Abstract
Toll-like receptor 4 (TLR4) contributes to cerebral ischemia/reperfusion (I/R) injury and is a potential target for the treatment of ischemic stroke. This experiment is to evaluate the effect of an exogenous TLR4 antagonist, TAK-242, against acute cerebral I/R injury. A mouse model of cerebral I/R was induced by transient middle cerebral artery occlusion. TAK-242 (3 mg/kg body weight) was injected intraperitoneally 1 hour after ischemia. Our results showed that the concentration of TAK-242 in plasma increased to 52.0 ng/mL 3 hours after injection, was maintained at 54.1 ng/mL 8 hours after injection, and decreased to 22.6 ng/mL 24 hours after injection. The concentration of TAK-242 in brain tissue increased to 26.1 ng/mL in ischemic hemisphere and 14.2 ng/mL in nonischemic hemisphere 3 hours after injection, and was maintained at the similar levels 24 hours after injection. We found that TAK-242 significantly reduced cerebral infarction compared with vehicle control, improved neurologic function, inhibited the phosphorylation of downstream protein kinases in TLR4 signaling pathway, and downregulated the expression of inflammatory cytokines. We conclude that TAK-242 is able to cross blood-brain barrier, blocks TLR4 signaling, mediates the expression of inflammatory cytokines, and protects the brain from acute damage induced by I/R.
Keywords: cerebral ischemia/reperfusion, neuroprotection, TAK-242, Toll-like receptors
Introduction
Cerebral ischemia is usually the result of the obstruction of brain arteries and lack of blood supply to the brain. Although therapies for the restoration of blood flow to brain tissue are effective, reperfusion in the ischemic brain leads to a cascade of pathophysiologic processes resulting in further damage known as ischemia/reperfusion (I/R) injury. A growing body of evidence suggests that cerebral I/R induces robust in situ inflammatory responses that contribute to further injury including neuronal death and white-matter damage.1, 2, 3
Toll-like receptors (TLRs) are a transmembrane pattern-recognition receptor family with important roles in the induction and regulation of immune/inflammatory responses. Toll-like receptor 4 is believed to be involved in several pathologies such as sepsis, cardiac diseases,4 and I/R injury.5, 6, 7 Our studies and others have revealed that TLR4-mediated signaling is activated after ischemia and contributes to increased inflammatory responses and further brain injury.7 Resatorvid (TAK-242), an exogenous synthetic antagonist for TLR4, is a small molecule which binds selectively to TLR4 and inhibits TLR4 signal transduction and its downstream signaling events.8, 9, 10 However, whether TAK-242 can pass through the blood-brain barrier (BBB) and inhibit neuroinflammation in ischemic brain has not been investigated. The present study evaluated the ability of TAK-242 to pass through the BBB, its neuroprotective effect, and its modulation on inflammatory cytokines after acute cerebral I/R in mice.
Materials and methods
Animals
Sixty-two male mice (C57BL/6J; age 12 weeks; body weight 25–30 g) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the Division of Animal Resources at Emory University. The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The animal care and experimental protocols were approved by the Emory University Institutional Animal Care and Use Committee.
In the experiment to evaluate the protective effect of TAK-242 against ischemia injury, mice were randomly assigned to four groups: (1) sham surgery treated with 1% dimethyl sulfoxide (DMSO, vehicle) as sham vehicle control (S, n=9; four mice were used for molecular analysis); (2) focal cerebral I/R treated with 1% DMSO as I/R plus vehicle control (I/R, n=17; five mice were used for molecular analysis); (3) sham surgery treated with TAK-242 (S-T, n=9; four mice were used for molecular analysis); and (4) focal cerebral I/R treated with TAK-242 (I/R-T n=17; five mice were used for molecular analysis).
In another experiment, 10 mice were used to evaluate the concentration of TAK-242 in plasma and brain tissue. One mouse (naive, without any treatment) was used as blank control, and 9 mice subjected to cerebral I/R treated with TAK-242 were used to evaluate the concentration of TAK-242 in plasma and brain tissue 3, 8 and 24 hours after intraperitoneal injection (three mice for each time point). The plasma, left cerebral hemisphere (ischemia), and right hemisphere (nonischemia) were harvested for detection of TAK-242.
Induction of Focal Cerebral Ischemia/Reperfusion
Focal cerebral I/R was induced by middle cerebral artery occlusion on the left side according to previously published methods.5 Briefly, mice were subjected to anesthesia by 5.0% isoflurane maintained by inhalation of 1.5% to 2% isoflurane driven by 100% oxygen flow. Mice were ventilated (110 breaths per minute with volume 0.5 mL) and body temperature was regulated at 37°C. Heart rate and partial pressure of oxygen were monitored during surgery. After the skin incision, the left common carotid artery, the external carotid artery, and the internal carotid artery were carefully exposed. Microvascular aneurysm clips were applied to the left common carotid artery and the internal carotid artery. A coated 6-0 filament (6023PK, Doccol Corp., Redlands, CA, USA) was introduced into an arteriotomy hole, fed distally into the internal carotid artery and advanced 11 mm from the carotid bifurcation. The internal carotid artery clamp was removed and focal cerebral ischemia started. After ischemia for 60 minutes, the filament and the common carotid artery clamp were gently removed (reperfusion starts). The collar suture at the base of the external carotid artery stump was tightened. The skin was closed, anesthesia was discontinued, and the animal was allowed to recover in a prewarmed cage. Sham control mice underwent a neck dissection and coagulation of the external carotid artery, but without middle cerebral artery occlusion. Ischemia and reperfusion conditions were confirmed by the regional cerebral blood flow (rCBF) detected by a laser Doppler cerebral flow meter (moorVMS-LDF, Moor Instruments, Delaware, DE, USA) before cerebral ischemia, during cerebral ischemia, and after reperfusion.
TAK-242 Treatment
TAK-242 was dissolved in DMSO and then diluted in sterile endotoxin-free water. The final concentration of DMSO was 1%. The dissolved TAK-242 or DMSO (1%) was injected intraperitoneally (3 mg/kg body weight) 1 hour after middle cerebral artery occlusion or sham surgical operation. This optimal dose was selected based on previous studies.8, 9, 10 The route of administration was modified to intraperitoneal injection.
Evaluation of Neurologic Score
All the mice were scored by an investigator who was masked using the method described previously with modification.5, 11 The scoring system ranges from 0 to 15, in which 0 is death because of cerebral I/R injury, and 15 is a perfect score. Briefly, the scoring system includes five principal tasks for survival mice: spontaneous activity over a 3-minute period (0 to 3), symmetry of movement (0 to 3), open-field path linearity (0 to 3), beam walking on a 3 cm × 1 cm beam (0 to 3), and response to vibrissae touch (1 to 3). If the mouse died from cerebral I/R injury, the animal received a 0 point.
Measurement of TAK-242 Concentration in Plasma and Brain Tissue
Three, 8, or 24 hours after the injection of TAK-242, the mice were anesthetized with 5.0% isoflurane driven by 100% oxygen flow. Blood (0.95 mL) was drawn from the left ventricle and immediately mixed with 0.05 mL sodium citrate. The samples were incubated at room temperature for 30 minutes and centrifuged at 8,000 r.p.m. The plasma was collected and stored at −80°C for future use. Immediately after the blood was drawn, the mice were perfused with ice-cold normal saline via the ascending aorta until the perfusion buffer was clear from the right atrium. The brains were removed and weighed. Brain tissues were homogenized with buffer and centrifuged at 14,000 r.p.m. for 10 minutes. Supernatants were collected and stored at −80°C. The concentrations of TAK-242 were detected by Intertek (EI Dorado Hill, CA, USA) using liquid chromatography with tandem mass spectrometry detection. Briefly, TAK-242 and internal standard (bromfenac) were extracted from 50 μL of mouse plasma or brain homogenate by liquid-liquid extraction using methyl tertiary-butyl ether. After evaporation to dryness and reconstitution, the extracts were analyzed by liquid chromatography with tandem mass spectrometry. Run times were ~5 minutes. The lower limit of quantitation for TAK-242 is 0.5 ng/mL for plasma, and 2 ng/g for brain homogenate. The calibration curve range is 0.5–2,000 ng/mL for plasma, and 2–4,000 ng/g for brain homogenate. The concentrations of TAK-242 were calculated based on the standard curve.
Assessment of Cerebral Infarct Size
The infarct size was determined as described previously.5 Twenty-four hours after I/R, mice were killed and perfused with ice-cold phosphate-buffered saline via the ascending aorta. Brains were removed and sectioned coronally into 2-mm-thick slices. The slices were stained with 2% 2,3,5-triphenyltetrazolium chloride solution at 37°C for 15 minutes followed by fixation with 10% formalin neutral buffer solution (pH 7.4). The infarct areas were traced and quantified with ImageJ software (NIH, Bethesda, MD, USA). Unstained areas (pale color) were defined as ischemic lesions. The area of infarction and the areas of both hemispheres were calculated for each brain slice. Edema index was calculated by dividing the total volume of the left hemisphere by the total volume of the right hemisphere. The actual infarct volume adjusted for edema was calculated by dividing the infarct volume by the edema index. Infarct volumes are expressed as percentage of the total brain volume±s.e.m.
Antibody Array
Six hours after cerebral ischemia, mice were killed and perfused with ice-cold phosphate-buffered saline via the ascending aorta. Ischemic cerebral hemispheres were removed and stored at −80°C. Proteins were prepared from ischemic cerebral hemispheres. Forty inflammatory cytokines were analyzed by antibody arrays (RayBio Cytokine Antibody Arrays—Mouse Inflammation Antibody Array G Series I, RayBiotech, Norcross, GA, USA). Briefly, the glass chips were air dried for 60 minutes and assembled into an incubation chamber and incubation frame. Blocking buffer (100 μL) was added into each well and the glass chips were incubated at room temperature for 30 minutes. After decanting the blocking buffer, 100 μL of each sample was added and incubated at 4°C overnight. The chips were washed five times with wash buffer I and then two times with wash buffer II at room temperature; 70 μL of diluted biotin-conjugated antibodies was added to each corresponding well, and the chips were incubated at 4°C overnight. The chips were washed as previously described and 70 μL of diluted Alexa Fluor 555–conjugated streptavidin was added to each subarray. The incubation chamber was covered with an adhesive film and aluminum foil, and incubated at 4°C overnight. The chips were washed two times with wash buffer I. The incubation frame and chamber were disassembled. The slides were taken out and placed in a 50-mL centrifuge tube, washed two times with wash buffer I at room temperature for 10 minutes each time, washed once with wash buffer II for 10 minutes, and rinsed with distilled H2O. The water droplets were removed by centrifuge at 1,000 r.p.m. for 3 minutes and then dried completely in air for at least 20 minutes while protected from light. The slides were scanned using a laser scanner (Axon GenePix, Molecular Devices, Sunnyvale, CA, USA) with a Cy3 channel. Signal data were collected and analyzed by a software from RayBiotech (Norcross, GA, USA).
Western Blots
Western blotting was performed as described previously.12 Briefly, proteins were extracted from the brain samples harvested 6 hours after cerebral ischemia, electrophoresed with sodium dodecyl sulfate polyacrylamide gel, and transferred onto Hybond ECL membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The ECL membranes were incubated with primary antibody followed by incubation with peroxidase-conjugated secondary antibodies. Signals were detected with the ECL system (Amersham Pharmacia Biotech) and quantified by scanning densitometry and computer-assisted image analysis. The primary antibodies used were anticyclooxygenase-2 (anti-COX-2), antimatrix metallopeptidase 9 (anti-MMP-9), anti-p-IκB kinase α/β (anti-p-IKKα/β), anti-IKKα, anti-p-inhibitor of nuclear factor kappa B alpha (anti-p-IκBα), anti-IκBα, and anti-beta actin (Cell Signaling Technology, Danvers, MA, USA).
Statistical Analysis
Continuous scale measurements were expressed as mean±s.e.m. for each group. Neurologic function (neurologic score) was analyzed by Kruskal-Wallis analysis of variance on ranks. After normality testing (Shapiro-Wilk method) and equal variance tests were passed, all pair-wise multiple comparison procedures (Dunn's method) were performed. A value of P<0.05 was considered to be significant. The concentration of TAK-242 was analyzed by repeated easures analysis of variance, and the size of infarction and the levels of inflammatory cytokines were analyzed by one-way analysis of variance. Pair-wise comparisons of group means (Holm-Sidak method) were used to compare group means after normality testing (Shapiro-Wilk) and equal variance tests were passed. A probability level of 0.05 or smaller was used to indicate statistical significance.
Results
Concentration of TAK-242 in Plasma and Brain Tissue
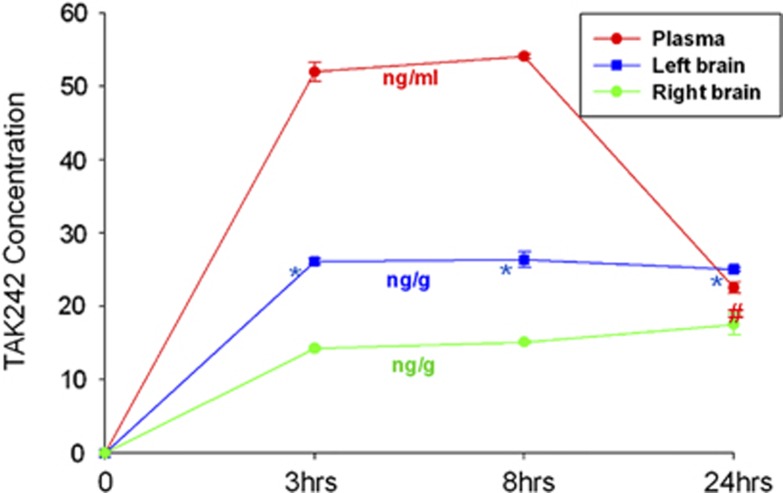
The concentrations of TAK-242 in plasma and brain tissue were measured at different time points after intraperitoneal injection using liquid chromatography with tandem mass spectrometry. Our results showed that the concentration of TAK-242 in plasma increased to 52.0 ng/mL 3 hours after treatment, was maintained at 54.1 ng/mL 8 hours after treatment, and decreased to 22.6 ng/mL 24 hours after treatment. The concentration of TAK-242 in brain tissue increased to 26.1 ng/mL (ipsilateral) and 14.2 ng/mL (contralateral) 3 hours after treatment, was maintained at 26.4 ng/mL (ipsilateral) and 15.1 ng/mL (contralateral) 8 hours after treatment, and was still maintained at 25.0 ng/mL (ipsilateral) and 17.5 ng/mL (contralateral) 24 hours after treatment (Figure 1).
Figure 1.
The concentration of TAK-242 in plasma and brain tissue. The figure shows the concentrations of TAK-242 in plasma (red line), the nonischemic hemisphere (green line, right brain), and the ischemic hemisphere (blue line, left brain) after the intraperitoneal injection (3 mg/kg body weight). *Compared with right brain, P<0.05. #Plasma vs right brain, P<0.05.
Infarct size and Neurologic Score
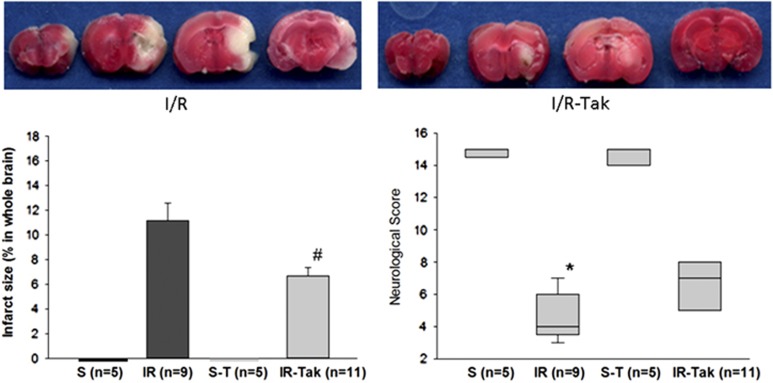
During ischemia, rCBF decreased to <80% of normal levels (before ischemia), and after reperfusion, rCBF recovered to >75% of normal, which confirmed the success of the animal model. Infarct size and neurologic score were measured 24 hours after cerebral I/R or sham surgery. Our data showed that sham surgery treated with vehicle (S) and sham surgery treated with TAK-242 (S-T) did not induce any cerebral infarction. The infarct sizes were 21.3% in the untreated I/R group (I/R) and 12.5% in the TAK-242-treated I/R group (I/R-T). TAK-242 treatment significantly reduced brain infarct size by 41% compared with the untreated mice (P<0.05; Figure 2).
Figure 2.
Brain infarct size and neurologic score 24 hours after cerebral I/R or sham surgery. The image shows that sham surgery treated with vehicle (S) and sham surgery treated with TAK-242 (S-T) did not induce any cerebral infarction. TAK-242 treatment significantly reduced brain infarct size (12.5%) compared with untreated mice (21.3% #P<0.05). TAK-242 treatment significantly improved neurologic function (6.73) compared with untreated mice (4.38; #P<0.05). A representative picture of 2,3,5-triphenyltetrazolium chloride (TTC) staining is shown on the top of the image. In addition, treatment with TAK-242 (S-T) did not induce significant neurobehavioral changes compared with sham surgery treated with vehicle (S). Cerebral I/R significantly reduced neurologic score (4.38) compared with sham surgery treated with vehicle (S, *P<0.05). The mice subjected to cerebral I/R treated with TAK-242 did not show significant difference from S-T, which indicated that TAK-242 treatment could improve neurologic function impaired by cerebral I/R.
Our data also showed that sham surgery treated with TAK-242 (S-T) did not induce significant neurobehavioral changes compared with sham surgery treated with vehicle (S). Cerebral I/R significantly reduced neurologic score (4.38) compared with sham surgery treated with vehicle (S) and sham surgery treated with TAK-242 (S-T, P<0.05; Figure 2). The mice subjected to cerebral I/R treated with TAK-242 did not show significant difference from S and S-T, which indicated that TAK-242 treatment could improve neurologic function impaired by cerebral I/R.
Protein Array
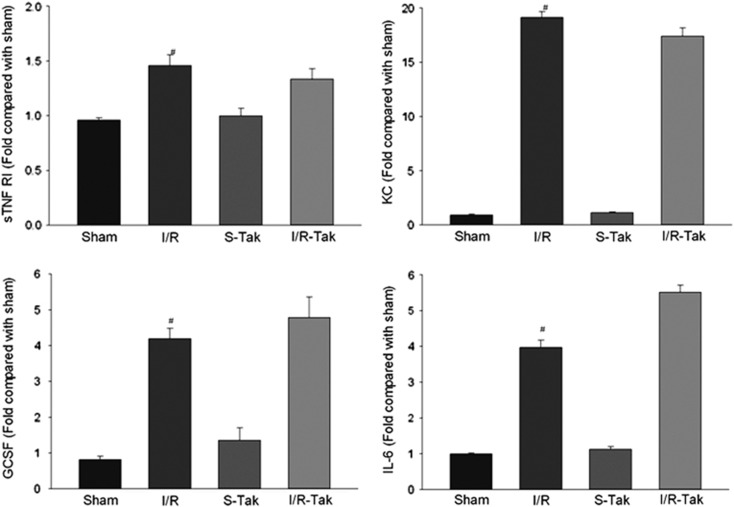
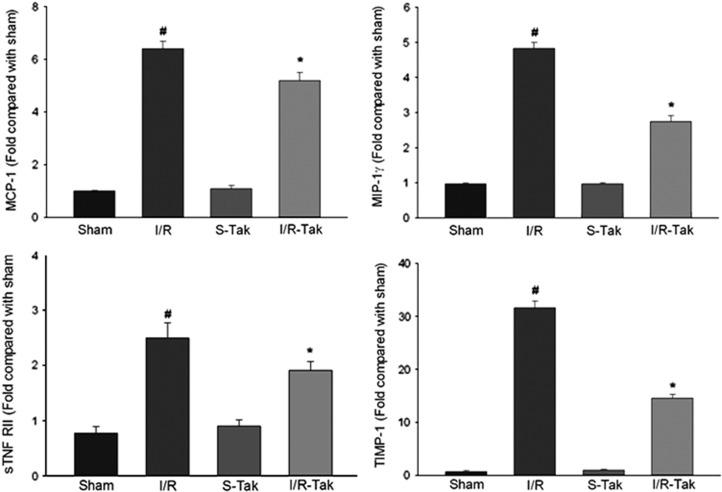
Levels of 40 inflammatory cytokines were detected 6 hours after cerebral I/R using an antibody array. Our data showed that the levels of soluble tumor necrosis factor receptor I, soluble tumor necrosis factor receptor II, chemokine (C-X-C motif) ligand 1 (CXCL1), granulocyte colony–stimulating factor, interleukin-6, monocyte chemotactic protein-1, macrophage inflammatory protein-1γ (MIP-1γ), and tissue inhibitor of metalloproteinases 1 (TIMP-1) significantly increased in ischemic brain compared with sham controls (P<0.05; Figures 3 and 4). Treatment with TAK-242 significantly reduced the levels of soluble tumor necrosis factor receptor II, monocyte chemotactic protein-1, MIP-1γ, and TIMP-1 compared with untreated controls (P<0.05; Figure 4).
Figure 3.
Levels of sTNF RI, KC, GCSF, and IL-6 in brain tissue 6 hours after cerebral I/R. The levels of sTNF RI, KC, GCSF, and IL-6 significantly increased in ischemic brain compared with sham controls (#compared with sham, P<0.05). GCSF, granulocyte colony–stimulating factor; I/R, ischemia/reperfusion; I/R-Tak, I/R treated with TAK-242; IL-6, interleukin-6; S-Tak, sham control with TAK-242; sham, sham control; sTNF RI, soluble tumor necrosis factor receptor-I; KC, chemokine (C-X-C motif) ligand.
Figure 4.
Levels of sTNF RII, MCP-1, MIP-1γ, and TIMP-1 in brain tissue 6 hours after cerebral I/R. Levels of sTNF RII, MCP-1, MIP-1γ, and TIMP-1 significantly increased in ischemic brain compared with sham controls (#compared with sham, P<0.05). Treatment with TAK-242 significantly reduced the levels of these cytokines compared with untreated controls (*compared with I/R, P<0.05). I/R, ischemia/reperfusion; I/R-Tak, I/R treated with TAK-242; MCP-1, monocyte chemotactic protein-1; MIP-1γ, macrophage inflammatory protein-1γ; S-Tak, sham control with TAK-242; sham, sham control; sTNF, soluble tumor necrosis factor; TIMP-1, tissue inhibitor of metalloproteinases 1.
Western Blots
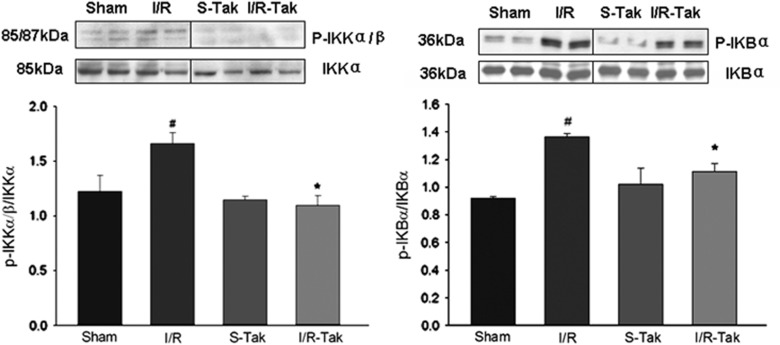
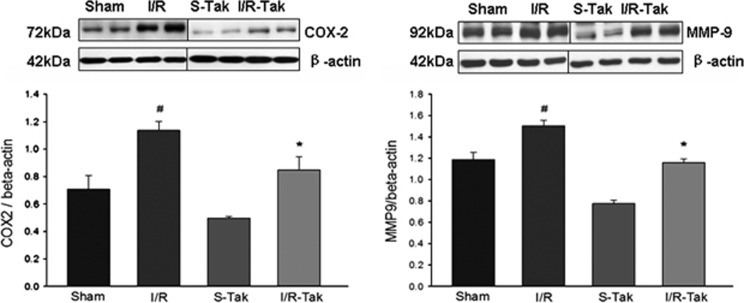
Our data showed that the phosphorylation of IKKα/β and IκBα significantly increased, and that the expression of COX-2 and MMP-9 were significantly upregulated in ischemic brain compared with sham controls (P<0.05; Figures 5 and 6). Treatment with TAK-242 significantly inhibited the phosphorylation of IKKα/β and IκBα, and the expression of COX-2 and MMP-9 compared with vehicle treated controls (P<0.05; Figures 5 and 6).
Figure 5.
Phosphorylation of IKKα/β, and IκBα in brain tissue 6 hours after cerebral I/R. The phosphorlyation of IKKα/β and IκBα significantly increased in ischemic brain compared with sham controls (#compared with sham, P<0.05). Treatment with TAK-242 significantly inhibited the phosphorylation of IKKα/β and IκBα compared with untreated controls (*compared with I/R, P<0.05). I/R, ischemia/reperfusion; I/R-Tak, I/R treated with TAK-242; IκBα, inhibitor of nuclear factor kappa B alpha; IKKα/β, IκB kinase α/β; S-Tak, sham control with TAK-242; sham, sham control.
Figure 6.
Expression of COX-2 and MMP-9 in brain tissue 6 hours after cerebral I/R. The expression of COX-2 and MMP-9 significantly increased in ischemic brain compared with sham controls (#compared with sham, P<0.05). Treatment with TAK-242 significantly downregulated the expression of COX-2 and MMP-9 compared with untreated controls (*compared with I/R, P<0.05). COX-2, cyclooxygenase-2; I/R, ischemia/reperfusion; I/R-Tak, I/R treated with TAK-242; MMP-9, matrix metallopeptidase 9; S-Tak, sham control with TAK-242; sham, sham control.
Discussion
Cerebral I/R triggers acute inflammation which aggravates cerebral ischemic injury. One of the important mechanisms of this process is the activation of the innate immune system. TLRs play important roles in the induction and regulation of immune/inflammatory responses. To date, 13 TLRs have been identified in mammals.13, 14 Each TLR can recognize its distinct ligands derived from various microorganisms or released from damaged tissues.15, 16 On activation by exogenous and endogenous ligands, TLRs recruit their adapter proteins, activate downstream kinases, and then induce the expression of inflammation-associated genes. Previous studies indicated that TLR4-mediated signaling was activated in ischemic brain, and that TLR4 deficit attenuated cerebral ischemic injury in TLR4 knockout mice.17, 18, 19, 20 Exogenous synthetic antagonists for TLR4, such as resatorvid (TAK-242), can block TLR4 signaling and inhibit the inflammatory responses in experimental sepsis.8 The specificity of TAK-242 was confirmed by several studies.8, 9, 10 TAK-242 binds selectively to TLR4, and is a selective inhibitor of signaling from the intracellular domain of TLR4. TAK-242 can disrupt the interaction of TLR4 with adapter molecules, and thereby inhibit TLR4 signal transduction and its downstream signaling events. TAK-242 represents a novel therapeutic approach to the treatment of TLR4-mediated diseases.21, 22 However, whether TAK-242 has neuroprotective effects on ischemic brain remains unknown. To investigate whether TAK-242 can pass through the BBB and protect the brain from cerebral I/R, we measured the concentration of TAK-242 in plasma and brain tissue, and evaluated ischemia-induced inflammation and subsequent brain damage in mice treated with TAK-242.
We used C57BL/6J mice to establish the model of cerebral I/R. Body temperature, heart rate, and partial pressure of oxygen were monitored during surgery, and there was no significant difference in these parameters between the TAK-242-treated and the untreated groups. Results from rCBF detected by a laser Doppler cerebral flow meter (moorVMS-LDF) showed that during ischemia, rCBF decreased to <80% of normal levels (before ischemia), and after reperfusion, rCBF recovered to >75% of normal, which confirmed the success of the model of cerebral ischemia and reperfusion.
The concentrations of TAK-242 in plasma and brain tissue were detected by liquid chromatography with tandem mass spectrometry. The results showing that the concentration of TAK-242 in plasma increased 3 hours after treatment, was maintained 8 hours after treatment, and decreased at 24 hours after treatment, indicated that TAK-242 injected intraperitoneally can be absorbed into blood circulation and is maintained up to 24 hours after injection (Figure 1). Interestingly, the concentration of TAK-242 in brain tissue also increased after the injection, indicating that TAK-242 can pass through the BBB in the ischemic and nonischemic brain, and be maintained in brain tissue for at least 24 hours after injection (Figure 1). Previous studies have indicated that the pharmacokinetics of TAK-242 depends on the species and organs—it differs, for example, in dogs and rats, and the distribution of TAK-242 is different in plasma and kidney.23, 24 However, the distribution of TAK-242 in mice plasma and brain after intraperitoneal injection has not been reported. In addition, in previous studies, the dosages of TAK-242 ranged from 1 to 10 mg/kg (including 3 mg/kg) in patients, guinea pigs, and mice via intravenous injection. Base on the previous reports, we modified the route of administration from intravenous injection to intraperitoneal injection. Our results confirmed that TAK-242 injected intraperitoneally could be absorbed into the blood stream, which was a new finding in the present study. Importantly, our data show for the first time that TAK-242 can cross the BBB and enter into ischemic and nonischemic hemispheres in mice after focal cerebral I/R induced by middle cerebral artery occlusion. Moreover, the concentration of TAK-242 in the ischemic hemisphere was significantly higher than that in the nonischemic hemisphere. This may be interpreted to indicate that cerebral I/R facilitates the capability of TAK-242 to cross the BBB, in the damaged brain.
To evaluate the neuroprotective effect of TAK-242 on ischemic brain, infarct size and neurologic function were measured 24 hours after cerebral I/R or sham surgery. As shown in Figure 2, our data showed that treatment with TAK-242 significantly reduced brain infarct size and significantly improved neurologic function showed that treatment with TAK-242 has a neuroprotective effect at the acute stage after cerebral I/R.
As described previously, in the TLR4-mediated pathway, activated TLR4 increases the phosphorylation of downstream kinase, p-IKKα/β, which then induces the phosphorylation of IκBα, and results in the nuclear translocation of nuclear factor kappa B and regulates immune/inflammatory responses.5 Our data showed that p-IKKα/β and p-IκBα significantly increased in ischemic brain tissue compared with sham controls, and treatment with TAK-242 inhibited the phosphorylation of IKKα/β and IκBα. The results showed that treatment with TAK-242 can block the activation of TLR4 signaling in brain tissue induced by cerebral I/R.
Our data also showed that, the levels of soluble tumor necrosis factor receptor I, soluble tumor necrosis factor receptor II, KC, granulocyte colony–stimulating factor, interleukin-6, monocyte chemotactic protein-1, MIP-1γ, and TIMP-1, and the expression of COX-2 and MMP-9 significantly increased in ischemic brain compared with sham controls (P<0.05, Figures 3, 4, 5, 6). Previous studies have shown that these cytokines are associated with ischemia-induced inflammatory responses, ischemic damage, and brain repair after cerebral I/R.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 For example, interleukin-6 is increased in the acute phase of stroke, and is considered a robust early marker for outcome in acute ischemic stroke.28 Monocyte chemotactic protein-1, MIP-1γ, and TIMP-1 play important roles in inflammatory processes and contribute to the pathogenesis of brain injury and neurologic dysfunction.31, 32, 33, 34 Moreover, MMP family and COX are mediated by TLR4 signaling and involved in the progression of neuronal damage after cerebral ischemia.34, 35 Our data showed that treatment with TAK-242 significantly reduced the levels of sTNF RII, monocyte chemotactic protein-1, MIP-1γ, TIMP-1, and the expression of MMP-9 and COX-2. The results show that administration of TAK-242 blocks TLR4-mediadted signaling and inhibits in situ inflammatory responses induced by cerebral I/R.
A limitation of the present study is that the tissues for assays on the concentrations of TAK-242 and the expression of inflammatory cytokines in brain tissue were harvested from entire cerebral hemisphere instead of specific brain tissues, such as cortex, striatum, and hippocampus. If the assays were performed in the individual cerebral structures, more detailed information about the distribution of TAK-242 in brain tissue and its effects on inflammatory responses in specific cerebral areas would be provided.
Conclusion
In conclusion, our data can be interpreted to show that TAK-242 can pass through the BBB and distribute in the nonischemic brain tissue and ischemic brain tissue in mice, and that administration of TAK-242 protects the brain from damage at the acute stage after cerebral I/R by inhibiting TLR4-mediated signaling and mediating the expression of inflammatory cytokines. These novel findings have the potential to lead to a new medication for ischemic stroke.
The authors declare no conflict of interest.
Footnotes
This work was supported by AHA National Program SDG 0830481N, National Natural Science Foundation of China (30970995; 81271268), Jiangsu Specially Appointed Professor Program, and Jiangsu Six Major Talent Summit Programs to FH, and partly by NIH 5R01NS048451 and 1R01HD061971 to DGS.
References
- Morioka T, Kalehua AN, Streit WJ. Progressive expression of immunomolecules on microglial cells in rat dorsal hippocampus following transient forebrain ischemia. Acta Neuropathologica. 1992;83:149–157. doi: 10.1007/BF00308474. [DOI] [PubMed] [Google Scholar]
- Stoll G. Inflammatory cytokines in the nervous system: multifunctional mediators in autoimmunity and cerebral ischemia. Rev Neurol. 2002;158:887–891. [PubMed] [Google Scholar]
- Yang GY, Gong C, Qin Z, Liu XH, Lorris BA. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1999;69:135–143. doi: 10.1016/s0169-328x(99)00007-8. [DOI] [PubMed] [Google Scholar]
- Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, et al. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–108. doi: 10.1016/j.brainres.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, et al. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- Kuno M, Nemoto K, Ninomiya N, Inagaki E, Kubota M, Matsumoto T, et al. The novel selective toll-like receptor 4 signal transduction inhibitor tak-242, prevents endotoxaemia in conscious Guinea-pigs. Clin Exp Pharmacol Physiol. 2009;36:589–593. doi: 10.1111/j.1440-1681.2008.05121.x. [DOI] [PubMed] [Google Scholar]
- Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Takeda K. Evolution and integration of innate immune recognition systems: the Toll-like receptors. J Endotoxin Res. 2005;11:51–55. doi: 10.1179/096805105225006687. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Gao Y, Fang X, Sun H, Wang Y, Yao LJ, Li JP, et al. Toll-like receptor 4-mediated myeloid differentiation factor 88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in hippocampal CA1 region in mice. Biol Pharm Bull. 2009;32:1665–1671. doi: 10.1248/bpb.32.1665. [DOI] [PubMed] [Google Scholar]
- Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Sha T, Iizawa Y, Ii M. Combination of imipenem and TAK-242, a Toll-like receptor 4 signal transduction inhibitor, improves survival in a murine model of polymicrobial sepsis. Shock. 2011;35:205–209. doi: 10.1097/SHK.0b013e3181f48942. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- Jinno F, Kakehi M, Takeuchi T, Tagawa Y, Kondo T, Asahi S. Investigation of the unique metabolic fate of ethyl (6R)-6- [N- (2-chloro-4-fluorophenyl) sulfamoyl] cyclohex-1-ene-1-carboxylate (TAK-242) in rats and dogs using two types of 14C-labeled compounds having different labeled positions. Arzneimittelforschung. 2011;61:458–471. doi: 10.1055/s-0031-1296228. [DOI] [PubMed] [Google Scholar]
- Jinno F, Takeuchi T, Tagawa Y, Kondo T, Itoh T, Asahi S. Differences in the pharmacokinetics of 4-amino-3-chlorophenyl hydrogen sulfate, a metabolite of resatorvid, in rats and dogs. Drug Metab Dispos. 2012;40:648–454. doi: 10.1124/dmd.111.043729. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Szymanowski A, Swahn E, Jonasson L. Soluble TNF receptors are associated with infarct size and ventricular dysfunction in ST-elevation myocardial infarction. PLoS One. 2013;8:e55477. doi: 10.1371/journal.pone.0055477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormstad H, Aass HC, Lund-Sørensen N, Amthor KF, Sandvik L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011;258:677–685. doi: 10.1007/s00415-011-6006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losy J, Zaremba J, Skrobański P. CXCL1 (GRO-alpha) chemokine in acute ischaemic stroke patients. Folia Neuropathol. 2005;43:97–102. [PubMed] [Google Scholar]
- Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-β induced experimental model of Alzheimer's disease. Pharmacol Biochem Behav. 2013;110C:46–57. doi: 10.1016/j.pbb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, et al. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28:3710–3717. doi: 10.1097/00003246-200011000-00029. [DOI] [PubMed] [Google Scholar]
- Waje-Andreassen U, Kråkenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm. 2005;2005:175–179. doi: 10.1155/MI.2005.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Paul R, Angele B, Popp B, Pfister HW, Koedel U. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 2006;8:974–983. doi: 10.1016/j.micinf.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Zhao JK, Guan FL, Duan SR, Zhao JW, Sun RH, Zhang LM, et al. Effect of focal mild hypothermia on expression of MMP-9, TIMP-1, Tau-1 and β-APP in rats with cerebral ischaemia/reperfusion injury. Brain Inj. 2013;27:1190–1198. doi: 10.3109/02699052.2013.804206. [DOI] [PubMed] [Google Scholar]
- Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, González-Falcón A, García-Cabrera M, Alvarez D, Al-Dalain S, Martínez G, et al. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]