Abstract
The blood–brain barrier (BBB) constitutes a major obstacle in brain drug delivery. Focused ultrasound (FUS) in conjunction with microbubbles has been shown to open the BBB noninvasively, locally, and transiently to allow large molecules diffusion. Neurturin (NTN), a member of the glial-derived neurotrophic factor (GDNF) family, has been demonstrated to have neuroprotective and regenerative effects on dopaminergic neurons in vivo using invasive drug delivery methods. The brain's ascending nigrostriatal pathway is severely damaged in Parkinson's disease (PD), and therefore the substantia nigra (SN) and striatal caudoputamen (CP) were selected as the target areas. The objective of the study was to investigate whether safe and efficient NTN delivery can be achieved through FUS-induced BBB opening via intravenous administration, and thus trigger the neuroregeneration cascade in the nigrostriatal pathway. After the optimization of FUS parameters and target locations in the murine brain, NTN bioavailability and downstream signaling were detected and characterized through immunostaining. FUS significantly enhanced the delivery of NTN compared with the direct injection technique, whereas triggering of the signaling cascade was detected downstream to the neuronal nuclei. These findings thus indicate the potential of the FUS method to mediate transport of proteins through the blood–brain barrier in a PD animal model.
Keywords: blood–brain barrier, drug delivery, focused ultrasound, neurotrophic factor, neurturin, Parkinson's disease
Introduction
To date, there are no central nervous system disease-modifying treatments, except for treatments limited to the symptomatic relief of such diseases, such as Alzheimer's and Parkinson's. Neurotrophic factors, such as members of the glial cell-derived neurotrophic family glial-derived neurotrophic factor (GDNF), neurturin (NTN), and brain-derived neurotrophic factor, are proteins with therapeutic potential in the treatment of central nervous system neurodegenerative diseases through neuroprotection and neuroregeneration. The blood–brain barrier (BBB) is the rate-limiting factor for the treatment of the central nervous system (CNS) diseases as it hinders drugs and other agents from reaching their end targets at the brain parenchyma.1, 2 Meanwhile, focused ultrasound (FUS) in conjunction with systemically administered microbubbles has been shown to be the sole noninvasive method to open the BBB locally and safely,3, 4, 5, 6 and to allow the penetration of molecules including pharmacologically relevant agents and drugs such as anticancer therapeutic drugs, therapeutic antibodies, neurotrophic factors, adeno-associated virus, and neural stem cells.7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Parkinson's disease (PD) is a neurodegenerative disorder where severe damage is observed in the dopaminergic neuronal cell bodies in the substantia nigra (SN), which are projecting their terminals in the caudate–putamen (CP).17 Previous studies have shown that GDNF has a strong therapeutic potential in rodents,18 non-human primates,19, 20, 21, 22 and PD patients in a phase I clinical trial23, 24, 25 with restoration of dopaminergic neurons and functional improvements; however, there have been conflicting findings in phase II clinical trials26, 27 and the studies were halted because of safety concerns, including but not limited to the presence of neutralizing antibodies to GDNF in some patients. As an alternative to GDNF, NTN has been demonstrated to have enormous neuroprotective and neuroregenerative effects on dopaminergic neurons and trophic effects on the parkinsonian brain through intrastriatal injections in rodent PD models,28, 29 convection-enhanced delivery in a non-human primate PD model,30 as well as intracranial lentiviral (AAV2) injection.31 AAV2-neurturin gene therapy in PD patients showed that both SN and CP have to be targeted as terminal fields to ensure maximal benefits,32 and therefore, in the more recent clinical trial,33 two infusions through one needle tract for the SN and three infusions through three needle tracts in the CP, passing through a single burr hole per hemisphere, were performed.
The aim of this study was the efficient in vivo delivery of systemically administered NTN via the safely, locally, and reversibly opened BBB through FUS to the brain parenchyma, followed by downstream bioactivity to the neurons in wild-type mice. In the first part of the study, FUS acoustic parameters and targeting sonication locations for efficient and safe BBB opening at the SN and CP were investigated and optimized using molecules with size similar to NTN (MW: 23.6 kDa), i.e., fluorescently tagged dextrans. Magnetic resonance imaging was used for the quantification of the BBB opening volume (VBBB), permeability mapping, i.e., transfer rate of gadolinium from blood plasma to the extravascular, extracellular space (Ktrans), as well as the reversibility timeline of the opening. In the second part, NTN was administered after FUS treatment, and brains were harvested for the assessment and quantification of the diffusion as well as the bioactivity through downstream signaling, and the outcome was also compared with NTN direct injection (DI) findings.
Materials and Methods
All animal experimental procedures were approved by the Columbia University Institutional Animal Care and Use Committee and Columbia University's Research and Compliance Administration System. All efforts were made to minimize animal suffering and to reduce the number of subjects used. As shown in Table 1, depending on each specific objective of this study, different methods and techniques were used in a total of 34 (n=34) wild-type adult male mice (strain: C57BL/6, Harlan Sprague Dawley, Indianapolis, IN, USA) weighing 20 to 25 g, with a minimum of two animals in one group and four in the other groups. Animals were individually housed under standard conditions (12-hour light/dark cycles, 22 °C), were fed a standard rodent chow (3 kcal/g; Harlan Laboratories, Indianapolis, IN, USA) and bi-distilled water, and had ad libitum access to their diets and drinking water.
Table 1. Study design.
| Objective | Number of animals | Son. loc. CP/SN | Agent or drug administered | Survival time | Method |
|---|---|---|---|---|---|
| Acoustic parameters and sonication locations optimization | n=5 n=5 & n=10 | 1/1 (PL: 5,000) 1/1 & 2/1 (PL: 10,000) | Gd-DTPA-BMA (6 mmol/g) & fluorescent dextrans (60 μg/g) | 1 hour or 1 week | MRI & fluorescence microscopy |
| NTN delivery & bioactivity assessment after FUS | n=6 n=6 | 1/1 2/1 | NTN (20 μg/g) or saline (iv 2 μL/g) | 1 hour | MRI & immunostaining |
| NTN delivery assessment after DI | n=2 | −/− | NTN (0.02 μg/g ~0.5 μg per mouse per target area) | 1 hour | Immunostaining |
Abbreviations: DI, direct injection; FUS, focused ultrasound; MRI, magnetic resonance imaging; PL, pulse length (cycles); Son. loc. CP/SN, number of sonication locations at caudate–putamen and substantia nigra; 1/1, one sonication location at CP and one sonication location at SN; 2/1, two sonication locations at CP and one sonication location at SN; −/−, no FUS.
Focused ultrasound
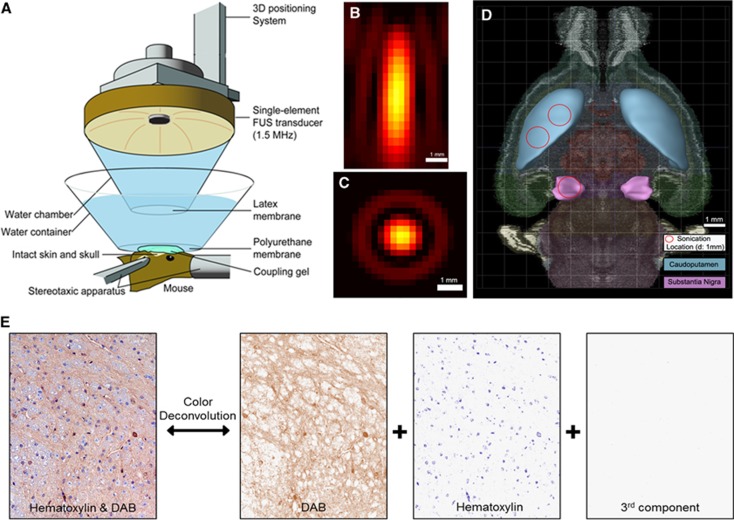
A single-element, spherical-segment FUS transducer (center frequency: 1.5 MHz, focal depth: 60 mm, radius: 30 mm; axial full-width half-maximum intensity: 7.5 mm, lateral full-width half-maximum intensity: 1 mm, Imasonic, Voray-sur-I'Ognon, France), driven by a function generator (Agilent, Palo Alto, CA, USA) through a 50-dB power amplifier (E&I, Rochester, NY, USA) as shown in Figures 1A and C was used to target the SN or the CP, as shown in Figure 1D. A needle hydrophone (HGL-0400, Onda, Sunnyvale, CA, USA) was used for the transducer calibration, which measured the acoustic beam profile in a tank filled with degassed water. A central void of the therapeutic transducer held a pulse-echo ultrasound transducer (center frequency: 10 MHz, focal depth: 60 mm, radius 11.2 mm; Olympus NDT, Waltham, MA, USA) used for alignment, with their two foci aligned. The imaging transducer was driven by a pulser–receiver (Olympus, Waltham, MA, USA) connected to a digitizer (Gage Applied Technologies, Lachine, QC, Canada). A cone filled with degassed and distilled water was mounted onto the transducer assembly. The transducers were attached to a computer-controlled three-dimensional positioning system (Velmex, Lachine, QC, Canada). A bolus of 1 μL/g of body mass polydisperse manufactured in-house34 microbubbles diluted in saline ( 8 × 108#/mL, mean diameter: 1.4 μm) was intravenously injected immediately preceding the sonication at each target, i.e., SN or CP. A 20-minute time interval was allowed between SN and CP targeting, to allow the microbubble concentration to be cleared from the circulation.35 Each animal was sonicated for 60 seconds, with a pulse repetition frequency of 10 Hz, with one or two sonication locations at each target to safely open the entire area of interest with acoustic parameters that do not cause damage, at peak negative acoustic pressure of 0.45 MPa36, 37 after accounting for 18% and 33% murine skull attenuation for the SN and CP, respectively.
Figure 1.
Methods, (A) experimental setup, (B) lateral focal beam profile, (C) axial focal beam profile, (D) axial brain section overlaid with the sonication locations targeted at the formations of interest, i.e., caudoputamen (CP) and substantia nigra (SN), adapted from Allen Brain Atlas. (E) An example of color deconvolution, which was used to calculate the contribution of each stain to the RGB image. All the samples were stained with diaminobenzidine (DAB) and hematoxylin, and, using this robust method, the DAB color could be extracted and used for qualitative and quantitative measurements. 3D, three-dimensional.
Acoustic parameters and sonication locations optimization
The pulse length (PL) tested varied between 5,500 cycles (3.3 ms) and 10,000 cycles (6.6 ms) to investigate which parameter would induce more efficient drug delivery to the areas of interest. It has been previously shown that the PL has an important role in the BBB opening characteristics, such as the permeability and the volume of opening,36 as well as the closing timeline.
To target the SN, the transducer, which was placed perpendicular to the brain surface, was aligned with the posterior fontanel, i.e., the sagittal suture's junction with the lambdoid suture, with a method described elsewhere4 and was then moved anteriorly 1.5 mm on the sagittal suture, and laterally 1.7 mm onto the left hemisphere, as shown in Figure 1D.
The CP is located in the striatum, which is a large area relative to the FUS focal size; it is therefore challenging to induce BBB opening in the entire CP using a single sonication. For example, it has been shown that the acoustic parameters used to increase the volume of opening with a single sonication can also increase the probability of damage.36, 37 For this reason, the effect of having two nonoverlapping s at that target was studied, each one at a relatively low PL, aiming at inducing sufficient BBB opening while staying within the safe acoustic parameters range. When FUS was applied only at one sonication location, the transducer focus was moved 4.5 mm anteriorly from the posterior fontanel and 2.5 mm laterally to the sagittal suture. When FUS was applied at two ipsilateral nonoverlapping sonication locations, the transducer focus was moved 4 mm anteriorly and 3 mm laterally for the first location, and was immediately moved after the first sonication 1 mm anteriorly and 1 mm laterally toward the sagittal suture for the second sonication location as shown in Figure 1D. There was no additional microbubble administration between the two sonication locations.
Fluorescence microscopy
In the first objective, animals were injected with a bolus of fluorescein-tagged dextrans (Invitrogen, Carlsbad, CA, USA) at a dosage of 6 μg/g of body mass, diluted in 100 μL of saline though the tail vein, immediately after sonication. Animals were transcardially perfused 1 hour after FUS with 30 mL phosphate-buffered saline (PBS) and 60 mL 4% paraformaldehyde. Heads were soaked in paraformaldehyde for 24 hours. Skulls were removed and the brains were fixed again in 4% paraformaldehyde for 24 hours and transferred to 10% (30 minutes), 20% (60 minutes), and 30% (24 hours) sucrose solutions for cryoprotection. Brain samples were then embedded in an optimal cutting temperature medium and were frozen using dry ice and isopentane. Frozen blocks were sectioned horizontally at 60-μm thickness for fluorescent imaging. Fluorescence images were acquired using a fluorescence microscope (BX61; Olympus, Melville, NY, USA) with a filter set at excitation and emission wavelengths of 490 and 525 nm, respectively.
Magnetic resonance imaging
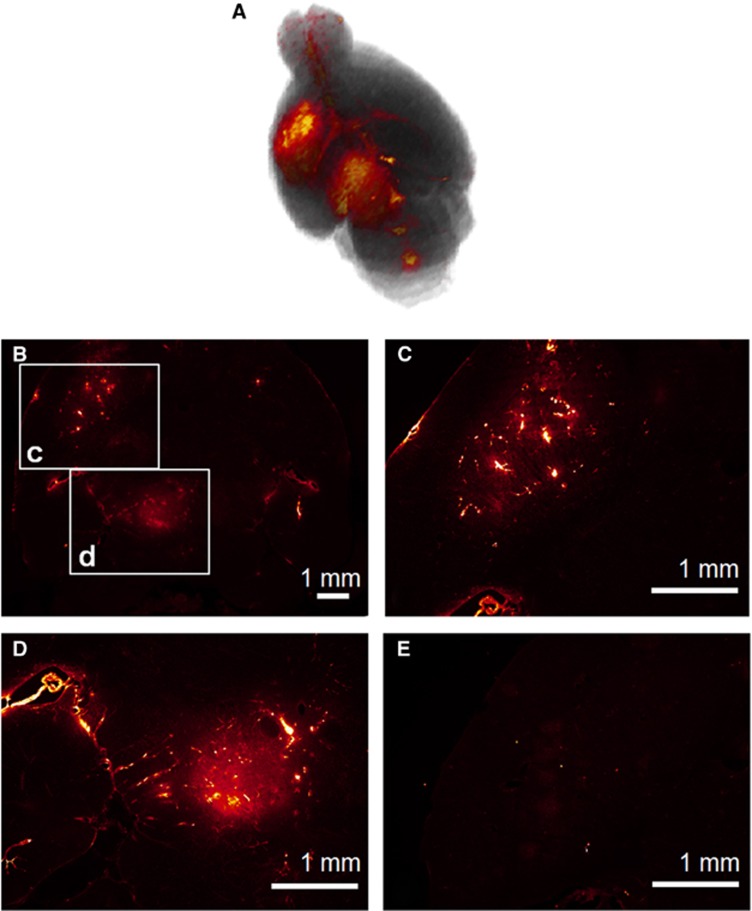
All mice were imaged using a 9.4T vertical bore microimaging magnetic resonance imaging (MRI) system (DRX400, Bruker, Biospin, Billerica, MA, USA). Each mouse was scanned 20 to 30 minutes after sonication, using a 30-mm-diameter 1H resonator. Isoflurane gas (1 to 2%) was used to keep the mouse anesthetized at 50 to 70 breaths/minute during the entire MRI procedure. On the day of sonication, dynamic contrast-enhanced MR imaging was performed using a two-dimensional FLASH T1-weighted sequence (40 acquisitions, 192 × 128 matrix size, in-plane resolution: 130 × 130 μm2, slice thickness: 600 μm, TR/TE=230/2.9 milliseconds). During the third acquisition of the dynamic sequence, a 0.30-mL nondiluted bolus of Gd-DTPA-BMA (Omniscan, 287 mg/mL) was injected intraperitoneally and was used as a tracer to depict the area of opening, as shown in Figure 2A. After completion of dynamic contrast-enhanced MR imaging, a post-contrast enhancement, T1-weighted two-dimensional FLASH high-resolution acquisition (TR/TE: 230/3.3 milliseconds, resolution 100 μm × 100 μm, slice thickness: 400 μm) was acquired. The scanning time of each dynamic contrast-enhanced and T1-weighted MRI image was 40 minutes and 4 minutes, respectively. Depending on the objective, T1-weighted MR imaging was repeated on a daily basis starting from the day of sonication (1 hour) and lasting up to 1 week after sonication.
Figure 2.
(A) Three-dimensional reconstructed T1-weighted magnetic resonance imaging showing gadolinium diffusion, (B–D) Horizontal fluorescence microscope images showing fluorescent 40 kDa dextran diffusion in the same brain. The area where the larger molecule diffused was smaller compared with gadolinium. A 10,000 cycles pulse length was necessary for the diffusion of the 40 kDa dextran. (E) With a 5,000 cycle, gadolinium could diffuse to the brain parenchyma but the 40 kDa dextrans could not.
Permeability mapping, volume quantification, and timeline
The general kinetic model38 was used to measure the BBB permeability following the general expression of
 |
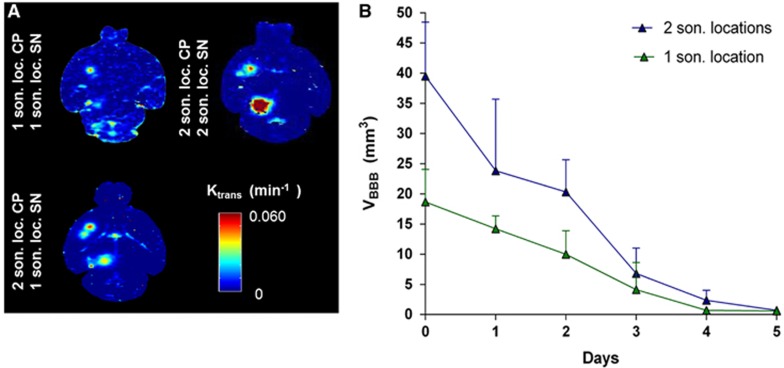
where Ct is the tracer concentration in the extravascular extracellular space at time t, Cp is the tracer concentration in the blood plasma and Ktrans and Kep are the transfer rate constants from the intravascular system to the extravascular extracellular space and backwards, respectively. The consecutive images from the dynamic contrast-enhanced MR imaging were entered in a custom algorithm implemented on Matlab ( MathWorks, Natick, MA, USA), with a method described elsewhere,39 and Ktrans maps were generated. The maps were then overlaid onto the MR images to provide information on the BBB opening permeability characteristics as shown in Figure 3A.
Figure 3.
(A) Horizontal permeability maps showing the difference between 1 and 2 nonoverlapping sonication locations (son. loc.) at each region of interest, i.e., caudoputamen (CP) and substantia nigra (SN); the optimal was 2 son. loc. at the CP and 1 son. loc. at the SN. (B) Quantification and reversibility of the opening volume (VBBB) using 2 nonoverlapping sonication locations VBBB was doubled without increase of the reversibility time.
The volume of opening (VBBB) and reversibility timeline were quantified by processing the high-resolution T1-weighted images with a custom algorithm in Matlab (MathWorks, Natick, MA, USA) as described elsewhere.37 A three-dimensional reconstruction of the opening in CP and SN is shown in Figure 2A. Thresholded voxels within the VOI with signal intensity of 2.5 standard deviations or above the signal intensity of a reference region in the non-sonicated area counted toward the opening volume, whereas the volume of contrast-enhanced vasculature was excluded. The longitudinal measurements, i.e., the closing timeline, are shown in Figure 3B.
Neurturin delivery
Neurturin administration
Human neurturin neurotrophic factor (NTN, Invitrogen, CA, USA) was injected via a tail vein bolus injection at dosage of 20 μg/g diluted in saline immediately after sonication and mice were survived for 1 hour to allow sufficient time for circulation and bioactivity effects (see Table 1).
To investigate the advantage of using FUS for NTN delivery to the brain, over the conventional method of DI, experiments with DI of 5 μg/g in the CP and 5μg/g in the SN, diluted in saline (9.8 mg/mL) with a total volume of 0.02 μL/g, were performed on two (n=2) mice (see Table 1). Using a stereotaxic frame, one hole for SN and one hole for CP were opened with the use of a surgical drill on the skull surface. To reach the SN, the hole through which the needle was inserted into the brain was 3.7 mm posterior to the bregma and 1.85 mm lateral, and it was injected 5 mm deep from the skull surface. To reach the CP, the hole was opened 1 mm posterior to the bregma and 2.2 mm lateral, and the needle was injected at a depth of 3 mm. The needle syringe was withdrawn 5 minutes after injection at each location to avoid potential backflow of NTN via the needle path outside the brain.
Tissue fixation and staining
The animals were killed and transcardially perfused with 30 mL PBS and 60 mL 4% paraformaldehyde. All the heads were soaked in paraformaldehyde for 24 hours. Skulls were removed, and the brains were fixed again in 4% paraformaldehyde for 6 days followed by conventional post-fixation procedures. Paraffin-embedded specimens were sectioned horizontally at a thickness of 6 to 10 μm covering the SN and CP formations.
For the detection of bioactivity and the downstream signaling cascade of NTN, four primary antibodies were selected: an antibody against human NTN (anti-NTN Ab8061, Abcam, Cambridge, MA, USA), an antibody against phosphorylated Ret (p-Ret rabbit, Tyr 1062, Santa Cruz Biotechnology, CA, USA) which is a receptor tyrosine kinase on the neuronal cell surface with NTN specificity, an antibody against phosphorylated ERK1/2 (Phospho-p44/42 MAPK (Erk1/2), Cell Signaling Technology, Beverly, MA, USA), which is a cytoplasmic protein kinase which promotes neuronal growth and differentiation, and finally an antibody against phosphorylated CREB (p-CREB-1 Antibody (Ser 133), Santa Cruz Biotechnology, Dallas, TX, USA) which is a transcription factor in the neuronal nucleus.
A standard protocol for deparaffinization and hydration was then followed. Sections were put in citrate buffer (1L, pH 6) in a pressure cooker and microwaved for 20 minutes, then let to cool down for 30 minutes at room temperature for antigen retrieval. Slides were then washed in dH2O one time and in PBS three times for 5 minutes each, incubated in 30% hydrogen peroxide in PBS for 10 minutes, and washed again in dH2O and PBS. Then 10%, 10%, 10%, and 5% normal goat serum was used for blocking for 20 minutes for human NTN, Ret, ERK1/2, and CREB, respectively. The blocking serum was then removed and the primary antibody to each section was added; anti-NTN (1:100), P-Ret(1:100 rabbit), p-ERK1/2 (1:200 rabbit), and phospho-CREB (1:200 goat). The slides were then washed in PBS three times for 5 minutes each. ABC reagent (A 1:50, B 1:50 in PBS 30 minutes before being mixed) were added for 30 minutes at room temperature. Slides were again washed in PBS three times for 5 minutes each. DAB solution was added to each section (DAKO North America, Carpinteria, CA, USA), and, as soon as the sections developed (brown stain), the slides were immersed in dH2O and counterstained with hematoxylin (dark blue/purple stain), were dehydrated, and mounted with coverslips.
Imaging and staining quantification
Bright-field images were acquired using a light microscope (BX61; Olympus, Melville, NY, USA) and were white corrected. The mean DAB intensity was computed using an algorithm implemented in Matlab (MathWorks, Natick, MA, USA) using the color deconvolution method40 for sections that had developed with DAB and were counterstained with hematoxylin, as shown in Figure 1E. DAB intensity was used for immunostaining detection of NTN diffusion through the BBB and bioavailability to different brain regions, and the areas of bioavailability thereafter as shown in Figure 4, as well as for the downstream molecules phosphorylation as shown in Figures 5, 6, 7.
Figure 4.
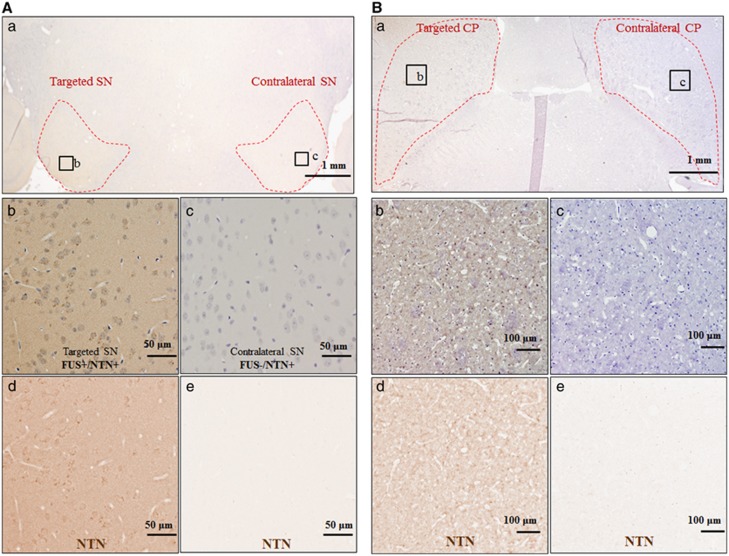
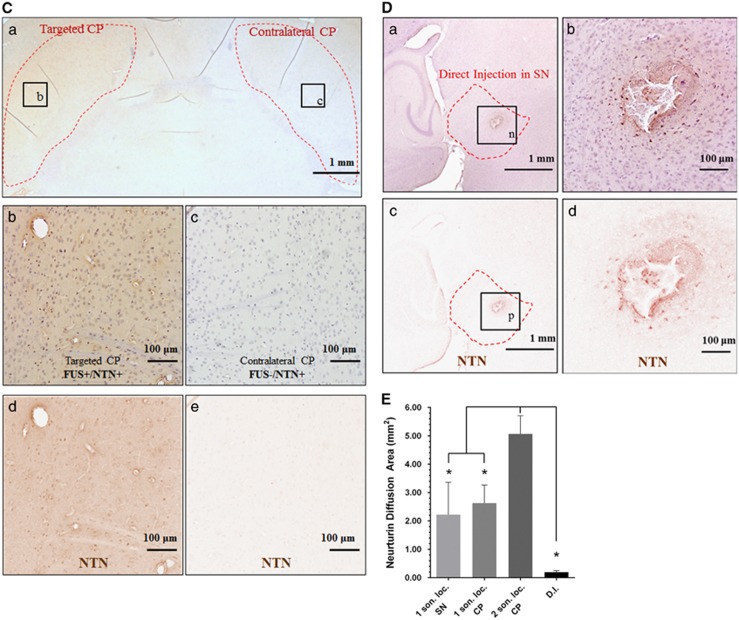
Horizontal section at the levels of SN or CP, which are outlined with red dotted lines, immunostained for NTN (DAB development—brown color), and counterstained with hematoxylin: (A): (a) sonicated SN (1 son. loc.) and contralateral SN, (b–c) higher magnification at sonicated and contralateral SN, respectively, (d–e) extraction of the DAB color only corresponding to NTN for (b–c), respectively. (B): (a) sonicated CP (1 son. loc. CP) and contralateral CP, (b–c) higher magnification at sonicated and contralateral SN, respectively, (d–e) extraction of the DAB color only corresponding to NTN for (b–c) respectively. (C): (a) sonicated CP (2 son. loc. CP) and contralateral CP, (b–c) higher magnification at sonicated and contralateral SN, respectively, (d–e) extraction of the DAB color only corresponding to NTN for (b–c), respectively. (D): (a) direct injection (DI) to the SN, (b) higher magnification at the DI site, (c–d) extraction of the DAB color only corresponding to NTN for (a) and (b), respectively. NTN was successfully delivered to the targeted CP and/or SN, while the contralateral areas did not show evidence of NTN just because of the intravenous circulation. (E): area quantification of the NTN bioavailability, asterisks denote significance (P<0.05). CP, caudoputamen; DAB, diaminobenzidine; NTN, neurturin; son. loc., sonicated location; SN, substantia nigra.
Figure 5.
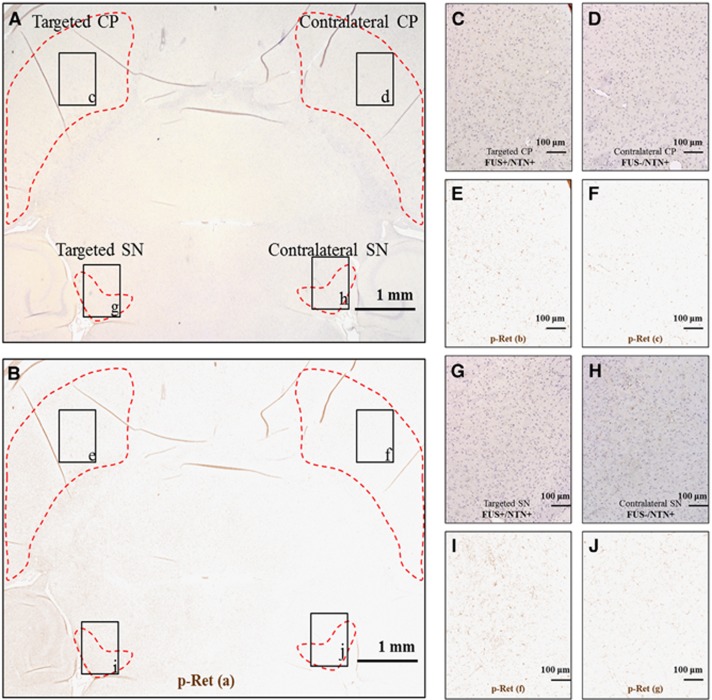
(A) Horizontal brain section, where the left CP and SN only were targeted with FUS, immonostained for p-Ret, and counterstained with hematoxylin, (B) is image (A) after the extraction of the DAB color only corresponding to p-Ret. (C, D, G, and H) are higher magnification at the targeted and contralateral CP and SN. (E, F) and (I, J) are the corresponding images after the extraction of the DAB color only corresponding to p-Ret. At the targeted with FUS side shown in (E) and (I), there is an increased p-Ret compared with the contralateral side shown in (F) and (J), respectively. Both sides were exposed to NTN through the systemic circulation for 1 hour after the intravenous administration. Immunostaining is detected in the neuronal bodies as well as in the dendrites and axon terminals, providing information on the location of the Ret receptor in the dopaminergic (DA) neurons. CP, caudoputamen; DAB, diaminobenzidine; FUS, focused ultrasound; NTN, neurturin; SN, substantia nigra.
Figure 6.
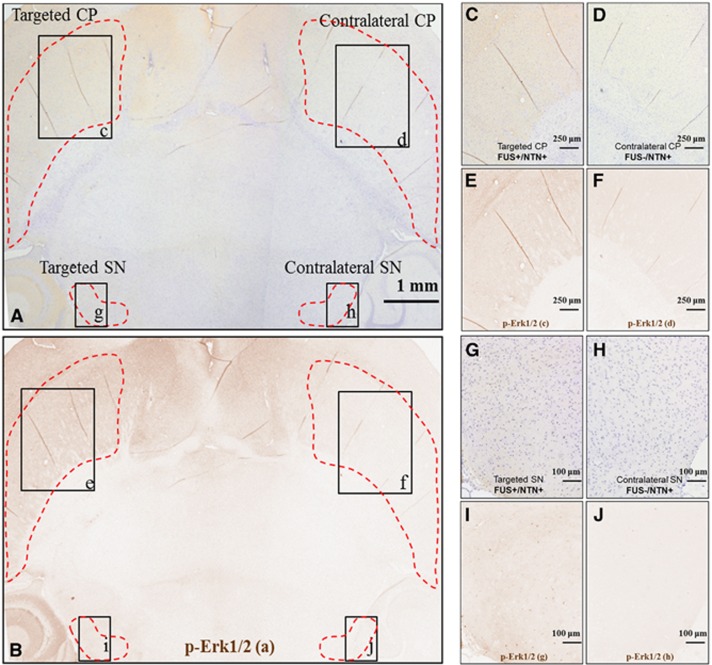
(A) Horizontal brain section, where the left CP and SN only were targeted with FUS, immonostained for p-Erk1/2, and counterstained with hematoxylin, (B) is image (A) after the extraction of the DAB color only corresponding to p-Ret. (C), (D), (G), and (H) are higher magnification at the targeted and contralateral CP and SN. (E), (F) and (I), (J) are the corresponding images after the the extraction of the DAB color only corresponding to p-Ret. At the targeted with FUS side shown in (E) and (I), there is an increased p-Ret compared with the contralateral side shown in (F) and (J), respectively. Both sides were exposed to NTN through the systemic circulation for 1 hour after the intravenous administration. Immunostaining for the phosphorylated cytoplasmic kinase Erk1/2 is detected in the neuronal axons mainly. CP, caudoputamen; DAB, diaminobenzidine; FUS, focused ultrasound; NTN, neurturin; SN, substantia nigra.
Figure 7.
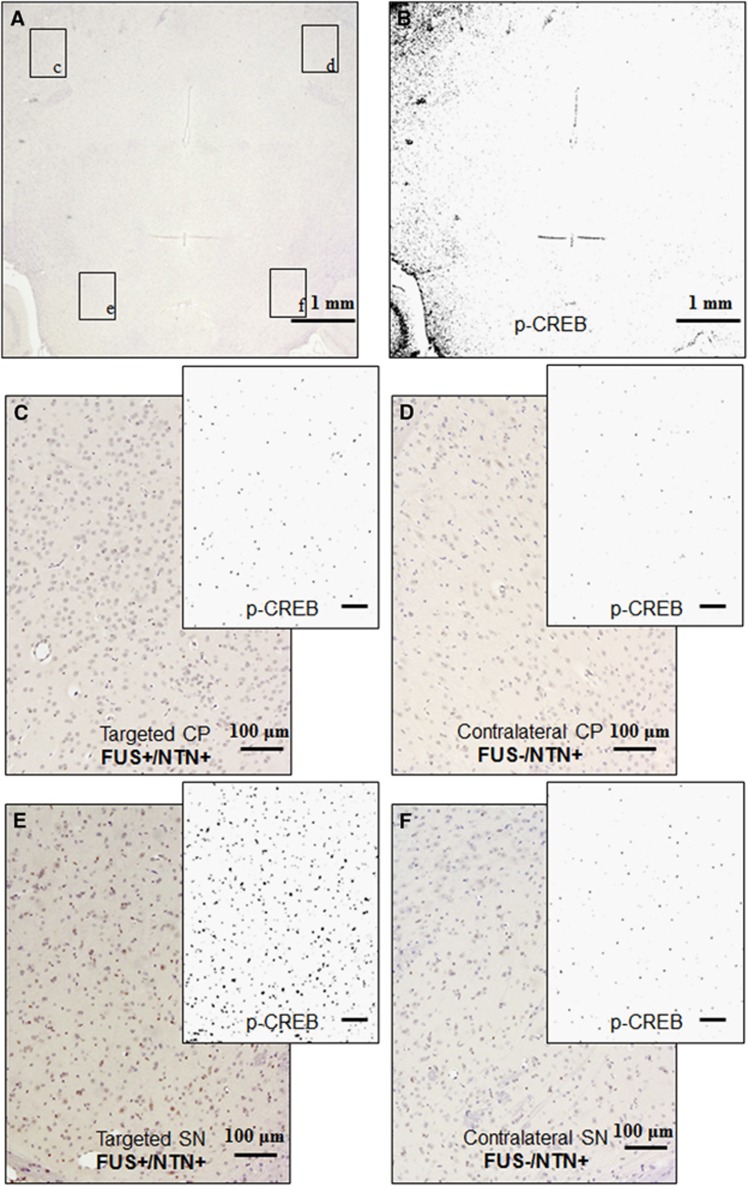
(A) Horizontal brain sections after sonication at the CP and SN, and NTN intravenous administration. (A) Horizontal brain section, where the left CP and SN only were targeted with FUS, immonostained for p-Erk1/2, and counterstained with hematoxylin, (B) is the binary image of (A) after the extraction of the DAB color only corresponding to p-Ret. (C), (D), (E), and (F) is higher magnification at the targeted and contralateral CP and SN, respectively, with the corresponding binary image after the extraction of the DAB color only. At the targeted with FUS side shown in (C) and (E), there is increased p-CREB compared with the contralateral side shown in (D) and (F). Both sides were exposed to NTN through the systemic circulation for 1 hour after the intravenous administration. Immunostaining for the phosphorylated CREB is detected in the neuronal nuclei only where the transcription factor is located. CP, caudoputamen; DAB, diaminobenzidine; FUS, focused ultrasound; NTN, neurturin; SN, substantia nigra.
Results
Optimization of acoustic parameters and targeting sonication locations for efficient drug delivery
The PL for efficient drug delivery was determined on the comparison of the diffusion of dextrans (40 kDa) and Gd-DTPA-BMA, which is a small tracer (574 Da), that was administered via intraperitoneal injection in the same animal each time. Even though the MRI contrast agent when used as an opening tracer showed diffusion in relatively large areas at both PLs in both the SN and CP as shown in Figure 2A, dextrans diffused in smaller areas as shown in Figures 2B and D and did not sufficiently diffuse in those areas for the shorter PL as shown in Figure 2E. For these reasons, the PL of 10,000 cycles was selected for more efficient drug delivery.
The design of a reversible BBB opening is critical for planning BBB opening for drug delivery. The results of the BBB opening characteristics (permeability, opening volume, and timeline) before and after optimizing the sonication locations at each target are shown in Figure 3. In Figure 3A, the permeability Ktrans maps are shown. The two sonication locations at the CP increased the affected area at the two distinct foci without dramatic increases in permeability, which are also often correlated with damage,36 while covering the entire region of interest. One sonication location at the SN was sufficient to be covered entirely.
In Figure 3B, it is shown that when two sonication locations are targeted, which are nonoverlapping, the overall opening volume is doubled, while the closing timeline is similar to that of one sonication location. The volume of opening after FUS could cover the entire CP with an average volume of opening 39.4±4.1 mm3 when two nonoverlapping sonication locations were targeted. A single sonication location target could provide coverage of the entire SN with an opening volume of 18.6±4.7 mm3, while the BBB-openings were monitored longitudinally and their closing, i.e., reversibility, timeline was found to be 4 to 5 days for both the CP and SN.
Neurturin bioavailability
The immunostaining of human NTN after FUS BBB opening is shown in Figure 4. In Figure 4A, an example of one sonication location at SN is shown, in Figure 4B an example of one sonication location at the CP is shown, and in Figure 4C an example of two sonication locations at the CP is shown. In Figure 4A(a), Figure 4B(a) and Figure 4C(a), the RGB low magnification ( × 1.25) images are shown, where the boundaries of the region of interest are outlined with a red dotted line, while the respective (b) and (c) are magnified at the targeted and contralateral regions. To better illustrate the bioavailability and diffusion of NTN, only the DAB channel, i.e., NTN, was extracted and is shown in (d) to (e) via color deconvolution, as was previously described and shown in Figure 1E. These findings indicate that the intravenously administered NTN diffused through the opened BBB into the brain parenchyma on the FUS-targeted side and, under high magnification, was detected in the extracellular space. NTN was not found to diffuse in the brain tissue in the nontargeted contralateral area after NTN circulation in the bloodstream, indicating highly localized delivery to the FUS-targeted areas only. NTN bioavailability was increased two-fold in the CP when two sonication locations were used, suggesting that two sonication locations are preferred over a single location for the whole CP formation coverage.
Immunostaining for NTN after DI in the SN is shown in Figure 4D, as indicated by a red dotted line in (a). The injection needle was inserted perpendicular to the plane of the figure. Under higher magnification, the area of NTN diffusion around the injection point is shown in (b). The DAB color for NTN was extracted from (a) and (b) and is shown in (c) and (d) where the diffusion area is better illustrated.
The area of bioavailability in horizontal sections corresponding to the center of the FUS beam at each target was directly calculated from the extracted DAB channel, i.e., NTN immunostaining after the color deconvolution (Figure 4E). NTN bioavailability in the CP was found to be 5.07 mm2±0.64 mm2 with two sonication locations used, compared with 2.63±0.64 mm2 with one sonication location, was targeted. For the SN, the NTN bioavailability area was measured to be 2.25±1.14 mm2. The protein bioavailability after FUS was also compared with that with DI of NTN to the CP and SN formations independently, with the latter found to be limited to an average area of 0.20±0.05 mm2 around the injection site. The use of FUS significantly increased the bioavailability of NTN compared with DI in all the cases studied. Using two sonication locations was found also to significantly increase the bioavailability by a two-fold increase compared with one sonication location, and by a 25-fold increase compared with DI.
Neurturin bioactivity and downstream signaling
The signaling cascade of NTN starting from the neuronal membrane where the Ret receptor is found, to the axons where the cytoplasmic Erk1/2 can be found, and further downstream to the nucleus where the CREB transcription factor is located, was also activated within 1 hour after intravenous administration as with immunostaining (Figures 5, 6, 7). No immunostaining was detected in the contralateral sides, except for some areas in the close vicinity of some blood vessels, potentially because of perivascular transport as reported during cerebral infusion or convection-enhanced delivery of neurotrophic factors.18 In addition, no immunostaining was observed because of FUS only (results not shown here).
In Figure 5A, an RGB horizontal brain section including both the targeted SN and CP and their contralateral sides is shown, where DAB staining is for phosphorylated Ret (p-Ret). The p-Ret only color is shown in Figure 5B. Under higher magnification as shown in Figures 5C, 5D and 5G, 5H and in the corresponding p-Ret only images are shown in Figures 5E, 5F and 5I, 5J, p-Ret expression was increased in the targeted areas compared with the contralateral side, and the immunostaining was detected in the neuronal bodies as well as in the dendrites and axon terminals, where the receptor is supposed to be located. There was increased p-Ret staining in the CP compared with the SN.
In Figure 6A, an RGB horizontal brain section including both the targeted SN and CP and their contralateral sides is shown, where DAB staining is for the phosphorylated kinase Erk1/2 (p-Erk1/2). The p-Erk1/2 only (brown color) is shown in Figure 6B. Under higher magnification as shown in Figures 6C, 6D and 6G, 6H and in the corresponding p-Erk1/2, only images are shown in Figures 5E, 5F and 5I, 5J, p-Erk1/2 expression was increased in the targeted areas compared with the contralateral side, while immunostaining was mainly detected along the neuronal axon fiber bundles. It should be noted that p-Erk1/2 was detected in terminals projecting from the SN to the CP in areas that exceeded the FUS focal spot size.
The effects of FUS with microbubbles-only in the downstream signaling cascade were also investigated. In Figure 7, an example for phosphorylated CREB (p-CREB) is shown where CP and SN were targeted with FUS while the contralateral side remained intact and NTN was administered. In Figure 7A, a horizontal brain section is shown, stained for p-CREB and counterstained with hematoxylin, while Figure 7B shows the corresponding p-CREB binary image after color deconvolution for DAB. In Figure 7A and Figure 7C, p-CREB immunostaining was increased in the targeted regions compared with their contralateral side (Figure 7B and Figure 7D). The effect is more evident in the SN where it is denser in the neuronal nuclei. Finally, FUS was detected to induce BBB opening but it was not detected to trigger the signaling cascade for p-CREB and no differences were detected between the FUS-targeted and the contralateral nontargeted sides (results not shown here).
Discussion
The objective of the study was to investigate whether safe and efficient NTN delivery could be achieved through FUS-induced BBB opening via intravenous administration. It was shown that the NTN was efficiently delivered through the safely and reversibly FUS-opened BBB in the caudoputamen and substantia nigra after intravenous systemic administration, while bioeffects via downstream signaling to neuronal nuclei were also detected within 1 hour. Intrastriatal injections of NTN in rodent models of PD have demonstrated neuroprotective and neuroregenerative effects on dopaminergic nigrostiatal function,28, 29 and most recently, intraputamenal infusion in the globus pallidus of MPTP-lesioned rhesus monkeys showed behavioral improvement and elevated locomotor activity levels.30 Nevertheless, in this study, it was shown that FUS in conjunction with microbubbles can induce high levels of NTN bioavailability through the safely opened BBB volume, and increased bioactivity, indicating the strong therapeutic potential for Parkinson's and other neurodegenerative diseases of this noninvasive drug delivery methodology.
Enhanced bioavailability of neurturin using focused ultrasound
Aside from the safety concerns associated with intracranial delivery methods, NTN tissue distribution enhancement to achieve trophic effects in the Parkinsonian brain using the aforementioned techniques remains a challenge. A critical advantage of using FUS for drug delivery, as shown in this study, is that it could induce BBB opening throughout the SN and CP with relatively large volumes (~20 mm3), while nonoverlapping sonication locations could be used to induce a two-fold increase in the opening volume (~40 mm3; Figure 3). The tissue distribution of the trophic factors is a critical variable to achieve optimal effects on dopaminergic function, as it has been previously shown that the volume distribution of GDNF, for example, significantly correlated with the motor function improvements.21 To this end, other studies21, 41 implemented multiport catheters implanted into the putamen to achieve greater tissue bioavailability, i.e., trophic factor distribution in larger tissue volumes. To better investigate the advantages of FUS over the conventional methods, in this study, the areas of delivery of NTN after FUS was compared with DI and was found to be significantly higher and able to cover the entire formation of CP or SN, whereas diffusion of NTN after DI was restricted around the injection site (Figure 4). It was shown in this study that FUS in conjunction with microbubbles is the only noninvasive technique that can be used for the treatment of large areas, thus achieving efficient trophic factor tissue bioavailability.
Here, the single application of both the FUS and the DI methods were compared. However, the DI injection method was not optimized for the purpose of this study. For example, the use of multiple injection sites instead of one could lead to better diffusion results. Therefore, the comparison reported in this study should be used qualitatively. A different study investigating the differences of the DI procedure would be necessary to reach conclusions on the exact difference between this technique and FUS.
Bioeffects of neurturin on neurons
In this study, it was shown for the first time that NTN could diffuse in the extracellular space after intravenous administration and trigger a downstream signaling cascade in neurons of the nigrostiatal pathway after FUS. First, successful phosphorylation of its receptor Ret was confirmed, and was found to be more pronounced in the CP (Figure 5) than in the SN, which could possibly be because of the receptor's abundance in the axon terminals rather than the dendrites. Then, successful phosphorylation of the cytoplasmic kinase Erk1/2 was detected, along the neuronal axons revealing the neuronal structure (Figure 6). Neurons of the thalamus did not show upregulation of p-Erk1/2, suggesting that mainly the dopaminergic neurons of the nigrostiatal pathway were affected by NTN in the extracellular space. It is important to note that the phosphorylation of Erk1/2 was detected in areas outside the FUS-targeted region, following the anatomical form of the neurons of the SN projecting their terminals in the CP. This may be because of the fact that the cytoplasmic kinase moved along the neuronal fibers of the neurons that responded to the NTN delivery in the CP and the SN, during the 1 hour survival post FUS. Finally, the phosphorylation of the CREB transcription nucleic factor was confirmed (Figure 7), and was more pronounced in the SN than in the CP, possibly because of the abundance of neuronal nuclei in the former area.
The contralateral areas were always found to have very low, if any, immunostaining intensity confirming that the intravenous administration of the NTN alone was not capable of having any effects to the brain tissue. Moreover, FUS alone, with the acoustic parameters used in the study, was not found to have any bioeffects on neurons, and did not cause upregulation of p-Ret, p-Erk1/2, or p-CREB.
Reversibility of blood–brain barrier opening and potential of repetitive focused ultrasound for long-term treatment
Regarding the reversibility of BBB opening, it was shown here that a maximum of 5 days is required for the BBB to be reinstated, in agreement with previous studies,36, 42 even for agents with small molecular size. It has also been shown that longitudinal sonications can be safely used to repeatedly induce reversible BBB opening in rodents36, 37 and non-human primates.43 In the intracranial delivery of NTN studies,28, 29, 30 administration of the trophic factor varied from a one-time single dose to recurring daily doses for 3 months to achieve neuroprotection or neuroregeneration in models of PD disease. In other words, the required pharmacological treatment dosage rate remains to be established. In the case of a long-term treatment, which may vary from subject to subject depending on the condition, the findings of FUS studies thus far have shown that the BBB could be repeatedly opened for drug delivery in a sterile environment at desired time intervals.
Collateral blood–brain barrier opening on adjacent areas
One challenge of using FUS for drug delivery in the murine brain is that because of the focal beam size, shape, and propagation pathway, it may not only affect a distinct region of interest in the brain, but also induce BBB opening in adjacent structures. For example, SN is a relatively small area located more ventrally in the brain, and as the focal length of the FUS acoustic beam was 7 mm, the entire coronal thickness of the murine brain was covered including the dorsal adjacent regions to the SN, such as some posterior regions of the thalamus and the hypothalamus, some small area of the dentate gyrus of the dorsal hippocampus and the cerebral cortex. Moreover, in studies such as this one where CP was targeted, one major challenge was to avoid FUS propagation through the ventricles. Leakage into the ventricles can lead to transportation of the drug to neighboring or more distant structures via the cerebrospinal fluid, whereas delivery of NTN beyond the desired target regions and outside the motor pathway could lead to potentially dangerous adverse effects and should be taken into consideration. Contrary to the FUS transducer used in this study for the murine brains, in a clinical setting, the focal size using FUS transducers designed for acoustic propagation through the human skull would be small relative to the brain and it could, therefore, be easier to further localize the effect of FUS onto the desired regions of interest only.
Other limitations
Limitations in this study included the limited number of mice used mainly because of the high cost of NTN acquisition. Because of this limitation, all animals were killed after the same survival time (1 hour) post sonication and injection to have a statistically significant number of repetitions of the experiment. Therefore, other effects could not be studied, such as extended circulation time in the brain and different dosages. Possibly, extended animal survival could have more intense immunostaining of NTN or increased diffusion of NTN inside the brain.
Conclusion
In this study, it was found that the BBB could be safely and reversibly disrupted in the caudate putamen and substantia nigra allowing the penetration of neurotrophic factors and subsequently triggering downstream effects. A PL of 10,000 cycles was found to be necessary for efficient protein delivery at 0.45 MPa . In addition, two sonication locations in close proximity were used to safely increase the BBB opening volume while covering the entire region of interest.
Neurturin delivery was shown feasible in both the caudate putamen and substantia nigra. Downstream signaling activation from its receptor to the nucleus was confirmed, and detected within 1 hour after intravenous administration. Neurturin delivery using FUS was shown to be more effective compared with administration to the brain through a DI showing enhanced delivery of the protein in the targeted areas.
Acknowledgments
The authors thank Oluyemi Olomulade (Biomedical Engineering), Iason-Zacharias Apostolakis (Biomedical Engineering, Columbia University), Hong Chen (Biomedical Engineering, Columbia University), and Cherry Chen (Biomedical Engineering, Columbia University) for their important input.
The authors declare no conflict of interest.
Footnotes
This study was supported by NIH grants R01 EB009041 and R01 AG038961 and the Kinetics foundation.
References
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Yang F-Y, Fu W-M, Yang R-S, Liou H-C, Kang K-H, Lin W-L. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption. Ultrasound Med Biol. 2007;33:1421–1427. doi: 10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Liu H-L, Wai Y-Y, Chen W-S, Chen J-C, Hsu P-H, Wu X-Y, et al. Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med Biol. 2008;34:598–606. doi: 10.1016/j.ultrasmedbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS One. 2008;3:e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W, et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung Y-S, Olumolade O, et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys Med Biol. 2012;57:N65–N81. doi: 10.1088/0031-9155/57/7/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shi Y, Lu L, Liu L, Cai Y, Zheng H, et al. Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS One. 2012;7:e52925. doi: 10.1371/journal.pone.0052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E-J, Zhang Y-Z, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163:277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Reinz E, Leuchs B, Kleinschmidt J, Fatar M, Geers B, et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol Ther Nucleic Acids. 2013;2:e73. doi: 10.1038/mtna.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Johnston L, Forsayeth J, Bankiewicz KS. Pharmacokinetics and bioactivity of glial cell line-derived factor (GDNF) and neurturin (NTN) infused into the rat brain. Neuropharmacology. 2010;58:1114–1121. doi: 10.1016/j.neuropharm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125 (Pt 10):2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Yoshimura R, Nakai K, Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson's disease. Brain Res. 2002;947:271–283. doi: 10.1016/s0006-8993(02)02934-7. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Björklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle. Eur J Neurosci. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Ding F, Walton AA, Surgener SP, et al. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of MPTP-lesioned rhesus monkeys. Cell Transplant. 2008;17:373–381. [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RAE, Stansell J, 3rd, Gasmi M, et al. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, et al. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80:1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feshitan JA, Chen CC, Kwan JJ, Borden MA. Microbubble size isolation by differential centrifugation. J Colloid Interface Sci. 2009;329:316–324. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- Sirsi S, Borden M. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiotaki G, Konofagou EE. Dependence of the reversibility of focused-ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:2257–2265. doi: 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiotaki G, Vlachos F, Tung Y-S, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med. 2011;67:769–777. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- Vlachos F, Tung Y-S, Konofagou EE. Permeability assessment of the focused ultrasound-induced blood-brain barrier opening using dynamic contrast-enhanced MRI. Phys Med Biol. 2010;55:5451–5466. doi: 10.1088/0031-9155/55/18/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, et al. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol Aging. 2002;23:881–889. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M, et al. Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab. 2012;32:1948–1958. doi: 10.1038/jcbfm.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet F, Tung Y-S, Teichert T, Ferrera VP, Konofagou EE. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo. PLoS One. 2011;6:e22598. doi: 10.1371/journal.pone.0022598. [DOI] [PMC free article] [PubMed] [Google Scholar]