Abstract
Hyperandrogenic syndrome (HAS) is associated with insulin resistance (IR) and type 2 diabetes. Muscle IR in type 2 diabetes is linked with defects in mitochondrial oxidative capacity. In vivo muscle mitochondrial function has not been studied in HAS, especially in youth, who are early in the disease process. Our goal was to measure muscle mitochondrial oxidative function and peripheral IR in obese youth with HAS. Obese girls without HAS [n = 22, age 15(13,17) yr, BMI Z-score 2.05 ± 0.37] and with HAS [n = 35, age 15(14,16) yr, BMI Z-score 2.18 ± 0.30] were enrolled. Mitochondrial function was assessed with 31phosphorus MR spectroscopy before, during, and after near-maximal isometric calf exercise, and peripheral IR was assessed with an 80 mU·m−2·min−1 hyperinsulinemic euglycemic clamp. Girls with HAS had higher androgens [free androgen index 7.9(6.6,15.5) vs. 3.5(3.0,4.0), P < 0.01] and more IR [glucose infusion rate 9.4(7.0, 12,2) vs. 14.5(13.2,15.8) mg·kg lean−1·min−1, P < 0.01]. HAS girls also had increased markers of inflammation including CRP, platelets, and white blood cell count and higher serum free fatty acids during hyperinsulinemia. Mitochondrial oxidative phosphorylation was lower in HAS [0.11(0.06,0.19) vs. 0.18(0.12,0.23) mmol/s, P < 0.05], although other spectroscopy markers of mitochondrial function were similar between groups. In multivariate analysis of the entire cohort, IR related to androgens, oxidative phosphorylation, and free fatty acid concentrations during hyperinsulinemia. These relationships were present in just the HAS cohort as well. Obese girls with HAS have significant peripheral IR, which is related to elevated androgens and free fatty acids and decreased mitochondrial oxidative phosphorylation. These may provide future options as targets for therapeutic intervention.
Keywords: hyperandrogenism, insulin resistance, mitochondria, obesity, hyperandrogenic syndrome
hyperandrogenic syndrome (HAS), also known as polycystic ovarian syndrome (PCOS), affects at least 10–15% of the female population in the United States, with a recent increase in prevalence associated with rising obesity rates (32). Women with HAS have a three- to fourfold increased risk of developing type 2 diabetes (T2D) compared with BMI-matched controls (37, 58). This increased risk is thought to be secondary to long-standing insulin resistance (IR) (37). There is also evidence of IR in youth with HAS (2). Similar to findings in T2D, peripheral IR as assessed with a hyperinsulinemic euglycemic clamp has been well documented in adults with HAS (7, 15), with similar but fewer data in youth (2). However, the mechanism of IR in HAS remains uncertain, and as a result the best treatments for HAS are also unclear.
A leading theory explaining IR in T2D includes defects in mitochondrial function in combination with excess serum free fatty acids (FFA), which can cause alterations in intracellular insulin signaling (52). Other potential mechanisms involve altered fat metabolism and inflammation, which have been documented in T2D and HAS but have not been examined in adolescents with HAS (14, 25, 29, 33, 54). However, data examining the role of mitochondria in IR in HAS are limited and conflicting. Muscle mitochondrial oxidation gene expression for oxidative enzymes such as citrate synthase, or β-hydroxyacyl-CoA dehydrogenase were similar between women with and without HAS despite worse IR in the women with HAS, and gene expression did not change with exercise training, whereas IR improved (26). Furthermore, neither stage 1 or stage 3 oxidation was different in biopsy samples from women with PCOS compared with controls, again despite worse IR in the women with HAS (48). Mitochondrial function in myotubes grown from women with HAS was identical to controls, whereas the same investigators found abnormalities in myotubes from T2D participants (19, 36). Conversely, Skov et al. (54, 55) did find a decrease in genes associated with oxidation phosphorylation in obese women with HAS compared with controls and also found that pioglitazone treatment increased oxidative phosphorylation genes along with insulin sensitivity. However, all of these studies are limited by the ex vivo nature of muscle biopsies, and no studies have been conducted in youth.
We hypothesized that obese youth with HAS would have IR, which would relate to established contributors to IR in other populations, including mitochondrial dysfunction, elevated FFA, and inflammation, as well as to androgens.
MATERIALS AND METHODS
Subjects
Fifty-seven female participants 12–20 yr of age were recruited from Weight Management and Endocrinology pediatric clinics at the Children's Hospital Colorado for a prospective, cross-sectional study. Participants included obese (BMI ≥95th %ile for age, but <275 lb due to equipment constraints) participants with and without HAS. After ruling out other causes of oligomenorrhea, HAS was defined per National Institutes of Health and recent Endocrine Society criteria, with adaptations for adolescents, so that all girls were at least 18 mo postmenarche (32). Hirsutism was scored by the Ferriman-Gallwey scale and acne on a score of 0 = none, 1 = mild, 2 = moderate, 3 = severe. All HAS diagnoses and exams were performed by pediatric endocrinologists (M. C. G Cree-Green and K. J. Nadeau). To help reduce variability, all participants were sedentary (defined as less than 3 h/wk of exercise, verified by standardized 3-day activity recall, and 7-day accelerometer recording, Actigraph, Pensacola, FL) and were not prescribed medications known to affect IR, blood pressure, or lipids, including metformin, hormonal contraception, or psychiatric medications. This study was approved by the University of Colorado Anschutz Medical Campus Institutional Review Board and the Children's Hospital Colorado Scientific Advisory Review Committee. Parental informed consent and participant assent were obtained from all participants less than 18 yr old and participant consent from those aged 18 yr and over.
Overall Study Design
Participants underwent a screening visit and an overnight inpatient stay, which included MRI imaging of the leg for maximal cross-sectional area (MCSA) and in-MRI exercise testing with phosphorus MRS (31P-MRS) to assess mitochondrial function in the afternoon. The next morning, a fasting blood draw for sex steroids, lipid panel, glycemic markers, insulin, FFA, inflammatory markers, and a CBC was performed, followed by a hyperinsulinemic euglycemic clamp. Volunteers consumed 3 days of an isocaloric diet (55% carbohydrate, 15% protein, 30% fat) prior to admission, and all subjects were free from acute illness. All testing was performed fasting, with no strenuous exercise for the 3 days prior and in the follicular phase of the menstrual cycle if possible. Body composition (Hologic, Waltham, MA) was assessed by standard DEXA methods (10).
Measure of Insulin Sensitivity
A hyperinsulinemic euglycemic clamp (80 mU·m−2·min−1 insulin) was performed fasting in the morning to measure IR, similar to our previously described methods (10, 40). Briefly, serum glucose concentrations were maintained at ∼95 mg/dl, based on serial blood samples drawn every 5 min, and analyzed at the bedside with a YSI (Yellow Springs Instrument, OH). Glucose infusion rate (GIR) during the last stage of the clamp was expressed as milligrams per kilogram per minute and milligrams per kilogram lean per minute. FFAs were assessed fasting prior to the clamp and again at the end of the clamp during steady state following hyperinsulinemia.
MRI and MRS
Imaging and spectroscopy acquisition.
All MRI and MRS equipment and procedures have been previously described (9, 10). In short, imaging and spectroscopy were performed on a General Electric (GE) 3T with HDx MRI (GE, Milwaukee, WI) running version 15M4 software. Our scanner was also equipped with the GE multinuclear spectroscopy hardware and research software upgrades and utilized a custom-built 1H/31P leg coil (Clinical MR Solutions, Brookfield, WI).
31P MRS exercise protocol.
Strength testing was done on a custom-built MR-compatible plantar flexion device with force measurement capability as previously described (3, 9, 31, 48). The force transducer box was connected to an external read-out stage (Omega Engineering, Stamford, CT), and finally to a laptop computer for constant recording of force throughout exercise with DAQ software (Labview, National Instruments, Austin, TX).
The 31P-MRS exercise protocol consisted of measurements during rest for 60 s, isometric plantar flexion exercise for 90 s at 70% maximal volitional contraction, and recovery for 5 min postexercise. We selected a 90-s isometric exercise bout, as this perturbation has been extensively modeled and utilized for assessing both aerobic and anaerobic processes (53). Force was monitored continuously throughout the exercise, with verbal feedback to help keep the force measurements within the target goal. The average force applied was recorded in kilograms. All participants were able to complete the exercise for 90 s at or near their personal target force of 70% maximal volitional contraction.
Spectroscopy Analysis
Peak positions and areas of interest [phosphocreatine (PCr), inorganic free phosphate (Pi), β-ATP (3 peaks), α-ATP (2 peaks), γ-ATP (2 peaks), and phosphomonoester] were determined by time domain fitting using jMRUi (30, 57) utilizing AMARES (A Method of Accurate, Robust and Efficient Spectral fitting), a nonlinear least-square-fitting algorithm using previously built prior-knowledge files (49). All exercise spectra were corrected for saturation using the fully relaxed spectra for that day. The jMRUi data were used to calculate the following metabolic variables as previously described (10, 43). Calculations included the rates of oxidative phosphorylation (OxPhos) following exercise, creatine kinase reaction, initial PCr synthesis (VPcr), maximal mitochondrial function (Qmax), and anaerobic glycolysis (AnGly). ADP, PCr, and Pi time constants were calculated via regression analyses with Sigmaplot (Systat Software, San Jose, CA).
Laboratory Analyses
Total testosterone was measured utilizing liquid chromatography-mass spectroscopy/mass spectroscopy and anti-mullerian hormone (AMH) via chemiluminescent assay by Esoterix Laboratories (Calabasas Hills, Ca). Free androgen index (FAI) was calculated as the ratio of total testosterone to sex hormone-binding globulin (SHBG). The remainder of analyses were performed by the University of Colorado Anschutz CTRC core laboratory. Insulin was measured by radioimmunoassay (Millipore, Darmstadt, Germany); FFAs were measured by ELISA (WaKo Chemicals, Richmond VA); hemoglobin A1c (A1C) was measured with a DCCT standardized potassium ferricyanide method (Siemens DCA Vantage); highly sensitive C-reactive protein (hs-CRP) was assessed via immunoturbidimetric assay (Beckman Coulter, Brea, CA); cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were assessed via enzymatic assays (Beckman Coulter); low-density lipoprotein cholesterol (LDL-C) levels were calculated with the Friedewald equation (21). A complete blood cell count and transaminases were analyzed by the Children's Hospital Colorado clinical laboratory using standard methods.
Statistical Analysis
The distribution of all variables was examined and results presented as means ± SD, median (minimum, maximum), or proportions, as appropriate. Group comparisons were made using χ2 or Fisher's exact test for proportions and the t-test or Kruskal-Wallis test for continuous variables. Multiple regression models were used to examine the associations between GIR (mg·lean kg−1·min−1) and FFA at the end of the clamp, and mitochondrial measures, including oxidative phosphorylation, FAI, ADP time constant, PCr time constant, and Qmax. Spearman correlation coefficients between the outcome (GIR) and the covariates were estimated, and only those covariates with a correlation significantly different from zero were considered for inclusion in the regressions. Two multivariable models were tested, each with a different marker of mitochondrial function: Model 1: GIR vs. end FFA, oxidative phosphorylation, and FAI; Model 2: GIR vs. end FFA, Qmax, and FAI. Both models were run with the entire sample of subjects, and then within the HAS group. For each covariate in each of the models, parameter estimates (β̂) and their associated standard errors are reported, along with an estimate of the squared semipartial correlation coefficient and the associated 95% confidence intervals. The squared semipartial correlation coefficient is a measure of the proportion of variance in the outcome that is explained by the covariate. All statistical analyses were performed with SAS Software version 9.4 (Cary, NC).
RESULTS
Of the 57 adolescents who were enrolled, 35 had HAS and 22 were obese controls. Participant demographics are shown in Table 1. The two groups had similar age and BMI z-score distributions. Per study design, girls with HAS had significantly more symptoms of HAS, including less frequent periods, a higher Ferriman-Gallwey score, and more acne as well as higher FAI and AMH. Several markers of inflammation were significantly higher in the girls with HAS, including hs-CRP, platelets, and AST (Table 1). Fasting markers of glucose and lipids were similar between groups, as was the HOMA-IR estimate of IR, although fasting insulin was significantly increased in the HAS group. Fasting FFA concentrations were significantly higher in HAS and inappropriately failed to fully suppress in response to hyperinsulinemia, indicating adipose IR. Furthermore, the GIR was significantly lower [9.4 (7.0,12.2) vs. 14.5 (13.2,15.8) mg·kg lean−1·min−1] in HAS, indicating muscle IR. The adipose and muscle IR occurred despite similar achieved serum glucose and insulin concentrations during the hyperinsulinemic euglycemic clamp.
Table 1.
Demographics and serum measurements
| HAS (n = 35) | Control (n = 22) | |
|---|---|---|
| Age (yr) | 15 (14,16) | 15 (13,17) |
| Race | ||
| White | 14 (40.0%) | 7 (31.8%) |
| Hispanic | 18 (51.4%) | 7 (31.8%) |
| Black | 3 (8.6%) | 7 (31.8%) |
| American Indian | 0 (0.0%) | 1 (4.6%) |
| BMI Z-score | 2.18 ± 0.30 | 2.05 ± 0.37 |
| Waist/hip ratio | 0.88 ± 0.08 | 0.91 ± 0.08 |
| Measures of HAS | ||
| Menarche (yr) | 12 (11,12) | 11 (10,12) |
| Menses per yr† | 3.1 (1.2,5.2) | 12.0 (12.0,12.0) |
| Ferriman-Gallwey score† | 7 (4,11) | 1 (0,2) |
| Acne† | 1 (1,2) | 1 (0,1) |
| Free androgen index† | 7.9 (6.6,15.5) | 3.5 (3.0,4.0) |
| AMH† | 5.9 (4.1,9.1) | 3.4 (2.0,5.4) |
| Clinical markers of glucose metabolism | ||
| Fasting glucose (mg/dl) | 87.0 (83.0,89.0) | 86.7 (85.0,90.0) |
| Fasting insulin (mmol/l)† | 27.0 (21.0,34.0) | 13.0 (2.7,20.0) |
| HOMA | 6.0 (4.2,7.1) | 4.3 (3.7, 5.2) |
| Hemoglobin A1C (%) | 5.4 (5.2,5.5) | 5.4 (5.2,5.5) |
| Inflammatory markers | ||
| AST (U/l) | 37 (29,44) | 33 (28,37) |
| ALT (U/l)* | 38 (28,43) | 32 (25,35) |
| hsCRP (mg/l)* | 2.6 (1.2,5.1) | 1.4 (0.5,2.3) |
| WBC (1K cell/μg) | 7.8 ± 1.6 | 7.1 ± 2.7 |
| Platelets (1K cell/μg)* | 280 ± 39 | 254 ± 46 |
| Lipids | ||
| Total cholesterol (mg/dl) | 165 ± 33 | 157 ± 35 |
| TG (mg/dl) | 122 (74,158) | 97 (87,152) |
| HDL (mg/dl) | 37 ± 6 | 40 ± 9 |
| LDL (mg/dl) | 114 ± 30 | 98 ± 26 |
| FFA baseline (mmol/l)* | 638 ± 138 | 543 ± 158 |
| Clamp measurements | ||
| FFA end clamp (mmol/l)† | 59 (36,87) | 32 (20,41) |
| Insulin end clamp (mmol/l) | 277 (233,322) | 239 (181,276) |
| Glucose end clamp (mg/dl) | 96 ± 4 | 95 ± 5 |
| GIR (mg·kg−1·min−1)† | 5.0 (4.1,6.8) | 7.9 (6.7,9.2) |
| GIR (mg·kg lean−1·min−1)† | 9.4 (7.0,12.2) | 14.5 (13.2,15.8) |
Data are expressed as means ± SD or median (25%,75%) for nonnormally distributed data.
HAS, hyperandrogenic syndrome; GIR glucose infusion rate.
P < 0.05; †P < 0.01.
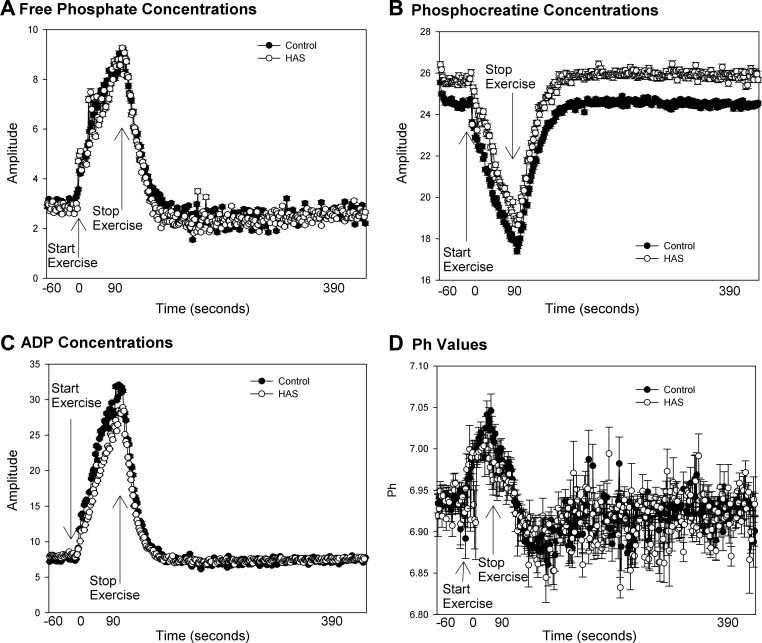
Group means of raw data from 31P-MRS, including PCr, free phosphate, ADP concentrations, and pH are shown in Fig. 1. Youth with HAS had similar changes in metabolite concentrations (Pi, Fig. 1A; PCr, Fig. 1B; ADP, Fig. 1C, and pH, Fig. 1D), with exercise indicating that the exercise intensity was similar between groups. Calculated measurements are show in Table 2. Per design, the force exerted during the exercise bout, normalized for leg area, was similar between groups. Nonmitochondrial sources of energy, including anaerobic glycolysis and the creatine kinase reaction, were similar between groups. Oxidative phosphorylation was significantly lower in HAS, whereas other measures of mitochondrial function were similar between the groups.
Fig. 1.
Average muscle metabolite concentrations before, during, and after 70% exercise. Average metabolic concentrations ± SD from hyperandrogenic syndrome (HAS) n = 3 and controls (n = 22) before, during, and after 70% exercise are shown. A: inorganic phosphate. B: phosphocreatine concentrations. C: ADP concentration. D: intracellular pH.
Table 2.
Muscle-specific measurements
| HAS (n = 35) | Control (n = 22) | |
|---|---|---|
| Force/area (kg/cm2) | 0.007 ± 0.001 | 0.007 ± 0.001 |
| Anaerobic glycolysis (mmol·l−1·s−1) | 0.26 (0.13,0.46) | 0.23 (0.14,0.61) |
| Creatine kinase ATP production (mmol·l−1·s−1) | 0.05 (0.03,0.09) | 0.08 (0.04, 0.12) |
| Oxidative phosphorylation (mmol·l−1·s−1)* | 0.11 (0.06,0.19) | 0.18 (0.12,0.23) |
| ME | 0.14 (0.10,0.21) | 0.15 (0.10,0.18) |
| ADP time constant (s) | 22 (19,26) | 21 (18,23) |
| PCr time constant (s) | 31 ± 8 | 29 ± 7 |
| VPCr (mMol/s) | 0.21 (0.15,0.32) | 0.27 (0.16,0.33) |
| Qmax (mMol/s) | 0.44 (0.31,0.58) | 0.54 (0.37,0.64) |
Data are expressed as means ± SD or median (25%,75%) for nonnormally distributed data.
P < 0.05.
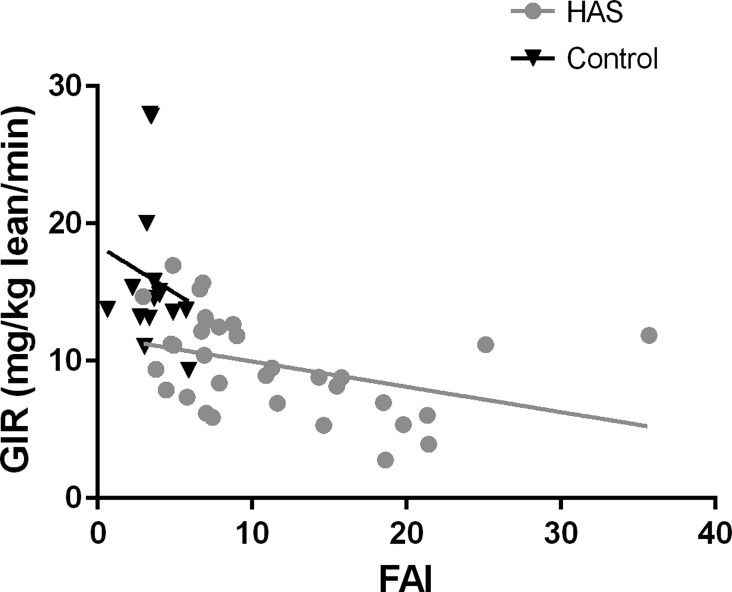
Results from the regression models are shown in Table 3. In Model 1, in all subjects, FFA end clamp, oxidative phosphorylation, and FAI were significantly associated with GIR. In Model 1, which included only HAS subjects, FFA end clamp was still significantly associated with GIR, and oxidative phosphorylation and FAI were borderline significant. In Model 2 with all subjects, GIR was significantly associated with FFA end clamp, and FAI, but not Qmax. In Model 2 with HAS-only subjects, only GIR was significantly associated with FFA end clamp. For all models, because the outcome (GIR) was log-transformed, parameter estimates have the following interpretation: the outcome changes by 100 × (parameter estimate)% for a one-unit increase in the covariate, whereas all other covariates are held constant. For example, in Model 1, for a one-unit increase in FFA end clamp, GIR will decrease by 6.7% (Fig. 2).
Table 3.
Multiple regression of insulin resistance model results
| Covariate | Parameter Estimate | SE of Parameter Estimate | P Value | Squared Semipartial Correlation and 95% CI |
|---|---|---|---|---|
| Model 1, all participants | ||||
| FFA end clamp | −0.0058 | 0.0009 | <0.0001 | 0.5534 (0.3454–0.6764) |
| OxPhos | 0.8007 | 0.2850 | 0.0074 | 0.0660 (0.0000–0.2301) |
| FAI | −0.0171 | 0.0059 | 0.0057 | 0.0614 (0.0000–0.2235) |
| Model 1, HAS participants | ||||
| FFA end clamp | −0.0056 | 0.0010 | <0.0001 | 0.5457 (0.2814–0.6888) |
| OxPhos | 0.7847 | 0.3871 | 0.0519 | 0.0440 (0.0000–0.2330) |
| FAI | −0.0129 | 0.0065 | 0.0560 | 0.0493 (0.0000–0.2417) |
| Model 2, all participants | ||||
| FFA end clamp | −0.0062 | 0.0010 | <0.0001 | 0.5534 (0.3454–0.6764) |
| Qmax | 0.0266 | 0.0417 | 0.5272 | 0.0073 (0.0000–0.1173) |
| FAI | −0.0178 | 0.0064 | 0.0077 | 0.0662 (0.0000–0.2304) |
| Model 2, HAS participants | ||||
| FFA end clamp | −0.0057 | 0.0010 | <0.0001 | 0.5457 (0.2814–0.6888) |
| Qmax | 0.0285 | 0.1525 | 0.8529 | 0.0020 (0.0000–0.1125) |
| FAI | −0.0118 | 0.0070 | 0.1013 | 0.0407 (0.0000–0.2275) |
Because the outcome (GIR) was log-transformed, parameter estimates have the following interpretation: outcome changes by 100 • (parameter estimate)% for a one-unit increase in the covariate while all other covariates are held constant.
Fig. 2.
Relationship between insulin resistance (IR) and free androgen index (FAI). Glucose infusion rate (GIR) as related to FAI is shown. Controls are shown in black triangles and HAS with gray circles. Relationship between FAI and IR in te entire cohort is shown by black line and in girls with HAS by gray line.
DISCUSSION
HAS is a common endocrinopathy, and obese women with HAS have a three- to fourfold increased risk of developing T2D, likely secondary to long-standing IR (37). We found that this process starts early in the development of HAS, as obese girls with HAS at an average age of 15 yr already had significant peripheral IR compared with BMI-similar obese controls. Akin to individuals with IR and other medical conditions, youth with HAS also had evidence of postexercise mitochondrial defects, specifically in oxidative phosphorylation. This is the first time that mitochondrial function has been studied in youth with HAS and the first published data assessing in vivo mitochondrial function in any age cohort of HAS. We also found a relationship between IR and mitochondrial function and FFA concentrations during hyperinsulinemia. We further found that androgen concentration related to IR across the entire cohort and that the degree of androgen elevation in girls with HAS tended to be related to IR.
The relationship between mitochondrial function and IR in HAS is similar to that seen in T2D, which is consistent with HAS as a precursor disease of T2D (52). We specifically found that the rate of postexercise oxidative phosphorylation, but not total maximal mitochondrial capacity, was related to IR. In addition, we and others have documented decreased mitochondrial function and alterations in lipid metabolism in nondiabetic populations with IR, including burn trauma, aging, and HIV medication-related lipodystrophy (11, 12, 46). Most recently we found that the IR seen in type 1 diabetes, and insulinopenic state, is also related to mitochondrial dysfunction following exercise (10). Finally, defects in mitochondrial function and insulin signaling are even found in those individuals with a genetic risk for T2D prior to development of clinical hyperglycemia, indicating that mitochondrial alterations can be seen before clinical hyperglycemia, as in our participants (39). These data add to the growing body of evidence linking mitochondrial function and IR regardless of the disease state.
Despite the consistent correlation in many studies between mitochondrial function and IR, the relationship between mitochondrial function and alterations in downstream insulin signaling appears to be indirect. We found that participants with HAS had elevated serum FFA concentrations during both fasting and hyperinsulinemia. FFA was the most related to IR of any of the variables examined, indicating that their IR is related to excess FFA delivery, in addition to mitochondrial dysfunction. Elevated FFA may contribute to the formation of diacylglycerol, which can interfere with downstream insulin signaling by excess serine phosphorylation (52). The specific change of increased serine phosphorylation of the insulin receptor has been documented in muscle from women with HAS (17), and it is possible that this mechanism is also occurring in our youth with HAS.
We found significant peripheral IR in obese girls with HAS, similar to other studies, with a discrepancy between clamp-measured IR and clinical measurements. Thirty to forty percent of obese women with HAS in their third decade have IR, but not yet T2D, compared with 5–10% in women without HAS (37). Overall, obese women with HAS are 2.5 times more likely, and nonobese women with HAS are 3.3 times more likely to have IR as assessed with hyperinsulinemic clamps or oral glucose tolerance tests (OGTT) compared with weight-matched controls (14–16, 38). The reported prevalence of IR and dysglycemia in young girls is much more varied, likely an artifact of the different methods of assessing IR (2, 22, 23, 44, 45). For example, Nur et al. (44) found that only 6 of 101 obese Hispanic girls with PCOS had IR as assessed by fasting insulin and glucose, and Fruzzetti et al. (22) found fasting glucose >110 mg/dl in only 2% of girls with HAS and varied BMI. In contrast, using OGTT, Palmert et al. (45) found that 9 of 27 girls of varied BMI had abnormal 2-h glucose, and Arslanian et al. (2) found similar results in 11 of 21 obese girls utilizing hyperinsulinemic clamps. Similarly, Fulghesu and coworkers (1, 23) found that post-glucose load insulin response was elevated in 38 of 79 girls with HAS despite identical fasting measures between HAS and controls. Thus, the prevalence of IR and dysglycemia in HAS teens may be similar to that of adult women, but it may only be detectable with postprandial or more intensive assessment methods such as the hyperinsulinemic clamp used in our study.
We found a relationship between IR and androgen concentrations in the entire cohort, and the trend maintained when only the HAS group was examined, indicating that the relationship was not solely driven by the extreme difference between the groups. Deleterious interactions between insulin and chronically elevated androgens likely contribute to unique facets of IR in HAS in women, as testosterone is associated with improvements in insulin sensitivity in men. Insulin acts on peripheral tissue and ovarian theca cells to enhance androgen production. Insulin can cause thecal cell hypertrophy, which indirectly increases androgens (6, 41, 42, 50, 59). Testosterone, in turn, can increase PKC-l activity, which also increases serine phosphorylation of the insulin receptor and its substrate, and administration of testosterone to adipocytes from women induces insulin resistance (8). Indeed administration of testosterone in a short-term experimental setting or in the more chronic therapy setting of female-to-male gender reassignment worsens IR in healthy premenopausal women (13, 47). Testosterone also increases central obesity in women, which is linked with increased visceral and hepatic fat, inflammatory markers, and IR, which are all in turn associated with mitochondrial dysfunction (4, 5, 24, 29, 33). In obese teens with IR, mitochondrial dysfunction begins in midpuberty (Tanner 3), and IR is associated with elevated androgens (20, 34, 35). It is notable that IR is present early in the development of HAS, when testosterone is elevated, also implicating a central role of androgens in IR in HAS.
Whereas we found significant abnormalities in oxidative phosphorylation, the other mitochondrial aspects studied were not abnormal in our population. One explanation may reflect the complex role of androgens at the level of the muscle. Testosterone is anabolic within the skeletal muscle and influences overall basal metabolic rates and the anabolic response to exercise, all contributing to sex differences in metabolism and exercise capacity (51). In keeping with these findings, mitochondrial function has been shown to vary by sex (28). Many mitochondrial studies account for this by selecting to study one sex or balancing sex distribution within groups (10, 27, 53). However, in adults with vascular disease, once protein and muscle weight were corrected for, there were no differences in mitochondrial function per se (56). It is thus not clear whether the increased androgens seen in HAS have an anabolic effect on muscle so that the ability to regenerate high-energy phosphate does not appear slowed. Indeed, the addition of additional androgens to myotubes from HAS participants increased gene expression but did not change insulin sensitivity, indicating that the muscle may not be the site of the primary defect (18). This is unique from the universal mitochondrial dysfunction seen in type 1 diabetes and T2D.
There are several limitations to broader application of our study's results. We chose an exercise paradigm to allow for maximal detection of differences between groups, and it was thus a short isometric exercise at a value close to maximal effort. Perhaps differences are more subtle and only seen at submaximal aerobic-type perturbations. However, in our study in youth with type 1 diabetes, we found identical results between submaximal and near-maximal perturbations. Additionally, we were limited by our DEXA and MRI to a weight of 275 lb, which excluded many girls with HAS. Thus, it may be that our population is relatively “healthy” in terms of obese HAS and underestimates the severity of disease. Our study may also have been underpowered to detect some mitochondrial outcomes. However, utilizing similar techniques we found differences between controls and youth with type 1 diabetes with a smaller sample size (10). Moreover, if differences are so subtle as to need a very large sample size, they may have minimal physiological or clinical relevance. Finally, we are assuming that all of the GIR is due to peripheral uptake. We (10) previously showed in youth that the contribution of endogenous rate of appearance of glucose at this insulin infusion rate and concentration is minimal.
In conclusion, we found that obese youth with HAS have significant IR, which is predominantly related to excess serum FFA in addition to muscle mitochondrial oxidative phosphorylation and excess androgens. We also confirmed the findings from previous studies with a gold standard method: that peripheral IR in obese youth is substantial yet difficult to detect utilizing common fasting clinical measures. Future work should explore the role of insulin sensitizers, direct mitochondrial stimulants, therapies to lower FFA, and medications that lower androgen concentrations on mitochondrial function in HAS. This additional understanding is necessary for the development of new and improved therapeutic options to prevent T2D and its complication in these high risk youth.
GRANTS
To K. J. Nadeau: NCRR K23 RR-020038-01, Colorado CTSI Co-Pilot Grant TL1 RR-025778, NIH/NIDDK 1R56 DK-088971-01, JDRF5-2008-291, ADA 7-11-CD-08. To M. Cree-Green: Thrasher Pediatric Research Foundation Mentored Pilot Grant, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR-025778, AHA 13CRP14120015, Pediatric Endocrinology Fellowship training grant, NIDDK T32 DK-063687, Pediatric Endocrine Society Women's Health Fellowship, BIRCWH K12 HD-057022. To J. E. Reusch: VA Merit Review BX002046, Center for Women's Health Research UCSOM; HL-014985 and TR-000154. This research was also supported by Adult CTRC NIH Grant M01 RR-00051, Pediatric CTRC NIH Grant 5MO1 RR-00069, and NIH/NCRR Colorado CTSI Grant UL1 RR-025780.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C.-G., B.R.N., M.S.B., J.E.R., and K.J.N. conception and design of research; M.C.-G., G.C., L.N., A.B., and M.S.B. performed experiments; M.C.-G., B.R.N., G.C., L.N., A.B., L.P., and K.J.N. analyzed data; M.C.-G., B.R.N., A.B., L.P., J.E.R., and K.J.N. interpreted results of experiments; M.C.-G. and L.P. prepared figures; M.C.-G. drafted manuscript; M.C.-G., B.R.N., G.C., L.N., A.B., M.S.B., L.P., J.E.R., and K.J.N. edited and revised manuscript; M.C.-G., B.R.N., G.C., L.N., A.B., M.S.B., L.P., J.E.R., and K.J.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants and their families for participating.
REFERENCES
- 1.Angioni S, Sanna S, Magnini R, Melis GB, Fulghesu AM. The quantitative insulin sensitivity check index is not able to detect early metabolic alterations in young patients with polycystic ovarian syndrome. Gynecol Endocrinol 27: 468–474, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86: 66–71, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bamman MM, Caruso JF. Resistance exercise countermeasures for space flight: implications of training specificity. J Strength Condition Res 14: 45–49, 2000. [PubMed] [Google Scholar]
- 4.Bjorntorp P. The android woman—a risky condition. J Intern Med 239: 105–110, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 20: 291–302, 1996. [PubMed] [Google Scholar]
- 6.Cara JF. Insulin-like growth factors, insulin-like growth factor binding proteins and ovarian androgen production. Horm Res 42: 49–54, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 94: 157–163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 192: 585–594, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Cree-Green M, Newcomer BR, Brown M, Hull A, West AD, Singel D, Reusch JE, McFann K, Regensteiner JG, Nadeau KJ. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med Sci Sports Exerc 46: 2030–2036, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes 64: 383–392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Green JM, Mlcak R, Aarsland A, Wolfe RR. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am J Surg 196: 234–239, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree MG, Newcomer BR, Herndon DN, Qian T, Sun D, Morio B, Zwetsloot JJ, Dohm GL, Fram RY, Mlcak RP, Aarsland A, Wolfe RR. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab (Lond) 4: 9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 83: 4420–4425, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18: 774–800, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 65: 499–507, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38: 1165–1174, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest 96: 801–810, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksen MB, Glintborg D, Nielsen MF, Jakobsen MA, Brusgaard K, Tan Q, Gaster M. Testosterone treatment increases androgen receptor and aromatase gene expression in myotubes from patients with PCOS and controls, but does not induce insulin resistance. Biochem Biophys Res Commun 451: 622–626, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen MB, Minet AD, Glintborg D, Gaster M. Intact primary mitochondrial function in myotubes established from women with PCOS. J Clin Endocrinol Metab 96: E1298–E1302, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab 94: 4923–4930, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. [PubMed] [Google Scholar]
- 22.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol 25: 335–343, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Fulghesu A, Magnini R, Portoghese E, Angioni S, Minerba L, Melis GB. Obesity-related lipid profile and altered insulin incretion in adolescents with polycystic ovary syndrome. J Adolesc Health 46: 474–481, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrom V, Flyvbjerg A, Hermann AP. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin and body composition in hirsute PCOS patients and controls. Eur J Endocrinol 155: 337–345, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Guo ZK. Intramyocellular lipid kinetics and insulin resistance. Lipids Health Dis 6: 18, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchison SK, Teede HJ, Rachon D, Harrison CL, Strauss BJ, Stepto NK. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 55: 1424–1434, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Item F, Heinzer-Schweizer S, Wyss M, Fontana P, Lehmann R, Henning A, Weber M, Boesiger P, Boutellier U, Toigo M. Mitochondrial capacity is affected by glycemic status in young untrained women with type 1 diabetes but is not impaired relative to healthy untrained women. Am J Physiol Regul Integr Comp Physiol 301: R60–R66, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 59: 89–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Res Med 14: 26–30, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier RL. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed 13: 14–27, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 98: 4565–4592, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. [DOI] [PubMed] [Google Scholar]
- 34.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 92: 430–436, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 91: 1714–1722, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Minet AD, Gaster M. ATP synthesis is impaired in isolated mitochondria from myotubes established from type 2 diabetic subjects. Biochem Biophys Res Commun 402: 70–74, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16: 347–363, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16: 347–363, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JEB. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 95: 513–521, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagamani M, Van Dinh T, Kelver ME. Hyperinsulinemia in hyperthecosis of the ovaries. Am J Obstet Gynecol 154: 384–389, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72: 83–89, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20: 336–346, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Nur MM, Newman IM, Siqueira LM. Glucose metabolism in overweight Hispanic adolescents with and without polycystic ovary syndrome. Pediatrics 124: e496–e502, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 87: 1017–1023, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79: 265–271, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Rabol R, Svendsen PF, Skovbro M, Boushel R, Schjerling P, Nilas L, Madsbad S, Dela F. Skeletal muscle mitochondrial function in polycystic ovarian syndrome. Eur J Endocrinol 165: 631–637, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. J Appl Physiol 87: 2068–2072, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Romualdi D, Giuliani M, Draisci G, Costantini B, Cristello F, Lanzone A, Guido M. Pioglitazone reduces the adrenal androgen response to corticotropin-releasing factor without changes in ACTH release in hyperinsulinemic women with polycystic ovary syndrome. Fertil Steril 88: 131–138, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF 3rd, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 371: 1131–1141, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Sirikul B, Hunter GR, Larson-Meyer DE, Desmond R, Newcomer BR. Relationship between metabolic function and skeletal muscle fatigue during a 90 s maximal isometric contraction. Physiol Appliq Nutr Metab 32: 394–399, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, Brusgaard K, Beck-Nielsen H, Hojlund K. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 56: 2349–2355, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Skov V, Glintborg D, Knudsen S, Tan Q, Jensen T, Kruse TA, Beck-Nielsen H, Hojlund K. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS One 3: e2466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson JR, Swanson SA, Casale GP, Johanning JM, Papoutsi E, Koutakis P, Miserlis D, Zhu Z, Pipinos II. Gastrocnemius mitochondrial respiration: are there any differences between men and women? J Surg Res 185: 206–211, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Boogaart A. 96 MRUI MANUAL V.3. A User's Guide to the Magnetic Resonance User Interface Software Package. Delft: Delft Technical Univ. Press, 1997. [Google Scholar]
- 58.Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE, Williams OD, Siscovick DS, Bibbins-Domingo K. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol 117: 6–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92: 10619–10623, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]