Abstract
Strategies to enhance weight loss with a high fat-to-lean ratio in overweight/obese older adults are important since lean loss could exacerbate sarcopenia. We examined how dietary protein distribution affected muscle protein synthesis during energy balance (EB), energy restriction (ER), and energy restriction plus resistance training (ER + RT). A 4-wk ER diet was provided to overweight/obese older men (66 ± 4 yr, 31 ± 5 kg/m2) who were randomized to either a balanced (BAL: 25% daily protein/meal × 4) or skewed (SKEW: 7:17:72:4% daily protein/meal; n = 10/group) pattern. Myofibrillar and sarcoplasmic protein fractional synthetic rates (FSR) were measured during a 13-h primed continuous infusion of l-[ring-13C6]phenylalanine with BAL and SKEW pattern of protein intake in EB, after 2 wk ER, and after 2 wk ER + RT. Fed-state myofibrillar FSR was lower in ER than EB in both groups (P < 0.001), but was greater in BAL than SKEW (P = 0.014). In ER + RT, fed-state myofibrillar FSR increased above ER in both groups and in BAL was not different from EB (P = 0.903). In SKEW myofibrillar FSR remained lower than EB (P = 0.002) and lower than BAL (P = 0.006). Fed-state sarcoplasmic protein FSR was reduced similarly in ER and ER + RT compared with EB (P < 0.01) in both groups. During ER in overweight/obese older men a BAL consumption of protein stimulated the synthesis of muscle contractile proteins more effectively than traditional, SKEW distribution. Combining RT with a BAL protein distribution “rescued” the lower rates of myofibrillar protein synthesis during moderate ER.
Keywords: aging, energy restriction, stable isotope
sarcopenia, the progressive loss of skeletal muscle mass with age, is associated with declines in strength and functional capacity, and increased risk for disability and mortality (21, 26). Progression of sarcopenic muscle loss occurs when the rate of muscle protein breakdown chronically exceeds muscle protein synthesis (MPS) (40). As potent sarcopenic “countermeasures,” protein ingestion and resistance exercise independently drive the synthesis of new muscle proteins and interact synergistically to stimulate protein synthetic rates (40). However, aging is associated with a reduced MPS response following both protein consumption (34) and resistance exercise (25). This phenomenon, termed “anabolic resistance,” has been implicated as an important factor in sarcopenic muscle loss (11, 24).
The dose of ingested protein required to maximally stimulate MPS in older adults remains to be clearly established. Nevertheless, MPS increases in a protein dose-response fashion, and doses in the range of 30–40 g of high-quality protein maximally stimulate MPS under resting conditions (34, 39, 41, 53). Older adults tend to consume protein in a “skewed” fashion, consuming almost 50% of their daily protein at the evening meal (45). Given that MPS is protein dose-responsive (34, 39, 41, 53), it has been speculated that a “balanced” per meal distribution of protein intake, with consumption of at least 30 g of protein/meal, would provide an optimal stimulation of MPS and potentially alleviate muscle loss (36, 47). The recommendation of an intake of 30 g protein/meal would necessitate a protein intake exceeding the current U.S.-Canadian recommended dietary allowance (RDA) of 0.8 g·kg−1·day−1. Nonetheless, numerous scientific committees have recommended higher protein intakes to support the maintenance/regain of muscle mass and function in older adults (3, 15, 47).
The prevalence of obesity is increasing among older adults and is often concomitant with a low skeletal muscle mass, a scenario termed “sarcopenic obesity” (4). A sustained energy deficit, achieved through a reduction in energy intake and/or increased energy expenditure, is a prerequisite for the loss of body mass; however, energy restriction (ER) alone results in weight loss comprised of both fat and lean body mass, with lean tissue generally accounting for ∼25% (50). The addition of exercise, particularly resistance training (RT), to a hypocaloric diet has been shown to be effective in retention of greater amounts of lean tissue (17, 27). Because ER-induced weight loss results in loss of fat and lean tissue this could lead to an “acceleration” of sarcopenic muscle loss. Thus, there is a need to identify strategies that facilitate simultaneous fat mass loss and muscle mass retention.
We examined the impact of dietary protein distribution on the synthesis of specific muscle protein fractions during periods of ER, with and without RT, compared with energy balance (EB) in 20 overweight/obese older men. We hypothesized that a balanced (BAL) distribution of dietary protein intake throughout the day would stimulate myofibrillar protein synthesis to a greater magnitude compared with a skewed (SKEW) distribution. Furthermore, we hypothesized that this effect would be enhanced after undertaking RT.
METHODS
Ethical approval.
This study was approved by the Hamilton Health Sciences Research Ethics Board and conformed to the standards for the use of human subjects in research as outlined by the Canadian Tri-Council Policy on the ethical use of human subjects in research (http://www.pre.ethics.gc.ca/pdf/eng/tcps2/TCPS_2_FINAL_Web.pdf). Each participant was informed of the purpose of the study, experimental procedures, and potential risks before written consent being provided.
Participants.
Twenty overweight and obese older adult men [age 66 ± 4 yr, body mass index (BMI) 31 ± 5 kg/m2] were recruited to participate in the study through posters and via local newspaper advertisements. Inclusion criteria were men, 60–75 yr of age, BMI between 27 and 40 kg/m2, nonsmokers, and generally healthy according to responses to a standard health screening questionnaire. Exclusion criteria included self-reported diabetes mellitus, cardiovascular disease, renal disease, gastrointestinal disease, musculoskeletal injuries, significant body mass loss in the 3-mo period before the study, vegetarianism, and use of medications known to interfere with muscle metabolism, including statins, β-blockers, hormone replacement therapy, antiarrhythmic drugs, oral hypoglycemic agents, and insulin. Inclusionary medications were low-dose aspirin (81 mg/day), type I and II 5 α-reductase inhibitors (avodart and propecia), xanthine oxidase inhibitors (allopurinol), calcium channel blockers, and selective serotonin reuptake inhibitors. Before commencing the study, participants completed a 5-day weighed food record (3 weekdays and 2 weekend days), and these were analyzed using a commercially available software program (Nutribase version 11.5; Cybersoft, Phoenix, AZ) to assess habitual dietary intake.
Study overview.
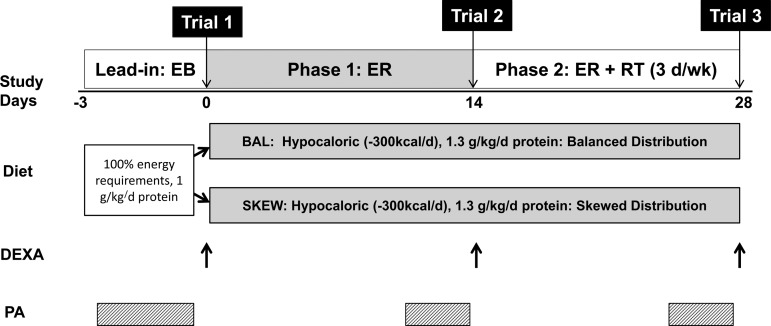
An overview of the study design is shown in Fig. 1. Participants were provided with a 3-day lead-in diet (days −3 to 0) designed to provide energy to maintain EB immediately before commencing a 4-wk hypocaloric feeding intervention with RT. Before entry, participants were randomly allocated to one of two groups (n = 10/group) matched for age and BMI (BAL or SKEW). In BAL, participants were provided with diets that evenly distributed dietary protein across their daily meals, and in SKEW the majority of protein was provided as part of the evening meal. The 4-wk intervention consisted of two × 2-wk phases. In weeks 1 and 2 all participants were in ER (Phase 1:ER) and continued their habitual physical activity. In weeks 3 and 4, while still energy restricted, all participants commenced a supervised RT program (Phase 2:ER + RT) in which they undertook whole body, progressive RT on 3 days/wk. The rates of MPS in response to a BAL or SKEW pattern of protein intake were measured at the conclusion of the energy balanced lead-in diet (trial 1, day 0), at the end of Phase 1:ER (trial 2, day 14), and at the end of Phase 2:ER + RT (trial 3, day 28). Participants were required to wear a pedometer and accelerometer (SenseWear v7.0; BodyMedia, Pittsburgh, PA) for 3 days immediately before each experimental infusion trial to monitor the number of daily steps and habitual physical activity.
Fig. 1.
Schematic overview of study design. DEXA, dual-energy X-ray absorptiometry; EB, energy balance; ER, energy restriction; ER + RT, energy restriction and resistance training; PA, physical activity assessed using accelerometry; SKEW, skewed; BAL, balanced.
Diets.
At baseline, each participant's energy requirement to maintain EB was calculated using the Mifflin St. Jeor equation (16, 30) with the appropriate activity factor, which was determined for each participant based on their response to a standard habitual physical activity questionnaire before intervention. During the 3-day lead-in phase, participants consumed a diet providing 100% of estimated energy requirements, including 1 g protein·kg−1·day−1. During this lead-in period, protein was distributed in a traditional skewed pattern (i.e., small amount of protein at breakfast and lunch and the majority of protein at the evening dinner meal). The macronutrient breakdown of the lead-in diet was 55% energy from carbohydrate, 15% from protein, and 30% from fat. The purpose of the lead-in diet was to ensure participants commenced the study in EB and to minimize the influence of interparticipant differences in habitual protein intake.
During the 4-wk energy-restricted period, participants in both groups consumed a diet providing 300 kcal/day less than their estimated energy requirements to maintain EB. The diets provided 1.3 g protein·kg−1·day−1, and dietary carbohydrate and fat were both manipulated within the ranges 50–55 and 20–25% of total energy intake, respectively, to achieve the target energy intake for each participant. Although the diets in both groups contained the same total amount of daily protein, they differed in the distribution pattern. In BAL, protein was evenly distributed across the four daily meals (∼25% of total protein intake at breakfast, lunch, dinner, and prebed snack), with each meal providing ≥30 g (≥0.33 g/kg) protein. In SKEW the majority of daily protein intake was provided with the evening dinner meal (∼7% at breakfast, ∼17% at lunch, ∼72% at dinner, and ∼4% prebed). In SKEW, the protein content of breakfast, lunch, and the prebed snack was <20 g (<0.22 g/kg), and dinner provided 70–110 g (∼0.94 g/kg), depending on each participant's daily protein requirement. In both groups, meals contained a variety of plant- and animal-based protein sources. In BAL, a ready-to-drink whey protein micelle (WPM) beverage (240 g: 25 g protein, 3 g carbohydrate, 0.6 g fat; Nestle, Lausanne, Switzerland) was consumed as part of breakfast and the prebed snack as a practical means of achieving target protein intakes at these meals, which are typically low in protein (43, 45). SKEW did not consume a WPM supplement throughout the intervention and therefore received their total daily protein intake from food sources only. To minimize any potential psychological influence of consuming a protein supplement in BAL, participants in SKEW consumed a protein-free, low-energy placebo drink (240 g: 0.2 g protein, 3 g carbohydrate, 0.6 g fat; Nestle) similar in appearance, smell, and taste to the WPM beverage, with breakfast and the prebed meal. Participants were not told their group allocation and were blinded to the composition of their assigned study beverage. Although complete blinding to a dietary intervention is difficult, we took several measures to minimize obvious differences between the diets. Provision of the study beverages with breakfast and the prebed snack allowed us to provide similar foods to both groups for these meals (i.e., breakfast cereals, milk, fruit, granola bars, juice, and nuts). Similar foods were also provided for lunch and dinner in both groups (i.e., skimmed milk and prepackaged frozen meals) although the serving sizes of meat within the meals differed between groups.
All study diets were designed by a research dietitian who met with each participant individually to customize meal plans in-line with their personal food preferences. To enhance compliance, participants were supplied with all of the food required for the duration of the study, which consisted of meals that required minimal preparation. The meal plans specified the time of day meals should be consumed (breakfast: 0700, lunch: 1200, dinner: 1700, prebed snack: 2200), and participants were instructed to mark food items that were consumed in a log. Eating food not provided by the study was discouraged, but, if this occurred, the participant logged the extra food that was consumed. The daily logs were returned by participants and checked by the research dietitian on a weekly basis.
Exercise training.
During weeks 3 and 4 participants underwent a progressive, low-load, high-volume RT program (31) consisting of three training sessions per week (6 sessions total) with at least 1 day between each session. Each session consisted of two upper body exercises (chest press, seated row; Hur) and three lower body exercises (leg press, leg extension, leg curl; Hur). Training sessions in week 3 consisted of two sets and in week 4 consisted of three sets (4 sets in the final training session) of each exercise performed to the point of volitional fatigue. A strength test was conducted at least 1 day before the start of the lead-in phase of the intervention to determine the maximum load that each participant could lift for 20–30 repetitions of each exercise. This load was used for the first set of each exercise in the first training session. Once a participant was able to complete more than 30 repetitions with a given load, the weight was increased to maintain each participant within the target repetition range of 20–30. Trained study personnel individually supervised each training session, verbally encouraged participants, and completed training logs detailing the load and repetitions for every session. All training sessions were performed in the morning before breakfast, and the final training session was performed 48 h before the third experimental infusion trial.
Anthropometrics and body composition.
Height was measured to the nearest 0.1 cm using a stadiometer, and body mass was assessed to the nearest 0.1 kg using a calibrated scale. Whole body dual-energy X-ray absorptiometry scans (QDR-4500A, software version 12.31; Hologic, Bedford, MA) were carried out after an overnight fast by the same trained technician to determine fat mass and (fat and bone free) lean mass. Appendicular skeletal muscle mass (ASMM) was measured as the sum of arm and leg lean mass.
Experimental infusion protocol.
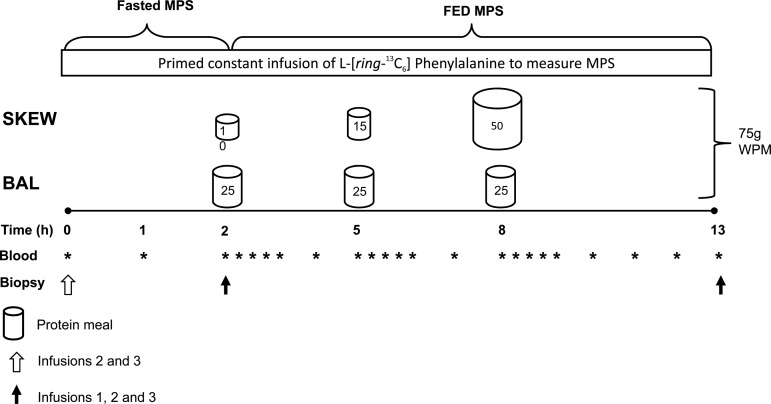
Participants reported to the laboratory via automobile or public transportation at ∼0600 following a 10- to 12-h overnight fast. Upon arrival a catheter was inserted in an antecubital vein to obtain a baseline blood sample before initiating a 0.9% saline drip to keep the catheter patent to allow for repeated arterialized blood sampling. Arterialized blood samples (13) were obtained repeatedly during the infusion trial by wrapping a heating blanket around the forearm. A second catheter was inserted into the antecubital vein of the opposite arm, and a primed, continuous infusion (0.05 μmol·kg−1·min−1, 2.0 μmol/kg prime) of l-[ring-13C6]phenylalanine (Cambridge Isotope Laboratories, Woburn, MA) was initiated and maintained for the next 13 h while the participants rested comfortably on a bed. Participants consumed a WPM supplement (Nestle) 2, 5, and 8 h following the onset of the primed constant infusion with the purpose of simulating a BAL or SKEW meal pattern. Both groups consumed a total of 75 g of WPM during each trial. In BAL, 25 g of WPM was consumed at 2 h (breakfast), 5 h (lunch), and 8 h (dinner). In SKEW, the WPM dose was 10 g at 2 h, 15 g at 5 h, and 50 g at 8 h. All protein “meals” were from original ready-to-drink beverages and were enriched to 4% with l-[ring-13C6]phenylalanine to minimize disturbances in isotopic equilibrium following amino acid ingestion (6). The spacing of 3 h between each protein “meal” was selected based on previous work showing that MPS is stimulated and returns to basal levels within 3 h of protein ingestion (2). The period following the “dinner” protein meal was extended to 5 h to ensure we did not “miss” the influence a potentially more prolonged stimulation of MPS in SKEW with the large dinner protein dose. Plasma samples were obtained before and 15, 30, 45, 60, 120, and 180 min after all meals and additionally 240 and 300 min after dinner during all trials. In trials 2 and 3, skeletal muscle biopsies were obtained directly before the initiation of the primed continuous infusion, at 2 h (immediately before ingestion of the first WPM feeding) and 13 h following infusion initiation. In trial 1 biopsies were obtained only at 2 and 13 h, and the fasted (0–2 h) fractional synthetic rate (FSR) was calculated based on the 13C enrichment of mixed plasma proteins obtained from the preinfusion blood sample and the skeletal muscle biopsy following 2 h of tracer incorporation (5). Muscle biopsies were obtained from the vastus lateralis muscle using a 5-mm Bergström needle adapted for manual suction under 2% xylocaine local anesthesia. The tissue samples were freed from visible fat and connective tissue and frozen immediately in liquid nitrogen for further analysis. Details of the infusion protocol are outlined in Fig. 2.
Fig. 2.
Schematic of the experimental infusion protocol performed in EB (trial 1), after 2 wk of ER (trial 2), and after 2 wk of ER + RT (trial 3). MPS, muscle protein synthesis (myofibrillar and sarcoplasmic); WPM, whey protein micelle drink.
Analytical procedures.
Blood glucose concentration was measured using a blood glucose meter (OneTouch Ultra 2; Lifescan, Milpitas, CA) within 2 min of blood collection. Plasma insulin concentration was measured using a commercially available immunoassay kit (ALPCO Diagnostics, Salem, NH). Plasma amino acid concentrations were analyzed via gas-chromatography-mass spectrometry using the Phenomenex EZfaast (Torrance, CA) amino acid analysis kit per the manufacturer's instructions. Plasma l-[ring-13C6]phenylalanine enrichment was determined as described previously (19).
Myofibrillar- and sarcoplasmic-enriched protein fractions were isolated as previously described (35). Amino acids were liberated by adding 1 M HCl and DOWEX (50WX8-200 resin Sigma-Aldrich) and heating at 110°C for 72 h, with vortex mixing every 24 h. Free amino acids were purified using DOWEX ion exchange chromatography and converted to their N-acetyl-n-propyl ester derivatives for analysis by gas chromatography combustion isotope ratio mass spectrometry (Hewlett Packard 6890, IRMS model Delta Plus XP; Thermo Finnagan, Waltham, MA) as described previously (35).
Calculations.
Total area under the concentration vs. time curve (TAUC) and concentration maximum (Cmax) were calculated for insulin and for amino acid data for each protein meal (breakfast: 2–5 h, lunch: 5–8 h, dinner: 8–13 h). The FSR of myofibrillar and sarcoplasmic protein were calculated using the standard precursor-product equation:
where Exb is the protein-bound enrichment from biopsy x according to the protocol (Fig. 2), Ep is the mean integrated plasma l-[ring-13C6]phenylalanine enrichment during the time period for determination of amino acid incorporation, and t is the tracer incorporation time in hours. The utilization of “tracer naïve” participants allowed us to use a preinfusion blood sample (i.e., a mixed plasma protein fraction) as the baseline enrichment (E1b) for calculation of basal (i.e., fasted) FSR in trial 1, an approach that has been validated (5). Trials 2 and 3 included a baseline muscle biopsy before the infusion began to account for changes in protein-bound enrichment from trial 1.
Statistical analysis.
All analyses were performed using SPSS (version 22.0, Chicago, IL). The Shapiro-Wilk test was used to check data for normality. If data were not normally distributed, values were transformed by using the square root or ln of the value. The statistical analysis was performed on transformed data, but nontransformed data are presented in graphic or tabular form for clarity. Mauchly's test of sphericity was used to assess homogeneity of variances, and if this assumption was violated the Greenhouse-Geisser correction of the degrees of freedom was used. Baseline characteristics (body composition, dietary intake parameters, physical activity level) were compared between groups using an unpaired t-test. Myofibrillar and sarcoplasmic FSR were analyzed separately using a two-factor (group × trial) mixed-model ANOVA for each feeding state (fasted and fed). Other variables were analyzed using a three-factor (group × trial × meal) mixed-model ANOVA, as appropriate. Significant main effects were further analyzed using simple planned contrasts. Tukey's post hoc test with a Bonferroni correction for multiple comparisons was performed whenever a significant interaction was found to isolate specific differences. Statistical significance was accepted when P ≤ 0.05. Results are presented as means ± SE in text and Figs. 1–4 and as means ± SD in Tables 1–4.
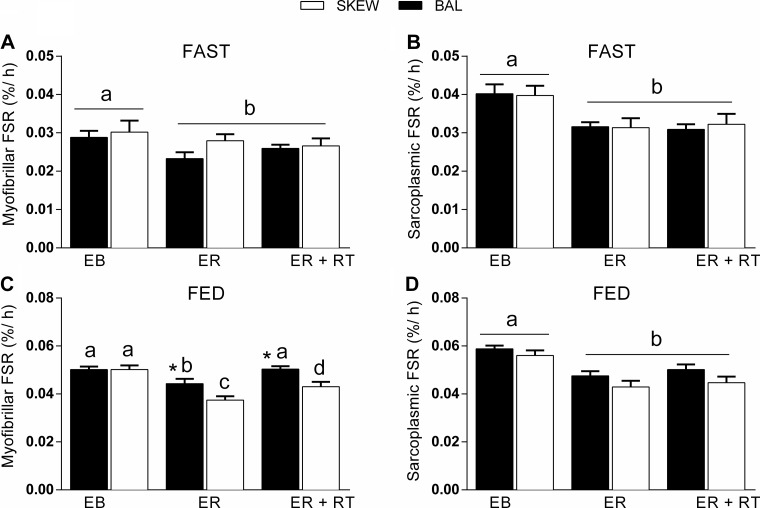
Fig. 4.
Myofibrillar (A and C) and sarcoplasmic (B and D) protein fractional synthetic rate (FSR, %/h) in the fasted (A and B; 0–2 h) and fed (C and D; 2–13 h) state in BAL and SKEW protein consumption groups in EB (trial 1), after 2 wk of ER (trial 2), and after 2 wk of ER + RT (trial 3). Note the difference in scales of the axes in A and B vs. C and D. Values are means ± SE, n = 20 (10 participants/group). Dissimilar letters demonstrate within-group differences (P < 0.05). *Different from SKEW in the same trial (P < 0.05).
Table 1.
Baseline participant characteristics
| BAL | SKEW | P | |
|---|---|---|---|
| Age, yr | 65 ± 3 | 66 ± 4 | 0.35 |
| Height, m | 1.76 ± 0.06 | 1.74 ± 0.05 | 0.46 |
| Body mass, kg | 97.2 ± 14.0 | 95.8 ± 13.9 | 0.82 |
| Body mass index, kg/m2 | 31.4 ± 4.8 | 31.4 ± 4.6 | 0.94 |
| Fat mass, kg | 29.1 ± 8.6 | 28.8 ± 9.0 | 0.93 |
| Lean body mass, kg | 66.8 ± 7.1 | 66.0 ± 5.1 | 0.77 |
| ASMM, kg | 28.2 ± 3.3 | 27.7 ± 2.7 | 0.75 |
| Fasting blood glucose, mM | 5.7 ± 0.5 | 5.6 ± 0.5 | 0.76 |
| HOMA | 3.8 ± 2.9 | 2.1 ± 1.1 | 0.21 |
| HbA1c, % | 5.0 ± 0.0 | 5.2 ± 0.2 | 0.48 |
| Steps per day | 8,535 ± 3,941 | 6,880 ± 3,899 | 0.49 |
Values are means ± SD; n = 10 participants/group. BAL, balanced; SKEW, skewed; ASMM, appendicular skeletal muscle mass, HOMA; homeostatic model assessment of insulin resistance.
Table 4.
TAUC and Cmax plasma ∑TAA, ∑EAA, and leucine in response to breakfast, lunch, and dinner consisting of BAL or SKEW distribution of protein intake
|
Trial 1 |
Trial 2 |
Trial 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BAL | SKEW | BAL | SKEW | BAL | SKEW | Trial | Meal × Group | Trial × Group | |
| TAUC | |||||||||
| TAA | 0.09 | 0.00 | 0.17 | ||||||
| Breakfast* | 11,004 ± 7,093 | 7,503 ± 1,866 | 9,566 ± 566 | 9,401 ± 1,103 | 8,847 ± 1,215 | 7,306 ± 547 | |||
| Lunch | 8,948 ± 2,639 | 8,127 ± 1,985 | 9,037 ± 1,448 | 9,248 ± 774 | 8,858 ± 1,074 | 7,876 ± 843 | |||
| Dinner*† | 14,027 ± 3,429 | 16,466 ± 3,810 | 14,386 ± 1,613 | 18,819 ± 1,799 | 14,130 ± 1,682 | 15,322 ± 1,626 | |||
| EAA$ | 0.01 | 0.00 | 0.19 | ||||||
| Breakfast*# | 3,774 ± 674 | 2,806 ± 769 | 4,252 ± 371 | 3,828 ± 318 | 3,806 ± 500 | 2,905 ± 197 | |||
| Lunch*# | 3,998 ± 1,029 | 3,297 ± 875 | 4,160 ± 787 | 3,983 ± 302 | 3,995 ± 407 | 3,347 ± 392 | |||
| Dinner*† | 6,248 ± 1,080 | 7,909 ± 2,124 | 6,631 ± 714 | 9,220 ± 732 | 6,395 ± 646 | 7,593 ± 949 | |||
| Leucine$ | 0.01 | 0.00 | 0.26 | ||||||
| Breakfast*† | 781 ± 110 | 489 ± 122 | 899 ± 113 | 670 ± 66 | 784 ± 104 | 500 ± 34 | |||
| Lunch*† | 941 ± 157 | 664 ± 174 | 975 ± 201 | 800 ± 80 | 898 ± 63 | 624 ± 63 | |||
| Dinner*† | 1,442 ± 191 | 1,918 ± 534 | 1,523 ± 198 | 2,189 ± 159 | 1,433 ± 182 | 1,821 ± 205 | |||
| Cmax | |||||||||
| TAA‡ | 0.00 | 0.00 | 0.04 | ||||||
| Breakfast* | 3,605 ± 657 | 3,218 ± 704 | 4,059 ± 265 | 4,176 ± 526 | 3,881 ± 615 | 3,100 ± 303 | |||
| Lunch | 3,859 ± 888 | 3,548 ± 677 | 3,914 ± 534 | 4,048 ± 445 | 3,729 ± 638 | 3,377 ± 284 | |||
| Dinner*# | 4,082 ± 925 | 4,193 ± 687 | 4,165 ± 653 | 5,059 ± 462 | 3,786 ± 313 | 4,186 ± 644 | |||
| EAA$ | 0.00 | 0.00 | 0.06 | ||||||
| Breakfast*# | 1,618 ± 256 | 1,318 ± 302 | 1,921 ± 159 | 1,838 ± 211 | 1,803 ± 295 | 1,326 ± 120 | |||
| Lunch*# | 1,832 ± 438 | 1,534 ± 312 | 1,933 ± 338 | 1,905 ± 230 | 1,769 ± 265 | 1,542 ± 149 | |||
| Dinner*† | 2,008 ± 349 | 2,155 ± 434 | 2,098 ± 325 | 2,658 ± 150 | 1,905 ± 154 | 2,182 ± 389 | |||
| Leucine$ | 0.00 | 0.00 | 0.16 | ||||||
| Breakfast*† | 367 ± 53 | 257 ± 50 | 436 ± 53 | 363 ± 67 | 403 ± 64 | 258 ± 27 | |||
| Lunch*† | 454 ± 102 | 336 ± 71 | 486 ± 83 | 426 ± 62 | 423 ± 49 | 330 ± 26 | |||
| Dinner*† | 499 ± 69 | 558 ± 116 | 542 ± 97 | 673 ± 48 | 479 ± 43 | 554 ± 91 | |||
Values are means ± SD; n = 10 participants/group. Cmax, concentration maximum; TAA, total amino acids; EAA, essential amino acids. Data were analyzed using a 3-factor (group × trial × meal) mixed-model ANOVA with simple planned contrasts and Tukey's post hoc test where appropriate. Plasma amino acids concentrations were measured immediately before and 15, 30, 45, 60, 120, and 180 min after all meals and additionally 240 and 300 min after dinner during trial 1 (energy balance), trial 2 (after 2 wk of energy restriction), and trial 3 (after 2 wk energy restriction + resistance training). Plasma amino acid concentrations are expressed as TAUC (nmol·ml−1·3 h−1 for breakfast and lunch, nmol·ml−1·5 h−1 for dinner) and Cmax (nmol/ml).
Different between groups.
Different from other meals within the same group.
Different from other meals in SKEW only.
Different from other trials for ∑EAA and leucine.
Different in trial 2 than other trials in SKEW only.
RESULTS
Participants.
Participant characteristics are shown in Table 1. All participants who commenced the intervention completed the study and were included in the final analysis. At baseline, there were no significant differences between groups for any of the anthropometric or descriptive variables examined. All participants had HbA1c levels below the Canadian Diabetes Association prediabetes diagnostic criteria of 6.0–6.4% (18). Baseline dietary intake before beginning the study is shown in Table 2. One participant in the SKEW group failed to return his diet record, and therefore baseline dietary intake data are reported for nine participants in that group. Both groups reported consuming protein, as expected, in a skewed pattern over the day in their habitual diets, eating the majority of protein at the evening meal. At baseline, BAL reported a slightly higher number of daily eating occasions during which ≥30 g of protein were consumed compared with SKEW (1.6 ± 0.7 vs. 1.0 ± 0.3 occasions/day, P = 0.034; Table 2). There were no other differences in baseline dietary intake variables between groups.
Table 2.
Baseline dietary intake measured by 5-day weighed diet record
| Balanced | Skewed | P Value | |
|---|---|---|---|
| Energy, kcal/day | 2,503 ± 719 | 2,203 ± 468 | 0.30 |
| Fat, g/day | 102 ± 36 | 87 ± 29 | 0.34 |
| Fat, g·kg body mass−1·day−1 | 1.1 ± 0.5 | 0.9 ± 0.3 | 0.36 |
| Fat, %total energy intake | 38 ± 7 | 35 ± 6 | 0.58 |
| CHO, g/day | 269 ± 97 | 246 ± 63 | 0.56 |
| CHO, g·kg body mass−1·day−1 | 2.8 ± 0.9 | 2.6 ± 0.7 | 0.65 |
| CHO, %total energy intake | 40 ± 6 | 42 ± 9 | 0.54 |
| Protein, g/day | 108 ± 24 | 95 ± 16 | 0.18 |
| Protein, g·kg body mass−1·day−1 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.23 |
| Protein, g·kg FFM−1·day−1 | 1.6 ± 0.3 | 1.4 ± 0.3 | 0.19 |
| Protein, %total energy intake | 18 ± 3 | 18 ± 4 | 0.99 |
| Alcohol, %total energy intake | 4 ± 5 | 4 ± 4 | 0.88 |
| Daily eating occasions | 4.8 ± 0.9 | 4.9 ± 1.2 | 0.82 |
| Eating occasions ≥30 g protein | 1.6 ± 0.7 | 1.0 ± 0.3 | 0.03 |
| Breakfast protein content, g | 16 ± 12 | 13 ± 5 | 0.32 |
| Lunch protein content, g | 30 ± 7 | 26 ± 12 | 0.36 |
| Dinner protein content, g | 54 ± 10 | 47 ± 12 | 0.17 |
| Prebed meal protein content, g | 3 ± 4 | 3 ± 4 | 0.75 |
Values are means ± SD; n = 10 (BAL) and 9 (SKEW). CHO, carbohydrate; FFM, fat-free mass.
Physical activity levels and RT variables.
There were no differences between groups in daily steps (BAL: 9,080 ± 790; SKEW: 6,782 ± 541), time spent engaged in moderate physical activity (BAL 1.9 ± 0.3; SKEW 1.7 ± 0.2 h/day), or average metabolic equivalents (BAL: 1.4 ± 0.1; SKEW: 1.4 ± 0.0) in the 3-day period before each experimental infusion trial (all P > 0.5). There were no differences between groups for the product of load (kg) × volume (no. of repetitions) for exercises performed during the training sessions (all P > 0.5; data not shown).
Changes in body composition and anthropometry.
A post hoc power calculation demonstrated that, with our sample size of n = 10/group, we only had 27% power to detect between-group differences in body composition changes. Therefore, the body composition data were pooled for analysis. Total body mass decreased over the 4-wk hypocaloric feeding intervention (P < 0.01), and body mass loss was greater in Phase 1:ER (pooled mean change: −2.5 ± 0.3 kg) than Phase 2:ER + RT (pooled mean change: −1.4 ± 0.2 kg; main effect for phase P < 0.01). Body fat decreased over the intervention (P < 0.001) with no difference between phases (Phase 1:ER −1.3 ± 0.2 kg, Phase 2:ER + RT −1.1 ± 0.2 kg; P = 0.75). Whole body lean mass decreased over Phase 1:ER (pooled mean change −1.1 ± 0.1 kg, P = 0.003) of the intervention; however, there were no further changes in Phase 2:ER + RT (pooled mean change −0.2 ± 0.1 kg, P = 0.64). Trunk lean mass decreased in a similar pattern to whole body lean mass with a loss occurring over Phase 1:ER (pooled mean change −0.8 ± 0.0 kg, P = 0.001) but no further change occurring during Phase 2:ER + RT (pooled mean change −0.2 ± 0.1 kg, P = 0.523). ASMM (legs and arms) was unchanged in both phases.
Plasma insulin.
Fasting insulin concentration and homeostatic model assessment of insulin resistance (HOMA-IR) were similar in BAL and SKEW in all trials. There was a trial × group interaction (P < 0.05) such that in BAL fasting insulin concentration and HOMA-IR were reduced in trial 2 and trial 3 compared with trial 1 (P < 0.01), whereas in SKEW fasting insulin and HOMA-IR decreased in trial 2 (P < 0.05) but returned to baseline levels in trial 3. There was a meal × group interaction for plasma insulin such that TAUC was greater in BAL than SKEW following breakfast and lunch (P < 0.05), but there was no difference between groups following the dinner meal (P = 0.49; Table 3).
Table 3.
Fasting plasma insulin concentrations and TAUC plasma insulin in response to breakfast, lunch, and dinner consisting of BAL or SKEW distribution of protein intake
|
Trial 1 |
Trial 2 |
Trial 3 |
||||||
|---|---|---|---|---|---|---|---|---|
| BAL$ | SKEW | BAL$ | SKEW | BAL$ | SKEW | Meal × Group | Trial × Group | |
| Fasting Insulin, μU/ml | 15.2 ± 2.8 | 8.4 ± 2.8 | 10.8 ± 2.4 | 7.1 ± 2.4$ | 10.0 ± 2.2 | 8.4 ± 2.2 | 0.03 | |
| TAUC | 0.00 | 0.04 | ||||||
| Breakfast*† | 87 ± 44 | 35 ± 21 | 66 ± 44 | 28 ± 12 | 62 ± 35 | 28 ± 11 | ||
| Lunch* | 68 ± 27 | 35 ± 20 | 56 ± 32 | 30 ± 16 | 44 ± 24 | 31 ± 15 | ||
| Dinner# | 93 ± 49 | 93 ± 54 | 75 ± 43 | 86 ± 41 | 63 ± 33 | 87 ± 42 | ||
Values are means ± SD; n = 10 participants/group. TAUC, total area under the concentration vs. time curve. Data were analyzed using a 3-factor (group × trial × meal) mixed-model ANOVA with simple planned contrasts and Tukey's post hoc test where appropriate. Plasma insulin concentrations were measured immediately before and 30 and 60 min after all meals and additionally 120 and 180 min after breakfast and lunch, and 240 min after dinner during trial 1 (energy balance), trial 2 (after 2 wk of energy restriction), and trial 3 (after 2 wk energy restriction + resistance training). Postprandial plasma insulin concentrations are expressed as TAUC (μU·ml−1·3 h−1 for breakfast and lunch, μU·ml−1·4 h−1 for dinner).
Different between groups.
Different from lunch in BAL only.
Different from other meals in SKEW only.
Different from other trials.
Plasma amino acids.
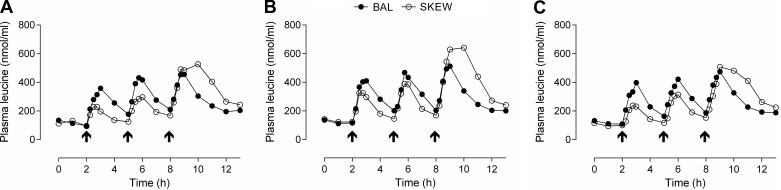
The TAUC and Cmax for plasma ∑total amino acids (TAA), ∑essential amino acids (EAA), and leucine in response to the three protein meals consumed during each experimental trial day are presented in Table 4. There was a meal × group interaction such that TAUC and Cmax for ∑TAA, ∑EAA, and leucine were greater in BAL than SKEW following breakfast and greater in SKEW than BAL following dinner (P < 0.05). TAUC and Cmax after lunch were also greater in BAL than SKEW for ∑EAA and leucine (P < 0.05). There was a main effect for trial for ∑EAA and leucine, and simple planned contrasts revealed that TAUC and Cmax were greater in trial 2 (ER) than trial 1 (EB; P < 0.01) and trial 3 (ER + RT; P < 0.01). There was a trial × group interaction for ∑TAA such that Cmax was greater in trial 2 than the other trials in SKEW (P < 0.01) but not in BAL (Table 4). Plasma concentrations of leucine over time are illustrated in Fig. 3. No statistical analysis was performed on the concentration vs. time data.
Fig. 3.
Plasma concentrations (nmol/ml) of leucine in the BAL and SKEW protein consumption groups in EB (trial 1, A), after 2 wk of ER (trial 2, B), and after 2 wk of ER + RT (trial 3, C). Arrows indicate a protein meal. Values are means (error bars omitted for clarity), n = 10 participants/group.
Muscle protein synthesis.
In the fasted state, there was a main effect for trial (P = 0.008) but not for group (P = 0.352) for myofibrillar FSR. Simple planned contrasts revealed that myofibrillar FSR was ∼14% lower in ER (trial 2) and ER + RT (trial 3) vs. EB (trial 1; P < 0.05; Fig. 4A). In the fed state, there were main effects for trial (P = 0.000) and group (P = 0.007) and a trial × group interaction for myofibrillar FSR (P = 0.035). Tukey's post hoc test revealed that myofibrillar FSR was lower in ER than EB in both groups (P = 0.000) but was ∼19% higher in BAL than SKEW (P = 0.014). In ER + RT, fed-state myofibrillar FSR increased above ER in both groups (P < 0.05) and in BAL was not different from EB (P = 0.903). In contrast, in SKEW myofibrillar FSR remained ∼14% lower than EB (P = 0.002) and ∼16% lower than BAL (P = 0.006, Fig. 4C). For sarcoplasmic FSR, in both the fasted and fed state, there was a main effect for trial (P = 0.000) but not for group. Simple planned contrasts revealed that sarcoplasmic FSR was reduced to a similar extent in ER and ER + RT compared with EB in both the fasted (∼22%) and the fed state (∼19%, P < 0.01, Fig. 4, B and D).
DISCUSSION
This study demonstrates for the first time that, during ER in overweight/obese older men, consumption of a balanced distribution of daily protein intake (i.e., 3 × 25 g evenly spaced doses of protein) acutely stimulated the synthesis of muscle contractile proteins more effectively than a skewed distribution of the same amount of protein (i.e., 10 g at breakfast, 15 g at lunch, 50 g at dinner). Furthermore, we show that combining RT with a balanced protein distribution restored depressed rates of myofibrillar protein synthesis during ER to those observed during EB.
The recommendation of ER in overweight older adults remains controversial (49), primarily because of concerns that weight loss to improve metabolic health in overweight older adults, while associated with health benefits, may exacerbate muscle loss (4, 49). Several studies have examined the effect of ER on MPS (1, 9, 37, 38, 46) with equivocal results. Such a lack of consistency between the results from studies may be the result of differences in dietary interventions, the methodologies used to assess MPS, the conditions under which MPS measurements were performed, and the characteristics of the population studied. Nonetheless, short-term studies have shown a decline in rested fasted MPS with ER (1, 38) and a reduced capacity to stimulate MPS (37). Similarly, in the present study we observed that short-term ER in older men was accompanied by a reduction in fasted- and fed-state MPS in both the myofibrillar and sarcoplasmic protein fractions. Because MPS is an energetically expensive process, the ER-induced downregulation of fasted- and fed-state MPS rates may reflect an adaptive response to conserve energy and protein reserves. Taken together, these data suggests that preservation of the MPS response during ER is a viable goal to support the maintenance of muscle mass while allowing for clinically indicated fat loss in a variety of populations.
Accumulating evidence indicates that consuming dietary protein at levels beyond the RDA of 0.8 g·kg−1·day−1 attenuates lean mass loss during periods of ER in young and older adults (27, 33, 52). The mechanism for this effect is unknown but may be through preservation of the anabolic sensitivity of skeletal muscle to protein-containing meals (37). Our data demonstrate that the quantity of protein consumed over the day is not the sole determinant of the potential to stimulate MPS in conditions of ER and suggest that the distribution of daily protein intake may also be important in attenuating the ER-induced decrement in myofibrillar protein synthesis when measured acutely. Indeed, although myofibrillar protein synthesis decreased with both patterns of protein feeding following 2 wk of ER (Phase 1) in the fed state, this reduction was less pronounced when protein intake was consumed in a balanced/even pattern across daily meals compared with the traditional dietary pattern of skewed protein intake at the evening meal (Fig. 4C).
We observed that a balanced pattern of protein consumption was even more effective when combined with RT, resulting in the restoration of myofibrillar protein synthesis to levels observed during EB. This synergistic effect of a balanced protein distribution and RT may be attributed to the fact that resistance exercise sensitizes the muscle protein synthetic machinery to protein feeding, resulting in an enhanced MPS response (7, 35), an effect that has also recently been demonstrated under conditions of ER in young adults (1). Our data extend these previous findings and show that, during ER, the synergistic effect of RT and protein feeding is still present 48 h after the last exercise bout.
In the present study the effect of daily protein distribution alone and in combination with RT was specific to the myofibrillar fraction, and we observed no influence of protein distribution pattern on the rate of sarcoplasmic protein synthesis. These findings are perhaps to be expected, since previous work has demonstrated that sarcoplasmic protein synthesis is less responsive to amino acid availability (14, 32) and resistance exercise than myofibrillar proteins (8, 35). Moreover, maintenance of myofibrillar protein synthetic rates is arguably of greater priority given that it is the loss of contractile proteins with aging that plays a greater role in the decrease in muscle mass and strength underpinning sarcopenia (40).
The superior capacity of a balanced protein distribution to stimulate myofibrillar protein synthesis over the day in ER, both in the presence and absence of prior resistance exercise, was likely the result of greater stimulation following breakfast and lunch in BAL than SKEW during the infusion trials. In support of this hypothesis, we observed that the TAUC and Cmax for ∑EAA and for leucine were greater in the BAL compared with the SKEW group following breakfast and lunch. An increase in plasma amino acid concentrations is a potent stimulator of MPS, an effect that is graded, saturable, and primarily attributable to the EAA (48). Furthermore, leucine plays a unique role as a key activator of the translation initiation process (23), and accumulating evidence supports the notion that a minimum concentration of leucine (systemic or intracellular) threshold must be surpassed to stimulate MPS in response to feeding (10, 34, 51). As such, the greater fed-state myofibrillar protein synthetic response in the balanced group compared with the skewed group during ER and ER + RT is likely attributable to the aminoacidemia during the infusion trials, rather than a chronic effect resulting from the consumption of daily protein intake in a balanced pattern throughout the 4-wk hypocaloric feeding period.
It could also be speculated that the greater postprandial insulinemia following the breakfast and lunch protein feedings in the balanced group compared with the skewed group may be, at least in part, responsible for the higher MPS in BAL during the ER and ER + RT infusion trials. Indeed, the feeding-induced rise in plasma insulin concentration represents a key factor driving postprandial perfusion, allowing subsequent amino acid delivery to the muscle and/or activating anabolic signaling (29). Nevertheless, the role of insulin appears to be permissive rather than stimulatory in the presence of hyperaminoacidemia (44). Gorissen et al. recently reported that carbohydrate coingestion with dietary protein did not modulate MPS in older adults despite an increase in plasma insulin concentrations from ∼18 μIU/ml (protein alone) to ∼65 μIU/ml (protein + carbohydrate) (20). Therefore, it appears unlikely that the peak insulin concentrations of ∼18–27 μIU/ml following the breakfast and lunch protein feedings in the skewed group would have limited the MPS response in the current study.
In contrast to the ER and ER + RT conditions, we observed no influence of protein distribution under conditions of EB. This is in agreement with recently published work by Kim and colleagues showing that 24-h mixed MPS was similar with an even (33:33:33% total protein at breakfast/lunch/dinner) vs. a skewed (15:20:65% total protein at breakfast/lunch/dinner) pattern of protein intake in older adults under conditions of EB (22). The latter findings, in addition to ours, are in contrast to a study by Mamerow et al. showing that the consumption of ∼30 g of protein at each mixed macronutrient meal stimulated 24-h mixed MPS to a greater extent than skewing protein intake toward the evening meal in younger adults in EB (28). It is difficult to explain the discrepancy between the results of these studies. However, one possible explanation may be the provision of an insufficient dose of protein per meal in the studies examining older adults. Whereas the exact amount of protein required to maximally stimulate MPS in older adults is unclear, previous estimations put the per meal dose at 30–40 g (39, 41, 42). Recently, we suggested a dose of ∼0.4 g protein·kg−1·meal−1 or 0.6 g protein·kg FFM−1·meal−1 in older persons (34). For the participants in the current study, this recommendation would be equivalent to ∼40 g/meal on average, with estimated optimal intakes of up to ∼48 g/meal in some of the heavier participants. Therefore, the per meal protein dose of 25 g of WPM (the decision on which was made before our previous results becoming available) consumed by the BAL group during our acute feeding protocol was likely insufficient to maximally stimulate MPS. Although the per meal protein intakes in the even/balanced groups in the study by Kim et al. were ∼0.3–0.5 g·kg−1·meal−1, protein was provided in the context of mixed macronutrient meals which, because of alterations in amino acid absorption and uptake kinetics, may further increase the quantity of protein required to maximally stimulate MPS (22, 51). In contrast, the 30 g dose of protein provided per meal in the Mamerow study equates to ∼0.39 g/kg, which is ∼60% higher than the 0.24 g·kg−1·meal−1 dose reported to maximally stimulate MPS in young adults (34) and was therefore likely sufficient even though protein was provided in mixed meals.
Intriguingly, that we observed greater myofibrillar protein synthesis over the day with a balanced protein distribution than a skewed distribution under conditions of ER and ER + RT in the present study, even despite a potentially suboptimal per meal protein dose, indicates that the distribution of daily protein likely becomes increasingly important in weight loss situations in older adults. Alternatively, that the influence of protein distribution on myofibrillar protein synthesis was specific to the ER conditions may relate to a potentially greater level of insulin resistance at baseline in the BAL group. Although we did not directly measure insulin resistance in the current study, fasting insulin concentration and HOMA-IR score were higher (although not statistically) in the BAL group compared with the SKEW group on trial 1 (EB). As such, it could be argued that the insulin-mediated increase in endothelial-dependent vasodilation and subsequent amino acid delivery to muscle may have been impaired to a greater extent in the BAL group, thus attenuating the MPS response (44). If this were the case, it is possible that it may account for our inability to detect a favorable effect of the BAL protein distribution on myofibrillar protein synthesis in trial 1 (EB), whereas during trial 2 (ER) and trial 3 (ER + RT) when fasting insulin concentration and HOMA-IR were more similar between groups (Table 3) a positive influence of BAL protein distribution on myofibrillar protein synthesis was apparent. In opposition to this notion, however, a number of studies to date report no influence of measures of insulin sensitivity and glycemic control on the postprandial MPS response (7, 8).
To date no study has examined the influence of protein distribution on MPS in conditions of ER, and further work is needed to elucidate the mechanisms as to why a balanced protein feeding pattern was beneficial. It will be important in future studies to confirm the influence of protein distribution on MPS during ER in the context of mixed meals comprised of real foods. An assumption of the constant labeled amino acid infusion technique used to measure MPS in the current study is that the tracer labeling in the precursor pool remains in a relative steady state during the infusion protocol. Because mixed meals have unpredictable amino acid absorption kinetics, we opted to feed isolated protein during the infusion trials to minimize disturbances in the precursor pool tracer enrichment. This study was designed to be a tightly controlled, “proof of principle” study to evaluate the MPS response to two different patterns of protein intake. As such, it should be noted that the metabolic responses during the infusion trials (insulinemia, aminoacidemia, etc.) do not fully reflect the BAL and SKEW diets participants were fed between trials.
In conclusion, we demonstrate that, during ER in older men, a balanced distribution of daily protein in amounts previously shown to increase MPS in elderly persons (12) acutely stimulated the synthesis of muscle contractile proteins more effectively than a skewed protein intake with consumption of the majority of protein in the evening meal. Combining RT with a balanced protein distribution rescued rates of myofibrillar protein synthesis during ER to the levels observed during EB. Although further work is required to determine whether our acute observations translate to mixed macronutrient meals and into a long-term functional response, we contend that the combination of RT and a balanced distribution of daily protein in the context of a higher protein diet may represent an effective strategy to allow for fat mass loss during ER without exacerbating sarcopenic muscle loss. In the face of the rising rates of obesity among the growing aging population, these results have potential implications for clinical practice in healthcare professionals working with community-dwelling and institutionalized older adults who have indications for weight loss.
GRANTS
This study was funded by an Australian Research Council Linkage Project grant (LP100100010) to J. A. Hawley and a National Science and Engineering Research Council of Canada grant to S. M. Phillips.
DISCLOSURES
Both Kassis and Karagounis are employees of Nestec SA a subsidiary of Nestle who was a linkage partner in this grant.
AUTHOR CONTRIBUTIONS
Author contributions: C.H.M., A.K., L.G.K., L.M.B., J.A.H., and S.M.P. conception and design of research; C.H.M., T.A.C.-V., C.J.M., N.M.K., and S.M.P. performed experiments; C.H.M., T.A.C.-V., C.J.M., N.M.K., J.A.H., and S.M.P. analyzed data; C.H.M., T.A.C.-V., C.J.M., N.M.K., L.M.B., and S.M.P. interpreted results of experiments; C.H.M. and S.M.P. prepared figures; C.H.M., L.M.B., J.A.H., and S.M.P. drafted manuscript; C.H.M., T.A.C.-V., C.J.M., N.M.K., A.K., L.G.K., L.M.B., J.A.H., and S.M.P. edited and revised manuscript; C.H.M., T.A.C.-V., C.J.M., N.M.K., A.K., L.G.K., L.M.B., J.A.H., and S.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tracy Rerecich and Todd Prior for technical and laboratory assistance and the study participants for their time and dedication.
REFERENCES
- 1.Areta JL, Burke LM, Camera DM, West DWD, Crawshay S, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab 306: E989–E997, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14: 542–559, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes 20: 412–419, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd NA, Groen BBL, Beelen M, Senden JMG, Gijsen AP, van Loon LJC. The reliability of using the single-biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism 61: 931–936, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Burd NA, West DW, Rerecich T, Prior T, Baker SK, Phillips SM. Validation of a single biopsy approach and bolus protein feeding to determine myofibrillar protein synthesis in stable isotope tracer studies in humans (Abstract). Nutr Metab (Lond) 8: 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Burd NA, West DWD, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 5: e12033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, Iglay HB. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring) 17: 1332–1339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churchward-Venne TA, Breen L, Donato Di DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99: 276–286, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Churchward-Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors 40: 199–205, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Churchward-Venne TA, Cotie LM, MacDonald MJ, Mitchell CJ, Prior T, Baker SK, Phillips SM. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am J Physiol Endocrinol Metab 307: E71–E83, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Copeland KC, Kenney FA, Nair KS. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab 263: E1010–E1014, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 33: 929–936, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 105: 775–789, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc 40: 1213–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorissen SHM, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJC. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab 99: 2250–2258, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159: 413–421, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NEP, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 308: E21–E28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136: 227S–S231S, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Koopman R, van Loon LJC. Aging, exercise, and muscle protein metabolism. J Appl Physiol 106: 2040–2048, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 42: 203–209, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr 135: 1903–1910, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Mamerow M, Mettler J. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 114: 876–880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meneilly GS, Elliot T, Bryer-Ash M, Floras JS. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab 80: 1899–1903, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell CJ, Churchward-Venne TA, West DWD, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113: 71–77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj a J, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci 66: 1218–1225, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol Ser A Biol Sci Med Sci 70: 57–62, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care 17: 5–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, Rood JC, Carbone JW, Combs GF, Young AJ. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J 27: 3837–3847, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC, Anderson JM, Ferrando AA, Wolfe RR, Rodriguez NR. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr 140: 745–751, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JMG, van Loon LJC. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 302: E992–E999, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 38: 120–125, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109: 1582–1586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tieland M, Borgonjen-Van den Berg KJ, van Loon LJC, de Groot LCPGM. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 51: 173–179, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 59: 2764–2771, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.USDAARS Food Surveys: data tables. Energy Intakes Percentages Energy From Protein, Carbohydrate, Fat Alcohol, By Gend. Age, What We Eat Am NHANES 2009–2010. http://www.ars.usda.gov/Services/docs.htm?docid=18349. [Google Scholar]
- 46.Villareal DT, Smith GI, Shah K, Mittendorfer B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity (Silver Spring) 20: 1780–1786, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 68: 677–681, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol 48: 1054–1061, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev 68: 375–388, 2010. [DOI] [PubMed] [Google Scholar]
- 51.West DWD, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94: 795–803, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 96: 1281–1298, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. [DOI] [PubMed] [Google Scholar]