Abstract
Excessive intake of high-caloric diets as well as subsequent development of obesity and diabetes mellitus may exert a wide range of unfavorable effects on the central nervous system (CNS). It has been suggested that one mechanism in this context is the promotion of neuroinflammation. The potentially harmful effects of such diets were suggested to be mitigated by physical exercise. Here, we conducted a study investigating the effects of physical exercise in a cafeteria-diet mouse model on CNS metabolites by means of in vivo proton magnetic resonance spectroscopy (1HMRS). In addition postmortem histologic and real-time (RT)-PCR analyses for inflammatory markers were performed. Cafeteria diet induced obesity and hyperglycemia, which was only partially moderated by exercise. It also induced several changes in CNS metabolites such as reduced hippocampal glutamate (Glu), choline-containing compounds (tCho) and N-acetylaspartate (NAA)+N-acetyl-aspartyl-glutamic acid (NAAG) (tNAA) levels, whereas opposite effects were seen for running. No association of these effects with markers of central inflammation could be observed. These findings suggest that while voluntary wheel running alone is insufficient to prevent the unfavorable peripheral sequelae of the diet, it counteracted many changes in brain metabolites. The observed effects seem to be independent of neuroinflammation.
Keywords: diabetes, exercise, 1HMRS, inflammation, obesity, spectroscopy
Introduction
Obesity is one of the major health burdens of modern societies and is associated with a variety of health-threatening sequelae such as diabetes mellitus (DM), cardiovascular diseases, and even cancers. Studies in humans and animals have also found evidence for an association of obesity and central nervous system (CNS) diseases such as cognitive decline and development of dementia.1 The exact underlying mechanism for this phenomenon has not yet been elucidated, though animal studies indicate that high-calorie diets may negatively affect structure and function of the hippocampus, a brain region critically involved in learning and memory.2 In addition, especially those individuals, who develop type 2 diabetes (T2DM) in the course of adiposity, seem to be more prone to the negative effects on the CNS level. The term subsuming the variety of different CNS changes that have been reported in these patients so far is diabetic encephalopathy. The underlying mechanisms of diabetic encephalopathies seem to be complex and may be attributable to a variety of factors such as insulin resistance, hyperinsulinemia and hyperglycemia resulting in vascular damage, brain atrophy, disturbed brain metabolism as well as an increased risk for the development of Alzheimer's disease.3
In this context, it has been further postulated before that central inflammation is a mechanism linking western diets, rich in energy and saturated fatty acids, with changes in brain integrity. A cell type directly involved in this process is the microglia, which has been reported to be activated by the consumption of such diets.4 Microglia activation is accompanied by morphologic changes of this distinct glia cell type and the release of pro-inflammatory cytokines.5
Exercise is an important factor for modifying peripheral risk factors of obesity such as energy metabolism, glucose use, insulin sensitivity, and inflammation.6 Voluntary exercise may further reverse neuroinflammation and restore the neuroplastic potential of the brain.7 Exercise is thus uniquely positioned to enhance brain health and function by reducing peripheral risk factors for lesions of the CNS and to directly affect brain integrity.
Progress in magnetic resonance imaging (MRI) techniques has led to a growing number of primarily human studies investigating structural cerebral changes in metabolic diseases such as DM and obesity.3
Proton magnetic resonance spectroscopy (1HMRS) has further been used to investigate central metabolite alterations in vivo, which may reflect a variety of neuropathologic processes such as neuroinflammation, or changes in neuronal integrity.8 Changes in the 1HMRS profiles may precede potentially irreversible and functionally relevant macro-morphologic disturbances and could hence provide a tool for early detection and prevention in this regard. In addition, it has the potential to detect disturbances in energy and neurotransmitter metabolism,9 which may be associated with diabetic encephalopathy as well.
As an example, the metabolic markers N-acetylaspartate (NAA) and N-acetyl-aspartyl-glutamic acid (NAAG) are believed to exist in significant quantities in neurons and neuronal processes, and therefore could represent markers of neuronal integrity, have repeatedly shown to be altered in patients with type 2 diabetes (T2DM).10, 11, 12 Though MRI studies in animals might be a suitable tool for experimentally disentangle the potential effects of DM and other components of the metabolic syndrome on brain integrity, studies on this topic are sparse13 and have instead mainly focused on models of insulin-dependent DM.14 So far, also no study has investigated the effects of physical exercise on the aforementioned changes in CNS metabolites in animal models of the metabolic syndrome.
In the present study, we aimed to investigate how consumption of a so-called cafeteria diet, which has been shown to be a robust model for the metabolic syndrome,15 is affecting brain metabolism, and if potential metabolic changes may be prevented by physical activity in the form of voluntary wheel running. The primary region of interest was the hippocampus, since it is widely involved both in cognitive and in affective processing. As a metabolically active brain region, it has been shown to be associated with the cognitive decline following bad metabolic health.16 On three cellular level, we hypothesized that the different diets are affecting glia-cell distribution and activity, potentially promoting inflammatory response under such conditions and that these changes in cell distribution and activity will be paralleled by specific metabolic patterns assessed by 1HMRS. As a control region, we chose the prefrontal cortex (PFC), to investigate whether potential chances would be a global or local phenomenon.
Materials and methods
All animal experiments were conducted according to the German federal animal welfare legislation and had been approved by the German animal welfare authorities (Regierungspräsidium, Karlsruhe, Germany). A total of 48 C57BL/6N male mice obtained at the age of 6 weeks from Charles River (Sulzfeld, Germany) were used to assess the effect of a high-caloric cafeteria diet in running and sedentary mice. Full details of the study had been approved by the German animal welfare authorities.
From the beginning, mice were kept single-housed in Macrolon type III cages in a temperature and humidity controlled room, on a 12-hour light–dark cycle with lights on at 1900 h. At the age of 8 weeks, running wheels were introduced into each cage and blocked in the sedentary group as described earlier. Water and food were available ad libitum. Simultaneously to wheel introduction half of the mice received a cafeteria diet additional to standard chow (ssniff R/M-H Extrudat, ssniff GmbH, Soest, Germany). The cafeteria diet consisted of commercially available candy bars, cookies, chocolate, cheese, processed meats, and crackers (Supplementary Table S1, Supplementary information). Foods were selected for energetic homogeneity and low evaporation within a 3-day period. In addition to the standard chow, mice in the cafeteria-diet group had the choice between two different snacks in addition to the standard diet provided throughout the experiment. We changed the cafeteria diet every 2 to 3 days. Food consumption was then determined by weighting the diet before and after feeding and further corrected for evaporation. Metabolizable energetic content of the different snacks was calculated according to the manufacturer's information. Bodyweight was assessed once-weekly in nonfasting animals during the dark phase.
We defined four experimental groups: standard chow sedentary (SS, n=12), standard chow runners (SR, n=12), cafeteria-diet sedentary (CS, n=12), and cafeteria-diet runners (CR, n=12). During the experiment two mice died, one due to unknown reasons and one during anesthesia. Therefore, group sizes are inhomogeneous (SS=12, SR=12, CS=11, and CR=11). After 9 to 10 weeks of running and diet consumption, magnetic resonance spectra of nonfasting mice were acquired in a 9.4-T horizontal bore animal scanner equipped with a cryogenic mouse brain coil (Bruker, Ettlingen, Germany).
Tissue Harvest
For tissue harvest, mice were anesthetized by intraperitoneal injection of ketamine and xylazine. After preparation for perfusion, an amount of 500 μL blood was taken from the left ventricle of the heart and subsequently mice were perfused transcardially with cooled sodium chloride. Brains were removed, split into halves and the randomly selected right and left hemisphere were postfixed for ~8 hours in 4% paraformaldehyde, and kept in phosphate-buffered saline (PBS) overnight. In all, 33-μm coronal sections were cut on a vibratome and kept at −20°C in cryoprotection solution until further processing. From the other hemisphere, hippocampus and frontal cortex were dissected on ice and stored at −80°C until further processing for real-time (RT)-PCR analysis. Intraabdominal fat pads were dissected according to the method of Johnson and Hirsch.17 After that, the skin of the animals was removed and all visible inguinal/subcutaneous fat was dissected. The animals were then weighted again and the difference before and after fat removal is further referred to as lean mass.
Measurement of Blood Glucose and Insulin Levels
For determination of fasting glucose, mice were food deprived for 6 hours before testing. Measurement of blood glucose took place at least 24 hours apart from administration of the mice for MRI routines, to avoid any interference of stress or tissue injury with brain metabolite levels. For the sampling procedure, mice were placed into a restraining tube. A blood sample was obtained by nicking the lateral tail vein using a sterile scalpel blade and a drop of blood was administered on a glucose test strip. Individual blood glucose levels were determined using a glucometer (Accu-Chek Aviva, Roche Diagnostics GmbH, Mannheim, Germany). Nonfasting glucose and insulin levels were determined in animals after scarification in ventricular blood. Plasma insulin concentrations were analyzed using commercially available kits (ALPCO, Salem, NH, USA) according to the manufacturer's instructions.
Immunohistochemistry
Every twelfth section of 6 to 8 animals per group was processed free-floating as described earlier.18, 19 Microglia cells were visualized using polyclonal rabbit IgG anti-Iba1 (ionized calcium-binding adapter molecule; 1:1,000; 019-19741, Wako Chemicals GmbH, Neuss, Germany). Sections were incubated in 0.6% H2O2 in Triton in Tris-buffered saline (Tris-Triton) for 30 minutes at room temperature to block endogenous peroxidase activity. Sections were preincubated in 2% normal serum in 0.2% Triton in Tris-buffered saline for 1 hour at room temperature and, subsequently, in primary antibody overnight at 4°C. Sections were rinsed and incubated with biotinylated secondary antibody (goat anti-rabbit IgG; diluted 1:300, VectastainEliteABC kit, Vector Laboratories, Burlingame, CA, USA). Rinses were performed between all steps using 0.05% (Tris-Triton), pH 7.4, or only Tris-buffered saline (pH 7.4). Finally, sections were incubated in avidin–biotin complex (VectastainEliteABC kit) for 20 minutes at room temperature, and stained using diaminobenzidine as a chromogen. Sections were further counterstained with hematoxylin solution (51275 Fluka, Buchs, Switzerland) for pyknotic cell evaluation as a marker of apoptosis.20 Slides were finally air dried overnight, dehydrated, coverslipped with Permountor mounting medium and examined with a ZeissAxioskop microscope (Carl Zeiss, Jena, Germany).
Stereology and Morphology
Quantitative analyses were performed as described. The investigator was blinded for the group allocation of the animal by Ben Abdallah et al.20 Briefly, pyknotic cells were counted in the subgranular zone of the dentate gyrus, using a × 40 magnification. Pyknotic cells were identified by strongly and homogeneously stained nuclei reflecting chromatin condensation and fragmentation of the nucleus. Total cell number was calculated by multiplying the number of cells counted by the inverse of the sample fractions.
The average total numbers of total dentate granule neurons and Iba-1-positive cells were estimated using the optical fractionator (StereoInvestigator software, MBF Bioscience, Williston, VT, USA). For granule cells, a counting frame sized 250 × 250 μm was placed over the dentate gyrus at intervals of 135 μm along the x-axis and 105 μm along the y-axis at × 40 magnification. Total numbers of cells were estimated by multiplying the number of cells counted with the inverse of the sampling fraction. Ionized calcium-binding adapter molecule 1-positive cells in the hippocampus were counted at × 40. Average cell numbers were calculated by using three sections representing medial, ventral, and dorsal hippocampus. In each of these sections, a counting area was applied, separately surrounding the CA1, CA3 region and the dentate gyrus. The Iba1-positive cells were also counted in the cortex region (Bregma+1.92). Cell numbers were calculated by application of a counting grid covering 250 × 250 μm at intervals of 135 μm along the x-axis and 105 μm along the y-axis by mean of the optical fractionator. Average cell numbers of each region were calculated by using the mean of three sections (medial, ventral, and dorsal hippocampus) of CA1, CA3, and DG, respectively, and are reported as average cell number per 10,000 μm2.
Real-Time PCR
Total RNA was prepared from the entire hippocampus and PFC samples from one hemisphere using TRIzol and the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany), in conjunction with on-column digestion for gDNA removal using DNAseI (Qiagen). For RT-PCR, cDNA was prepared with the SuperScript II First-Strand cDNA synthesis kit for RT-PCR (Invitrogen, Carlsbad, CA, USA) using random hexamer primer and analyzed in duplicate using the QuantiFastSYBR Green PCRKit (Qiagen). The experiments were performed in a LightCycler2.0 (Roche Diagnostics) with the following PCR settings: initial activation step at 95°C for 5 minutes followed by 40 cycles of denaturation at 95°C for 10 seconds and combined annealing/extension at 60°C for 30 seconds. At the end of every run, a melting curve was measured to ensure the quality of PCR products. The oligonucleotide primer sets were synthesized from Sigma-Aldrich GmbH (St Louis, MO, USA). The primer set for each gene is listed in the Supplementary information (Supplementary Table S2).
Magnetic Resonance Spectroscopy Methods
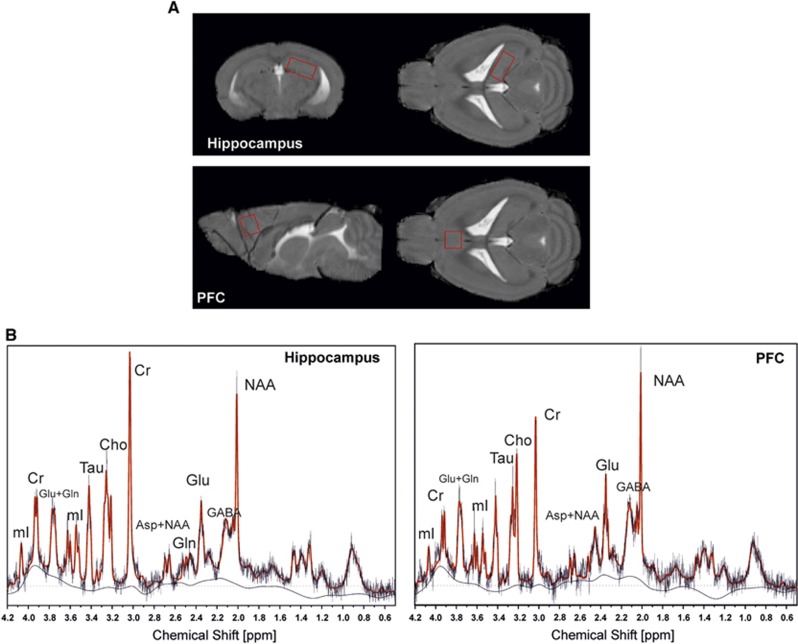
All magnetic resonance experiments were performed at a 9.4-T horizontal bore animal scanner equipped with a cryogenic mouse brain coil (Bruker). Mice were anesthetized by a gas mixture of O2: 50% and air: 50% with approximately 1.5% isoflurane. Respiration rate was monitored throughout the experiment. Body temperature was maintained at 36°C by warm water circulation and an external coil-heater and verified by a rectal thermosensor. The 1HMR spectra were acquired using a point resolved spectroscopy (PRESS) sequence at a short echo time of 10 ms and a repetition time of 4 seconds from a 3.2-μL volume (2.2 × 1.2 × 1.2 mm3) placed in the right hippocampus and from a 2.5-μl volume (1.6 × 1.2 × 1.3 mm3) placed in the right PFC. Figures 1A and 1B, show the location of the two spectroscopy voxel. Both sequences were performed with 256 averages resulting in a scan time of 17 minutes 04 seconds for each spectrum. Additionally, the voxel was angulated to exclude any partial volume. To minimize chemical shift displacement artifacts, the PRESS sequence was modified to deliver the slice selective excitation and refocusing pulses with a frequency shift of −2 ppm referred to the water peak. An additional one shot unsuppressed water signal was acquired with no frequency shift, which was used for water scaling. Spectral quantification of the in vivo spectra was obtained from LCModel (LCModel, ver. 6.2-0R; http://www.s-provencher.com/), a user-independent fitting routine, for which we measured a reference phantom data set consisting of 13 different metabolite signals acquired at the same scanner with the same acquisition parameters. Concentration values were referenced to the unsuppressed water signal from the same voxel and corrected for voxel tissue compartment using an in-house algorithm (SegSpec v6.4)18 and are reported in institutional units (i.U.).
Figure 1.
Voxel location and representative spectra. Voxel localization (A) and typical spectra at 9.4 T (point resolved spectroscopy (PRESS), 3.2 μL, echo time (TE)=10 ms, repetition time (TR)=4 seconds, 256 averages) (B). Both spectra are overlaid by the LC Model fit-curve. Peak resonances are N-acetylaspartate (NAA), creatine and phosphocreatine (Cr), choline-containing compounds (Cho), myo-inositol (mI), glutamate (Glu), glutamine (Gln), Glu+Gln (Glx), taurine (Tau), and γ-aminobutyric acid (GABA).
In total, 13 metabolites were quantified by means of 1HMRS: myo Inositol (mI), creatine and phosphocreatine (Cr), glutamine (Gln), glutamate (Glu), NAA, NAAG, NAA+NAAG (total NAA, tNAA), taurine (Tau), aspartate(Asp), γ-aminobutyric acid (GABA), glycerophosphocholine and phosphocholine (choline-containing compounds tCho), glucose (Glc), and lactate (Lac).
Results
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). All data are reported as means±s.e.m. Differences between subgroups were detected using two-factorial analysis of variance (ANOVA) followed by Bonferroni's post hoc analysis where applicable. Pearson correlation coefficients with two-tailed P values were used to assess the relationship between brain metabolites and metabolic variables of our model. Significance was evaluated at a probability of 5% or less.
Total Energy Intake
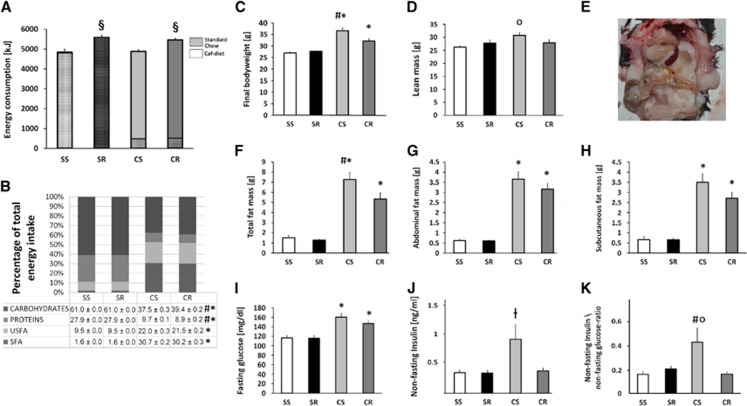
Running was the main factor for total energy intake (F(1,42)=61.1, P<0.001) but not cafeteria diet. Post hoc analysis revealed that CR mice consumed significantly more energy in total (5,460±72.8 Kilojoule (kJ)) than CS mice (4,886±68.7 kJ) (P<0.001), while the SR group (5,582±103.7 kJ) had a similar energy consumption compared with CR mice and significantly higher consumption than its sedentary counterpart (SS, 4,789±94.0 kJ, P<0.001). Over the whole study period, CS mice in average only acquired 286±2.4 kJ of energy from the consumption of standard chow and CR mice 302±3.3 kJ. Thus corresponded to 4.6% of total energy intake in CS and 6.1% in CR, a difference that was not significant (P=0.416) (Figure 2A).
Figure 2.
The metabolic phenotype. Total energy intake: Cafeteria running (CR) mice consumed significantly more energy in total than cafeteria sedentary (CS) mice (P<0.001), while the standard running (SR) group had a similar energy consumption compared with CR mice and significantly higher consumption than its sedentary counterpart (P<0.001). Though animals from both caf groups also consumed the offered standard chow, the total relative energy intake from that source was minimal (A). Caf animals acquired more energy from the intake of total fat than animals in the standard chow groups (P<0.001). CS animals also consumed relatively more total fat than their exercising counterparts (CR) (P=0.012). The relative total intake of saturated fatty acid (SFA) in caf animals was higher than in the standard chow groups (P<0.001). There was no significant difference between CS and CR animals while caf animals consumed significantly more SFA than did standard chow mice (P<0.001). The relative total intake of SFA in caf animals was higher than in the standard chow groups (P<0.001). Caf animals consumed significantly more unsaturated fatty acid (USFA) than did standard chow mice (P<0.001). Caf animals acquired significantly less energy from the intake of protein than animals in the standard chow groups (P<0.001). CS animals consumed relatively more protein than their exercising counterparts (CR) (P<0.001). CR consumed relatively less energy derived from carbohydrates as did mice on standard chow (P<0.001). CR mice also consumed significantly more carbohydrates than did their sedentary counterparts (P<0.001) (B). Bodyweight: SR mice had a mean total body weight of 27.7±0.3 g which was not significantly different from standard sedentary (SS) animals 26.9±0.4 g. In average, CR animals weighted 32.1±1.0 g, which was significantly higher than SS and SR mice (P<0.001) but also significantly lower than CS mice whose average weight of 36.6±1.1 g was significantly higher than that of all other groups (P<0.001) (C). Lean mass did only significantly differ between CS and SS animals (P=0.01) (D). CS mice (panel (E) shows excessive intraabdominal fat accumulation in an CS mouse) had significantly more total body fat than did CR mice (P=0.01) and both caf groups had a higher fat mass than did the mice on standard chow. SS and SR did not differ in terms of total body fat (F). CS and CR mice showed a significantly higher abdominal fat mass than did mice on standard chow (P<0.001). There were no significant differences within the two diet groups (G). CS and CR mice showed a significantly higher subcutaneous fat mass than did mice on standard chow (P<0.001). There were no significant differences within the two diet groups (H). Fasting glucose in SR animals was comparable to that of SS animals (n.s.). CS animals had the highest fasting glucose followed by CR mice. While both cafeteria groups did not differ significantly (P=0.683), fasting glucose of cafeteria-diet mice was much higher than in animals receiving standard chow (CR versus (SR and SS), P=0.003; CS versus (SR and SS), P<0.001) (I). In post hoc analysis, CS animals had significantly higher insulin levels than SR (P=0.032) but there was only a trend for higher insulin levels in CS versus CR mice (P=0.061). There was no significant difference between SS and SR mice (J). For the insulin/glucose ratio in post hoc analysis, there was a significant difference between CR and CS mice (P=0.023) and in CS versus SS animals (P=0.045) (K). (Symbols indicate significant differences between subgroups: §SR+CS versus SS+CS; *CS+CR versus SS+SR; #CS versus CR; OCS versus SS, ┼CS versus CR). Errorbars indicate s.e.m.'s. Statistical significant differences between the groups were determined by one-way analysis of variance followed by Bonferroni post hoc test, P≤0.05 was taken to indicate statistical significant differences.
Energy Consumption
Total fat
For the percentage of energy derived from total fat with regard to total energy intake, there was a significant effect of running (F(1,46)=5.6, P=0.022) as well as for cafeteria diet (F(1,46)=40323, P<0.001) and an interaction effect of running × cafeteria (F(1,46)=5.6, P=0.022). In post hoc analysis cafeteria animals (CR 51.7±0.3%, CS 52.7±0.3%) acquired more energy from the intake of total fat than animals in the standard chow groups (SR 11.1±0%)(P<0.001). The CS animals also consumed relatively more total fat than their exercising counterparts (CR) (P=0.012).
Saturated and Unsaturated Fatty Acids
By looking at saturated (SFA) and unsaturated (USFA) fatty acids separately the relative total intake of SFA in caf animals (CR 30.2±0.3%, CS 30.7±0.2%) was higher than in the standard chow groups (SR 1.6±0.0%) (F(1,46)=23212.9, P<0.001). There was no effect of running, so in post hoc comparison there was no significant difference between CS and CR animals while cafeteria animals consumed significantly more SFA than standard chow mice (P<0.001) (Figure 2B).
The relative total intake of USFA in cafeteria animals (CR 21.5±0.7%, CS 22.0±0.3%) was higher than in the standard chow groups (9.5±0.0%) (F(1,46)=4898, P<0.001). As for SFA there was also no effect of running and in post hoc comparison there was also no significant difference between CS and CR animals while cafeteria animals consumed significantly more USFA than mice on standard chow (P<0.001) (Figure 2B).
Protein
For the percentage of energy derived from total protein with regard to total energy intake there was a significant effect of running (F(1,46)=22.5, P<0.001) as well as for cafeteria diet (F(1,46)=42631, P<0.001) and an interaction effect of running × cafeteria (F(1,46)=22.5, P<0.022). In post hoc analysis cafeteria animals (8.9±0.2%, CS 9.7±0.1%) acquired significantly less energy from the intake of proteins than animals in the standard chow groups (27.9±0.0%) (P<0.001). CS animals consumed relatively more protein than their exercising counterparts (CR) (P<0.001) (Figure 2B).
Carbohydrates
There was a significant effect of running (F(1,46)=23.9, P<0.001) and diet (F(1,46)=14619, P<0.001) on the relative total intake of energy derived from carbohydrates as well as an interaction effect of running × cafeteria (F(1,46)=23.9, P<0.001). In post hoc analysis, CR (39.4±0.2%) and CS (37.5±0.3%) animals consumed relatively more energy derived from carbohydrates as did mice on standard chow (P<0.001). The CR mice also consumed significantly more carbohydrates than SR mice (P<0.001) (Figure 2B).
Bodyweight
The final bodyweight was significantly influenced by diet (F(1,42)=83.68, P<0.001) and running (F(1,42)=8.57, P=0.005). In post hoc analyses, SR mice had a mean total body weight of 27.7±0.3 g that was not significantly different from SS animals 26.9±0.4 g. In average, CR animals weighted 32.1±1.0 g, which was significantly higher than SS and SR mice (P<0.001) but also significantly lower than CS mice whose average weight of 36.6±1.1 g was significantly higher than that of all other groups (P<0.001) (Figure 2C).
Body Composition
There was a significant effect for diet on lean mass (F(1,41)=5.9, P=0.02), while running had no effect in this regard. There was also an interaction effect for running × diet (F(1,41)=5.4, P=0.024). In post hoc analysis, lean mass did only significantly differ between CS and SS animals (P=0.01) (Figure 2D). Diet (F(1,41)=140.9, P<0.001), as well as running (F(1,41)=7.2, P=0.01), had a significant main effect on total body fat. Post hoc analysis revealed that CS mice had significantly more total body fat than did CR mice (7.3±0.5 g versus 5.3±0.6 g; P=0.01) and both cafeteria groups had a higher fat mass than mice on standard chow (SR 1.3±0.9 g; SS 1.5±0.2 g). The SS and SR animals did not differ in terms of total body fat (Figure 2F). Only diet (F(1,35)=213.2, P<0.001) but not running had a significant main effect on abdominal fat mass. In post hoc analysis, CS (3.2±0.3 g) and CR (3.7±0.3 g) mice showed a significantly higher abdominal fat mass than did mice on standard chow (both 0.6±0.02 g) (P<0.001). There were no significant differences within the two diet groups (Figure 2G). Diet (F(1,35)=101.7, P<0.001) but not running had a significant main effect on subcutaneous fat mass. In post hoc analysis, CS (3.5±0.4 g) and CR (2.7±0.3 g) mice had a significantly higher subcutaneous fat mass than mice on standard chow (both 0.7±0.1 g) (P<0.001). There were no significant differences within the two diet groups (Figure 2H).
Effects of Diet and Running on Fasting Glucose
There was a significant effect of diet on fasting glucose (F(1,42)=83.68, P<0.001), while running had no effect in this regard. Fasting glucose in SR animals (115.8±4.8 mg/dL) was comparable to that of SS animals (116.3±5.8 mg/dL) (n.s.). The CS animals had the highest fasting glucose with 160.1±6.9 mg/dL followed by CR mice with 146±5.4 mg/dL. While both cafeteria groups did not differ significantly (P=0.683), fasting glucose of cafeteria-diet mice was much higher than in animals receiving standard chow (CR versus (SR and SS), P=0.003; CS versus (SR and SS), P<0.001) (Figure 2I).
Effects of Diet and Running on Insulin Levels
There was a significant main effect of diet (F(1,20)=8.23, P=0.011) and running (F(1,20)=4.5, P=0.05) on nonfasting insulin levels. In post hoc analysis, CS (0.9±0.27 ng/mL) animals had significantly higher insulin levels than SR (03.±0.05 ng/mL) mice (P=0.032), but there was only a trend for higher insulin levels in CS versus CR mice (0.43±0.05 ng/mL) (P=0.061). There was no significant difference between SS and SR mice (both 0.3±0.05 ng/mL) (Figure 2J).
For the insulin/glucose ratio, as a measure of insulin resistance, there was only a trend for the factors diet (F(1,20)=3.9 P=0.058) and running (F(1,20)=3.9 P=0.064) observed, but there was an interaction effect running × diet (F(1,20)=6.2 P=0.024). In post hoc analysis, there was a significant difference between CR (1.7±0.02) and CS mice (0.4±0.12) (P=0.023) and in CS versus SS animals (0.2±0.03) P=0.045) (Figure 2K).
Magnetic Resonance Spectroscopy
After visual inspection, we discarded five of the obtained spectra due to bad spectral quality ending up in a total sample size for the hippocampal region of 41 (SS=11, SR=9, CS=10, and CR=11). Additionally, only metabolite concentrations estimated with a CRLB (Cramér-Rao lower bound) of ≤20% were taken into account. Statistical analysis was performed using SPSS 21.0 (SPSS Inc.). All data are reported as means±s.e.m. Due to the CRLB criterion, we investigated first those metabolites which fulfilled this criterion for all subjects namely tNAA, Glu, Gln, Tau, tCho, tCr, and mI. A multivariate ANOVA was performed for these metabolites with running and cafeteria as factors. Since the NAAG peak could not be resolved in sufficient fit quality in all spectra tNAA is reported. We hypothesized that Glc and Lac should be influenced by exercise and cafeteria, but since both resonances could not be determined with a CRLB of ≤20% for all animals univariate ANOVAs for Glc and Lac were performed. Furthermore, to find group specific differences in a Bonferroni post hoc test, we performed the same univariate and multivariate ANOVA again but with group (SS/SR/CS/CR) as a factor. Significance was evaluated at a probability of 5% or less and both regions were expected as independent. The statistical analysis yielded the following results in the two investigated brain regions.
Hippocampus
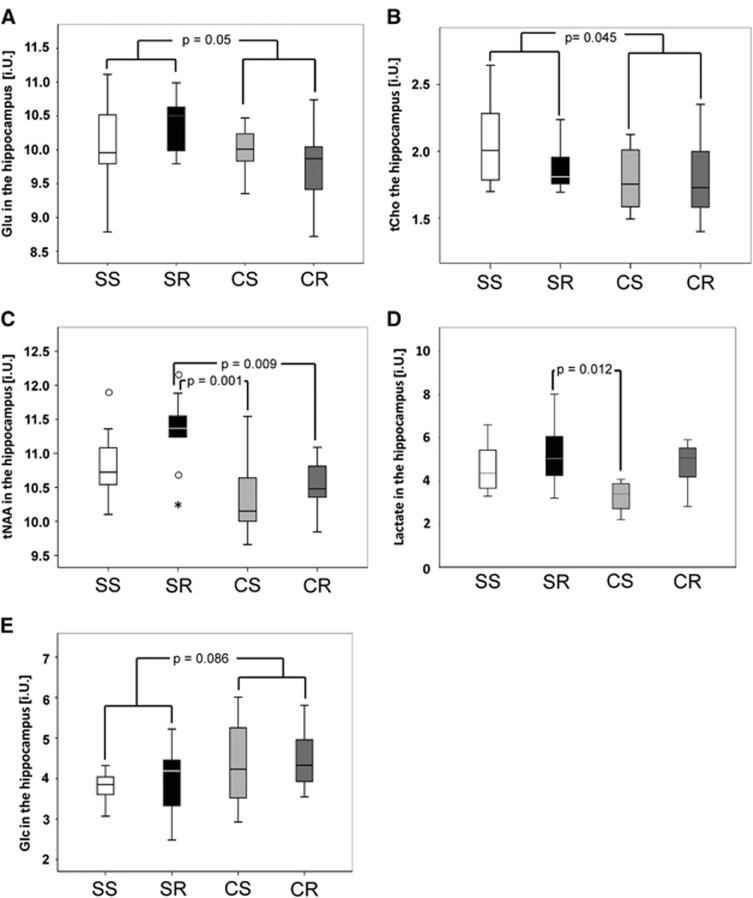
In the hippocampal region, both running (F(7,31)=2.56, P=0.034) and cafeteria diet (F(7,31)=4.66, P=0.001) showed significant effects in the performed MANOVA (NSS=11, NSR=9, NCS=10, and NCR=11). The tests of between-subject effects yielded significant results for tNAA (P=0.046) while Gln (P=0.058) and tCr (P=0.082) showed a trend. All these metabolites were increased by the factor running. For cafeteria diet, the tests of between-subject effects showed significant decreasing effects for Glu (P=0.05), tCho (P=0.045), and tNAA (P<0.001). In post hoc analyses, tNAA in CR (10.5±0.1 i.U.) and CS mice (10.4±0.2 i.U.) was lower than in SR mice (11.3±0.2 i.U.) (P=0.009 and P=0.001, respectively). Furthermore, there were no significant subgroup differences regarding Glu and tCho (Figures 3A to 3C).
Figure 3.
Effects of running and cafeteria diet on hippocampal metabolite concentrations (institutional units, i.U.). For cafeteria diet, the tests of between-subject effects showed significant decreasing effects for total N-acetylaspartate (tNAA) (P<0.001), glutamate (Glu) (P=0.05) (A), and choline-containing compounds (tCho) (P=0.045) (B). In post hoc analyses, tNAA in cafeteria running (CR) (10.5±0.1 i.U.) and cafeteria sedentary (CS) mice (10.4±0.2 i.U.) was lower than in standard running (SR) mice (11.3±0.2 i.U.) (P=0.009 and P=0.001, respectively) (D). For lactate, the post hoc Bonferroni comparison yielded a significant difference between SR (5.3±0.4 i.U.) and CS (3.3±0.4 i.U.) groups (P=0.012) (C). Regarding increased brain Glc levels there was only a trend (F(1,30)=3.15, P=0.086) for cafeteria diet (E). There were no further group differences with regard to the other brain metabolites investigated (N=10 to 12/group). SS, standard sedentary.
In an univariate ANOVA (NSS=10, NSR=8, NCS=7, and NCR=8) running (F(1,29)=8.04, P=0.008) as well as cafeteria diet (F(1,29)=4.69, P=0.039) had effects on hippocampal lactate levels. While running increased lactate levels and would survive correction for multiple comparison up to a factor of 6, cafeteria diet showed a trend toward lower lactate levels that does not survive correction for multiple comparison. The post hoc Bonferroni comparison yielded a significant difference between SR (5.3±0.4 i.U.) and CS (3.3±0.4 i.U.) groups (P=0.012) (Figure 3D)
Regarding increased brain Glc levels there was only a trend (F(1,30)=3.15, P=0.086) for cafeteria diet (NSS=8, NSR=6, NCS=10, and NCR=10) (Figure 3E).
Prefrontal Cortex
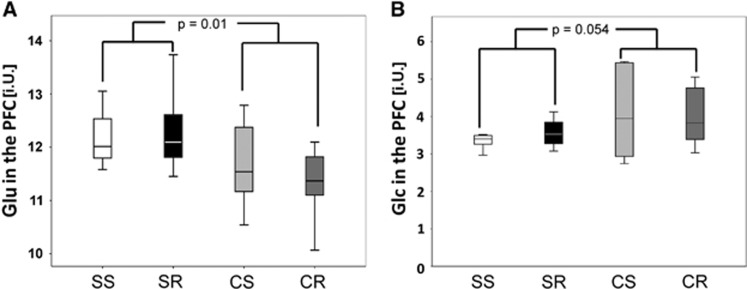
As a control region to see whether observable effects are limited to the hippocampus or whether they can also be found in other regions of the brain we also investigated the PFC. Performing the same MANOVA as in the hippocampus (NSS=10, NSR=10, NCS=11, and NCR=11) yielded no significance for the factor running (P=0.967) but a highly significant effect for cafeteria diet (P<0.001) with a significant effect on Glu (P=0.01) in the tests of between-subject effects and a trend for tCho (P=0.081). In post hoc analyses, CR (11.3±0.6 i.U.) mice showed lower Glu levels than SR (12.3±0.7 i.U.) mice (P=0.027) (Figure 4A). As in the hippocampal voxel, there was only a trend seen in terms of higher glucose levels for cafeteria diet (F(1,24)=3.5, P=0.054) in an univariate ANOVA (NSS=6, NSR=6, NCS=6, and NCR=10) which would also not survive the first multiple comparison (Figure 4B). The Lac level was not significantly influenced neither by running nor by diet.
Figure 4.
Effects of running and cafeteria-diet on brain metabolite concentrations in the prefrontal cortex (PFC) (institutional units, i.U.). For metabolites in the PFC, MANOVA yielded no significance for the factor running (P=0.967) but a highly significant effect for cafeteria diet (P<0.001) with a significant effect on glutamate (Glu) (P=0.01). In post hoc analyses, CR (11.3±0.6 i.U.) mice showed lower Glu levels than SR (12.3±0.7 i.U.) mice (P=0.027) (A). As in the hippocampal voxel, there was only a trend seen in terms of higher glucose levels for cafeteria diet (F(1,24)=3.5, P=0.054) in an univariate ANOVA, which would also not survive the first multiple comparison (B). The Lac level was not significantly influenced neither by running nor by diet. CR, cafeteria running; CS, cafeteria sedentary; SR, standard running; SS, standard sedentary.
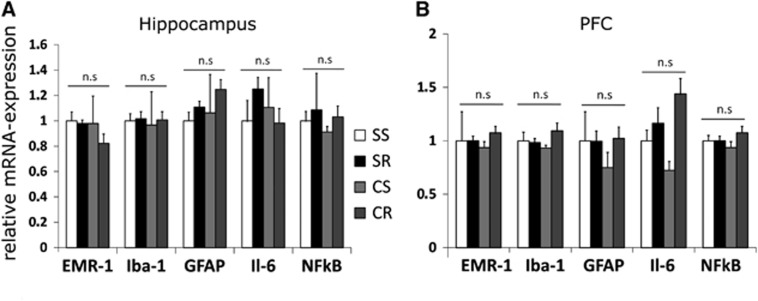
mRNA Expression
Relative mRNA expression in the hippocampus as well as in the PFC for the microglia markers Iba-1 and EMR1 did not significantly differ between the four groups. We also did not find any significant group differences with regard to inflammatory processes by means of Il-6 and NfκB expression, mRNA for TNF-α was nondetectable neither in hippocampus nor in PFC samples (Figure 5).
Figure 5.
mRNA expression in the hippocampus and the prefrontal cortex (PFC). Relative mRNA expression in the hippocampus (A) as well as in the PFC (B) for the microglia markers ionized calcium-binding adapter molecule (Iba-1) and EMR1 did not significantly differ between the four groups. We also did not find any significant group differences with regard to inflammatory processes by means of Il-6 and NfkB expression, mRNA for TNF- α was nondetectable neither in hippocampus nor in PFC samples. Errorbars indicate s.e.m.'s (N=8 to 12/group). Statistical significant differences between the groups were determined by one-way analysis of variance followed by Bonferroni post hoc test, P≤0.05 was taken to indicate statistical significant differences. CR, cafeteria running; CS, cafeteria sedentary; SR, standard running; SS, standard sedentary.
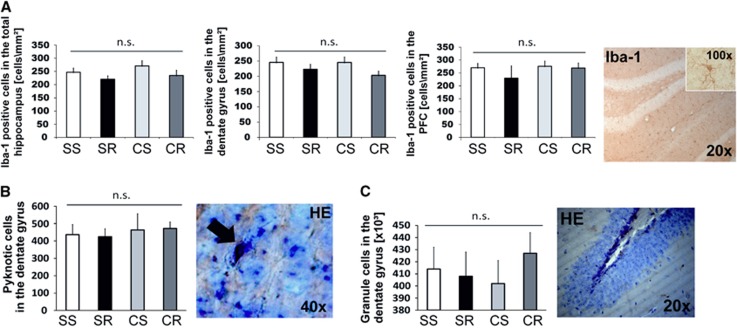
Effects of Diet and Running on Microglia and Neurons in the Hippocampus
We found no significant effect of running or cafeteria diet on Iba-1-positive cells in the hippocampus or PFC. There was only a trend for a negative effect of running on the number of Iba-1-positive cells in the dentate gyrus of the hippocampus (F(1,18)=3.5, P=0.075) as well as in the total hippocampus (F(1,18)=3.0, P=0.099). In the cortical region, no group differences were observed (Figure 6A).
Figure 6.
Effects of running and cafeteria diet on microglia and neurons. There was only a trend for a negative effect of running on the number of ionized calcium-binding adapter molecule (Iba-1)-positive cells in the dentate gyrus of the hippocampus (F(1,18)=3.5, P=0.075) as well as in the total hippocampus (F(1,18)=3.0, P=0.099). In the cortical region, no group differences were observed (A). (*P<0.001 versus CR; P<0.05 versus CR). There were no significant group differences with regard to pyknotic cells detectable in the granule cell layer (B, × 40) or estimated total granule cell number in the dentate gyrus (C, × 20). Errorbars indicate s.e.m.'s (N=6 to 8/group). Statistical significant differences between the groups were determined by one-way analysis of variance followed by Bonferroni post hoc test, P≤0.05 was taken to indicate statistical significant differences. CR, cafeteria running; CS, cafeteria sedentary; SR, standard running; SS, standard sedentary.
There were also no significant differences with regard to pyknotic cells detected in the granule cell layer or estimated total granule cell number in the dentate gyrus (Figures 6B and 6C).
Magnetic resonance Spectroscopy, Histologic Markers, and PCR-Results
Among all animals, there was a significant negative correlation of the number of pyknotic cells in the granule cell layer of the dentate gyrus and hippocampal tCho levels (Pearson correlation coefficient (R2)=0.325, P=0.011) (Figure 7). There were no significant associations with the neuronal marker tNAA with total neuron count in the granule cell layer of the hippocampus or with the number of pyknotic cells. There was also no significant association with any marker of central inflammation by means of RT-PCR and tNAA (data not shown).
Figure 7.
Correlations of metabolites with histologic markers of the hippocampus and hyperglycemia. There was a significant negative correlation of the number of pyknotic cells in the granule cell layer of the dentate gyrus and hippocampal GPC+PCh levels (Pearson correlation coefficient (R2)=0.325, P=0.011) (N=6 to 8/group). CR, cafeteria running; CS, cafeteria sedentary; SR, standard running; SS, standard sedentary.
Discussion
In the present study, mice with a voluntary high-caloric cafeteria diet gained significantly more weight and developed hyperglycemia and hyperinsulinemia during the course of the study. As intended by the composition of the cafeteria diet, these animals consumed significantly more fat than those animals receiving standard chow. In contrast, standard chow was composed of more proteins and had higher carbohydrate content and especially a higher proportion of polysaccharides. Interestingly, as in our free choice paradigm the cafeteria animals had the chance to choose how much of the corresponding snack they preferred to consume, there were small but highly significant differences with regard to the relative amount of macronutrient intake between CS and CR mice. While over the whole study period CR mice consumed more carbohydrates (mostly consisting of readily available mono- and disaccharides), the opposite was true for relative protein intake. This might be due to the fact that exercising animals prefer the directly available energy derived from the digestion of carbohydrates over protein digestion to satisfy their higher energy needs.21
Cafeteria diet induced obesity irrespective of total energy consumption, an effect that had been described before for western style diets.22 While weight gain per se was ameliorated by concomitant physical activity as was total body fat, this was not true for abdominal fat mass. Other aspects of the metabolic syndrome, such as fasting glucose, in these mice were not positively affected by exercise though there was an improvement in terms of the indicator of insulin resistance determined by the insulin/glucose ratio that has been observed before.23 This indicates that diets rich in saturated fats and sugars have detrimental effects on metabolic health not only by increasing body weight, though this may depend on the investigated rodent strain and time point of exercise introduction.24
Proton Magnetic Resonance Spectroscopy Findings
We could show that cafeteria diet decreased Glu levels in the hippocampus as well as in the PFC. Glu is the primary excitatory neurotransmitter in the mammalian brain. The Glu signal detected in 1HMRS is however most likely primarily attributable to the intracellular content, as the amount of extracellular Glu in contrast represents only a minor amount of the signal. Intracellular and extracellular Glu levels are however closely connected by the Glu–Gln cycle, which describes the crosstalk between neurons and astrocytes in terms of glutamate metabolism.25 In a study investigating subjects with T2DM compared with healthy controls, there was a significant decrease in glutamine and glutamate concentrations in the subcortical region investigated only in those detectable who were additionally also suffering from major depression.26
As a potential mechanism it has been proposed that in T2DM there might be an impairment in terms of glutamate–glutamine cycling.25 Astrocytes in contrast to neurons are capable of net synthesis of glutamate and glutamine from glucose in the tricarboxylic acid cycle, which in turn can be derived from their intracellular glycogen storages. Glutamine is then passed to the glutamatergic neuron, which subsequently transforms it into glutamate for neurotransmission purposes. Detectable changes in intracellular Glu levels may therefore also have an impact on neuronal communication.
How this mechanism might be affected by T2DM has been investigated in a study based on a zucker diabetes fatty rat model which used 13C-labeled glucose to visualize brain metabolism. Sickmann et al25 could show in this study that in these diabetic animals there is a decrease in brain glycogen storages, an impairment in tricarboxylic acid cycle activity and a concomitant decrease in Glu. They could further show that glycogen storages are differentially distributed not only between various brain regions but also between different rodent strains, potentially accounting for brain-site-specificity of their findings. Though it has been showed that glycogen storages are important to sustain glutamatergic signaling,9 it is still not clear how such findings may contribute a further mechanism to cognitive impaired in diabetic patients in addition to the structural alterations that have already been observed.
Though an effect of the lower protein intake in the cafeteria-diet group cannot be excluded, the content of Glu in the diet and its peripheral uptake is unlikely to contribute to group differences, as uptake of Glu and Gln is well controlled by specific transporters at the blood brain barrier level. At rest, the brain uptake of these amino acids from blood has been shown to be minimal and, additionally, intense exercise is not significantly altering this uptake to the brain.27 It is therefore more likely that changes in CNS levels of these amino acid are due to differences in central metabolism than due to alterations in peripheral uptake.
Cafeteria diet also decreased total choline levels in the hippocampus. tCho in our study was further negatively correlated with the number of pyknotic cells as a surrogate for the number of apoptotic cells. It has been reported before that apoptosis is associated with reduced Cho signals.28 While PCho is a biosynthetic precursor of the membrane-forming Phophytidylcholine (PtdCho), GPCho is a breakdown product of PtdCho. They can serve as a marker for cellular membrane turnover and Cho signals have been suggested to be decreased by neuronal death.
In human studies, Cho and Cho/Cr ratio levels have been repeatedly shown to be altered in T2DM. While some studies reported on an increase in Cho levels, i.e., in the occipital gray matter29, 30 or the parietal white matter,30 others studies even found an inverse relationship.10 Interestingly, in one study Cho/Cr ratios were particularly increased in those with poor glycemic control (HbA1C>10%) and HbA1C levels were further inversely correlated with frontal cortical and parietal white-matter Cho/Cr ratios.10 This might indicate that findings on Cho levels may depend on severity of glucose intolerance.
In contrast, in streptozotocin-induced diabetic rats under hyperglycemic conditions it has been reported that there is an increase in tCHo14 which seems, however, to be reversible upon reestablishment of euglycemia. Due to the fast reversibility of changes in tCho in rodent model of T1DM by the reversal of hyperglycemia, we cannot exclude that our findings on tCho are a temporal phenomenon as well. However, this may also point to the different etiologies of diabetic encephalopathies between type 1 and type 2 diabetics.3
Furthermore, cafeteria diet had a highly significant decreasing effect on tNAA levels in the hippocampus. The metabolite NAA has been frequently investigated in T2DM, and most studies showed decreased levels of NAA/Cr ratios.11, 30, 31 However, others showed no association between diabetes and NAA levels.26, 32 In a recent study in type 2 diabetics, it was further showed that there is a decrease in NAA which is more pronounced in those individuals with diabetic retinopathy as an early marker of microvascular damage.12
In humans studies, it has been demonstrated that markers for the degree of hyperglycemia such as fasting glucose and HbA1c levels are negatively correlated with NAA/Cr ratios in the brain, though these findings may depend on brain region investigated.11, 33 However, it has been described before that creatine itself might change in insulin-dependent diabetic conditions,13, 14 though, this is not a constant finding in T2DM.34 However, as a limitation, most of these studies have not focused on the hippocampus as a target region. In our study, the NAA signals were referenced to the unsuppressed water signal instead, to avoid any interference with this phenomenon.
While cafeteria diet influences metabolites in both regions investigated, voluntary running only had an effect on metabolites in the hippocampus.
Site specificity has been explained by differences in vascularization making some distinct brain structures more prone to hypoxia than others.
Decrease in tNAA was counteracted by voluntary wheel running, which is in line with findings in humans, where higher fitness levels of aged adults led to an increase in NAA levels, indicating increased neuronal viability in the frontal cortex.35
While running had a positive effect on hippocampal lactate levels, the opposite was true for cafeteria diet. It has been shown before that brain activity36 as well as acute physical activity in humans lead to an increase in brain lactate levels.37 This is explained by the findings, that, although carbohydrates are the main energy source of the brain, a significant amount of energy is provided by central glycogenolysis38 or even by utilization of peripheral lactate.39 In case of vigorous exercise, blood lactate rises and is taken up by the brain following a concentration gradient.40 The decrease in lactate levels in the caf animals would be in accordance with the idea of the aforementioned decreased hippocampal glycogen storages in T2DM.
The limitation of changes in lactate to the hippocampus can be explained by the fact that there is a twofold to threefold higher amount of glycogen stores in this brain region which are metabolized to lactate, and may potentially also adapt to an increased demand following neuronal activity during chronic exercise.38 Taken together, these results support the idea that cerebral lactate increases in case of increased blood level and is further enhanced by neuronal activation. As we did not measure lactate levels simultaneously in our study we can however only speculate on the primary underlying mechanism of our findings.
We could not confirm that the diet regimen used in this study was capable of inducing markers of central inflammation. There were no differences neither with regard to inflammatory markers nor with regard to microglia distribution. This may be explained by the fact that a change in microglia cell numbers as well as in activity following high-caloric diets is highly dependent on fat content and composition of the corresponding diet ingested and that only a diet containing as much as 60% fat is sufficient to induce central inflammation.41 The average fat content in our study was 51% (SFA 21% and USFA 30%) ranging from 50% to 55%. There were also no differences with regard to total granule cells and pyknotic cells in the dentate gyrus, though it has been reported before that voluntary wheel running may reduce cell death in a similar model after 6 weeks of voluntary wheel running. 42 However, the animals in the present study were older and the plastic changes induced by physical activity in the hippocampus have been suggested to decrease over time.43
Strength and Limitations
A strength of our study is that it is the first of its kind investigating the effects of an obesity-inducing western style diet in conjunction with a model of physical exercise on central metabolites by means of 1HMRS.
Though there are several 1HMRS studies in humans with T1DM and T2DM to our knowledge there is only one other study that has investigated these effects in a rodent model representing a type 2 diabetes-like condition, which is limited by its low sample size.13 Our findings do not support the idea that neuroinflammation is involved in the observed in vivo changes in brain metabolite levels. Metabolite changes reported to be associated with neuroinflammation, i.e., increased mI, tCho, and tCr were not observed. An additional strength of our study is that we investigated two brain regions separately, as earlier studies in rats have only used a single voxel, encompassing several brain regions, not allowing investigating for distinct effects on the hippocampus.
One might argue that the voluntary wheel running paradigm as well as the free choice cafeteria-diet model lack standardization, however, we chose these models, as forced running as well as many predefined commercially available high-fat or western diets lack diversity and may hence result in increased stress levels in contrast to a highly palatable cafeteria diet.44 This would have interfered with our initial hypothesis that central metabolites might be altered due to diet-induced central inflammation. To meet these limitations, we evaluated in detail the consumption of the different nutrients in our animals and closely monitored wheel running behavior to include it in our models.
To do so, it was inevitable to house the animals individually which has been suggested to change behavior and may also affect CNS processes, mainly attributable to impoverishment of the environment. However, our animals were living in large cages with environmental enrichment (bedding material, blocked or free running wheels) to mitigate an effect of impoverishment. Single housing is routinely performed in wheel running studies45 and it has also been shown that single housing does not necessarily induce changes in any major immuno-endocrine category under nonstressed conditions.46
Another limitation is that we did not determine blood glucose and lactate in parallel to MRI measurements, as we were afraid that the corresponding tissue lesions would interfere with the metabolite profiles. Finally, we cannot completely rule out that differences in body composition might have interfered with kinetics of anesthetics, which might hence have influenced brain lactate levels. This is of importance as volatile anesthetics47 such as isoflurane may alter neuronal activity and hence brain metabolism in particular with regard to central glucose and lactate levels and it has also been shown to increase peripheral lactate levels.48 Finally, the determination of nonfasting insulin is not optimal, what was attributable to our design. Keeping in mind that many changes in CNS metabolites in rodent models of T1DM were reversible upon achievement of euglycemia, this should also be a focus of further studies on T2DM in this regard and may further help to dissect the effects of obesity alone on CNS metabolites.
It would further be of interest, if the detection of early CNS metabolite alterations in the long run is predictive for the development of macro-morphologic changes or cognitive decline as part of diabetic encephalopathies. In multiple sclerosis, i.e., the disease in which 1HMRS procedures as a diagnostic tool has been extensively studied, changes in metabolites within lesion-free white-brain matter may predict disease progress.8 This is of particular importance as CNS disturbances following diabetic conditions are mostly irreversible as soon as they are detectable by conventional MRI procedures.3 Proton magnetic resonance spectroscopy studies may therefore have the potential to help at the prevention of such irreversible sequelae, by uncovering the need for intensified treatment modalities.
Conclusion
Voluntary cafeteria diet induces obesity and diabetes symptoms, which are paralleled by significant changes in CNS metabolite levels. We could show that voluntary wheel running alone is insufficient to prevent the unfavorable peripheral sequelae of this diet; however, it counteracted or attenuated changes in brain metabolites in regard that there were no significant differences between SS and CR mice. The observed effects seem to be independent of neuroinflammation.
MK Auer has received an unrestricted research grant by MSD Sharp & Dohme, Haar, Germany, to perform this study during a research fellowship at the Central Institute of Mental Health, Mannheim, Germany.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Sima AA. Encephalopathies: the emerging diabetic complications. Acta Diabetol. 2010;47:279–293. doi: 10.1007/s00592-010-0218-0. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chen YC, Yu L, Chen HI, Jen CJ, Huang AM, et al. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem. 2007;103:2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x. [DOI] [PubMed] [Google Scholar]
- Caramanos Z, Narayanan S, Arnold DL. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain. 2005;128:2483–2506. doi: 10.1093/brain/awh640. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Walls AB, Schousboe A, Bouman SD, Waagepetersen HS. Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem. 2009;109:80–86. doi: 10.1111/j.1471-4159.2009.05915.x. [DOI] [PubMed] [Google Scholar]
- Sahin I, Alkan A, Keskin L, Cikim A, Karakas HM, Firat AK, et al. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complications. 2008;22:254–260. doi: 10.1016/j.jdiacomp.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhou J, Sha L, Li Y, Qu X, Liu L, et al. Metabolite differences in the lenticular nucleus in type 2 diabetes mellitus shown by proton MR spectroscopy. AJNR Am J Neuroradiol. 2013;34:1692–1696. doi: 10.3174/ajnr.A3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Geng H, Zhang Z, Zhu X, Meng Q, Sun X, et al. Brain metabolite alterations demonstrated by proton magnetic resonance spectroscopy in diabetic patients with retinopathy. Magn Reson Imaging. 2014;32:1037–1042. doi: 10.1016/j.mri.2014.04.020. [DOI] [PubMed] [Google Scholar]
- van der Graaf M, Janssen SWJ, van Asten JJA, Hermus ARMM, Sweep CGJ, Pikkemaat JA, et al. Metabolic profile of the hippocampus of Zucker Diabetic Fatty rats assessed by in vivo 1H magnetic resonance spectroscopy. NMR Biomed. 2004;17:405–410. doi: 10.1002/nbm.896. [DOI] [PubMed] [Google Scholar]
- Duarte JMN, Carvalho RA, Cunha RA, Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J Neurochem. 2009;111:368–379. doi: 10.1111/j.1471-4159.2009.06349.x. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity. 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, et al. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2477. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- Böttiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, et al. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Mol Brain Res. 1999;65:135–142. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Zörner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes Brain Behav. 2003;2:3–10. doi: 10.1034/j.1601-183x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NMB, Slomianka L, Lipp H-P. Reversible effect of X-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus. 2007;17:1230–1240. doi: 10.1002/hipo.20358. [DOI] [PubMed] [Google Scholar]
- Oudot F, Larue-Achagiotis C, Anton G, Verger P. Modifications in dietary self-selection specifically attributable to voluntary wheel running and exercise training in the rat. Physiol Behav. 1996;59:1123–1128. doi: 10.1016/0031-9384(95)02175-2. [DOI] [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, Keeney BK, Hannon RM, Dlugosz EM, Garland T. Effects of early-life exposure to Western diet and wheel access on metabolic syndrome profiles in mice bred for high voluntary exercise. Genes Brain Behav. 2013;13:322–332. doi: 10.1111/gbb.12098. [DOI] [PubMed] [Google Scholar]
- Benrick A, Wallenius V, Asterholm IW. Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp. Physiol. 2012;97:1224–1235. doi: 10.1113/expphysiol.2012.065508. [DOI] [PubMed] [Google Scholar]
- Wagener A, Schmitt AO, Brockmann GA. Early and late onset of voluntary exercise have differential effects on the metabolic syndrome in an obese mouse model. Exp Clin Endocrinol Diabetes. 2012;120:591–597. doi: 10.1055/s-0032-1321727. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab. 30:1527–1537. doi: 10.1038/jcbfm.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajilore O, Haroon E, Kumaran S, Darwin C, Binesh N, Mintz J, et al. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2006;32:1224–1231. doi: 10.1038/sj.npp.1301248. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Ide K, Cai Y, Quistorff Br, Secher NH. The intent to exercise influences the cerebral O2/carbohydrate uptake ratio in humans. J Physiol. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LR, Huuse EM, Bathen TF, Goa PE, Bofin AM, Pedersen TB, et al. Assessment of early docetaxel response in an experimental model of human breast cancer using DCE-MRI, ex vivo HR MAS, and in vivo1H MRS. NMR Biomed. 2010;23:56–65. doi: 10.1002/nbm.1426. [DOI] [PubMed] [Google Scholar]
- Geissler A, Fründ R, Schölmerich J, Feuerbach S, Zietz B. Alterations of cerebral metabolism in patients with diabetes mellitus studied by proton magnetic resonance spectroscopy. Exp Clin Endocrinol Diabetes. 2003;111:421–427. doi: 10.1055/s-2003-44289. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ross B. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992;184:123–130. doi: 10.1148/radiology.184.1.1319074. [DOI] [PubMed] [Google Scholar]
- Kario K, Ishikawa J, Hoshide S, Matsui Y, Morinari M, Eguchi K, et al. Diabetic brain damage in hypertension: role of Renin-Angiotensin System. Hypertension. 2005;45:887–893. doi: 10.1161/01.HYP.0000163460.07639.3f. [DOI] [PubMed] [Google Scholar]
- Tiehuis A, van der Meer F, Mali W, Pleizier M, Biessels G, Kappelle J, et al. MR spectroscopy of cerebral white matter in type 2 diabetes; no association with clinical variables and cognitive performance. Neuroradiology. 2010;52:155–161. doi: 10.1007/s00234-009-0598-4. [DOI] [PubMed] [Google Scholar]
- Mangia S, Kumar AF, Moheet AA, Roberts RJ, Eberly LE, Seaquist ER, et al. Neurochemical profile of patients with type 1 diabetes measured by 1H-MRS at 4T. J Cereb Blood Flow Metab. 2013;33:754–759. doi: 10.1038/jcbfm.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Ekka M, Sharma U, P R, Pandey R, Jagannathan N. Assessment of changes in brain metabolites in Indian patients with type-2 diabetes mellitus using proton magnetic resonance spectroscopy. BMC Res Notes. 2014;7:41. doi: 10.1186/1756-0500-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Sutton BP, Prakash RS, Voss MW, Chaddock L, et al. Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. 2012;2:32–41. doi: 10.1002/brb3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, Lavoie SP, Copeland LE, Kile SJ, Richards AL, et al. Brain lactate responses during visual stimulation in fasting and hyperglycemic subjects: a proton magnetic resonance spectroscopy study at 1.5 Tesla. Psychiat Res Neuroim. 2006;148:47–54. doi: 10.1016/j.pscychresns.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Buonocore MH, Tanase C. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H-MRS study. NeuroImage. 2011;57:1324–1330. doi: 10.1016/j.neuroimage.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK. Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab. 2005;26:731–750. doi: 10.1038/sj.jcbfm.9600256. [DOI] [PubMed] [Google Scholar]
- van Hall G, Strømstad M, Rasmussen P, Jans Ø, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol. 2004;554:571–578. doi: 10.1113/jphysiol.2003.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NMB, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Zeeni N, Daher C, Fromentin G, Tome D, Darcel N, Chaumontet C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress. 2013;16:211–219. doi: 10.3109/10253890.2012.708952. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28:540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Jacob Z, Li HF, Makaryus R, Zhang SN, Reinsel R, Lee H, et al. Metabolomic profiling of children's brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiology. 2012;117:1062–1071. doi: 10.1097/ALN.0b013e31826be417. [DOI] [PubMed] [Google Scholar]
- Horn T, Klein J. Neuroprotective effects of lactate in brain ischemia: dependence on anesthetic drugs. Neurochem Int. 2013;62:251–257. doi: 10.1016/j.neuint.2012.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.