO2 at HA. This finding was in contrast to ventilatory and MAP reactivities to CO2, both of which increased at HA, but seemingly not due to the change in acid-base buffering, but rather to sensitization by background hypoxia. Importantly, we report evidence that cerebral autoregulation to hypercapnia-induced increases in MAP is effective at HA, a finding that challenges a number of previous studies suggesting the opposite but that were confounded by the use of transcranial Doppler ultrasound and largely spontaneous analysis of dynamic autoregulation between MAP and MCAv. Indeed, MCAv likely underestimated cerebrovascular responses at HA, consistent with a number of recent reports that the vessel diameter is not, in fact, static as is so often assumed.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was carried out within the framework of the Ev-K2-CNR Project in collaboration with the Nepal Academy of Science and Technology, as foreseen by the Memorandum of Understanding between Nepal and Italy and thanks to contributions from the Italian National Research Council. The authors are grateful to the other members of the UBC International Research Expedition to Mt. Everest for invaluable help with logistical planning and implementation of this research study. The authors are grateful to Professor G Atkinson, PhD, FRCS for feedback on the statistical analyses associated with this study.
Supplementary Material
References
- Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN, Subudhi AW. Cerebral blood flow at high altitude. High Alt Med Biol. 2014;15:133–140. doi: 10.1089/ham.2013.1138. [DOI] [PubMed] [Google Scholar]
- Willie CK, Smith KJ, Day TA, Ray LA, Lewis NC, Bakker A, et al. Regional cerebral blood flow in humans at high altitude: gradual ascent and 2 wk at 5,050m. J Appl Physiol (1985) 2014;116:905–910. doi: 10.1152/japplphysiol.00594.2013. [DOI] [PubMed] [Google Scholar]
- Bradley RD, Semple SJ. A comparison of certain acidbase characteristics of arterial blood, jugular venous blood and cerebrospinal fluid in man, and the effect on them of some acute and chronic acid-base disturbances. J Physiol. 1962;160:381–391. doi: 10.1113/jphysiol.1962.sp006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinghaus JW, Chiodi H, Eger EI, Brandstater B, Hornbein TF. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res. 1966;19:274–282. doi: 10.1161/01.res.19.2.274. [DOI] [PubMed] [Google Scholar]
- Milledge JS, Sorensen SC. Cerebral arteriovenous oxygen difference in man native to high altitude. J Appl Physiol. 1972;32:687–689. doi: 10.1152/jappl.1972.32.5.687. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Shaw AD, Smith KJ, Willie CK, Ikeda K, Graham J, et al. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci. 2014;126:661–670. doi: 10.1042/CS20130343. [DOI] [PubMed] [Google Scholar]
- Moller K, Paulson OB, Hornbein TF, WNJM Colier, Paulson AS, Roach RC, et al. Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J Cereb Blood Flow Metab. 2002;22:118–126. doi: 10.1097/00004647-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Sorensen SC, Lassen NA, Severinghaus JW, Coudert J, Zamora MP. Cerebral glucose metabolism and cerebral blood flow in high-altitude residents. J Appl Physiol. 1974;37:305–310. doi: 10.1152/jappl.1974.37.3.305. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PJ, Alexander SC, Smith TC, REIVICH M, Wollman H. Effects of hypoxia and normocarbia on cerebral blood flow and metabolism in conscious man. J Appl Physiol. 1967;23:183–189. doi: 10.1152/jappl.1967.23.2.183. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Brown WD, Stanley C, Stone CK, Nickles RJ, et al. The brain at high altitude: hypometabolism as a defense against chronic hypoxia. J Cereb Blood Flow Metab. 1994;14:671–679. doi: 10.1038/jcbfm.1994.84. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Monge C, Stanley C, Brown WD, Stone CK, et al. Sherpa brain glucose metabolism and defense adaptations against chronic hypoxia. J Appl Physiol (Bethesda, Md: 1985) 1996;81:1355–1361. doi: 10.1152/jappl.1996.81.3.1355. [DOI] [PubMed] [Google Scholar]
- Folbergrová J, Norberg K, Quistorff B, Siesjö BK. Carbohydrate and amino acid metabolism in rat cerebral cortex in moderate and extreme hypercapnia. J Neurochem. 1975;25:457–462. doi: 10.1111/j.1471-4159.1975.tb04350.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart JJ, Yezhuvath US, Gu H, et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab. 2010;30:1094–1099. doi: 10.1038/jcbfm.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590:3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Ainslie PN, Stembridge M, Day TA, Bakker A, Lucas SJ, et al. Resting pulmonary haemodynamics and shunting: a comparison of sea-level inhabitants to high altitude Sherpas. J Physiol. 2014;592:1397–1409. doi: 10.1113/jphysiol.2013.266593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJE, Burgess KR, Thomas KN, Donnelly J, Peebles KC, Lucas RAI, et al. Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050m. J Physiol. 2011;589:741–753. doi: 10.1113/jphysiol.2010.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen CJ, Semple SJ, Smyth MG, Gelfand R. H+ and pCO2 as chemical factors in respiratory and cerebral circulatory control. J Appl Physiol. 1961;16:473–484. doi: 10.1152/jappl.1961.16.3.473. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Brain extracellular pH: the main factor controlling cerebral blood flow. Scand J Clin Lab Invest. 1968;22:247–251. doi: 10.3109/00365516809167060. [DOI] [PubMed] [Google Scholar]
- Fencl V, Vale JR, Broch JA. Respiration and cerebral blood flow in metabolic acidosis and alkalosis in humans. J Appl Physiol. 1969;27:67–76. doi: 10.1152/jappl.1969.27.1.67. [DOI] [PubMed] [Google Scholar]
- Ainslie P, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Subudhi AW, Fan JL, Evero O, Bourdillon N, Kayser B, Julian CG, et al. AltitudeOmics: cerebral autoregulation during ascent, acclimatization, and re-exposure to high altitude and its relation with acute mountain sickness. J Appl Physiol (1985) 2013;116:724–729. doi: 10.1152/japplphysiol.00880.2013. [DOI] [PubMed] [Google Scholar]
- Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, Macrae BA, et al. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol. 2012;303:H658–H671. doi: 10.1152/ajpheart.00328.2012. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W.Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy Neurosurgery 199332737–741.discussion 741-2. [PubMed] [Google Scholar]
- Hansen JM, Pedersen D, Larsen VA, Sanchez-del-Rio M, Alvarez Linera JR, Olesen J, et al. Magnetic resonance angiography shows dilatation of the middle cerebral artery after infusion of glyceryl trinitrate in healthy volunteers. Cephalalgia. 2007;27:118–127. doi: 10.1111/j.1468-2982.2006.01257.x. [DOI] [PubMed] [Google Scholar]
- Fan JL, Subudhi AW, Evero O, Bourdillon N, Kayser B, Lovering AT, et al. AltitudeOmics: enhanced cerebrovascular reactivity and ventilatory response to CO2 with high altitude acclimatisation and re-exposure. J Appl Physiol (1985) 2013;116:911–918. doi: 10.1152/japplphysiol.00704.2013. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Sperling B, Severinghaus JW, Lassen NA. Augmented hypoxic cerebral vasodilation in men during 5 days at 3,810m altitude. J Appl Physiol. 1996;80:1214–1218. doi: 10.1152/jappl.1996.80.4.1214. [DOI] [PubMed] [Google Scholar]
- Rupp T, Esteve F, Bouzat P, Lundby C, Perrey S, Levy P, et al. Cerebral hemodynamic and ventilatory responses to hypoxia, hypercapnia, and hypocapnia during 5 days at 4,350m. J Cereb Blood Flow Metab. 2014;34:52–60. doi: 10.1038/jcbfm.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen GF, Krins A, Basnyat B. Cerebral vasomotor reactivity at high altitude in humans. J Appl Physiol (Bethesda, Md: 1985) 1999;86:681–686. doi: 10.1152/jappl.1999.86.2.681. [DOI] [PubMed] [Google Scholar]
- Cohen PJ, Wollman H, Alexander SC, Chase PE, Behar MG. Cerebral carbohydrate metabolism in man during halothane anesthesia: effects of Paco2 on some aspects of carbohydrate utilization. Anesthesiology. 1964;25:185–191. doi: 10.1097/00000542-196403000-00013. [DOI] [PubMed] [Google Scholar]
- Miller AL, Hawkins RA, Veech RL. Decreased rate of glucose utilization by rat brain in vivo after exposure to atmospheres containing high concentrations of CO2. J Neurochem. 1975;25:553–558. doi: 10.1111/j.1471-4159.1975.tb04367.x. [DOI] [PubMed] [Google Scholar]
- Hertz MM, Paulson OB. Transfer across the human blood-brain barrier: evidence for capillary recruitment and for a paradox glucose permeability increase in hypocapnia. Microvasc Res. 1982;24:364–376. doi: 10.1016/0026-2862(82)90023-1. [DOI] [PubMed] [Google Scholar]
- Jain V, Langham MC, Floyd TF, Jain G, Magland JF, Wehrli FW, et al. Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab. 2011;31:1504–1512. doi: 10.1038/jcbfm.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SP, Krasney JA. Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J Cereb Blood Flow Metab. 1995;15:115–123. doi: 10.1038/jcbfm.1995.13. [DOI] [PubMed] [Google Scholar]
- van Hulst RA, Lameris TW, Haitsma JJ, Klein J, Lachmann B. Brain glucose and lactate levels during ventilator-induced hypo- and hypercapnia. Clin Physiol Funct Imaging. 2004;24:243–248. doi: 10.1111/j.1475-097X.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.