Abstract
Adipose tissue fatty acid storage varies according to sex, adipose tissue depot, and degree of fat gain. However, the mechanism(s) for these variations is not completely understood. We examined whether differences in adipose tissue glycerol-3-phosphate acyltransferase (GPAT) might play a role in these variations. We optimized an enzyme activity assay for total GPAT and GPAT1 activity in human adipose tissue and measured GPAT activity. Omental and subcutaneous adipose tissue was collected from obese and nonobese adults for measures of GPAT and GPAT1 activities, ex vivo palmitate storage, acyl-CoA synthetase (ACS) and diacylglycerol-acyltransferase (DGAT) activities, and CD36 protein. Total GPAT and GPAT1 activities decreased as a function of adipocyte size in both omental (r = −0.71, P = 0.003) and subcutaneous (r = −0.58, P = 0.04) fat. The relative contribution of GPAT1 to total GPAT activity increased as a function of adipocyte size, accounting for up to 60% of GPAT activity in those with the largest adipocytes. We found strong, positive correlations between ACS, GPAT, and DGAT activities for both sexes and depots (r values 0.58–0.91) and between these storage factors and palmitate storage rates into TAG (r values 0.55–0.90). We conclude that: 1) total GPAT activity decreases as a function of adipocyte size; 2) GPAT1 can account for over half of adipose GPAT activity in hypertrophic obesity; and 3) ACS, GPAT, and DGAT are coordinately regulated.
Keywords: omental fat, subcutaneous fat, fat distribution, glycerol-3-phosphate acyltransferase 1
body fat distribution plays an important role in the development of the comorbidities associated with obesity. In general, adults with greater amounts of visceral fat have more risk for chronic disease than those with a lesser proportion of visceral fat (14). Although regional balances of fatty acids (uptake vs. release) should determine whether one depot expands at the expense of another, interindividual differences in regional lipolysis (8, 13, 24) and meal fat storage (21, 26, 31) do not appear to explain differences in body fat distribution. We found that direct free fatty acid (FFA) storage rates in subcutaneous fat are greater in women than men and that the sex-specific variations in direct FFA storage into adipocyte triacylglycerol (TAG) are consistent with body fat distribution patterns (16, 18, 28). On average, the protein content (CD36 and fatty acid transport protein 1) and enzyme activity [acyl-CoA synthetase (ACS) and diacylglycerol-acyltransferase (DGAT)] are greater in omental than subcutaneous adipose tissue depots (1). We previously observed that femoral, but not abdominal, subcutaneous adipose tissue ACS and DGAT activities are greater in women than men (16). Because our findings do not completely explain the sex differences in regional fatty acid storage rates (1, 10, 11, 18), we examined a previously unexplored step in the human adipocyte TAG synthesis pathway, glycerol-3-phosphate acyltransferase (GPAT).
TAG synthesis is achieved through two major pathways: the glycerol phosphate pathway that is employed by most cells (29) and the monoacylglycerol pathway. GPAT enzymes are responsible for acylation of glycerol 3-phosphate, the first committed step in TAG synthesis through the glycerol phosphate pathway (29). There are four mammalian isoforms of GPAT: GPAT1-4.GPAT1 and GPAT2 are found in the outer mitochondrial membrane while GPAT3 and GPAT4 are found in the endoplasmic reticulum. GPAT1 is the only N-ethylmaleimide (NEM)-insensitive isoform, and thus adding NEM to the assay is used to measure GPAT1 activity. GPAT1 is prevalent in liver (30–50% total activity) and reportedly less so in adipose tissue (∼10% of total activity) and is the only GPAT isoform regulated in an insulin-dependent manner via sterol regulatory element-binding protein-1 (SREBP-1) (29, 32).
The trafficking of fatty acids to TAG in adipose tissue is the most benign form of fatty acid storage; there are major sex and depot differences in fatty acid storage rates (1, 18, 28). Fatty acids enter adipocytes via both passive diffusion (flip-flop) and facilitated transport. When plasma FFA concentrations are high/high normal they enter most tissues rapidly (10), probably independent of proteins such as CD36 that facilitate transport through the plasma membrane (12). However, when FFA concentrations are low, CD36 enhances tissue FFA uptake (10). Once inside the cell, fatty acids are activated/trapped via the action of ACS (15). The next step in the triglyceride synthesis pathway is conversion to lysophosphatidic acid via the action of GPAT (6, 20, 29, 32), followed by a series of steps culminating in the conversion of diacylglycerol (DG) to TAG via the action of DGAT (22).
We have reported that adipocyte CD36 content (16, 18), ACS (27), and DGAT activity (18, 27) correlate with rates of FFA storage in subcutaneous fat in some depots under some conditions. However, because plasma FFA concentrations are even better predictors of the direct FFA storage rates (16, 18), and because plasma FFA concentrations can differ dramatically between individuals, teasing out whether any of these fatty acid storage factors is truly rate-limiting is challenging. To overcome this problem, we studied adipose tissue from a variety of volunteers incubated at high and low palmitate concentrations with the goal of determining if different fatty acid storage factors could play predominant roles in regulating FFA storage under different concentration conditions. We also aimed to determine if GPAT enzyme activity varies by depot, by sex, and by adipocyte size in a manner that might account for some of the variations we have observed in adipose tissue fatty acid storage rates. We developed a reliable GPAT enzyme activity assay for human adipose tissue and tested the hypothesis that GPAT activity in omental fat would differ from abdominal subcutaneous fat and that men would differ from women.
MATERIALS AND METHODS
Preparation of whole tissue extract and total pellet samples.
Samples from each participant were run as both a whole tissue extract (WTE) and total mitochondrial/microsomal pellet sample. For the WTE preparation ∼250 mg of flash-frozen adipose tissue were placed in 4 μl/mg standard homogenization buffer (20 mM Tris·HCl, pH 7.4, 1 mM EDTA, and 255 mM sucrose) with antiprotease tablets (Roche, Indianapolis, IN) and homogenized using 1.4-mm zirconium oxide beads in an Omni Bead Ruptor (Omni, Kennesaw, GA). Following homogenization, samples were centrifuged at 600 g for 10 min at 4°C, and the supernatant was collected and used for the GPAT assay. In addition, the fat cake was collected, and the lipid content was extracted using a 2:1 chloroform-methanol solution, dried, and weighed. The information regarding tissue weight, lipid weight, and protein content was used to express enzyme activity data in different formats as needed. To obtain the total mitochondrial/microsomal pellet sample ∼500 mg of flash-frozen adipose tissue were homogenized, and the fat cake was collected as described above. Following homogenization, the samples were centrifuged at 37,500 revolutions/min for 1 h at 4°C, and pellets were resuspended in standard homogenization buffer (4 μl/mg) for use in the GPAT assay.
GPAT assay.
All samples were prepared in duplicate: with or without 20 mM NEM (Thermo Scientific, Rockford, IL). Each assay was performed on samples that had only been frozen and thawed one time. One hundred microliters from a total pellet or 200 μl from WTE (isolation described above) were added to the assay buffer made up of reaction mixture (75 mM Tris, 1 mg/ml BSA, 4 mM MgCl2, 8 mM NaF, 1 mM DTT, and 60 μM palmitoyl-CoA) and 1 mM or 1,000 μCi/mM [14C]glycerol 3-phosphate (PerkinElmer, Boston, MA). The samples were incubated in the assay buffer for 25 min at 37°C; the reaction was stopped by adding a 1:2 chloroform-methanol mixture with 1% perchloric acid and placing the samples on ice for 5 min. After 5 min an additional 1 ml of chloroform and 1 ml of 1% percholoric acid were added, and the samples were vortexed. The samples were then centrifuged for 5 min, and the upper methanolic phase was removed and discarded. The remaining phase was washed three times with 1% percholoric acid, with the upper layer phase removed following each wash. Samples were then dried using an air evaporator system (Organomation, Berlin, MA), and the 14C-labeled lysophosphatidic acid was measured as disintegrations per minute using a multipurpose scintillation counter (Beckman Coulter, Pasadena, CA). GPAT activity was then calculated as picomole of lysophosphatidic acid (14C-labeled product) synthesized. Because both [14C]lysophosphatidic acid and [14C]phosphatidic acid (PA) can be present in the organic phase, the radioactivity in the organic phase may reflect GPAT and, to an unknown extent, AGPAT activity. However, because 14C-labeled product is the obligate precursor of PA, the assay results are an accurate reflection of GPAT activity. Briefly, the protein concentration, volume of buffer used for the homogenization of tissue, and the disintegrations per minute were used to calculate picomole 14C-labeled product per milligram tissue or milligram lipid per minute as follows:
where x (dpm) is the average disintegrations per minute of one sample counted minus the disintegrations per minute of the blank, y is the disintegrations per microcurie isotope, z (μCi/mmol) is millimole glycerol 3-phosphate used, and 1,0002 is the conversion factor.
To calculate the activity rate in picomole 14C-labeled product per milligram lipid per minute, the activity rate per milligram tissue was divided by the lipid content of the tissue as measured by the lipid extraction. The inter- and intra-assay coefficients of variation (CV) were <10%.
Participants.
The study was approved by the institutional review board of the Mayo Clinic. Written, informed consent was obtained from all study participants. Adipose tissue samples (1–4 grams) were collected by excision from 32 participants undergoing elective abdominal surgery at Mayo Clinic. None of participants had acute illnesses, and the intravenous fluids they received during surgery consisted of 0.9% NaCl. We excluded patients taking medications that could possibly affect adiposity or lipid metabolism. Likewise, we did not include patients with intra-abdominal inflammatory conditions or advanced malignancies.
Measurement of adipocyte size.
The size of adipocytes was measured in total adipose tissue using digital photomicrographs and an automated software program as previously described (30). An average of 300 cells/sample was measured to calculate the average adipocyte size.
Measurement of direct FFA storage.
Adipose tissue from both subcutaneous and omental depots was incubated in high-palmitate media [0.5 mM [3H]palmitate (NET-043; PerkinElmer), 10 mM glucose, 4% BSA, final specific activity = 0.5 μCi/μmol] and low-palmitate media (0.05 mM [3H]palmitate, 10 mM glucose, 4% BSA, final specific activity = 5.0 μCi/μmol). [14C]mannitol (NEC-314; PerkinElmer) was added to both the high- and low-palmitate media as an extracellular marker. We conducted a series of pilot studies to determine the duration of incubation resulting in readily detectable [3H]palmitate in adipose lipids while remaining within the time frame of linear accumulation of tracer. The optimal protocol was to incubate 50–80 mg of adipose tissue in the respective media for 1 h at 37°C. Following incubation, lipid was extracted from the adipose tissue, and the incubation media was placed in a multipurpose scintillation counter (Beckman Coulter) for counts of 3H- and 14C-labeled incubation medium and to calculate the ratio of 3H/14C. The 14C was used to account for any contamination of the sample with extracellular [3H]palmitate.
Measurement of lipid fractions.
Fractionation of lipids was performed using conditioned Supleclean LC-NH2 tubes (Supelco, St. Louis, MO) per the manufacturer's instructions. Briefly, the lipid extraction was diluted in chloroform and passed through a conditioned LC-NH2 tube no. 1. The fatty acids were then eluted utilizing 2% acetic acid in diethyl ether, and neutral lipids were eluted from 2:1 chloroform-isopropanol. Next, reconstituted samples were placed in a LC-NH2 tube no. 2, and triglycerides were eluted with hexane containing 1% diethyl ether and 10% methylene chloride. Last, 15% ethyl acetate in hexane and 2:1 chloroform-methanol were used to elute diglycerides (DG) and monoglycerides, respectively, from LC-NH2 tube no. 2. All of the lipid fractions were collected, dried, and placed in the scintillation counter for counts of [3H]palmitic acid and [14C]mannitol.
ACS assay.
The conversion of [3H]palmitate to its CoA derivative was measured using the method described by Hall et al. (9). The inter- and intra-assay CV is <10%.
DGAT assay.
We used the method from Coleman (5), which was modified slightly to use the cytosol fraction of the adipose tissue homogenate. To isolate the cytosolic fraction from the tissue homogenate, the samples were centrifuged at 37,500 revolutions/min for 1 h at 4°C, and the supernatant was collected. In addition, we used 20 μl of 10.0 mmol/l (rather than 2.0 mmol/l) 1,2-dioleoyl-sn-glycerol (Sigma D-0138 FW:621; Sigma-Aldrich, St. Louis, MO) in the reaction mixture.
CD36.
A sandwich enzyme-linked immunosorbent assay was used to measure the CD36 content of adipose tissue as previously described (2).
Calculations and statistics.
Descriptive statistics were performed using a one-way ANOVA with a Tukey posttest to compare the participant characteristics among groups. A Wilcoxon-Rank-Sum test with a two-tailed P value was used to determine the difference in GPAT activity among groups. To define the relationship between GPAT activity and adipocyte size, univariate regression analyses were performed. All statistical analyses were performed using JMP software (Cary, NC). Univariate regression analyses were used to test for correlations between palmitate storage and fatty acid storage factors. Univariate regression analyses were also used to test for correlations between the fatty acid storage factors. Multiple linear regression analysis was used to assess independent predictors of regional palmitate storage rates within a depot and sex. All data are reported as means ± SD or SE. Treatment groups with the same letter are not significantly different from each other.
RESULTS
Protein and substrate concentrations.
To determine the optimal range of sample to be assayed, we measured GPAT enzyme activity using a number of different samples with ranges of protein content from 5 to 80 μg. GPAT activity was plotted as picomole 14C-labeled product per milligram tissue per minute vs. tissue protein amount. The GPAT activity was constant between 20 to 35 μg of protein (data not shown). We therefore selected ∼25 μg of protein for each experimental sample to achieve activity rates within the linear range.
We also examined palmitoyl-CoA concentrations ranging from 20 to 160 μM to determine the optimal amount of substrate for human adipose GPAT enzyme activity. Between 40 and 80 μM of palmitoyl-CoA GPAT activity was constant and maximal (pmol 14C-labeled product·mg tissue−1·min−1), whereas palmitoyl-CoA concentrations below 40 and above 80 μM yielded lesser 14C-labeled product generation rates, similar to previously reported results (4). Therefore, 60 μM of palmitoyl-CoA was used for the experiments discussed in the current manuscript.
Adipose WTE yields greater GPAT activity than an adipose tissue pellet.
Because we previously found that DGAT enzyme activity was not different between adipocyte mitochondrial/microsomal pellet fraction and adipose WTE (11), and because of the simpler preparation of a WTE sample, we tested whether there are differences in GPAT activity between these two sample preparation approaches. In contrast with our findings for DGAT activity, GPAT activity per milligram tissue was significantly greater when the assay was performed using WTE than when back-calculated using the mitochondrial/microsomal pellet from the same samples. Total omental GPAT activity was approximately threefold greater and GPAT1 activity was approximately twofold greater using WTE than using the pellet results (P < 0.05). Likewise, total subcutaneous GPAT and GPAT1 activity calculated using WTE were ∼2.5- and 3-fold greater, respectively, than when using the mitochondrial/microsomal pellet (P < 0.05). We observed greater GPAT activity in WTE samples similar to previous investigators (4, 25). Because WTE had consistently, significantly greater (P < 0.05) total GPAT and GPAT1 activity than the mitochondrial/microsomal pellet in both adipose tissue depots, we elected to present GPAT activities using the WTE preparation results.
We assessed and controlled for intra- and interassay variation by creating a quality control (QC) sample that was included with every assay. To accomplish this we obtained a large sample of waste adipose tissue from a patient undergoing surgery and homogenized the entire sample. The homogenized tissue was divided into 250-mg aliquots and frozen at −70°C until used. In addition to performing two complete assay runs with only the QC sample, two QC samples were included with each subsequent assay. We were able to achieve a CV of 2.7% for the intra-assay and a CV of 6.4% for the interassay CV.
Participant characteristics.
Participant characteristics are outlined in Table 1. The study cohort consisted of 32 participants, 17 males and 15 females. Males and females were well matched for age and, within groups, by body mass index (BMI). As anticipated, the average adipocyte size was greater for obese than nonobese participants (P < 0.0001).
Table 1.
Participant characteristics
| Male (n = 17) |
Female (n = 15) |
||||
|---|---|---|---|---|---|
| Nonobese (n = 10) |
Obese (n = 7) |
Nonobese (n = 8) |
Obese (n = 7) |
P Value | |
| Age, yr | 54 ± 17 | 46 ± 6 | 39 ± 14 | 54 ± 6 | 0.45 |
| BMI, kg/m2 | 25.1 ± 3.4 | 44.1 ± 7.1 | 22.2 ± 3.9 | 39.6 ± 4.3 | 0.41 |
| Omental adipocyte size, μg lipid/cell | 0.55 ± 0.39* | 1.11 ± 0.38 | 0.24 ± 0.3* | 0.84 ± 0.37 | 0.11 |
| Subcutaneous adipocyte size, μg lipid/cell | 0.66 ± 0.35* | 1.05 ± 0.19 | 0.39 ± 0.34* | 1.16 ± 0.42 | 0.5 |
Data are shown as means ± SD; n, no. of subjects. BMI, body mass index. Participant characteristics of all four study groups: two male groups (nonobese and obese) and two female groups (nonobese and obese). P value represents the difference between male and female groups (nonobese and obese combined).
P < 0.0001 vs. same depot in the obese group.
Total GPAT and GPAT1 activity variations by adipose tissue depot.
Both total GPAT and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) was ∼40% greater in omental tissue than subcutaneous abdominal tissue (P < 0.01, Table 2). However, the proportion of GPAT activity accounted for by GPAT1 was not significantly different between the two depots (Table 2). When data are expressed as picomole 14C-labeled product per milligram protein per minute, total omental GPAT activity was greater than subcutaneous adipose tissue GPAT activity (P < 0.01, Table 2). The GPAT1 activity was not significantly different between depots (P = 0.08).
Table 2.
Total GPAT and GPAT1 activity variations by adipose tissue depot and sex
| Male Nonobese (n = 10) |
Male Obese (n = 7) |
Female Nonobese (n = 8) |
Female Obese (n = 7) |
P Value Between Sexes | |
|---|---|---|---|---|---|
| Omental: total GPAT, pmol 14C-labeled product·mg lipid−1·min−1 | 12.1 ± 7.4 | 4.2 ± 1.3 | 23.5 ± 14.9 | 6.14 ± 4.0 | 0.121 |
| Omental: GPAT1 (pmol 14C-labeled product·mg lipid−1 ·min−1 | 2.8 ± 1.1 | 1.1 ± 0.6 | 4.1 ± 3.2 | 1.9 ± 1.1 | 0.169 |
| Omental GPAT1/total | 0.33 ± 0.19 | 0.27 ± 0.09 | 0.20 ± 0.10 | 0.31 ± 0.14 | 0.471 |
| SQ: total GPAT, pmol 14C-labeled product·mg lipid−1 ·min−1 | 5.3 ± 4.2 | 0.87 ± 0.55 | 12.3 ± 5.4 | 2.8 ± 092 | 0.028 |
| SQ: GPAT1, pmol 14C-labeled product·mg lipid−1 ·min−1 | 1.3 ± 0.78 | 0.28 ± 0.19 | 2.1 ± 1.6 | 0.96 ± 0.41 | 0.028 |
| SQ GPAT1/total | 0.27 ± 0.05 | 0.33 ± 0.10 | 0.23 ± 0.14 | 0.34 ± 0.10 | 0.644 |
| Omental: total GPAT, pmol 14C-labeled product·mg protein−1 ·min−1 | 508 ± 262 | 464 ± 144 | 776 ± 240 | 526 ± 128 | 0.041 |
| Omental: GPAT1, pmol 14C-labeled product·mg protein−1 ·min−1 | 151 ± 80 | 122 ± 48 | 146 ± 74 | 170 ± 108 | 0.478 |
| SQ: total GPAT, pmol 14C-labeled product·mg protein−1 ·min−1 | 451 ± 306 | 190 ± 139 | 581 ± 99 | 341 ± 89 | 0.075 |
| SQ: GPAT1, pmol 14C-labeled product·mg protein−1 ·min−1 | 110 ± 59 | 63 ± 48 | 134 ± 94 | 135 ± 86 | 0.087 |
Values represent means ± SD; n, no. of subjects. Whole tissue extract glycerol-3-phosphate acyltransferase (GPAT) activity normalized to mg lipid or mg protein. SQ, abdominal subcutaneous. P value is the difference between male and female participants.
Sex differences in GPAT and GPAT1 activity.
Total GPAT and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) as well as the proportion of GPAT1 for omental tissue were not significantly different between males and females (Table 2). Abdominal subcutaneous tissue from females had ∼35% greater total GPAT activity than tissue from males (P = 0.03). In addition, abdominal subcutaneous adipose tissue GPAT1 activity was ∼45% greater in samples from females compared with males (P = 0.03). There was not a significant sex difference in the proportion of total GPAT activity accounted for by GPAT1 activity (Table 2). When expressed as picomole 14C-labeled product per milligram protein per minute, total omental GPAT activity was significantly greater in females than males (P = 0.04, Table 2). However, there was no difference in omental GPAT1 activity or subcutaneous GPAT activity between the sexes.
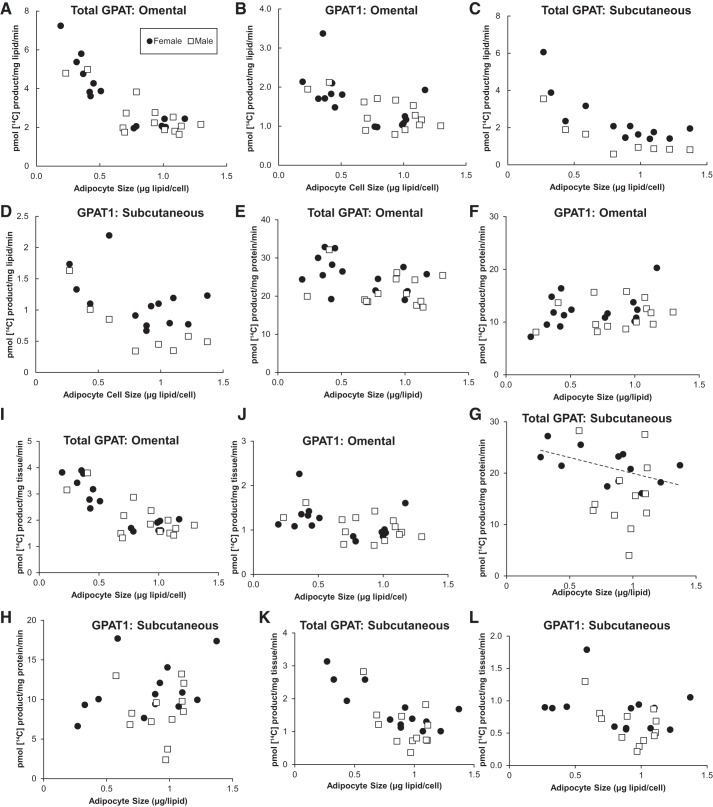
GPAT activity and adipocyte size.
Total omental GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) was negatively correlated with adipocyte size for both females (r = −0.81, P = 0.0003) and males (r = −0.69, P = 0.006) (Fig. 1A). Omental GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) was negatively correlated with adipocyte size for both males (r = −0.57, P = 0.04) and females (r = −0.54, P = 0.04) (Fig. 1B).
Fig. 1.
Omental glycerol-3-phosphate acyltransferase (GPAT) activity. Relationship between GPAT activity and adipocyte size in omental and subcutaneous adipose tissue samples. LPA, lysophosphatidic acid. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution; n = 28 subjects. A: omental total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1). B: omental GPAT1 activity (pmol/14C-labeled product·mg lipid−1·min−1). C: subcutaneous total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1). D: subcutaneous GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1). E: omental total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1). F: omental GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1). G: subcutaneous total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1). H: subcutaneous GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1). I: omental total GPAT activity (pmol 14C-labeled product·mg tissue−1·min−1). J: omental GPAT1 activity (pmol 14C-labeled product·mg tissue−1·min−1). K: subcutaneous total GPAT activity (pmol 14C-labeled product·mg tissue−1·min−1). J: subcutaneous GPAT1 activity (pmol 14C-labeled product·mg tissue−1·min−1).
For subcutaneous adipose tissue samples, total GPAT activity was negatively correlated with adipocyte size for both males (r = −0.76, P = 0.04) and females (r = −0.73, P = 0.005) (Fig. 1C). Subcutaneous adipose tissue GPAT1 activity was negatively correlated with adipocyte size for males (r = −0.79, P = 0.002) and females (r = −0.85, P = 0.003) (Fig. 1D).
With the exception of total GPAT in subcutaneous adipose tissue collected from females, there was no correlation between GPAT activity and adipocyte size when expressed as picomole 14C-labeled product per milligram protein per minute (Fig. 1, E–H).
When expressed as picomole 14C-labeled product per milligram tissue per minute, there was a negative correlation between total GPAT activity and adipocyte size for both the omental and subcutaneous adipose tissue depots for both sexes (r = −0.057 to −0.86) (Fig. 1, I and K). For the omental adipose tissue, there was a statistically significant negative correlation between GPAT1 activity (pmol 14C-labeled product·mg tissue−1·min−1) and adipocyte size for females (r = −0.36, P = 0.02), whereas the relationship in males did not reach statistical significance (r = −0.48, P = 0.09) (Fig. 1J). For subcutaneous adipose tissue samples, there was a negative correlation between GPAT1 activity (pmol 14C-labeled product·mg tissue−1·min−1) and adipocyte size for males (r = −0.57, P = 0.006), but not females (r = −0.27, P = 0.45) (Fig. 1L).
Proportion of GPAT1 activity.
While we observed a decrease in GPAT1 activity as a function of adipocyte size, the decrease in the other GPAT isoforms was even greater, leading to an increase in the proportion of GPAT1 as a function of adipocyte size. For the omental depot, the smallest 10% of adipocytes had GPAT1 accounting for an average of 12% (SD = 4%) of total GPAT activity. On the contrary, the largest 10% of adipocytes had GPAT1 account for an average of 51% (SD = 11%) of total GPAT activity. We found similar results for the subcutaneous depot: the smallest 10% of adipocytes had GPAT1 that accounted for an average of 14% (SD = 7%) of total GPAT activity and the largest 10% of adipocytes had GPAT1 account for an average of 42% (SD = 7%) of total GPAT activity. This relationship between adipocyte size and percentage of GPAT1 activity was continuous; the correlation between these two variables was significant for the data from women for omental (r = 0.62, P = 0.01) and abdominal subcutaneous (r = 0.67, P = 0.008) depots. Although the relationship was not statistically significant for the data from men, multivariate regression analysis that included sex as a variable suggested no significant effect of sex on the relationship of GPAT1 proportion and adipocyte size (P = 0.38).
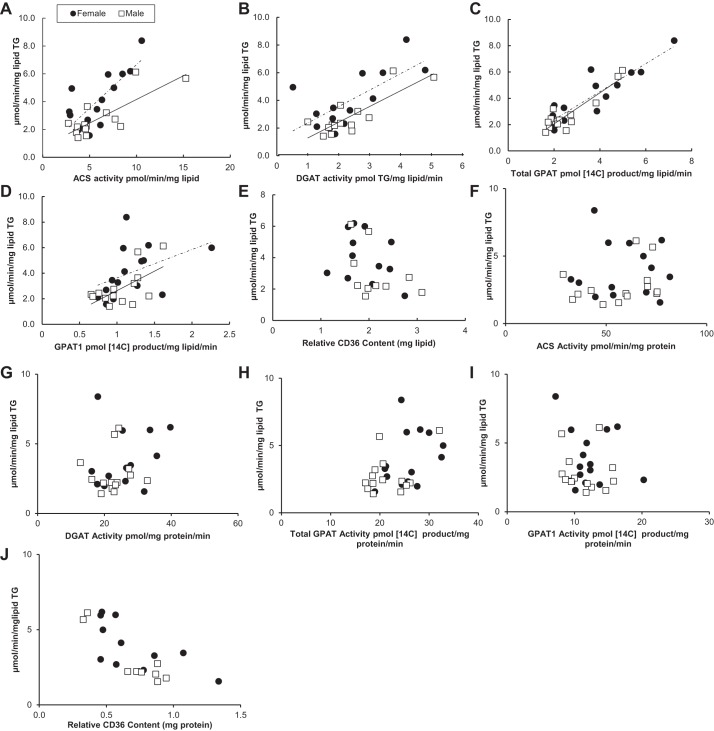
Relationship between fatty acid storage factors and storage rates into TAG at high palmitate concentrations.
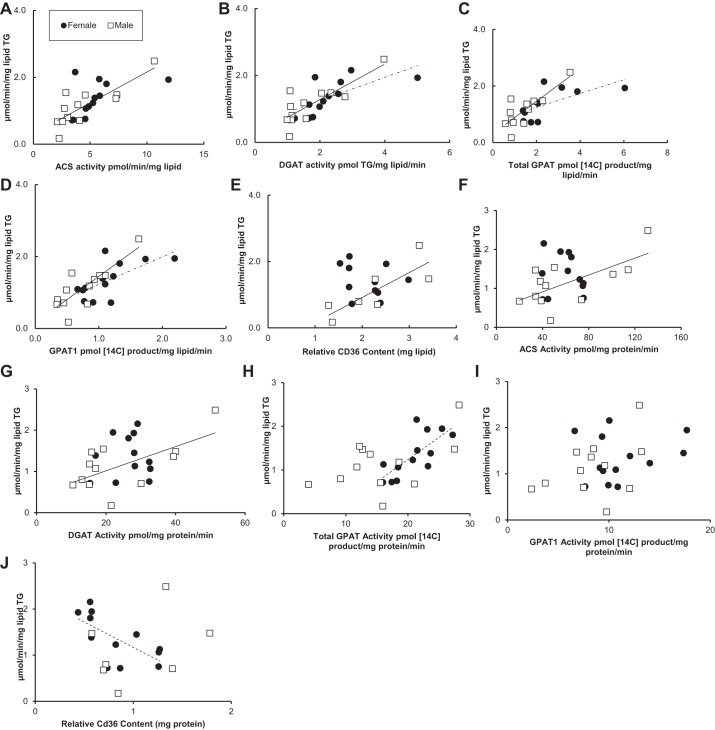
The relationship between palmitate storage rates into TAG and omental adipose tissue fatty acid storage factors at high palmitate concentrations is shown in Fig. 2. Palmitate storage rates in omental fat from both sexes correlated positively with omental ACS, DGAT, total GPAT, and GPAT1 activity (r values 0.67–0.90) (Fig. 2, A–D). Palmitate storage rates into adipocyte TAG were significantly greater in women than men relative to ACS (P = 0.009) or DGAT activity (P = 0.03). In contrast, there was no significant sex difference in the relationship between palmitate storage rates into TAG and omental adipose tissue total GPAT or GPAT1 activity. We found no relationship between the relative CD36 content (per mg lipid) and palmitate storage rates into TAG for omental adipose tissue obtained from either females (r = −0.31, P = 0.32) or males (r = −0.43, P = 0.22) (Fig. 2E). There was no relationship between the fatty acid storage factors and storage rates into TAG when the fatty acid storage factor data were expressed as milligram protein per minute (Fig. 2, F–J).
Fig. 2.
Relationship between omental fatty acid storage factors and storage rates at high palmitate concentrations. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between acyl-CoA synthetase (ACS) activity (pmol·min−1·mg lipid−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.77, P = 0.0007) and for men (r = 0.80, P = 0.0006); n = 28. B: relationship between diacylglycerol-acyltransferase (DGAT) activity [pmol triglyceride (TG)·mg lipid−1·min−1] and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.70, P = 0.006) and for men (r = 0.82, P = 0.0003); n = 28. C: relationship between total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.90, P = 0.0001) and for men (r = 0.91, P = 0.0001); n = 28. D: relationship between GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.67, P = 0.0001) and for men (r = 0.75, P = 0.0001); n = 28. E: relationship between CD36 relative content (mg lipid) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.31, P = 0.32) and for men (r = −0.43, P = 0.22); n = 22. F: relationship between ACS activity (pmol·min−1·mg protein−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.21, P = 0.51) and for men (r = 0.28, P = 0.33); n = 28. G: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.2, P = 0.94) and for men (r = 0.04, P = 0.9); n = 28. H: relationship between total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.37, P = 0.2) and for men (r = 0.39, P = 0.16); n = 28. I: relationship between GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.26, P = 0.38) and for men (r = 0.13, P = 0.66); n = 28. J: relationship between CD36 relative content (mg protein) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.66, P = 0.39) and for men (r = −0.87, P = 0.22); n = 22.
Palmitate storage rates into TAG in abdominal subcutaneous adipose tissue samples from men and women were also positively correlated with ACS, DGAT, total GPAT, and GPAT1 activity (r values 0.55–0.87) at high palmitate concentrations (Fig. 3, A–D). ACS and DGAT were positively correlated with storage rates in subcutaneous adipose tissue samples from females when data are expressed as milligrams protein per minute (Fig. 3, F and G). Palmitate storage rates in abdominal subcutaneous fat from males was positively correlated with relative CD36 content (mg lipid) (r = 0.77, P = 0.04), whereas we did not find a similar relationship when testing abdominal subcutaneous fat from females (r = −0.41, P = 0.18) (Fig. 3E). However, when data are expressed as milligram protein per minute we found a negative relationship between CD36 and TAG storage rates for female participants only (Fig. 3J).
Fig. 3.
Relationship between subcutaneous fatty acid storage factors and storage rates at high palmitate concentrations. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.55, P = 0.07) and for men (r = 0.87, P = 0.0002); n = 28. B: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.58, P = 0.05) and for men (r = 0.87, P = 0.0002); n = 28. C: relationship between total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.69, P = 0.009) and for men (r = 0.85, P = 0.0005); n = 28. D: relationship between GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.64, P = 0.02) and for men (r = 0.85, P = 0.0005); n = 28. E: relationship between CD36 relative content (mg lipid) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.41, P = 0.18) and for men (r = −0.77, P = 0.04); n = 22. F: relationship between ACS activity (pmol·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.02, P = 0.96) and for men (r = 0.72, P = 0.008); n = 28. G: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.06, P = 0.85) and for men (r = 0.72, P = 0.009); n = 28. H: relationship between total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.5, P = 0.83) and for men (r = 0.55, P = 0.06); n = 28. I: relationship between GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.11, P = 0.72) and for men (r = 0.46, P = 0.13); n = 28. J: relationship between CD36 relative content (mg protein) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.56, P = 0.057) and for men (r = 0.36, P = 0.43); n = 22.
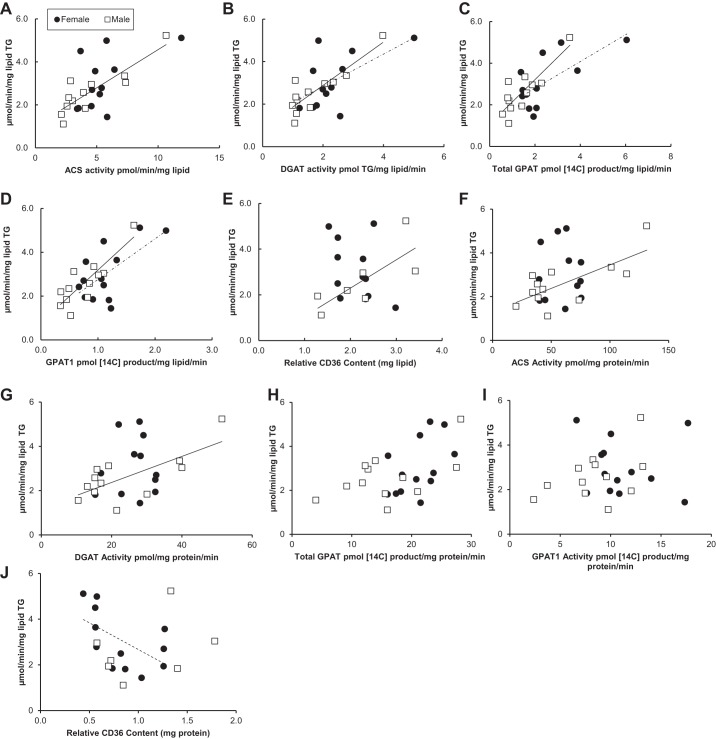
Relationship between fatty acid storage factors and storage rates into TAG at low palmitate concentrations.
Figures 4 and 5 display the relationship between palmitate storage rates into TAG and fatty acid storage factors at low palmitate concentrations. Omental palmitate storage rates were correlated positively with omental ACS, DGAT, total GPAT, and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) and under conditions of low palmitate concentrations for males (r values 0.65–0.86) (Fig. 4, A–D). We did not observe a significant correlation between the storage factors (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates into TAG (Fig. 4, F–J). The correlations between fatty acid storage factors (ACS, DGAT, GPAT1) in omental fat from females and palmitate storage into TAG did not reach statistical significance, with the exception of total GPAT activity (mg lipid) (r = 0.58, P = 0.02) (Fig. 4, A–D). We found no relationship between omental palmitate storage rates and relative CD36 content (per mg lipid) for females (r = 0.13, P = 0.93) or males (r = −0.35, P = 0.88) (Fig. 4E).
Fig. 4.
Relationship between omental fatty acid storage factors and storage rates at low palmitate concentrations. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.36, P = 0.18) and for men (r = 0.65, P = 0.01); n = 28. B: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.44, P = 0.1) and for men (r = 0.70, P = 0.006); n = 28. C: relationship between total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.58, P = 0.02) and for men (r = 0.86, P = 0.0001); n = 28. D: relationship between GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.42, P = 0.12) and for men (r = 0.68, P = 0.008); n = 28. E: relationship between CD36 relative content (mg lipid) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.13, P = 0.93) and for men (r = −0.35, P = 0.88); n = 22. F: relationship between ACS activity (pmol·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.28, P = 0.33) and for men (r = 0.26, P = 0.37); n = 28. G: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.22, P = 0.46) and for men (r = 0.09, P = 0.75); n = 28. H: relationship between total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.16, P = 0.58) and for men (r = 0.49, P = 0.07); n = 28. I: relationship between GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.26, P = 0.38) and for men (r = −0.11, P = 0.7); n = 28. J: relationship between CD36 relative content (mg protein) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.35, P = 0.29) and for men (r = −0.77, P = 0.01); n = 22.
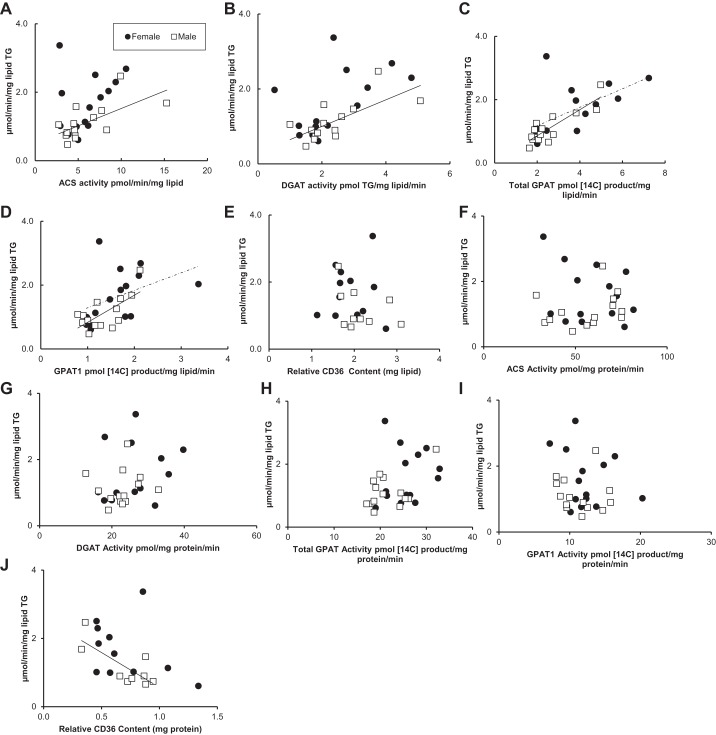
Fig. 5.
Relationship between subcutaneous fatty acid storage factors and storage rates at low palmitate concentrations. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.51, P = 0.09) and for men (r = 0.82, P = 0.001); n = 28. B: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.67, P = 0.02) and for men (r = 0.81, P = 0.0001); n = 28. C: relationship between total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.66, P = 0.015) and for men (r = 0.81, P = 0.001); n = 28. D: relationship between GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.66, P = 0.014) and for men (r = 0.82, P = 0.001); n = 28. E: relationship between CD36 relative content (mg lipid) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.27, P = 0.39) and for men (r = 0.81, P = 0.03); n = 22. F: relationship between ACS activity (pmol·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.13, P = 0.68) and for men (r = 0.66, P = 0.02); n = 28. G: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.09, P = 0.77) and for men (r = 0.64, P = 0.02); n = 28. H: relationship between total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.74, P = 0.004) and for men (r = 0.51, P = 0.09); n = 28. I: relationship between GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = 0.2, P = 0.51) and for men (r = 0.42, P = 0. 71); n = 28. J: relationship between CD36 relative content (mg protein) and palmitate storage rates (μmol·min−1·mg lipid−1) for women (r = −0.66, P = 0.02) and for men (r = 0.38, P = 0.4); n = 22.
The palmitate storage rates in abdominal subcutaneous adipose tissue from males were positively correlated with ACS, DGAT, and total GPAT and GPAT1 activities (r values 0.81–0.82) (Fig. 5, A–D). There was also a positive correlation between palmitate storage rates and DGAT, total GPAT, and GPAT1 for females (r values 0.66–0.67) (Fig. 5, A–D). However, the relationship between ACS activity and palmitate storage rates into TAG did not reach statistical significance (r = 0.51, P = 0.09) for females (Fig. 5A). When storage factor data are expressed as picomole 14C-labeled product per milligram protein per minute, we observed a positive correlation between ACS and DGAT activity and palmitate storage into TAG for females but not for males (Fig. 5, F and G). We did not observe a significant correlation between total GPAT, GPAT1, or CD36 and palmitate storage into TAG when data are expressed as picomole 14C-labeled product per milligram protein per minute (Fig. 5, F–J). There was a positive correlation between palmitate storage and relative CD36 content (per mg lipid) of abdominal subcutaneous fat from males (r = 0.81, P = 0.03) but not females (r = −0.27, P = 0.39) (Fig. 5E), similar to the results observed with high palmitate concentrations.
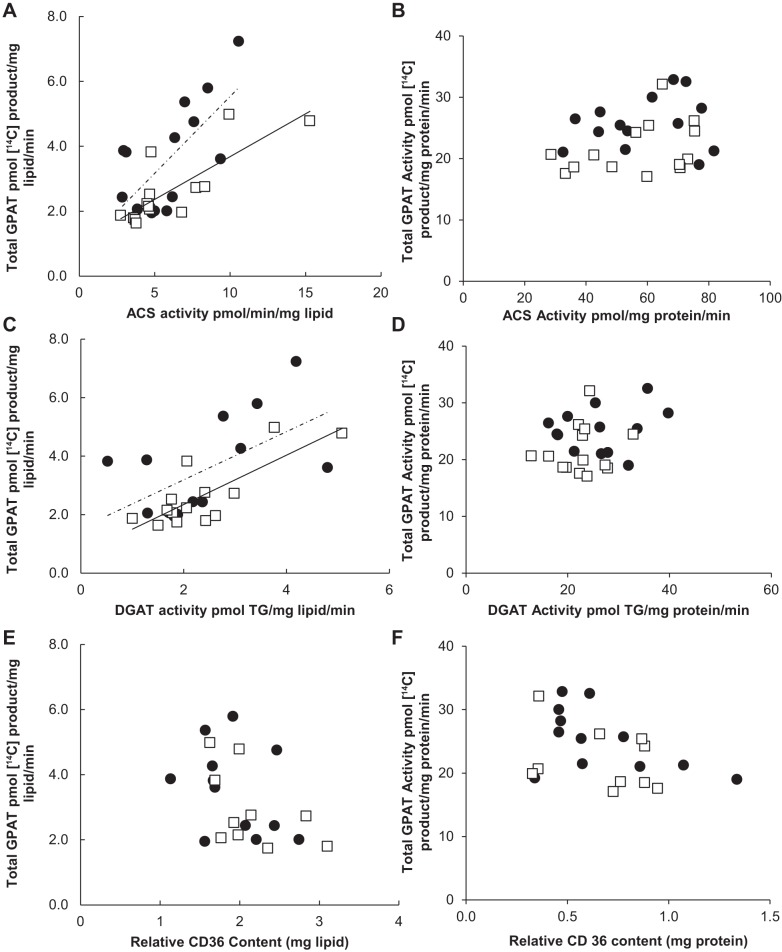
Correlation between omental depot GPAT activity and fatty acid storage factors.
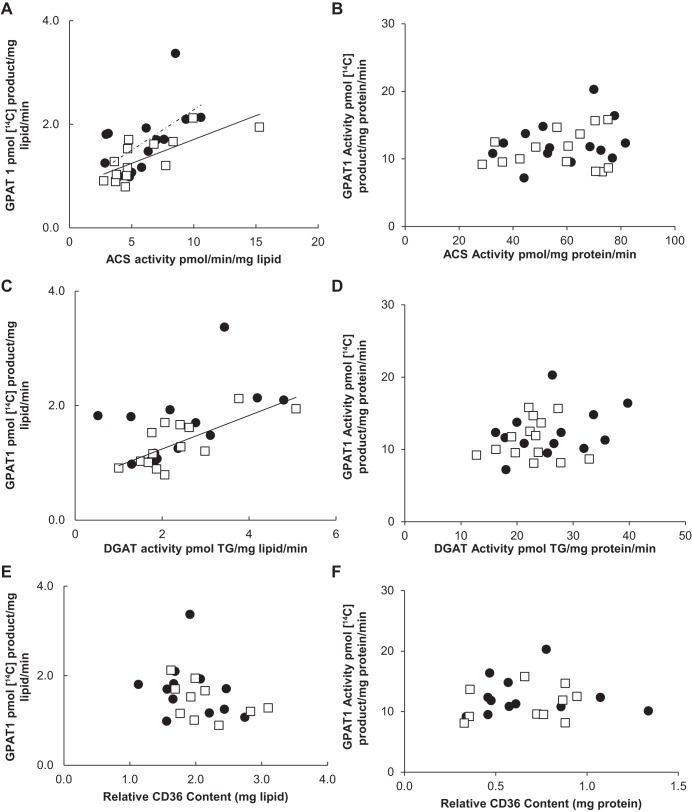
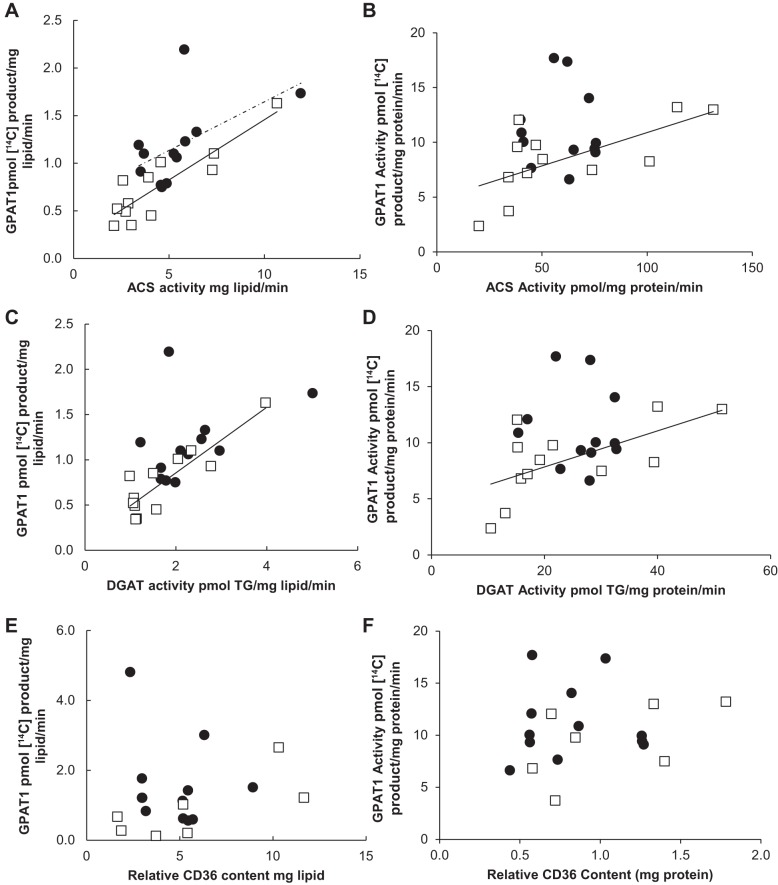
Omental ACS and DGAT activity (mg lipid) was positively correlated with total GPAT activity for both women and men (r values 0.58–0.80) (Fig. 6, A and C). In addition, there was a significant difference between the sexes (P = 0.03) in the relationship between total GPAT and ACS activity; total GPAT was greater for any given level of ACS or DGAT activity in women compared with men. There was no relationship, however, between relative CD36 content (mg lipid or mg protein) and total GPAT activity from the omental depot for either females (r = −0.38, P = 0.32) or males (r = −0.52, P = 0.17) (Fig. 6, E and F). We found similar results when comparing ACS, DGAT, and CD36 with GPAT1. Omental ACS and DGAT (mg lipid) were positively correlated with GPAT1 for both sexes (r values 0.52–0.74) (Fig. 7, A and C). Again, there was no relationship between GPAT1 and CD36 (mg lipid) for either sex (Fig. 6E). When data are expressed as milligram protein, there was no significant correlation between total GPAT or GPAT1 and any of the storage factors (Figs. 6 and 7).
Fig. 6.
Correlation between total GPAT activity and fatty acid storage factors in omental tissue. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·mg lipid−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.69, P = 0.004) and for men (r = 0.80, P = 0.0006); n = 25. B: relationship between ACS activity (pmol·mg protein−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = 0.15, P = 0.61) and for men (r = 0.37, P = 0.19); n = 25. C: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.58, P = 0.03) and for men (r = 0.79, P = 0.0007); n = 25. D: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = 0.33, P = 0.52) and for men (r = 0.18, P = 0.53); n = 25. E: relationship between relative CD36 content (mg lipid) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = −0.38, P = 0.32) and for men (r = −0.52, P = 0.17); n = 21. F: relationship between relative CD36 content (mg protein) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.50, P = 0.09) and for men (r = −0.33, P = 0.36); n = 21.
Fig. 7.
Correlation between GPAT1 activity and fatty acid storage factors in omental tissue. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.60, P = 0.02) and for men (r = 0.74, P = 0.003); n = 25. B: relationship between ACS activity (pmol·mg protein−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = 0.28, P = 0.33) and for men (r = 0.16, P = 0.58); n = 25. C: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.52, P = 0.06) and for men (r = 0.72, P = 0.003); n = 25. D: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = 0.33, P = 0.26) and for men (r = 0.05, P = 0.86); n = 25. E: relationship between relative CD36 content (mg lipid) and total GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = −0.20, P = 0.52) and for men (r = −0.45, P = 0.19); n = 21. F: relationship between relative CD36 content (mg protein) and total GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.002, P = 0.99) and for men (r = 0.2, P = 0.58); n = 21.
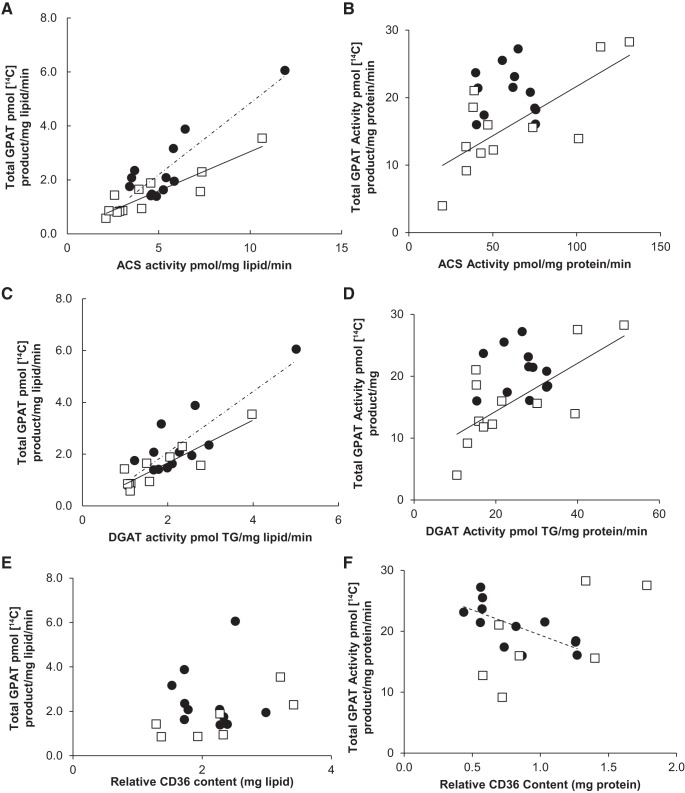
Correlation between subcutaneous adipose tissue GPAT activity and fatty acid storage factors.
Abdominal subcutaneous tissue total GPAT activity was positively correlated with ACS and DGAT activity (mg lipid) for both women and men (r values 0.85–0.91) (Fig. 8, A and C). As with omental adipose tissue, there was a significant sex difference (P = 0.03) in that total GPAT activity relative to ACS or DGAT activity was greater in women than men. There was no relationship between total GPAT activity and CD36 for females (r = −0.06, P = 0.86), but there was a positive correlation between total GPAT activity and CD36 for males (r = 0.75, P = 0.05) (Fig. 8E). For tissue obtained from women, there was a positive correlation between total GPAT and both ACS and DGAT (mg protein/min) (Fig. 8, B and D).
Fig. 8.
Correlation between total GPAT activity and fatty acid storage factors in subcutaneous tissue. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.88, P = 0.0002) and for men (r = 0.91, P = 0.0001); n = 25. B: relationship between ACS activity (pmol·mg protein−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.09, P = 0.78) and for men (r = 0.75, P = 0.005); n = 25. C: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.85, P = 0.0005) and for men (r = 0.89, P = 0.0001); n = 25. D: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.06, P = 0.84) and for men (r = 0.72, P = 0.009); n = 25. E: relationship between relative CD36 content (mg lipid) and total GPAT activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = −0.06, P = 0.86) and for men (r = 0.75, P = 0.05); n = 18. F: relationship between relative CD36 content (mg protein) and total GPAT activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.7, P = 0.01) and for men (r = 0.69, P = 0.08); n = 18.
Abdominal subcutaneous adipose tissue ACS and DGAT activity (mg lipid) was positively correlated with GPAT1 activity for males (r values 0.88 and 0.89, respectively). This relationship did not reach statistical significance for females (Fig. 9, A and C). There was a significant difference between the sexes (P = 0.02) in the relationship between GPAT1 and ACS activity (Fig. 9A). There was no relationship between subcutaneous adipose tissue GPAT1 activity and relative CD36 content (mg lipid) for either sex (Fig. 9E). For tissue obtained from males, there was a positive correlation between GPAT1 and both ACS and DGAT (mg protein/min) (Fig. 9, B and D).
Fig. 9.
Correlation between total GPAT activity and fatty acid storage factors in subcutaneous tissue. Solid circles, females; open squares, males. Square root-transformed values were used to achieve normal distribution. A: relationship between ACS activity (pmol·min−1·mg lipid−1) and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.54, P = 0.07) and for men (r = 0.87, P = 0.0002); n = 25. B: relationship between ACS activity (pmol·mg protein−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.03, P = 0.92) and for men (r = 0.65, P = 0.02); n = 25. C: relationship between DGAT activity (pmol TG·mg lipid−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = 0.40, P = 0.2) and for men (r = 0.89, P = 0.0001); n = 25. D: relationship between DGAT activity (pmol TG·mg protein−1·min−1) and GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = −0.12, P = 0.72) and for men (r = 0.63, P = 0.03); n = 25. E: relationship between relative CD36 content (mg lipid) and total GPAT1 activity (pmol 14C-labeled product·mg lipid−1·min−1) for women (r = −0.24, P = 0.46) and for men (r = 0.72, P = 0.07); n = 18. F: relationship between relative CD36 content (mg protein) and total GPAT1 activity (pmol 14C-labeled product·mg protein−1·min−1) for women (r = 0.005, P = 0.99) and for men (r = 0.53, P = 0.21); n = 18.
DISCUSSION
We previously reported that the greater abdominal subcutaneous fatty acid storage rates in women than in men could not be accounted for by greater amounts of the fatty acid storage factors DGAT, ACS, and CD36 in women (1, 16, 17). We suspected that there were as yet unmeasured fatty acid storage factor(s) that might explain these sex differences in fatty acid storage. Our goals were to develop a reliable GPAT activity assay for human adipose tissue and to test the hypothesis that GPAT, the first step toward TAG synthesis, may be responsible for these observed variations in storage rates. We found that 1) total GPAT and GPAT1 activity in abdominal subcutaneous fat, but not omental fat, is greater in females than males; 2) total GPAT and GPAT1 activities per unit adipose tissue lipid decrease as a function of adipocyte size in both the omental and the subcutaneous depots; 3) total GPAT and GPAT1 activity is greater in omental than subcutaneous adipose tissue; 4) GPAT1 accounted for up to 60% of GPAT activity in humans with the largest adipocytes (typically those who are obese); 5) there is a strong, positive intercorrelation between the fatty acid storage factors (ACS, GPAT, and DGAT) for both adipose depots and sexes; 6) palmitate storage rates in both adipose tissue depots from both sexes were positively correlated with the activities of all three enzymes, suggesting that there is no one rate-limiting enzyme in the TAG synthesis pathway; 7) GPAT activity is greater in WTE than the microsomal pellet; and 8) relative to ACS and DGAT, GPAT activity is greater in females than males.
We present GPAT activity data both from the perspective of whole tissue physiology (expressed per mg adipose tissue lipid) and from a cellular perspective (expressed per mg protein). Assuming that adipocytes are unlikely to increase total cellular protein in direct proportion to lipid content, we would expect a large increase in GPAT and other fatty acid storage factor activities per milligram protein if maximal adipose tissue fatty acid storage rates could remain as great in physiological terms across the fivefold range of average adipocyte size we observed. However, GPAT activity per milligram protein remained constant across the entire range of adipocyte size, indicating that, per unit adipose tissue mass, individuals with large adipocytes may have lesser rates of fatty acid storage per unit tissue than those with small adipocytes. Indeed, when GPAT activity is expressed as picomole 14C-labeled product per milligram tissue, we observed a significant negative correlation between total GPAT activity and adipocyte size (Fig. 1, I and K).
Men and women have similar DGAT or ACS activity in abdominal subcutaneous adipose tissue (1), whereas we found that abdominal subcutaneous adipose tissue GPAT activity (pmol 14C-labeled product/mg lipid) was significantly greater in women than men (Table 2). These findings suggest the possibility that the sex differences in GPAT activity contribute to the greater rates of direct FFA storage in abdominal subcutaneous adipose tissue in women compared with men. However, the exact cause for these sex differences requires further study.
We previously found that adipocyte CD36, DGAT, and ACS are significantly greater in omental than abdominal subcutaneous adipose tissue (1). Consistent with these findings, GPAT activity was also significantly greater in omental than abdominal subcutaneous adipose tissue (Table 2). These findings are consistent with the greater direct FFA storage rates in the omental depot compared with the subcutaneous depot (1).
In the present study, obese participants, or those with increased adipocyte size, have GPAT1 activity that accounts for 30–60% of total GPAT activity in both adipose depots. These results are surprising, since GPAT1 has been thought to account for about 10% of total GPAT activity in adipose tissue (7). While whole tissue GPAT1 decreases as a function of adipocyte size, the other isoforms of GPAT decrease to an even greater extent. One possible explanation for the lesser reductions in GPAT1 in obese individuals is the regulation of GPAT1 by insulin and SREBP-1c. The partial rescue of GPAT1 in obesity may be explained by the increase in circulating insulin concentrations; insulin can upregulate GPAT1 (3). These results raise questions about whether GPAT1 inhibitors are potential therapeutic targets to improve fatty acid metabolism in the adipose tissue of obese patients; however, it is also possible that GPAT1 inhibition would lead to harmful ectopic fat accumulation. Of note, in a diet-induced obesity mouse model, administration of the small-molecule GPAT inhibitor FSG67 leads to 1) decreased adiposity; 2) increased fatty acid oxidation; and 3) reduced dietary intake (19). We speculate that effects on appetite overwhelm any negative effects of inhibition of GPAT or GPAT1 in adipose tissue.
Based on the findings that ACS, GPAT, and DGAT activities were highly intercorrelated, we were unsurprised that they all were also correlated with fatty acid storage rates at both high and low palmitate concentrations (Figs. 2–5). These results are similar to previous studies analyzing the relationship of ACS and DGAT to fatty acid storage rates under high palmitate conditions (1, 18). To the best of our knowledge, no other studies have compared these fatty acid storage factors with palmitate storage rates under low palmitate concentrations. Our results suggest that at least these three fatty acid storage enzymes are regulated in an integrated fashion and can regulate fatty acid storage across a range of FFA concentrations. Although we cannot be certain of the regulatory elements of these fatty acid storage enzymes, PPARγ could be responsible (reviewed in Ref. 23), and further research into the role of these proteins in TAG synthesis is necessary.
We found that the WTE method of tissue preparation allows for greater recovery of GPAT activity than the microsomal pellet method. These results are similar to previous studies (4, 25). In particular, Rider and Saggerson found low palmitoyl-CoA concentrations; adding the cytosol to the pellet resulted in a lower GPAT activity compared with the activity measured in the pellet only, whereas at high concentrations it was enhanced. This suggests the existence of proteins or cofactors in the cytosol that can influence GPAT. We could not discount the possibility that WTE GPAT activity is a better reflection of the maximal tissue activity than that measured in the pellet fraction.
One of the limitations of this study is that we did not have measures of insulin sensitivity in our patients. Insulin resistance is one of the main comorbidities associated with obesity, and insulin upregulates GPAT1 activity (7). Determining the relationship between these factors and levels of total GPAT and GPAT1 may aid in identifying the pathophysiology of obese adipose tissue that leads to insulin resistance and hyperinsulinemia. In addition, we selected the low total palmitate concentration of 0.05 mM, expecting that the lower unbound palmitate concentration would uncover a relationship between CD36 and palmitate storage rates as facilitated transport becomes more important. If this were the case, we would expect that CD36 would have been a stronger predictor of palmitate storage at low palmitate concentrations than ACS, GPAT, or DGAT. Instead, except for abdominal subcutaneous fat from men, the intracellular enzymes responsible for activation and TAG synthesis were the better predictors. This suggests to us that, under the conditions of these experiments, the low palmitate concentration we used was sufficiently great that fatty acids could enter the cells independent of CD36 even at “low” palmitate concentrations in the subcutaneous adipose depot. Indeed, a subsequent study conducted by our group found that a modest elevation in extracellular FFA concentrations bypasses the CD36 facilitation of FFA into adipose tissue (10).
In summary, we have shown here that both total GPAT and GPAT1 activities vary by adipose tissue depot and sex, since they are significantly increased in omental tissue and in women. Total adipose GPAT and GPAT1 activity per unit lipid decreases as a function of adipocyte size regardless of depot or sex. We also found a significant positive correlation between GPAT1 proportion and adipocyte size. There were strong positive correlations between ACS, DGAT, total GPAT, and GPAT1 for both adipose tissue depots and sexes and strong positive relationships between these fatty acid storage factors and palmitate storage into TAG. We found a significant difference among the sexes in ACS and DGAT relative to palmitate storage, but not GPAT. All of the fatty acid storage factors appear to be coordinately regulated and intercorrelated with fatty acid storage rates. This leads us to suggest that, generally speaking, there is no single “rate-limiting” enzyme for adipocyte fatty acid storage. Focusing on a single step in the fatty acid storage pathway could be misleading, but future research examining the inhibition of GPAT1 could prove promising.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-45343, DK-40484, and DK-50456.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.M.-B., L.C., and M.D.J. analyzed data; M.M.-B. and M.D.J. prepared figures; M.M.-B. and M.D.J. drafted manuscript; M.M.-B. and M.D.J. edited and revised manuscript; M.M.-B., L.C., E.O., D.H., and M.D.J. approved final version of manuscript; L.C., E.O., D.H., and M.D.J. conception and design of research; L.C., E.O., and D.H. performed experiments; M.D.J. interpreted results of experiments.
ACKNOWLEDGMENTS
We are grateful of Darlene Lucas (from Mayo Clinic) for technical assistance and help with data collection.
REFERENCES
- 1.Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, Taler SJ, Nygren J, Thorell A, Bogachus LD, Turcotte LP, Bernlohr D, Jensen MD. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes 60: 2300–2307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res 52: 408–415, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagdade JD, Bierman EL, Porte D Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 46: 1549–1557, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates EJ, Saggerson ED. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J 182: 751–762, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol 209: 98–104, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Progr Lipid Res 43: 134–176, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Baro MR, Lewin TM, Coleman RA. Regulation of triglyceride metabolism. II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action. Am J Physiol Gastrointest Liver Physiol 292: G1195–G1199, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48: 1586–1592, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 278: 43008–43013, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Hames KC, Vella A, Kemp BJ, Jensen MD. Free fatty acid uptake in humans with CD36 deficiency. Diabetes 63: 3606–3614, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity 17: 1129–1134, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Curr Opin Clin Nutr Metab Care 5: 139–145, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96: 2297–2303, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S57–S63, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansara MS, Mehra AK, Von Hagen J, Kabotyansky E, Smith PJ. Physiological concentrations of insulin and T3 stimulate 3T3–L1 adipocyte acyl-CoA synthetase gene transcription. Am J Physiol Endocrinol Metab 270: E873–E881, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating FFA in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 60: 2032–2040, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab 96: 541–547, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsari C, Mundi MS, Ali AH, Jensen MD. Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes 61: 329–338, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhajda FP, Aja S, Tu Y, Han WF, Medghalchi SM, El Meskini R, Landree LE, Peterson JM, Daniels K, Wong K, Wydysh EA, Townsend CA, Ronnett GV. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 301: R116–R130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewin TM, Wang S, Nagle CA, Van Horn CG, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the metabolic fate of exogenous fatty acids in hepatocytes. Am J Physiol Endocrinol Metab 288: E835–E844, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Marin P, Rebuffe-Scrive M, Bjorntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 20: 158–165, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Meegalla RL, Billheimer JT, Chen D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransfarase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun 298: 317–323, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53: 124–144, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rider M, Saggerson E. A trypsin-sensitive, heat-labile, N-ethylmaleimide-sensitive factor in adipocyte post-microsomal supernatant which affects the assay of adipocyte glycerol phosphate acyltransferase activities. Biochemistry 214: 247–255, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: Gender effects in non-obese humans. Am J Physiol Endocrinol Metab 279: E455–E462, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Santosa S, Jensen MD. Effects of male hypogonadism on regional adipose tissue fatty acid storage and lipogenic proteins. PloS One 7: e31473, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56: 1369–1375, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 44: 1795–1801, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Votruba SB, Jensen MD. Short-term regional meal fat storage in nonobese humans is not a predictor of long-term regional fat gain. Am J Physiol Endocrinol Metab 302: E1078–E1083, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendel AA, Cooper DE, Ilkayeva OR, Muoio DM, Coleman RA. Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J Biol Chem 288: 27299–27306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]