Abstract
Stability of myogenic tone in middle cerebral arteries (MCA) is essential for adequate control over penetration of pressure waves into the distal portion of the cerebral microcirculation. Because the increased pulse pressure observed in advanced aging is associated with cerebromicrovascular injury, the effect of aging on myogenic response of mouse MCAs was determined. Aging did not affect the myogenic constriction in response to static increases in pressure, whereas it significantly impaired pulsatile pressure-induced myogenic tone. Impaired myogenic adaptation of MCAs to pulsatile pressure may allow high pressure to penetrate the distal portion of the cerebral microcirculation, contributing to microvascular damage.
Keywords: autoregulation, cerebral blood flow, pulse wave, Windkessel
Introduction
With advancing age the large elastic arteries (aorta and carotids) undergo significant stiffening, resulting in decreased Windkessel function and increased pulse pressure waves.1, 2 In elderly patients increased pulse pressure waves were shown to penetrate the cerebral microcirculation, likely contributing to white matter damage3, 4 and development of vascular cognitive impairment (VCI).5, 6, 7
In healthy young individuals the cerebral microcirculation is uniquely protected by the proximal resistance arteries of the circle of Willis and the pial arterial network, which contribute significantly to moment-to-moment regulation of cerebrovascular resistance.8 In addition to their well-characterized role in steady-state myogenic autoregulation of cerebral blood flow (CBF; reviewed in the study by Tan et al9), myogenic response of these arteries also plays a central role in dampening of pulse pressure waves, protecting the downstream microcirculation from pressure-induced injury (reviewed in the study by Vrselja et al10). Although increasing evidence from clinical4, 11 and experimental studies12, 13 suggest that aging may impair local vasoregulatory mechanisms, age-related alterations in myogenic adaptation of cerebral arteries to pulsatile pressure remain elusive.
The present study was designed to test the hypothesis that aging impairs myogenic adaptation of cerebral arteries to pulsatile pressure. To test our hypotheses, myogenic constriction in response to static and pulsatile intraluminal pressure was compared in middle cerebral arteries (MCAs) isolated from young and aged C57BL/6 mice.
Materials and Methods
Animals
Young (3 months old, n=8) and aged (24 months old, n=8) male C57BL/6 mice were purchased from the National Institute of Aging. All mice were maintained under specific pathogen-free barrier conditions. Water and normal laboratory diet were available ad libitum. All procedures were approved by the Institutional Animal Use and Care Committees of the participating institutions in accordance with the ARRIVE guidelines.
Pressure-Induced Constriction of Cerebral Arteries
Mice were killed and decapitated, and segments of the MCAs were isolated from the brains, as previously reported.13 Segments of MCAs were mounted onto two glass micropipettes in an organ chamber in oxygenated Krebs' buffer (pH=7.4). The hydrodynamic resistance of the cannulas was equal and the inflow and outflow pressures were controlled by a pressure servo-control system (Living Systems Instrumentation, Burlington, VT, USA). A custom-built pulsatile pressure generator device was used to generate sinusoidal pulsatile pressure with adjustable amplitude and frequency. The internal and external diameters of the pressurized MCAs was measured by videomicroscopy and continuously recorded using the automated edge detection function of the Ionoptix Microfluorimeter System (Ionoptix, Milton, MA, USA), as previously described.12 After an equilibrium period of 1 hour, changes in diameter to stepwise increases in static intraluminal pressure (from 20 to 140 mm Hg, in 20 mm Hg steps, 5 minutes each step) were assessed under no-flow conditions. Then, pulsatile pressure (pulse pressure frequency: 450 per minute, which corresponds to the lower end of the physiological range for heart rate in mice; pulse pressure amplitude: 40 mm Hg, which corresponds to the higher end of the physiological range for pulse pressure amplitude in mice) was initiated and changes in diameter over the range of 20 to 140 mm Hg mean pressure values were recorded. At the end of each experiment, maximal vasodilation was achieved by incubating the vessels with a Ca2+-free Krebs solution containing the L-type calcium channel inhibitor nifedipine (10−5 mol/L). Changes in MCA diameter in response to increases in static and pulsatile pressure were obtained.
Calculations
Myogenic tone (%) was calculated according to the following formula: ((DP−DA)/DP) x 100, where DP is passive diameter (obtained in the absence of Ca2+) and DA is active diameter of the vessels at a given intraluminal pressure value. Strain (ɛ) of the vessel wall was calculated according to the following formula: ɛ=(DP–D0)/D0), wherein DP is the passive lumen diameter at a given intraluminal pressure and D0 is the passive lumen diameter at 5 mm Hg. Stress (σ; dyn/cm2) was calculated according to the following formula: σ=P × DP/(2 × WT), where DP is passive lumen diameter, WT is wall thickness and P is intraluminal pressure (1 mm Hg=1,334 dyn/cm2). Stress–strain data for each artery was fitted to the curve σ=σ0 × e(β.ɛ) wherein σ0 is the stress at 5 mm Hg and β is the slope of the tangential elastic modulus versus stress relation. Thus, the β value is an index of distensibility, the higher the value of β the lower the arterial distensibility. Incremental distensibility (%/mm Hg) was calculated according to the following formula: [(D1−D0)/(D1 × PΔ)] × 100, where D0 is the passive diameter before the pressure step, D1 is the passive diameter after the pressure step and PΔ is the pressure step (20 mm Hg). Segmental vascular hydrodynamic resistance (R; GPa × s/m3) was calculated according to the following formula R=(8 × η × L)/(π × r4), where η is viscosity of the aqueous buffer at 37°C, L is the unit length of the vascular segment (1 mm) and r is the radius of the vascular segment (r=DA/2).
Quantitative Real-Time RT–PCR
A quantitative real time reverse transcription (RT–PCR) technique was used to analyze messenger RNA expression of genes that are involved in the regulation of pressure-induced myogenic constriction (Table 1) using a Strategen MX3000 platform (Agilent Tech., Santa Clara, CA, USA), as previously reported.12, 13 In brief, total RNA was isolated from MCAs with a Mini RNA Isolation Kit (Zymo Research, Orange, CA, USA) and was reverse transcribed using Superscript III RT (Invitrogen, Life Sciences, Grand Island, NY, USA). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Hprt, Ywhaz, B2m, and Actb were determined and a normalization factor was calculated based on the geometric mean for internal normalization.
Table 1. Age-related changes in mRNA expression of ion channels and intracellular factors involved in the mechanotransduction of pressure in middle cerebral arteries isolated from young (3 months old) and aged (24 months old) mice.
| Symbol | Name | Age-related change in mRNA expression (fold change) |
|---|---|---|
| Cacna1c | Voltage-gated (L-type) Ca2+ channel | 0.67±0.04* |
| Trpc6 | Transient receptor potential canonical channel-6 | NS12, 13 |
| Trpc3 | Transient receptor potential canonical channel-3 | NS12, 13 |
| Trpc7 | Transient receptor potential canonical channel-7 | NS12, 13 |
| Trpm4 | Transient receptor potential cation channel sub-family m-4 | NS12 |
| Kcnb1 | Voltage-gated K+ channel, shab-related sub-family | NS |
| Kcna2 | Voltage-gated K+ channel, shaker-related sub-family, member 25 | NS |
| Kcna5 | Voltage-gated K+ channel, shaker-related sub-family, member 5 | 0.63±0.1* |
| Kcnj8 | Inwardly-rectifying K+ channel, sub-family J, member 8 | NS |
| Kcnma1 | Large conductance Ca2+-activated K+ channel α-subunit | NS |
| Kcnmb1 | Large conductance Ca2+-activated K+ channel β-subunit | NS |
| Cyp4a12 | Cytochrome P450 4A12 ω-hydroxylase producing 20-HETE | NS13 |
| Sphk1 | Sphingosine kinase 1 | NS |
| Rock1 | Rho kinase | NS |
| Pla2g4 | Phospholipase A2 | NS |
Statistical Analysis
Data were analyzed by t-test or two-way analysis of variance for repeated measures followed by Bonferroni multiple comparison test, as appropriate. Statistical comparisons were performed by Prism 5.0 for Windows (Graphpad Software, La Jolla, CA, USA), and were considered significant at P<0.05. Data are expressed as mean±s.e.m.
Results
Aging Impairs Myogenic Constriction of MCAs to Pulsatile Pressure
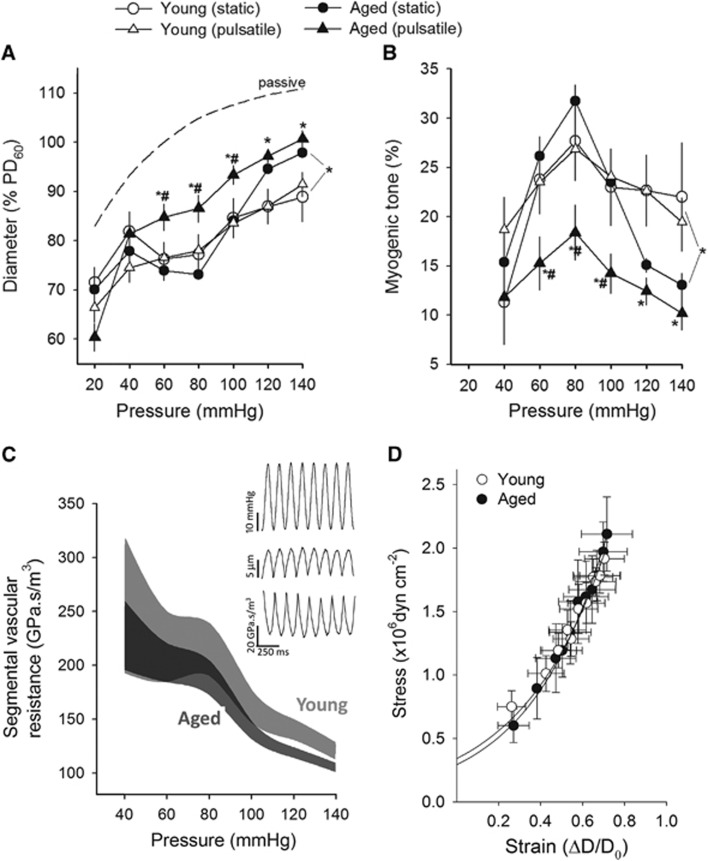
Figure 1A shows that myogenic constriction developed in isolated MCAs at static intraluminal pressures of 20–140 mm Hg. In MCAs of young control mice, increases in static intravascular pressure elicited significant myogenic constriction, and myogenic tone (Figure 1B) was maintained at almost the same level over the pressure range studied, which overlaps the autoregulatory range of CBF. In MCAs of aged control mice, increases in static intravascular pressure (up to ~100 mm Hg) elicited similar myogenic constriction as observed in young vessels. However, at higher pressures (140 mm Hg) myogenic tone tended to decrease and arteries dilate gradually (Figures 1A and 1B).
Figure 1.
Aging impairs myogenic adaptation to pulsatile pressure in mouse middle cerebral arteries (MCAs). (A): Steady-state changes in diameter of MCAs isolated from young and aged mice in response to stepwise increases in intraluminal pressure, developed in the absence (‘static') and presence of pressure pulsatility (‘pulsatile' pulse pressure amplitude: 40 mm Hg, frequency: 450 per min; mean pressure values are plotted). Note that the presence of pressure pulsatility significantly decreases myogenic constriction of MCAs of aged mice. Vascular diameters are expressed as percentage of the of maximally dilated passive diameter of each vessel at 60 mm Hg. Data are represented as mean ±s.e.m. (n=8; *P<0.05 versus young pulsatile, #P<0.05 versus aged static). (B) Effect of pulsatile pressure on the myogenic tone of MCAs of young and aged mice. Data are represented as mean ±s.e.m. (n=8; *P<0.05 versus young pulsatile, #P<0.05 versus aged static). (C) Calculated segmental vascular hydrodynamic resistance of young (light gray) and aged (dark gray) MCAs exposed to pulsatile pressure, plotted as a function of mean intraluminal pressure. Inset: original traces showing simultaneous recordings of the pulsatile pressure signal and changes in the diameter and calculated segmental hydrodynamic resistance of a cannulated MCA. (D) There were no significant age-related differences in the stress–strain relationship of the MCAs. Data are represented as mean±s.e.m. (n=8 in each group).
In MCAs from young mice, pulsatile pressure induced similar myogenic constriction to that induced by static pressure (Figure 1A) and the myogenic tone was maintained at the same level up to ~140 mm Hg (Figure 1B). In contrast, MCAs of aged mice exposed to pulsatile pressure developed a significantly decreased myogenic constriction (Figure 1A). The pressure-passive diameter curves were similar in MCAs from each group. We found that pulsatile pressure lead to slight pulsatile changes in the diameter of myogenically active MCAs (Figure 1C). These small changes in the vascular diameter translate to significant changes in the calculated segmental hydrodynamic resistance (being inversely proportional to the radius to the fourth power (r4)). Figure 1C depicts the range of calculated segmental vascular hydrodynamic resistance in isolated MCAs from young and aged mice exposed to pulsatile pressure as a function of mean intraluminal pressure. Note that decreased myogenic constriction in response to pulsatile pressure results in a downward shift in the pressure-segmental vascular hydrodynamic resistance curve in aged arteries.
Passive Characteristics of the MCAs
In addition to the active pressure-induced vasoconstriction, passive mechanical characteristics of the vascular wall may also affect the ability of MCAs to protect the microcirculation. For example, changes in the distensibility of the vascular wall would alter the characteristics of the pressure wave that reaches the microcirculation.10 However, in the present study no significant age-related differences in the wall stress/strain relationship (Figure 1D, β=2.2±0.2 and 2.4±0.2 for young and aged, respectively, not significant ) and incremental distensibility (not shown) of mouse MCAs were observed.
Age-Related Changes in Vascular Gene Expression
Aging was associated with downregulated cerebrovascular expression of voltage-gated L-type Ca2+ channels, whereas it did not affect significantly the messenger RNA expression of other ion channels and intracellular factors involved in the mechanotransduction of pressure in MCAs (Table 1).
Discussion
Increased microvascular injury is thought to play a central role in the pathogenesis of vascular cognitive impairment in older individuals.5 It is thought that mechanisms that lead to penetration of high pressure in the vulnerable distal portion of the cerebral microcirculation significantly increase the risk for vascular cognitive impairment.
Here, we show for the first time that aging is associated with impaired myogenic adaptation of isolated MCAs to pulsatile pressure. If aged MCAs in vivo also exhibit similarly decreased myogenic constriction in response to physiologic pulsatile pressure, it is likely associated with significant decline in vascular hydrodynamic resistance in the proximal larger resistance arteries. Together, this may impose greater burden on the distal portion of the cerebral microcirculation. Importantly, a recent study demonstrated that increased pulse pressure in elderly individuals lead to increased pulsatility of CBF.3 This observation is compatible with the view that the protection of the aging microcirculation against increases in pulsatile pressure is impaired. Recent evidence suggest that a strong relationship exists between impaired cerebrovascular hemodynamics assessed by transcranial Doppler ultrasound and loss of cerebral white matter structural integrity in elderly individuals.4 Analysis of the pulsatility index and dynamic cerebral autoregulation in these patients further support the view that aging may impair local vasoregulatory mechanisms intrinsic to the vascular wall of cerebral arteries leading to impaired protection against pulse waves.4 We posit that age-related increases in pulse pressure1 combined with impaired myogenic protection may contribute significantly to increased propensity of the aged brain for pressure-induced microvascular injury, including disruption of the blood–brain barrier, microvascular rarefaction,13 and the development of spontaneous intracerebral hemorrhages.14 The molecular mechanisms responsible for impaired myogenic adaptation to pulsatile pressure in aged MCAs, including the possible role of dysregulation of L-type Ca2+ channels, remain unknown and should be elucidated in future studies.
Limitations of the Study
At present it remains unknown how the myogenic adaptation of young and aged arteries differ at different combinations of physiologically relevant pulse pressure frequencies and amplitudes. Also, in theory, small changes in shear force acting on the endothelial layer can happen during application of pulsatile pressure to the cannulated arteries even under no-flow conditions, because of cyclical changes in the intraluminal volume. Although these changes are likely small, we cannot exclude the possibility that they contribute to the mechanosensitive release of vasoactive mediators of the endothelial cells and thereby to the regulation of vascular tone.
Collectively, on the basis of our findings we recommend that experimental studies on myogenic mechanisms in MCAs should always consider the effect of pressure pulsatility.15 Furthermore, our findings that pulsatile pressure-induced relatively small changes in MCA diameter may be associated with significant changes in segmental vascular resistance have important implications for the design of clinical studies, emphasizing the importance of measuring blood flow velocity in larger cerebral arteries simultaneously with the vascular diameter.
The authors declare no conflict of interest.
Footnotes
This work was supported by the American Heart Association (to PT, ST, ZT, AC, and ZU), the American Federation for Aging Research (to AC), the Run to Remember Award (to NMA), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, and WES), the Hungarian National Science Research Fund (OTKA) K 108444 and grants: Developing Competitiveness of Universities in the South Transdanubian Region, ‘Identification of new biomarkers..', SROP-4.2.2.A-11/1/KONV-2012–0017, and ‘Complex examination of neuropeptide ..'SROP-4.2.2.A-11/1/KONV-2012-0024" to AK, the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU); the National Institute on Aging (AG031085 to AC; AG038747 to WES), the Arkansas Claude Pepper Older Americans Independence Center at the University of Arkansas Medical Center (to AC), and the Ellison Medical Foundation (to WES).
References
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Tarumi T, Ayaz Khan M, Liu J, Tseng BM, Parker R, Riley J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34:971–978. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, et al. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab. 2014;34:228–234. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Tan CO, Hamner JW, Taylor JA. The role of myogenic mechanisms in human cerebrovascular regulation. J Physiol. 2013;591:5095–5105. doi: 10.1113/jphysiol.2013.259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrselja Z, Brkic H, Mrdenovic S, Radic R, Curic G. Function of circle of Willis. J Cereb Blood Flow Metab. 2014;34:578–584. doi: 10.1038/jcbfm.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani S, Bacci M, Ungar A, Prati P, Di Serio C, Geppetti P, et al. Abnormal pressure passive dilatation of cerebral arterioles in the elderly with isolated systolic hypertension. Hypertension. 2006;48:1143–1150. doi: 10.1161/01.HYP.0000248533.58693.c4. [DOI] [PubMed] [Google Scholar]
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, et al. Role of 20-HETE,TRP channels & BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection Aging Cell 2015(in press). [DOI] [PMC free article] [PubMed]
- van de Vosse FN, Stergiopulos N. Pulsewave propagation in the arterial tree. Annu Rev Fluid Mech. 2011;43:467–499. [Google Scholar]