Abstract
Current functional neuroimaging methods are not adequate for high-resolution whole-brain visualization of neural activity in real time. Here, we show imaging of fast hemodynamic changes in deep mouse brain using fully noninvasive acquisition of five-dimensional optoacoustic data from animals subjected to oxygenation stress. Multispectral video-rate acquisition of three-dimensional tomographic data enables simultaneous label-free assessment of multiple brain hemodynamic parameters, including blood oxygenation, total hemoglobin, cerebral blood volume, oxygenized and deoxygenized hemoglobin, in real time. The unprecedented results indicate that the proposed methodology may serve as a powerful complementary, and potentially superior, method for functional neuroimaging studies in rodents.
Keywords: cerebral hemodynamics, functional neuroimaging, neuroimaging, optoacoustic/photoacoustic tomography, real time
Introduction
Cerebral hemodynamic changes are closely linked to neuronal activity by neurovascular coupling,1 and neuroimaging methods capable of assessing the concentrations of oxygenized (HbO) and deoxygenized (HbR) hemoglobin, cerebral blood volume (CBV), or cerebral blood flow greatly contribute to a better understanding of the mechanisms underpinning this phenomenon.2, 3 Yet, the current imaging approaches are limited in their capacity of simultaneously monitoring all these hemodynamic parameters with an adequate spatio-temporal resolution.3, 4 The mainstay of whole-brain functional neuroimaging, the blood oxygenation level-dependent functional magnetic resonance imaging, offers noninvasive endogenous measurements of brain anatomy and function but struggles with resolving the cerebral microvasculature.1 Furthermore, the temporal resolution of the method is considered insufficient to fully resolve neurovascular responses,5 while additional limitations stem from its basic contrast mechanism that purely relies on changes in HbR. Other imaging techniques such as positron emission tomography and X-ray computed tomography are likewise not capable of real-time volumetric imaging and further require the application of exogenous contrast agents and exposure to ionizing radiation. Furthermore, the high costs associated with the whole-body clinical imaging methods limit their applicability in basic neuroscience research. Recently, functional neuroimaging of stimulus-evoked brain activation in the somatosensory cortex of the rat was showcased using pure ultrasound (US) imaging.6 While this method offered high spatio-temporal resolution, it was only sensitive to CBV and cerebral blood flow and further required an invasive approach using a cranial window.
Optical imaging methods are becoming increasingly attractive for in vivo brain imaging, mainly due to their unique capabilities to distinguish between HbO and HbR. Thereby, a large variety of biomedical optical techniques have been so far attempted for neuroimaging.3 Here, the main limitation is photon scattering that confines the effective imaging depth of even most advanced optical microscopy methods to superficial brain vasculature, also necessitating removal of the scalp and the skull.3 In turn, the intense light scattering in skin, skull, and brain itself crumbles the spatial resolution of the macroscopic optical imaging approaches, such as near-infrared spectroscopy and diffuse optical tomography.3, 7 Other epifluorescence imaging approaches are similarly affected by the intense light scattering thus can only deliver planar two-dimensional (2D) projection images lacking the depth resolution and limiting penetration to cortical vasculature.8
Among other available optical imaging techniques optoacoustics combines the benefits of both optics and US as it provides the highly specific functional and molecular contrast of photons while not suffering resolution degradation because of photon scattering in deep tissues.9 Although optoacoustic imaging has recently shown powerful performance for functional neuroimaging in rodents,5 significant limitations yet remain in terms of invasiveness,5 inadequate penetration,10 and lack of three-dimensional (3D) imaging capacity in real time,11 hindering practical applications. More importantly, multispectral measurements have not yet been exploited to their full potential in simultaneous monitoring of multiple hemodynamic parameters. In this work, we show unprecedented performance in volumetric imaging of spectrally-distinctive absorbers and fast hemodynamic changes in the brain using fully noninvasive acquisition of five-dimensional (5D) optoacoustic data (with 5D being three spatial dimensions, time, and multispectral information12). In particular, it is made possible, for the first time to our knowledge, to record and simultaneously quantify blood oxygenation (SO2), total hemoglobin (HbT), and CBV in deep brain structures and in real time.
Materials and methods
Animal preparation and breathing gas stimulation paradigm
Eight-week-old Female athymic nude-Foxn1nu mice (Harlan Laboratories LTD, Itingen, Switzerland) were used for imaging, in full compliance with the institutional guidelines of the Institute for Biological and Medical Imaging and with approval from the Government District of Upper Bavaria under animal protocol reference number 55.2.1.54-2532-95-12. Furthermore, the manuscript was written in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Animals were anesthetized with isoflurane (1.5% to 2.5% v/v) in 100% O2. Physiologic parameters, including blood oxygenation, heart rate, and body temperature were continuously monitored throughout the experiments. The latter was kept constant using a rectal thermometer and a feedback-controlled heating pad (PhysioSuite, Kent Scientific, Torrington, CT, USA). A custom-designed stereotactic mouse head holder (Narishige International Limited, London, UK) to avoid animal motion was positioned parallel to the surface of the imaging probe (Figure 1A). Optoacoustic recordings started under hyperoxic conditions (100% O2) and the inhaled gas was changed between hyperoxia and normoxia (medical air, 20% O2) every 2 minutes for a total duration of 10 minutes. The gas mixture was controlled manually using a multi-gas flowmeter (UNO, Zevenaar, The Netherlands) keeping the isoflurane level constant in the inhaled gas.
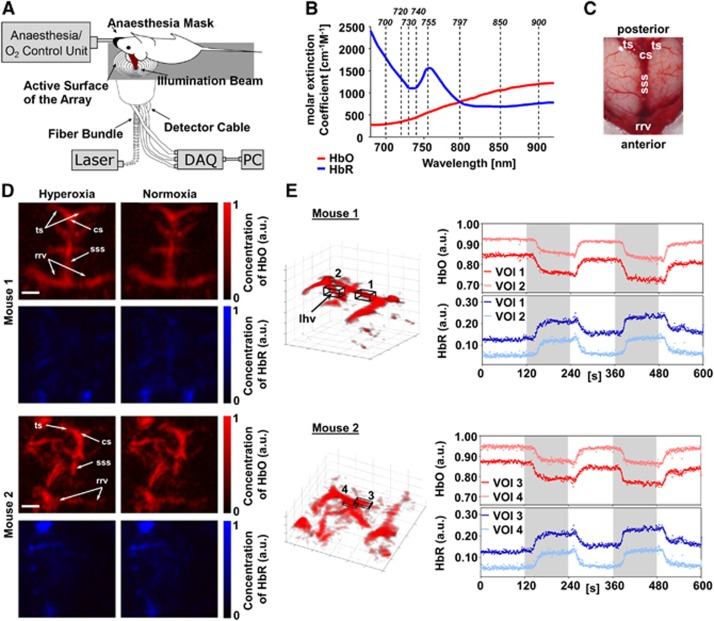
Figure 1.
Five-dimensional (5D) imaging of mouse brain oxygenation under hyperoxia and normoxia. (A) Experimental setup. The mouse head is fixed inside a custom-made stereotactical frame to minimize motion artifacts and the mouse is placed in a supine position on top of the spherical array optoacoustic probe for volumetric imaging. DAQ: Data acquisition system. (B) Extinction spectra of oxygenized (HbO) and deoxygenized (HbR) hemoglobin in the near-infrared range. Indicated are the eight wavelengths (dotted lines) that were used to acquire multispectral data sets. (C) Postmortem photograph of a mouse brain. Rostral rhinal vein (rrv), superior sagittal sinus (sss), confluence of sinuses (cs), transverse sinus (ts). (D) Maximum intensity projection images (along the depth direction) of HbO and HbR distribution in the mouse brain under hyperoxia and normoxia. The head of the second mouse was angled in relation to the surface of the array probe, revealing more lateral vasculature of the brain. The main identifiable veins are indicated (for abbreviations, see Figure 1C). Scale bars are 2 mm. (E) 3D visualization of HbO under hyperoxia. Volumes of interest (VOIs) were placed inside the anterior part of the sss and the longitudinal hippocampal vein (lhv). Time courses of HbO and HbR inside the VOIs are shown normalized to their respective total hemoglobin in all vessel voxels of a given frame (dots) along with the moving average over 5 frames (lines). The concentrations of HbO and HbR follow the changes from hyperoxia (white background) to normoxia (gray background) and vice versa. The benefits of the 5D information (multispectral three-dimensional data in real time), provided by the system, can be best appreciated in the Supplementary Video 2, in which changes of HbO and HbR in the whole brain are displayed over time.
Optoacoustic tomography system and imaging paradigm
The experimental system used for real-time acquisition of multispectral volumetric optoacoustic image data has been previously described.12 In short, a short-pulsed (~10 ns) light beam from a custom-made optical parametric oscillator laser (Innolas Laser GmbH, Krailling, Germany) guided through a fiber-bundle (CeramOptec GmbH, Bonn, Germany) is used as an excitation source. The pulse repetition frequency of the laser can be tuned to 50 Hz while it further possesses a unique fast tuning capability for precise per-pulse tuning of the excitation wavelengths within the entire near-infrared spectrum (700 to 900 nm). The generated US waves are then picked up at different locations around the imaged volume by means of a 256-element detection array (4 MHz central frequency, Imasonic SaS, Voray, France) and are simultaneously digitized with a parallel data acquisition system. In the current experiments, a total of eight wavelengths (Figure 1B) were used, thereby an entire multispectral dataset can be acquired within 0.8 second.
Data analysis
The acquired data were analyzed using Matlab (version 2013a, Mathworks Inc., Natick, MA, USA). In a first step, the signals were deconvolved with the impulse response of the transducer elements and then band-pass filtered between 80 KHz and 8 MHz. Volumetric data were reconstructed using a parallel GPU-based implementation of the 3D back-projection formula.13 The reconstructed volume was corrected for light fluence attenuation using exponential decay function along the depth direction.14 The distributions of HbO and HbR were retrieved by spectral processing of optoacoustic images obtained at eight discrete wavelengths, as shown in Figure 1B. Linear least square fitting (LSQ) unmixing was applied on a per voxel basis using the known molar extinction coefficient spectra of the main blood chromophores, namely, HbO and HbR.9 Yet, when unmixing the data acquired from pigmented black mice (Supplementary Figure 1), an additional contribution of melanin was accounted for in the unmixing procedure. Moreover, extinction spectra of deep-seated chromophores may also become affected by wavelength-dependent light attenuation.15 This effect can be partially accounted for by means of a blind unmixing algorithm such as vertex component analysis, which determines the actual spectra of absorbing components from the acquired data and then applies LSQ to these components.15 The map of blood oxygen saturation SO2 was subsequently calculated voxel-wise via SO2=HbO/(HbO+HbR).
Cerebral blood volume was estimated as the number of voxels for which the total hemoglobin (HbT=HbO+HbR) signal is higher than a given threshold. HbT is taken from images reconstructed at 797 nm (isosbestic point of hemoglobin, Figure 1B) and the threshold is established as a heuristically-determined factor times the standard deviation of the background. Specifically, the CBV was estimated as the number of voxels where the signal exceeds by a factor of at least 8.5 times the standard deviation of the background noise.
Results
A total of 750 unmixed frames were recorded during the experiments. The spatial resolution of the system (~200 μm) enables visualizing 3D distributions of major cerebral veins and their oxygenation status in real time. For comparison, an annotated postmortem photograph of a mouse brain is shown (Figure 1C). Two different head positions were imaged in two different mice to show visualizations of the cerebral vasculature (Figures 1D and 1E). However, position of the mouse head with respect to the detection array can be readily varied during the measurement to improve visualization of the different parts of the brain, as shown in Supplementary Video 1. The true benefits of the 5D information (i.e., 3D multispectral data in real time) can be best visualized in Supplementary Video 2, in which quantitative changes of HbO and HbR in the whole brain are displayed over time. Furthermore, the multispectral approach not only allows identifying hemodynamic parameters but also facilitates analysis and spectral unmixing under more difficult experimental conditions, e.g., when imaging skin-pigmented mice commonly used as models for neurodegenerative diseases (Supplementary Figure 1). In this case, the multispectral data acquisition allows enhancing the contrast between cerebral vessels and the background, ultimately leading to a higher contrast-to-noise in resolving vascular structures of the 5D approach over single-wavelength measurements.
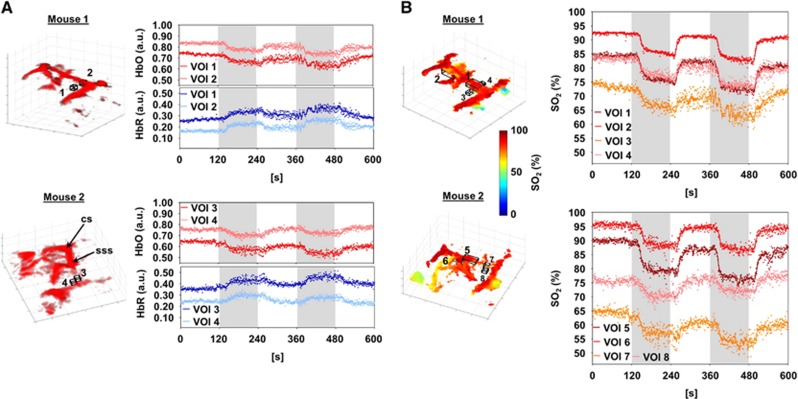
Patterns of changes of HbO and HbR following the breathing gas stimulation paradigm can be readily analyzed in specific volumes of interest (VOIs) located in the superior sagittal sinus and in deep cerebral veins, e.g., the longitudinal hippocampal vein (Figure 1E). The percentile changes in both mice are consistent with those observed in magnetic resonance imaging for major cerebral veins under hyperoxic challenges.16 Furthermore, VOIs placed outside the major vessels in the cortex allowed analyzing changes in HbO and HbR that followed the breathing gas stimulation paradigm (Figure 2A).
Figure 2.
Analysis of mouse brain hemodynamics. (A) Volume of interest (VOI) 1, 2 and VOI 3, 4 for mouse 1 and 2, respectively, were placed outside the major vessels inside the cortex. Variations of oxygenized (HbO) and deoxygenized (HbR) hemoglobin inside these voxels are shown here as time-dependent curves (for details and abbreviations, see Figure 1E). (B) Blood oxygenation (SO2) levels in the voxels were determined on a voxel-by-voxel basis from the spectrally unmixed data. Segmentation of major vessels was performed by thresholding the data acquired at 797 nm. The generated volumetric vessel mask was applied to assess the SO2 levels in the same VOIs as used in Figure 1E and SO2 changes could be monitored in the sss (mouse 1: VOI 1; mouse 2: 5) and lhv (mouse 1: VOI 2; mouse 2: VOI 6). For the three-dimensional (3D) visualization only voxels exceeding the signal threshold were considered. See Supplementary Video 3 for a dynamic visualization. In addition, SO2 changes were analyzed in the same VOIs from Figure 2A, placed outside the major vessel inside the cortex (mouse 1: VOI 3, 4; mouse 2: VOI 7, 8). In this case, no threshold was applied to the VOIs.
Vessel (hemoglobin) related voxels can be separated from background noise and subresolution voxels by applying the threshold criteria. A volumetric mask corresponding to the CBV can then be generated from positively identified voxels. This mask is further used to analyze changes in vessel SO2 (Figure 2B). This capacity is also dynamically visualized in Supplementary Video 3. Owing to the multispectral nature of our imaging paradigm, SO2 can directly be measured in any VOI or in single voxels from the spectrally-unmixed data. Figure 2B shows the analysis of SO2 changes in the VOIs placed outside major vessels roughly inside the cortex.
Discussion
Optoacoustic imaging has recently shown unique performance for measuring, visualizing, and quantifying cerebral hemodynamic changes related to functional activity.5 While state-of-the-art optoacoustic methods have prompted new prospects in neuroscience, the full capacity of the technology for evaluating brain activity has not yet been unveiled. Recent technical advances have enabled here the completely noninvasive acquisition of truly three-dimensional data from deep mouse brain and in real time,12 rendering simultaneous information on the underlying SO2, HbT and CBV with high temporal resolution. Multiple wavelengths covering the near-infrared spectrum of HbO and HbR were further used for a more accurate unmixing of the tissue chromophores, which has greatly facilitated analysis of fast oxygenation dynamics and further allowed to separate optoacoustic signals from melanin-related skin pigmentation in the 3D data sets.
The measured changes of ~10% in venous SO2 are in good accordance with previously reported values.16 While optical imaging methods generally experience intense light scattering in deep tissues, hindering extraction of depth-resolved 3D information with an adequate spatial resolution, the performance of our optoacoustic approach is not directly affected as the image reconstruction is based on propagation of the generated US waves, which undergo ~1,000 times less scattering in biologic tissues as compared with light.17 Even though, it was previously suggested to partly overcome light scattering problems in optical imaging by Monte Carlo simulations of light propagation,18 the latter approach has only shown effectiveness in resolving structures with comparable spatial resolution at mesoscopic depths of below 2 mm, while visualization of only several imaging planes in the depth direction was shown. Deeper penetration would naturally deteriorate the spatial resolution performance as light transforms from quasi-ballistic into fully diffusive regime.19 Clearly, Monte Carlo simulations are also very computationally extensive, making them inapplicable for real-time visualizations. In contrast, due to the true 3D nature of the acquired data and nearly isotropic spatial resolution across a large field of view of ~1 cm3 covering the entire mouse brain,12 our method made it possible to visualize optical contrast deeper in the brain by, e.g., resolving the hippocampal longitudinal vein and its deeper lying branching at depths between 3 and 4 mm without degradation of spatial resolution (see Supplementary Videos 2 and 3 and HbR data pertaining VOI 2 in Figure 1E). Furthermore, our approach is fully noninvasive (skin and skull intact) whereas optical studies with comparable spatial resolution required invasiveness—skin removal and skull thinning.18 Thus, the relatively simple setup also allowed here freely moving the mouse head and visualizing the reconstructed images in 3D during the data acquisition (Supplementary Video 1). While US-based neuroimaging was similarly capable of visualizing the whole rodent brain, this was also achieved invasively by removing the skull, while imaging was limited to single transverse imaging planes and measurements of CBV and cerebral blood flow only.6 With respect to the temporal resolution of our system, it is mainly limited by the pulse repetition rate of the laser. While the data presented in the current study was acquired at a frame rate of 10 Hz, the same laser and acquisition hardware can support imaging speeds of up to 50 volumetric frames per second if faster dynamics are to be tracked.6, 18
Most importantly for the purpose of functional neuroimaging, we have shown feasibility of analyzing hemodynamic changes outside major vessels. In this respect, given the currently available spatial resolution of the system in the range of 200 μm, one may consider two cases: (1) The imaging voxel fully lies inside a blood vessel and thus directly reflects hemodynamic changes in the vessel (e.g., voxels located within the superior sagittal sinus); (2) The voxel only partially comprises vasculature, i.e., an unknown percentage of its volume contains blood vessels that are smaller than the resolution of the system, as is the case for most of the recorded signals outside major vessels. These two cases are common in all macroscopic imaging modalities, whose spatial resolution does not allow resolving microvasculature, and we were similarly able to follow hemodynamic changes under the above partial volume assumptions (Figure 2B). Other more biologically relevant stimuli such as electrical forepaw stimulation were shown to be detectable using optoacoustics,5, 10 albeit only two-dimensionally and with much lower temporal resolution. In both cases relative changes in SO2 of ~5% were observed. Changes in this range can be clearly distinguished by our system under partial volume conditions (Figure 2B). In fact, in contrast to other imaging modalities, optoacoustic signals are mainly generated by light absorption in blood, which is distributed everywhere in tissues. Thus, one should expect that any hemodynamic change would result in corresponding optoacoustic signal changes, even if the particular imaging system is not able to accurately resolve all the microvascular structures responsible for these changes.
Several issues remain to be addressed. First, significant light absorption in the brain can have a profound influence on the local light fluence and hence on the amplitude of signals recorded from deep tissues. Blood oxygenation values can additionally be affected by wavelength-dependent light attenuation, which scales with depth. These effects were partially compensated here with the first order exponential correction factor14 but more accurate modeling of light distribution is expected to further improve data quantification. Furthermore, the sensitivity of our method to different types of functional stimuli needs to be determined. Finally, efforts need to be invested into improving the spatial resolution of the system which currently hinders visualization of cerebral vasculature smaller than the major arteries and veins. It is however highly anticipated that the tremendous ongoing developments in the 3D detection array technology will soon make it possible to analyze the important functional parameters also in smaller vessels.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
The research leading to these results has received funding from the European Research Council under grant agreement ERC-2010-StG-260991.
Supplementary Material
References
- Blockley NP, Griffeth VE, Simon AB, Buxton RB. A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed. 2013;26:987–1003. doi: 10.1002/nbm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Sakadzic S, Srinivasan VJ, Yaseen MA, Nizar K, Saisan PA, et al. Frontiers in optical imaging of cerebral blood flow and metabolism. J Cereb Blood Flow Metab. 2012;32:1259–1276. doi: 10.1038/jcbfm.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers ZB, Jain V, Englund EK, Langham MC, Wehrli FW. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J Cereb Blood Flow Metab. 2013;33:1514–1522. doi: 10.1038/jcbfm.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao LD, Lin CT, Shih YY, Duong TQ, Lai HY, Wang PH, et al. Transcranial imaging of functional cerebral hemodynamic changes in single blood vessels using in vivo photoacoustic microscopy. J Cereb Blood Flow Metab. 2012;32:938–951. doi: 10.1038/jcbfm.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M. Functional ultrasound imaging of the brain. Nat Methods. 2011;8:662–664. doi: 10.1038/nmeth.1641. [DOI] [PubMed] [Google Scholar]
- Liao LD, Tsytsarev V, Delgado-Martinez I, Li ML, Erzurumlu R, Vipin A, et al. Neurovascular coupling: in vivo optical techniques for functional brain imaging. Biomed Eng Online. 2013;12:38. doi: 10.1186/1475-925X-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MB, Chen BR, Burgess SA, Hillman EM. Ultra-fast multispectral optical imaging of cortical oxygenation, blood flow, and intracellular calcium dynamics. Optics Express. 2009;17:15670–15678. doi: 10.1364/OE.17.015670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razansky D, Distel M, Vinegoni C, Ma R, Perrimon N, Koster RW, et al. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nat Photonics. 2009;3:412–417. [Google Scholar]
- Yao J, Xia J, Maslov KI, Nasiriavanaki M, Tsytsarev V, Demchenko AV, et al. Noninvasive photoacoustic computed tomography of mouse brain metabolism in vivo. Neuroimage. 2013;64:257–266. doi: 10.1016/j.neuroimage.2012.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Wang B, Ji L, Jiang H. 4-D photoacoustic tomography. Sci Rep. 2013;3:1113. doi: 10.1038/srep01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deán-Ben XL, Razansky D. Adding fifth dimension to optoacoustic imaging: Volumetric time-resolved spectrally enriched tomography. Light Sci Appl. 2014;3:e137. [Google Scholar]
- Dean-Ben XL, Ozbek A, Razansky D. Volumetric real-time tracking of peripheral human vasculature with GPU-accelerated three-dimensional optoacoustic tomography. IEEE Trans Med imaging. 2013;32:2050–2055. doi: 10.1109/TMI.2013.2272079. [DOI] [PubMed] [Google Scholar]
- Laufer J, Zhang E, Raivich G, Beard P. Three-dimensional noninvasive imaging of the vasculature in the mouse brain using a high resolution photoacoustic scanner. Appl Opt. 2009;48:D299–D306. doi: 10.1364/ao.48.00d299. [DOI] [PubMed] [Google Scholar]
- Deán-Ben XL, Deliolanis NC, Ntziachristos V, Razansky D. Fast unmixing of multispectral optoacoustic data with vertex component analysis. Optics Lasers Eng. 2014;58:119–125. [Google Scholar]
- Bulte D, Chiarelli P, Wise R, Jezzard P. Measurement of cerebral blood volume in humans using hyperoxic MRI contrast. J Magn Reson Imaging. 2007;26:894–899. doi: 10.1002/jmri.21096. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu H, Wang LV. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat Photonics. 2011;5:154. doi: 10.1038/nphoton.2010.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.