Abstract
Thromboxane A2, an arachidonic acid-derived eicosanoid generated by thromboxane synthase (TBXAS), plays critical roles in hemostasis and inflammation. However, the contribution of thromboxane A2 to obesity-linked metabolic dysfunction remains incompletely understood. Here, we used in vitro and mouse models to better define the role of TBXAS in metabolic homeostasis. We found that adipose expression of Tbxas and thromboxane A2 receptor (Tbxa2r) was significantly upregulated in genetic and dietary mouse models of obesity and diabetes. Expression of Tbxas and Tbxa2r was detected in adipose stromal cells, including macrophages. Furthermore, stimulation of macrophages with interferon-γ or resistin factors known to be upregulated in obesity induced Tbxas and Tbxa2r expression. Mice lacking Tbxas had similar weight gain, food intake, and energy expenditure. However, loss of Tbxas markedly enhanced insulin sensitivity in mice fed a low-fat diet. Improvement in glucose homeostasis was correlated with the upregulated expression of multiple secreted metabolic regulators (Ctrp3, Ctrp9, and Ctrp12) in the visceral fat depot. Following a challenge with a high-fat diet, Tbxas deficiency led to attenuated adipose tissue fibrosis and reduced circulating IL-6 levels without adipose tissue macrophages being affected; however, these changes were not sufficient to improve whole body insulin action. Together, our results highlight a novel, diet-dependent role for thromboxane A2 in modulating peripheral tissue insulin sensitivity and adipose tissue fibrosis.
Keywords: adipose tissue, eicosanoid, thromboxane, fibrosis, obesity, diabetes, C1q/tumor necrosis factor-related protein
arachidonic acids released from the plasma membrane by phospholipases can be converted to eicosanoids, a class of lipids that includes the prostaglandins, thromboxane, and leukotrienes (28, 50). These signaling lipids play pivotal and pleotropic roles in wide-ranging physiological processes, including development, tissue homeostasis, immunity, inflammation, and reproduction (15). Dysregulated production of eicosanoids underlies the pathogenesis of many diseases, such as allergic inflammation, atherosclerosis, and cardiovascular disease (36, 43, 51, 56). Consequently, enzymes involved in the synthesis of eicosanoids are major targets of pharmaceutical drugs, including the widely prescribed nonsteroidal anti-inflammatory drugs (NSAID) (6, 44).
Whereas leukotrienes are generated through the lipoxygenase pathway, the prostanoids (prostaglandins and thromboxane) are generated through the cyclooxygenase (COX) pathway (15). COX-1 and COX-2 are the two major enzyme isoforms that convert arachidonic acid to prostaglandin H2 (PGH2), which in turn serves as a substrate for the synthesis of other prostaglandins and thromboxane (50). Whereas COX-1 is widely and constitutively expressed, COX-2 is the inducible isoform with a more restricted tissue distribution (12); both of these enzymes are targeted by NSAID such as aspirin (46, 59).
Recent studies using cyclooxygenase-2 (Cox-2)-deficient mice showed that arachidonic acid-derived eicosanoids such as 15d-PGJ2 are important for adipocyte differentiation in vivo (16). Loss of COX-2 reduces body weight in aged (>8 mo old) mice and attenuates adipose tissue inflammation (16). The COX-2 enzyme, normally found at very low levels in immune cells, is induced by inflammatory stimuli. Through enzymatic and nonenzymatic pathways, COX-2-derived PGH2 is further converted to other prostanoids with distinct functions, including PGD2, PGE2, PGI2, PGJ2, PGF2α, and thromboxane A2 (60). Thus, in Cox-2-deficient mice, synthesis of multiple types of prostanoids may be affected. As such, the contribution of specific eicosanoids to obesity-linked metabolic dysfunctions remains incompletely defined.
Thromboxane synthase (TBXAS) catalyzes the conversion of PGH2 to thromboxane A2 (11, 22, 33, 49). Because of its short half-life (∼30 s) and rapid conversion to the inactive form (thromboxane B2), thromboxane A2 acts locally in an autocrine or paracrine fashion (47). By binding to the G protein-coupled thromboxane A2 receptor (TBXA2R) on vascular endothelial and smooth muscle cells, thromboxane A2 regulates hemostasis by modulating platelet aggregation and vasoconstriction (24, 47). Indeed, targeted deletion of Tbxas or Tbxa2r in mice results in hemostasis defects (54, 68). Although platelets produce thromboxane in clotting blood, the major source of thromboxane in inflammation is derived from immune cells (23, 57). Mice lacking TBXA2R have reduced inflammatory response to tissue injury (55). Excess thromboxane A2 has also been linked to atherosclerosis (27, 31), glomerulonephritis (42), and hypertension (8, 13). In humans, serum levels of thromboxane B2 (a stable metabolite of thromboxane A2) are found to be significantly elevated in obese subjects relative to lean individuals (18); paradoxically, in morbidly obese (average BMI of 49) but insulin-sensitive subjects, serum thromboxane B2 levels are found to be lower than in healthy lean individuals (18). In the context of diabetes, both type 1 and type 2 diabetic individuals have higher serum thromboxane B2 levels (41); the production of thromboxane B2 is also correlated with fasting plasma glucose and hemoglobin A1c (Hb A1c). In studies involving obese females with elevated thromboxane levels, weight loss or pioglitazone treatments also result in significant reduction of urinary thromboxane levels (4, 9). Although the role of thromboxane in obesity-linked metabolic dysregulation has not been examined, the correlative studies in humans prompted us to further explore its metabolic function in the context of obesity and diabetes.
One of the hallmarks of obesity is the striking recruitment of proinflammatory macrophages into adipose tissue and the ensuing inflammatory sequela (21, 25, 64, 67). Elevated expression of proinflammatory cytokines such as TNFα, IL-1β, and IL-6 by infiltrated macrophages contributes to chronic low-grade inflammation and adipose tissue insulin resistance and dysfunction (25). Given that thromboxane A2 is produced by activated macrophages (7, 57) and has potent proinflammatory activity (56), this study aimed to uncover the role of this lipid mediator in obesity-linked metabolic dysfunction using a whole body knockout mouse model devoid of the key terminal enzyme (TBXAS) that synthesizes thromboxane A2.
EXPERIMENTAL PROCEDURES
Mice.
All animal experiments were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. Male wild-type (WT), leptin-deficient obese (ob/ob), and Tbxas+/− mice (all on a C57BL/6J genetic background) were obtained from The Jackson Laboratory (Bar Harbor, ME) and were allowed to acclimatize to the animal facility for ≥1 wk. Tbxas−/− knockout (KO) mice and Tbxas+/+ WT littermate controls were generated by crossing Tbxas+/− heterozygous breeding pairs. The genotypes of Tbxas WT, heterozygous, and KO mice were confirmed by PCR (68). Age- and sex-matched mice of both sexes were used in all studies unless otherwise stated. Male and female Tbxas WT and KO mice from 4 to 24 wk old were used. Laboratory mice consumed a standard chow diet (no. 5001; Lab Diet, St. Louis, MO), had free access to water, and were housed in polycarbonate cages on a 12-h light-dark photocycle. Four-week-old C57BL/6J male mice or Tbxas WT and KO mice were fed a high-fat diet (HFD; 60 kcal% derived from fat, D12492; Research Diets, New Brunswick, NJ) or a matched control low-fat diet (LFD; 10 kcal% derived from fat, D12450B; Research Diets) for 12–14 wk. Body weights of Tbxas WT and KO mice were measured weekly. To assess direct insulin action in vivo, a subset of WT and KO mice from each diet group was injected with saline control or insulin (1 U/kg of body wt) via intraperitoneal (ip) route 15 min before euthanization. Epididymal white adipose tissue (eWAT), liver, and skeletal muscle were quickly removed for RNA and protein extraction or for histological study. Tissues were collected, weighed, snap-frozen in liquid nitrogen, and stored at −80°C. Blood samples were collected for serum analysis. The fed, fasted, refed experiments in WT C57BL/6J male mice were performed as described previously (39).
Isolation of primary adipocytes and stromal vascular cells from adipose tissue.
Twenty-week-old C57BL/6J WT male mice fed a standard laboratory chow were used to obtain primary adipocytes and stromal vascular cells [stromal vascular fraction (SVF)] from epididymal white adipose tissue, as described previously (65).
Cell culture.
Mouse 3T3-L1 fibroblasts were cultured and differentiated into adipocytes, as described previously (63). Mouse RAW264.7 macrophage cells (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS (Invitrogen) and antibiotics. RAW264.7 macrophages or differentiated 3T3-L1 adipocytes were stimulated with PBS control, IL-1β (2 ng/ml), IL-6 (2 ng/mL), TNFα (2 ng/ml), IFNγ (50 ng/ml), or resistin (100 ng/ml) for 6 h. Total RNA was isolated, reverse-transcribed, and subjected to quantitative real-time PCR (qRT-PCR) analysis of Tbxas and Tbxa2r expression.
RNA isolation and real-time PCR analysis.
Total RNA was isolated using Trizol reagent (Life Technologies, Carlsbad, CA) and reverse-transcribed using GoScript Reverse Transcriptase (Promega, Madison, WI). Ten nanograms of cDNA from each sample was analyzed by quantitative real-time PCR on a CFX Connect system (Bio-Rad Laboratories, Hercules, CA). Samples were analyzed in 25-μl reactions using SYBR Green PCR master mix per the manufacturer's instructions (Applied Biosystems/Life Technologies). Expression was normalized to 18S rRNA for mouse samples. Primer sequences are listed in Table 1. The primer sequences used to assess Ctrp1, Ctrp3, Ctrp9, and Ctrp12 gene expression in adipose tissue have been described previously (62, 65, 66).
Table 1.
Primers for quantitative real-time PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Tbxas | TACCATAGTGACTGTGACTCTGC | GGTGCCTGATGCCCAACTT |
| Tbxa2r | GTGGTCTTCGGGCTCATATTC | CCCACGAGCTGAACCATCAT |
| 18S rRNA | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA |
| F4/80 | CCCCAGTGTCCTTACAGAGTG | GTGCCCAGAGTGGATGTCT |
| Cd11c | CTGGATAGCCTTTCTTCTGCTG | GCACACTGTGTCCGAACTCA |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Col1 | GTGCTCCTGGTATTGCTGGT | AAGGACCATCCCACTGTCTG |
| Col3 | GGGTTTCCCTGGTCCTAAAG | CCTGGTTTCCCATTTTCTCC |
| Col6 | GATGAGGGTGAAGTGGGAGA | CAGCACGAAGAGGATGTCAA |
Tbxas, thromboxane synthase; Tbxa2r, thromboxane A2 receptor; Col1, -3, and -6, collagen type 1, 3, and 6, respectively.
Cell lysate preparation and Western blotting.
Frozen mouse tissues were thawed and minced in ice-cold lysis buffer (20 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, and 10% glycerol) supplemented with PhosSTOP phosphatase inhibitor cocktail (Roche, Basel, Switzerland) and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Tissues were further disrupted and homogenized with a Benchmark D1000 tissue homogenizer. Tissue lysates were centrifuged at 10,000 g for 20 min at 4°C to remove insoluble materials. Protein concentration was determined using the Bradford protein assay (Sigma-Aldrich). Twenty micrograms of protein lysate was separated on 10% Bis-Tris NuPAGE gels (Invitrogen). Western blots and quantifications were carried out as described previously (62). Phospho-Akt (Thr308) and Akt antibodies were obtained from Cell Signaling Technology.
Histology.
Formalin-fixed, paraffin-embedded white adipose tissue sections were stained with hemotoxylin and eosin at the Pathology Core facility at the Johns Hopkins University School of Medicine. Images were captured with a Zeiss Axioplan upright microscope with a Zeiss Axiocam color CCD camera (Carl Zeiss Microscopy, Thornwood, NY). Masson's trichrome staining (AML Laboratories, Baltimore, MD) was performed on paraffin-embedded tissue sections to visualize collagen deposition in the extracellular matrices of adipose tissue.
Glucose and insulin tolerance tests.
Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed as described previously (39) on Tbxas WT and KO mice fed a HFD or LFD for 10–20 wk. For GTT, mice were fasted overnight before ip injection of 1 g glucose/kg body wt. Blood was collected via tail bleed before and after injection, and glucose concentrations were measured using a glucometer (BD Biosciences, San Jose, CA) at the indicated time point. For ITT, food was removed 2 h before ip injection with 1 U insulin/kg body wt. Blood glucose concentrations were measured at the indicated time points.
Body composition analysis.
Body composition of Tbxas WT and KO mice was measured using a whole body EchoMRI NMR instrument (Echo Medical Systems, Waco, TX) housed at the Molecular and Comparative Pathobiology Phenotyping Core facility at the Johns Hopkins University School of Medicine. EchoMRI analyses measured fat mass, lean mass, and water content.
Indirect calorimetry.
Tbxas WT and KO mice (n = 10/group) were used for simultaneous assessments of daily body weight change, energy intake (corrected for spillage), and whole body metabolic profile in an open-flow indirect calorimeter [Comprehensive Laboratory Animal Monitoring System (CLAMS); Columbus Instruments, Columbus, OH]. Data were collected for 3 days to confirm acclimation to the calorimetry chambers (stable body weights and food intakes), and data from day 4 in CLAMS were analyzed. Rates of oxygen consumption (V̇o2; ml·kg lean mass−1·h−1), carbon dioxide production (V̇co2), physical activity, and food intake were measured as described previously (37, 38). Average metabolic values were calculated per subject and averaged across subjects for statistical analysis.
Blood chemistry analysis.
Tail vein blood samples were collected in the morning (10 AM) from mice that were fasted for 15 h during the dark cycle (7 PM-10 AM). Samples were allowed to clot on ice and then centrifuged for 10 min at 10,000 g. Serum samples were stored at −80°C. Serum insulin, leptin, adiponectin, IL-6, IL-10, TNFα (Millipore, Billerica, MA), and monocyte chemoattractant protein-1 (MCP-1; R & D Systems, Minneapolis, MN) levels were measured by ELISA according to the manufacturer's instructions. Serum triglycerides and nonesterified free fatty acids were measured using a Wako kit. Serum thromboxane B2 (a stable metabolite of thromboxane A2) was measured using an ELISA kit (CSB-E08048m; Cusabio) according to the manufacturer's instructions.
Statistical analysis.
Two-way ANOVA and Student's t-tests were used to determine significant differences between groups. Statistical analyses were performed with GraphPad Prism software (GraphPad Prism, San Diego, CA), and values were considered significant at P < 0.05. All data are presented as means ± SE.
RESULTS
Metabolic perturbations alter Tbxas and Tbxa2r expression in mice.
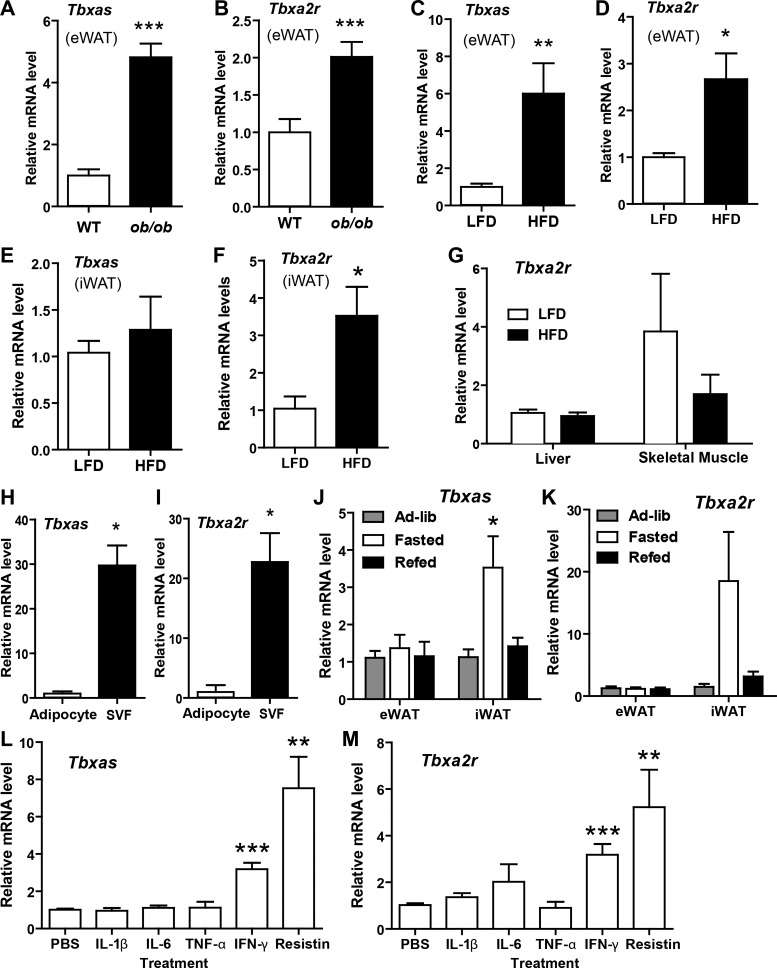
To establish the physiological relevance of thromboxane A2 to metabolism, we first examined whether changes in metabolic states affect the expression of Tbxas and Tbxa2r. In a genetic model of severe obesity, i.e., leptin-deficient ob/ob mice, Tbxas and Tbxa2r expression was upregulated in epididymal white adipose tissue (Fig. 1, A and B). In a diet-induced obese (DIO) mouse model more akin to the common form of human obesity, the expression of Tbxas was upregulated in visceral (epididymal) but not subcutaneous (inguinal) white adipose tissue (Fig. 1, C and E). The expression of thromboxane receptor (Tbxa2r), however, was similarly upregulated in both visceral (epididymal) and subcutaneous (inguinal) white adipose tissue of DIO mice (Fig. 1, D and F) mouse models. In contrast to adipose tissue, the expression of Tbxa2r in liver and skeletal muscle was not different between mice fed an LFD or HFD (Fig. 1G). As expected based on their distribution in platelets and immune cells (22, 57), most Tbxas and Tbxa2r transcripts found in adipose tissue were produced by stromal cells (referred to as the stromal vascular fraction SVF) rather than mature adipocytes (Fig. 1, H and I). Interestingly, acute metabolic changes, such as an overnight fast, also induced Tbxas and Tbxa2r expression in subcutaneous (inguinal) but not visceral (epididymal) fat depots (Fig. 1, J and K).
Fig. 1.
Relative expression of thromboxane synthase (Tbxas) and thromboxane A2 receptor (Tbxa2r) in mice and a macrophage cell line. A–F: expression of Tbxas and Tbxa2r mRNA expression in epididymal white adipose tissue (eWAT) of lean wild-type (WT; n = 10) and leptin-deficient obese (ob/ob; n = 10) mice (A and B); eWAT (C and D), subcutaneous (inguinal) white adipose tissue (iWAT; E and F), liver, and skeletal muscle (Tbxa2r only; G) of mice fed a low-fat diet (LFD; n = 7) or high-fat diet (HFD; n = 7); and isolated primary adipocytes or cells of the stromal vascular fraction (SVF; n = 3) (H and I). J and K: expression of Tbxas and Tbxa2r mRNA expression in eWAT and iWAT of chow-fed male mice under fasted, refed, or ad libitum conditions (n = 10/group). L and M: expression of Tbxas (L) and Tbxa2r (M) mRNA in cultured RAW264.7 macrophage cells treated with recombinant IL-1β (2 ng/ml), IL-6 (2 ng/ml), TNFα (2 ng/ml), IFNγ (50 ng/ml), or resistin (100 ng/ml) for 6 h (n = 6). Expression data were normalized to 18S rRNA in each sample. All data are expressed as means ± SE. *P < 0.05 compared with ad libitum group; **P < 0.01; ***P < 0.001.
Proinflammatory cytokines upregulate Tbxas and Tbxa2r expression in macrophages in vitro.
In the obese state, local expression of proinflammatory cytokines is upregulated in adipose tissue (21, 25, 64). We examined whether the same factors that promote inflammation and insulin resistance would alter the expression of Tbxas and Tbxa2r in macrophages, which are known to infiltrate adipose tissue in the obese state (21, 64, 67) and serve as a major source of thromboxane A2 in inflammation (56). RAW264.7 macrophages stimulated with recombinant IFNγ or resistin for 6 h significantly upregulated expression of both Tbxas and Tbxa2r (Fig. 1, L and M). No effects were seen when cells were treated with IL-1β, IL-6, and TNFα. Consistent with macrophages, rather than adipocytes, being a major producer of thromboxane A2 in adipose tissue, expression of Tbxas and Tbxa2r was low and unchanged in 3T3-L1 adipocytes upon differentiation (data not shown). Tbxas and Tbxa2r expression in differentiated adipocytes was also unaffected by treatment with IL-1β, IL-6, TNFα, IFN-γ, or resistin (data not shown).
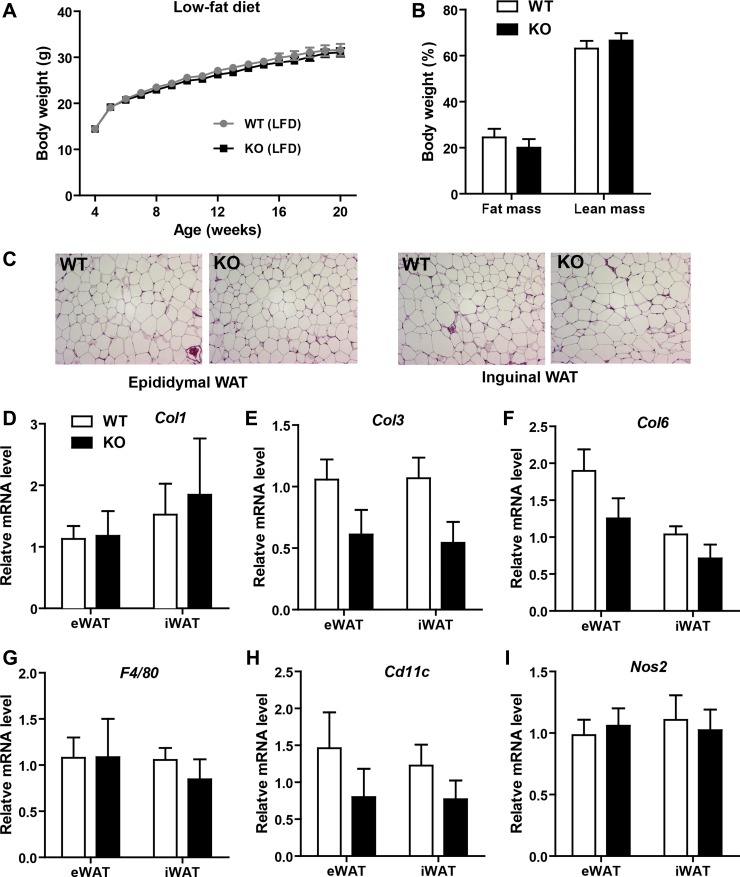
Impact of Tbxas deficiency on body weight, adiposity, and adipose tissue histology of mice fed a LFD.
A Tbxas-deficient mouse model (68) was used to determine the contribution of Tbxas to local (adipose tissue) and systemic energy balance in normal or pathophysiological contexts of diet-induced obesity. Although thromboxane A2 is the primary physiological agonist for TBXA2r (24), other molecules such as PGH2, isoprostanes, and hydroxyeicosatetranenoic acids are also potent agonists for TBXA2r (3, 17); furthermore, epoxyeicosatrienoic acids can act as endogenous antagonists of TBXA2r (5). Based on this consideration, we chose to use Tbxas, rather than Tbxa2r, KO mice to address the contribution of thromboxane A2 to metabolic homeostasis. Four-week-old Tbxas WT and KO mice were fed an HFD or LFD for 20 wk. We observed no differences in body weight gain over time between WT or KO mice fed an LFD (Fig. 2A). Body composition analysis using NMR indicated no differences in fat or lean mass between the two groups of mice (Fig. 2B). Visceral (epididymal) and subcutaneous (inguinal) white adipose tissue histologies were not different between WT and KO animals (Fig. 2C). Although the gross morphology of adipose tissue looked comparable, we examined the expression of fibrotic (Col1, Col3, and Col6), adipose macrophage (F4/80 and Cd11c), and proinflammatory macrophage M1 (Nos2) marker gene expression in both the visceral and subcutaneous fat depots of Tbxas WT and KO mice (Fig. 2, D–I) and did not observe significant differences between the two groups.
Fig. 2.
Metabolic parameters of Tbxas knockout (KO) mice fed a LFD. A: body weight gain over time of WT and Tbxas KO male mice fed a LFD (n = 10). B: NMR body composition analysis of fat and lean mass in WT and KO mice (n = 10). C: adipose tissue histology (hematoxylin and eosin stain) of WT and KO mice. D–I: expression of collagen (Col)1 (D), Col3 (E), Col6 (F), F4/80 (G), Cd11c (H), and Nos2 (I) in eWAT and iWAT of Tbxas WT and KO mice (n = 10). Expression data were normalized to 18S rRNA in each sample. All data are expressed as means ± SE.
Tbxas deficiency improves peripheral tissue insulin action in mice fed a LFD.
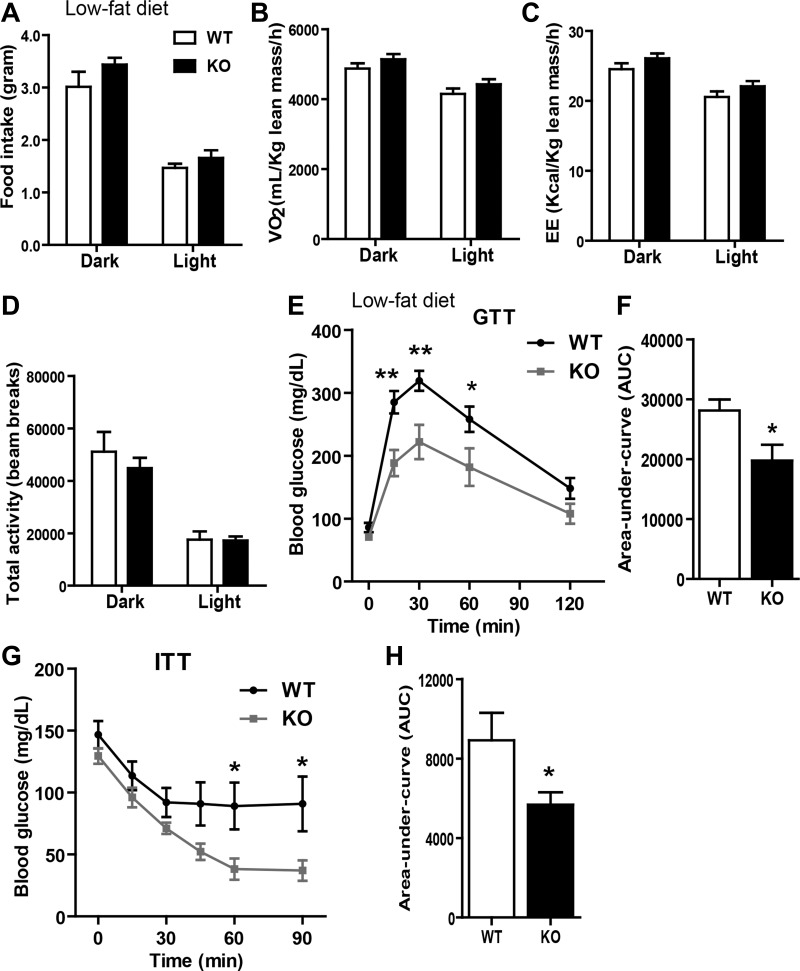
We next performed indirect calorimetry analysis on LFD-fed mice. No differences were observed in food intake, V̇o2, energy expenditure, or physical activity levels between WT and KO mice (Fig. 3, A–D). Despite similar body weight and adiposity, KO mice had enhanced insulin sensitivity, as indicated by a much greater rate of glucose disposal in the peripheral tissue (Fig. 3, E and F); the magnitude of insulin secretion during GTT was not significantly different between the two groups (data not shown). Improved insulin action in the Tbxas KO mice was further confirmed by insulin tolerance tests (Fig. 3, G and H). Next, we injected insulin into a separate cohort of LFD-fed WT and KO animals to directly assess the activation of insulin signaling in three major metabolic tissues. Before insulin administration, we observed an approximately twofold increase in basal Akt phosphorylation (a metric of insulin signaling) in the adipose tissue but not liver or skeletal muscle of KO mice relative to WT controls (data not shown). At 15 min after insulin administration we observed a robust insulin-stimulated Akt phosphorylation in all three tissues in both Tbxas WT and KO mice. Although the levels of Akt phosphorylation were higher in the KO mice (after normalization to total Akt), the difference fell short of being statistically significant, and this may be attributed to a small sample size (n = 3) and the length of insulin stimulation (15 min).
Fig. 3.
Tbxas deficiency improves glucose homeostasis in LFD-fed mice. A: food intake measurements of LFD-fed Tbxas WT and KO mice (n = 10). B–D: indirect calorimetry analysis of whole body oxygen consumption rate (V̇o2; B), energy expenditure (EE; C), and physical activity (D) of Tbxas WT and KO mice (n = 10). E: blood glucose levels in Tbxas WT and KO mice subjected to an intraperitoneal glucose tolerance test (GTT; n = 8). F: quantification of cumulative glucose clearance [area under the curve (AUC)], as shown in E. G: blood glucose levels in Tbxas WT and KO mice subjected to an insulin tolerance test (ITT; n = 8). H: quantification of cumulative glucose clearance (AUC), as shown in G. All data are expressed as means ± SE. *P < 0.05; **P < 0.01.
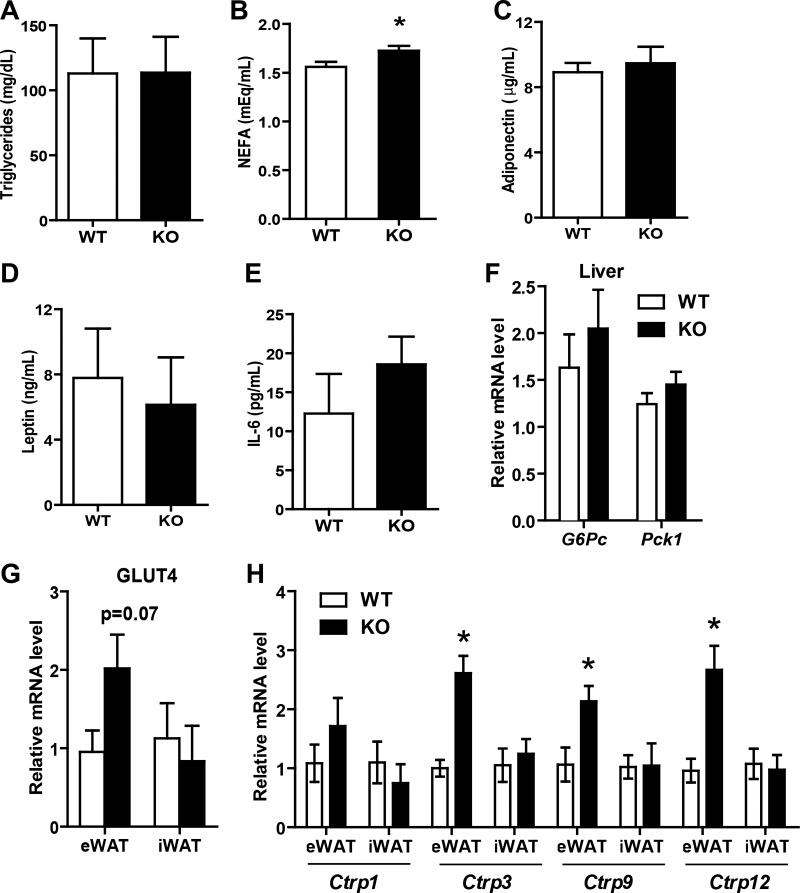
Enhanced insulin sensitivity in LFD-fed KO mice, as judged by GTTs and ITTs, appeared to be independent of serum triglyceride, adiponectin, leptin, and IL-6 levels (Fig. 4, A–E). Only serum nonesterified fatty acid (NEFA) levels were modestly higher in KO mice compared with WT controls (Fig. 5B). Examination of hepatic gluconeogenic gene (G6Pc and Pck1) expression revealed no differences between WT and KO mice (Fig. 4F). Expression of insulin-responsive glucose transporter 4 (Glut4) gene was higher in the visceral (epididymal) adipose tissue of Tbxas KO mice (Fig. 4G). We have shown previously that secreted proteins of the C1q family, the C1q/TNF-related proteins (CTRPs), play important roles in regulating insulin sensitivity and glucose and lipid metabolism in vivo (37–40, 61, 62). Therefore, we also examined the expression of Ctrp in adipose tissue of Tbxas WT and KO mice; we focused our analysis on Ctrp1, Ctrp3, Ctrp9, and Ctrp12 based on what was described previously in vivo metabolic functions (37–40, 61, 62). Three of the Ctrp transcripts were upregulated in visceral (epididymal) but not subcutaneous (inguinal) white adipose tissue of Tbxas KO mice (Fig. 4H).
Fig. 4.
Serum metabolite and adipokine levels and metabolic gene expression in Tbxas KO mice fed a LFD. A–E: fasting serum triglycerides (A), nonesterified fatty acids (NEFA; B), adiponectin (C), leptin (D), and IL-6 (E) in LFD-fed Tbxas WT and KO mice (n = 10). F: expression of gluconeogenic genes (G6Pc and Pck1) in the liver of WT and KO mice (n = 10). G and H: expression of glucose transporter 4 (Glut4; G) and C1q/TNF-related protein (Ctrp; H) in eWAT and iWAT of WT and KO mice (n = 10). All data are expressed as means ± SE. *P < 0.05.
Fig. 5.
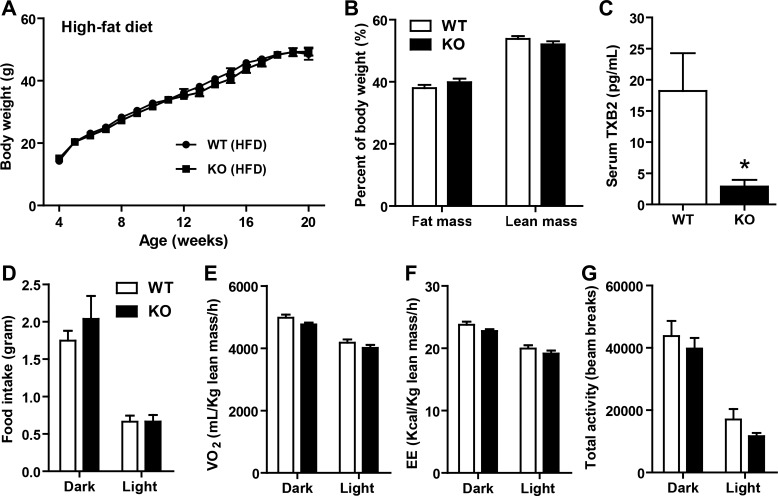
Metabolic parameters of Tbxas KO mice fed a HFD. A: body weight gain over time of WT and Tbxas KO male mice fed a HFD (n = 10). B: NMR body composition analysis of fat and lean mass in WT and KO mice (n = 10). C: serum thromboxane B2 (TBX2) in WT and KO mice (n = 10). D: food intake measurements of HFD-fed Tbxas WT and KO mice (n = 10). E–G: indirect calorimetry analysis of whole body V̇o2 (E), EE (F), and physical activity (G) of Tbxas WT and KO mice (n = 10). All data are expressed as means ± SE. All data are expressed as means ± SE. *P < 0.05.
Tbxas deficiency attenuates adipose tissue fibrosis in HFD-fed mice.
We next subjected Tbxas WT and KO mice to metabolic stress induced by high-fat feeding. When Tbxas KO mice were challenged with a HFD for a period of 16 wk, we observed no differences in body weight or fat and lean mass between WT and KO mice (Fig. 5, A and B). As expected from TBXAS-deficient mice, serum thromboxane B2, a stable metabolite of thromboxane A2, was largely if not completely abolished compared with WT controls (Fig. 5C). The lowest assay detection limit for thromboxane B2 in mouse serum is 2.4 pg/ml; therefore, we could not distinguish the apparent residual thromboxane B2 seen in the Tbxas KO mouse sera (∼2.8 pg/ml) from background signals. As with the LFD-fed groups, we also performed indirect calorimetry analysis on the HFD-fed animals. Tbxas KO mice fed a HFD also had similar food intake, metabolic rate (V̇o2), energy expenditure, and physical activity levels compared with WT controls (Fig. 5, D–G).
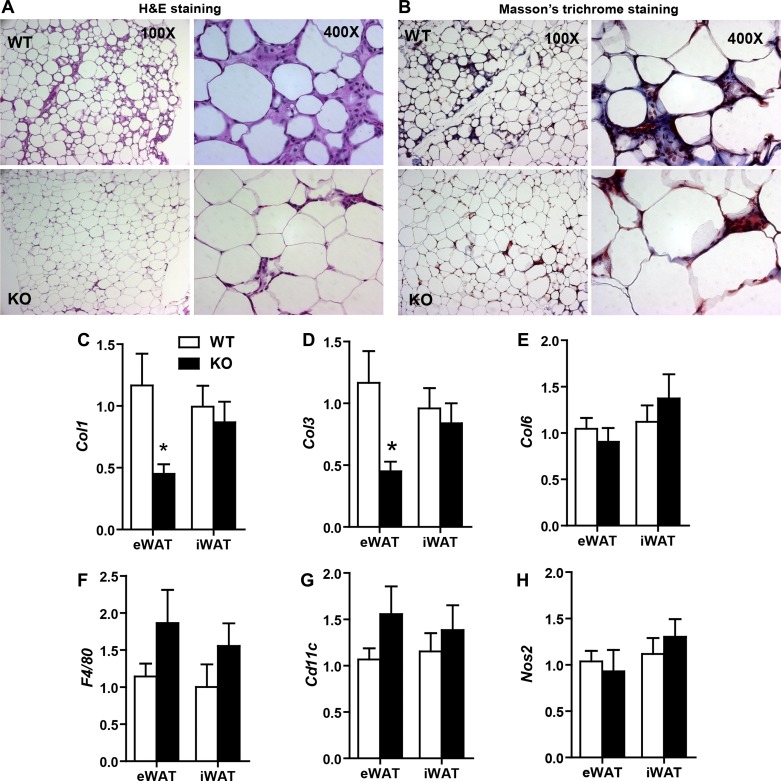
Since macrophage-derived thromboxane A2 acts locally due to its short half-life (20, 47), we further examined the local milieu of white adipose tissue. The extracellular matrix (ECM) plays an important role in adipose tissue expansion in response to excess caloric intake (26, 52). Diet-induced obesity also results in adipose tissue hypoxia and fibrosis (19). Therefore, we examined the histology and expression of multiple ECM markers in both the visceral (eWAT) and subcutaneous [inguinal WAT (iWAT)] fat depots of Tbxas WT and KO mice. Histological analysis of adipose tissue sections revealed differences between Tbxas WT and KO mice (Fig. 6, A and B). KO animals had better preservation of adipose tissue architecture, with reduced numbers of stromal vascular cells interspersed among adipocytes (Fig. 6A); the majority of the nonadipocytic cells are infiltrated immune cells, especially macrophages, as has been shown previously (64, 67). Adipose histology also demonstrated decreased deposition of fibrotic collagens (indicated by Masson's trichrome stain) compared with WT animals (Fig. 6B). Trichrome staining specifically highlights the presence of fibrillar collagens I and III, yielding a blue stain. Whereas trichrome staining of HFD-fed WT mice contained pronounced trichrome-positive “streaks” interspersed among the adipocytes, adipose tissue from KO mice revealed only thin collagen sheets surrounding each adipocyte. In support of the histology data, expression of adipose fibrosis-promoting collagen genes Col1 and Col3 was significantly reduced in eWAT (Fig. 6, C and D), whereas the adipose expression of Col6 (Fig. 6E) and Hif-1α (not shown) did not differ between the two groups of mice.
Fig. 6.
Tbxas deficiency attenuates fibrosis in adipose tissue of HFD-fed mice. A and B: representative histology images of formalin-fixed, paraffin-embedded tissue sections from epididymal adipose tissues of HFD-fed Tbxas WT and KO mice stained with hematoxylin and eosin (A) or Masson's trichrome (B), which allows visualization of collagen deposition (blue) in extracellular matrices of adipose tissue. Images are shown at ×100 and ×400 magnification. C–H: expression of Col1 (C), Col3 (D), Col6 (E), F4/80 (F), Cd11c (G), and Nos2 (H) in eWAT and iWAT white adipose tissues of Tbxas WT and KO mice (n = 10). Expression data were normalized to 18S rRNA in each sample. All data are expressed as means ± SE. *P < 0.05.
Diet-induced obesity is known to result in macrophage infiltration into the adipose compartment (64, 67). Therefore, we determined whether there is any difference in adipose macrophages between Tbxas WT and KO animals. Expression of macrophage-specific markers F4/80 and Cd11c was not significantly different in the adipose tissue of WT and KO mice (Fig. 6, F and G). The expression of Nos2, a marker of the proinflammatory M1 macrophage subtype, was also not different between WT and KO mice (Fig. 6H), nor were there differences in the adipose expression of Tnfα (data not shown). Thus, loss of Tbxas in mice had no apparent effect on the inflammatory state of adipose tissue in mice fed an HFD. In HFD-fed mice, we also examined the expression of Ctrp1, Ctrp3, Ctrp9, and Ctrp12 in eWAT and did not observe any differences between WT and KO mice (data not shown).
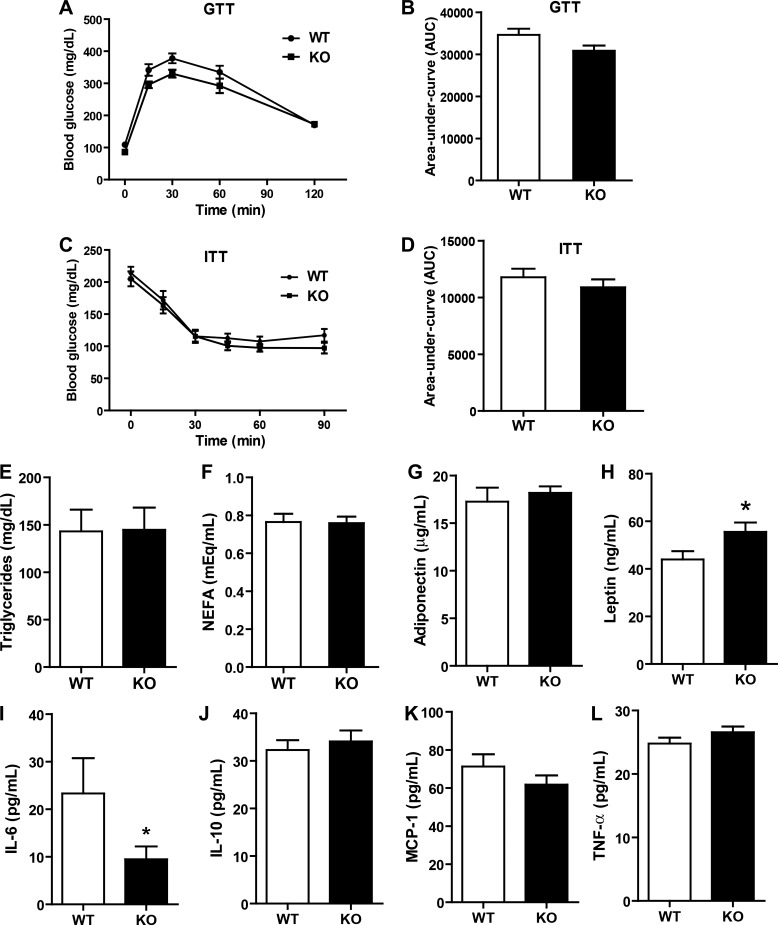
To examine the effects of Tbxas deficiency on glucose metabolism, we measured fasting (5-h fast) blood glucose concentrations every other week in Tbxas WT and KO mice. No differences were seen between the two groups over a 14-wk period (data not shown). In contrast to KO mice fed an LFD, we observed no differences in GTT and ITT between Tbxas WT and KO mice fed a HFD (Fig. 7, A–D), nor were there any differences in serum triglycerides, NEFA, adiponectin, IL-10, MCP-1, or TNFα (Fig. 7, E–G and J–L). Interestingly, serum leptin levels were higher and IL-6 levels lower in the KO group relative to WT controls (Fig. 7, H and I). Thus, enhanced insulin sensitivity seen in the LFD-fed Tbxas KO animals was abrogated when mice were subjected to high-fat feeding.
Fig. 7.
Tbxas deficiency has no metabolic impact in HFD-fed mice. A: blood glucose levels in Tbxas WT and KO mice subjected to an intraperitoneal GTT (n = 8). B: quantification of cumulative glucose clearance (AUC), as shown in A. C: blood glucose levels in Tbxas WT and KO mice subjected to an ITT (n = 8). D: quantification of cumulative glucose clearance (AUC), as shown in C. E–I: fasting serum triglycerides (E), NEFA (F), adiponectin (G), leptin (H), IL-6 (I), IL-10 (J), monocyte chemoattractant protein (MCP-1; K), and TNFα (L). All data are expressed as means ± SE. *P < 0.05.
DISCUSSION
In the present study, we sought to uncover the role of thromboxane A2 in a dietary model of obesity. We provide evidence that thromboxane A2, generated by TBXAS, contributes to whole body insulin sensitivity and obesity-linked adipose tissue fibrosis. Although loss of Tbxas had no impact on food intake, weight gain, adiposity, or energy expenditure, it enhanced insulin action in the peripheral tissue of mice fed an LFD. When challenged with an HFD, TBXAS deficiency helped attenuate adipose tissue fibrosis without any apparent effect on infiltrating macrophages. The expression of Tbxas and Tbxa2r in white adipose tissue was markedly increased in genetic and dietary mouse models of obesity. Our mouse expression data on Tbxa2 are consistent with recent human studies showing that serum thromboxane B2 levels are elevated in obese and/or diabetic subjects (18, 41) and that weight reduction from decreased caloric intake or pioglitazone treatment leads to reduced urinary thromboxane B2 levels (4, 9). Furthermore, our genetic loss-of-function studies in Tbxas KO mice provide functional evidence to support a role for thromboxane in modulating peripheral tissue insulin sensitivity and glucose homeostasis. Thus, our findings underscore the clinical relevance of thromboxane A2 to metabolic dysregulation in humans.
Leptin and ghrelin are among the many circulating hormones whose levels can be altered by fasting and refeeding. Plasma leptin levels are very low in fasted mice and increased upon refeeding (1). When leptin levels are low, as in the fasted state, the expression of both Tbxas and Tbxa2r in white adipose tissue is increased. Consistent with this, in leptin-deficient ob/ob mice, we observed a significant increase in the expression of both Tbxas and Tbxa2r. Although leptin levels are inversely correlated with Tbxas and Tbxa2r expression, we do not know whether leptin directly regulates the expression of these two genes. The leptin-deficient mice are hyperphagic and morbidly obese; thus, obesity may be responsible in part for the upregulated expression of Tbxas and Tbxa2r. In support of this, we also observed an increase in Tbxas and Tbxa2r expression in diet-induced obese mice.
In the obese state, large numbers of macrophages infiltrate adipose tissue (21, 25, 64, 67). Activated macrophages (30, 35) and to a lesser extent adipocytes produce and secrete proinflammatory cytokines. These factors in turn create a state of chronic low-grade inflammation within the adipose compartment, leading to impaired insulin action and adipocyte dysfunction (21, 25, 64, 67). Some of these proinflammatory cytokines (e.g., resistin) also induce Tbxas expression in macrophages. Recent studies suggest that macrophage populations are heterogeneous and consist of multiple subtypes (29, 30, 32). M1-type macrophages promote inflammation, whereas M2-type macrophages play an anti-inflammatory role through the cytokines they secrete (32, 34). In the context of obesity, M1-type macrophages are recruited into the adipose compartment (30). However, adipose tissue function and systemic insulin sensitivity can be significantly improved when macrophages are polarized toward the M2-type phenotype (35). Adipocytes secrete many adipokines (e.g., leptin, adiponectin, and resistin) that help maintain energy homeostasis (45). When adipocyte function is compromised by chronic local inflammation due to DIO, whole body metabolism is affected (25, 34). Since TBXAS-generated thromboxane A2 has proinflammatory activity (56) and acts locally, it may exacerbate the inflammatory state in adipose tissues. However, in Tbxas KO mice fed a LFD or HFD, we observed no differences in the inflammatory state of adipose tissue compared with WT controls.
Since adipose tissue specializes in triglyceride storage, its coordinated expansion in response to excess caloric intake is crucial to maintain lipid homeostasis and prevent aberrant lipid deposition in nonadipose tissues (e.g., liver and skeletal muscle) that can promote insulin resistance (58). HFD-induced fibrosis in the adipose compartment compromises adipose tissue expandability and hence, its capacity to handle excess dietary lipids (26, 53). In HFD-fed Tbxas KO mice, expression of fibrotic collagens (Col1 and Col3) is decreased, leading to reduced collagen deposition in the ECM. Reduced adipose tissue fibrosis, however, was not sufficient to improve whole body glucose and insulin tolerance in HFD-fed Tbxas KO mice compared with WT controls.
Because COX-derived PGH2 can be converted to different prostaglandins and thromboxane, we compared and contrasted our present findings with two recent studies, one of which involved the use of Cox-2 KO mice (16) and another that involved transplanting Cox-1+/+ and Cox-1−/− bone marrow cells into lethally irradiated WT recipient mice to reconstitute the hematopoetic compartment (48). The use of whole body Cox-2 KO mice demonstrates the importance of eicosanoids in adipocyte differentiation in vivo (16). In chow-fed Cox-2 KO mice, reduced body weight and fat mass is attributed in part to reduced adipogenesis resulting from decreased production of 15-deoxy-Δ(12,14)-PGJ2, an activating ligand for PPARγ that plays a critical transcriptional role in orchestrating the adipogenic program (14). The levels of other prostaglandins, including PGD2, PGE2, PGF2α, and 6-keto-PGF1α, are not different in the adipose tissue explants of Cox-2 WT and KO mice (16). We do not know whether thromboxane A2 was affected in Cox-2-deficient mice (16). The striking differences in body weight between Cox-2 WT and KO mice emerged when these animals aged (>8 mo); this was attributed in part to increased energy metabolism without changes in food intake of Cox-2 KO animals (16). In contrast to the Cox-2 KO animals, Tbxas WT and KO mice fed a LFD or HFD did not differ in food intake, body weight, or adiposity, although the study duration described here was less than 4 mo.
Despite the striking differences in body weight and adiposity, there appear to be no differences in nonfasting blood glucose, triglycerides, and cholesterol levels between Cox-2 WT and KO mice (16). Since glucose and insulin tolerance tests were not performed, we do not know whether the Cox-2 KO mice have improved glucose homeostasis. In contrast to the Cox-2 KO mice, loss of TBXAS improved insulin sensitivity significantly in mice fed a LFD compared with WT controls, as judged by both glucose and insulin tolerance tests. Since circulating levels of leptin, adiponectin, and IL-6 were not different between Tbxas WT and KO mice, improvements in insulin action in Tbxas KO mice were independent of these adipokines known to modulate whole body insulin sensitivity (45). Hepatic expression of gluconeogenic genes (G6Pc and Pck1) was not different between Tbxas WT and KO mice; hence, improved glucose homeostasis was likely not due to changes in hepatic glucose output. However, we did observe a higher expression of insulin-responsive Glut4 expression in the adipose tissue of KO mice. Furthermore, we observed a significant increase in the expression of Ctrp3, Ctrp9, and Ctrp12 in the visceral fat depot of LFD-fed KO animals. Given the known positive metabolic function of CTRPs in vivo (37–40, 61, 62), these observed changes may account in part for the improved glucose metabolism seen in the LFD-fed KO mice. Interestingly, the beneficial effects of improved insulin sensitivity seen in the LFD-fed Tbxas KO mice were largely masked or negated in the KO animals challenged with a HFD; this effect was independent of fasting serum triglycerides, NEFA, adiponectin, and TNFα levels and is likely due to other metabolic processes dysregulated by chronic high-fat feeding.
In addition to the adiposity phenotype, chow-fed Cox-2 KO mice also have a striking reduction in the expression of macrophage (Cd68) and inflammatory (Tnfα) markers in adipose tissue (16). In contrast to the COX-2-deficient animals, the expression of markers associated with macrophage number (F4/80 and Cd11c) and polarization (Nos2) was not different in the visceral (epididymal) or subcutaneous (inguinal) fat depots of Tbxas WT and KO mice.
In a study by Saraswathi et al. (48), mice that were transplanted with Cox-1−/− bone marrow cells had reduced immune cell-derived thromboxane B2 (a stable metabolite of thromboxane A2) and elevated fasting blood glucose, triglycerides, and cholesterol levels. Higher blood glucose was attributed partly to reduced expression and circulating levels of adiponectin as well as increased gluconeogenic gene expression in the kidney. Intriguingly, gluconeogenic gene expression was decreased in the liver of mice transplanted with Cox-1−/− bone marrow cells, which was opposite to that observed in the kidney of these animals. Insulin signaling was also unexpectedly enhanced in the skeletal muscle of mice receiving Cox-1−/− bone marrow cells. Although fasting blood glucose is higher, fasting plasma insulin and HOMA-IR (an index of insulin resistance) are not different between mice transplanted with Cox-1+/+ or Cox-1−/− bone marrow cells (48). Because glucose and insulin tolerance tests were not performed, we do not know whether these mice have altered whole body insulin sensitivity. A second major finding is the observation that mice transplanted with Cox-1−/− bone marrow cells have reduced inflammatory marker gene expression without alteration in macrophage number in the adipose tissue, suggesting a possible polarization of adipose tissue macrophages toward the proinflammatory M1 phenotype. In contrast to the Saraswathi et al. (48) study, we found that circulating levels of adiponectin were not different between Tbxas WT and KO mice fed either a LFD or HFD, nor was there any alteration in adipose tissue macrophage number or M1/M2 polarization. It would be interesting in future studies to determine whether there are any phenotypic differences between mice transplanted with Cox-1−/− or Tbxas−/− bone marrow cells. Transplanting TBXAS-deficient bone marrows into lethally irradiated mice will provide additional evidence to further confirm that macrophage-derived thromboxane A2 is indeed responsible for the observed metabolic phenotypes reported in the present study.
It is clear that the absence of thromboxane A2, due to TBXAS deficiency, results in phenotypes that are distinct from mice lacking COX-2 throughout the whole body or mice reconstituted with immune cells devoid of COX-1 enzyme. In studies by both Ghoshal et al. (16) and Saraswathi et al. (48), mice were fed a standard laboratory chow diet, and the animals had not been metabolically challenged with a HFD. We do not know whether the COX-deficient mice would develop a more pronounced or different metabolic phenotypes when subjected to high-fat feeding. In our study, Tbxas KO mice were fed either an HFD or a control LFD. Both diets were matched for all of the micronutrients, and the macronutrients (carbohydrates and fat) were also derived from the same source. The control LFD used in the present study had a lower percent fat content compared with standard laboratory chow. Thus, the comparisons made between TBXAS- and COX-deficient mice should be tempered by these considerations. In summary, our study provides evidence for the physiological relevance of thromboxane A2 in modulating insulin action and adipose tissue health, functions that are dependent on dietary context.
GRANTS
This work was supported, in whole or in part, by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-084171 (to G. W. Wong). X. Lei and S. Y. Tan were supported by a postdoctoral and predoctoral fellowship, respectively, from the American Heart Association. Q. Li was supported by a predoctoral fellowship from the China Scholarship Council. The human adipose tissue collection was supported by the Mid-Atlantic Nutrition Obesity Research Center through a grant from the NIDDK (P30-DK-072488).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.L. and G.W.W. conception and design of research; X.L., Q.L., S.R., S.Y.T., and M.M.S. performed experiments; X.L., Q.L., S.R., S.Y.T., M.M.S., and G.W.W. analyzed data; X.L., Q.L., S.R., S.Y.T., M.M.S., and G.W.W. interpreted results of experiments; X.L. and Q.L. prepared figures; X.L., Q.L., and G.W.W. drafted manuscript; S.R., S.Y.T., and M.M.S. edited and revised manuscript; J.C.M. and W.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Susan Aja for help with the indirect calorimetry studies.
REFERENCES
- 1.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation 101: 2833–2840, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Basili S, Pacini G, Guagnano MT, Manigrasso MR, Santilli F, Pettinella C, Ciabattoni G, Patrono C, Davi G. Insulin resistance as a determinant of platelet activation in obese women. J Am Coll Cardiol 48: 2531–2538, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther 328: 231–239, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs—differences and similarities. N Engl J Med 324: 1716–1725, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Brune K, Glatt M, Kalin H, Peskar BA. Pharmacological control of prostaglandin and thromboxane release from macrophages. Nature 274: 261–263, 1978. [DOI] [PubMed] [Google Scholar]
- 8.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327: 70–75, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA 288: 2008–2014, 2002. [DOI] [PubMed] [Google Scholar]
- 10.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56: 1655–1661, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Diczfalusy U, Falardeau P, Hammarstrom S. Conversion of prostaglandin endoperoxides to C17-hydroxy acids catalyzed by human platelet thromboxane synthase. FEBS Lett 84: 271–274, 1977. [DOI] [PubMed] [Google Scholar]
- 12.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J 12: 1063–1073, 1998. [PubMed] [Google Scholar]
- 13.Fitzgerald DJ, Rocki W, Murray R, Mayo G, FitzGerald GA. Thromboxane A2 synthesis in pregnancy-induced hypertension. Lancet 335: 751–754, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803–812, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Ghoshal S, Trivedi DB, Graf GA, Loftin CD. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J Biol Chem 286: 889–898, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146: 834–845, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graziani F, Biasucci LM, Cialdella P, Liuzzo G, Giubilato S, Della Bona R, Pulcinelli FM, Iaconelli A, Mingrone G, Crea F. Thromboxane production in morbidly obese subjects. Am J Cardiol 107: 1656–1661, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 72: 2994–2998, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92: 2240–2247, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Haurand M, Ullrich V. Isolation and characterization of thromboxane synthase from human platelets as a cytochrome P-450 enzyme. J Biol Chem 260: 15059–15067, 1985. [PubMed] [Google Scholar]
- 23.Higgs GA, Moncada S, Salmon JA, Seager K. The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol 79: 863–868, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 349: 617–620, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest 114: 784–794, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis RA, Austen KF. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest 73: 889–897, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem 285: 15333–15345, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta JL, Lawson D, Mehta P, Saldeen T. Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis. Proc Natl Acad Sci USA 85: 4511–4515, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261: 558–560, 1976. [DOI] [PubMed] [Google Scholar]
- 34.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol 6: 275–297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447: 1116–1120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med 357: 1841–1854, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem 287: 1576–1587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–G224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R522–R533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, Di Renzo L, Trifiro E, Mattiello T, Pitocco D, Liuzzo G, Ghirlanda G, Crea F. COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J 30: 1279–1286, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Remuzzi G, Imberti L, Rossini M, Morelli C, Carminati C, Cattaneo GM, Bertani T. Increased glomerular thromboxane synthesis as a possible cause of proteinuria in experimental nephrosis. J Clin Invest 75: 94–101, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986–1000, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336: 973–979, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci USA 72: 3073–3076, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuelsson B, Goldyne M, Granstrom E, Hamberg M, Hammarstrom S, Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem 47: 997–1029, 1978. [DOI] [PubMed] [Google Scholar]
- 48.Saraswathi V, Ramnanan CJ, Wilks AW, Desouza CV, Eller AA, Murali G, Ramalingam R, Milne GL, Coate KC, Edgerton DS. Impact of hematopoietic cyclooxygenase-1 deficiency on obesity-linked adipose tissue inflammation and metabolic disorders in mice. Metabolism 62: 1673–1685, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen RF, Tai HH. Immunoaffinity purification and characterization of thromboxane synthase from porcine lung. J Biol Chem 261: 11592–11599, 1986. [PubMed] [Google Scholar]
- 50.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69: 145–182, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res 50, Suppl: S423–S428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest 102: 1994–2001, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas DW, Rocha PN, Nataraj C, Robinson LA, Spurney RF, Koller BH, Coffman TM. Proinflammatory actions of thromboxane receptors to enhance cellular immune responses. J Immunol 171: 6389–6395, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 108: 15–23, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tripp CS, Leahy KM, Needleman P. Thromboxane synthase is preferentially conserved in activated mouse peritoneal macrophages. J Clin Invest 76: 898–901, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231: 232–235, 1971. [DOI] [PubMed] [Google Scholar]
- 60.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38: 97–120, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E779–E790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu IS, Lin SR, Huang CC, Tseng HY, Huang PH, Shi GY, Wu HL, Tang CL, Chu PH, Wang LH, Wu KK, Lin SW. TXAS-deleted mice exhibit normal thrombopoiesis, defective hemostasis, and resistance to arachidonate-induced death. Blood 104: 135–142, 2004. [DOI] [PubMed] [Google Scholar]