Abstract
Objectives
Sialolithiasis, or salivary stones, is not a rare disease of the major salivary glands. However, the aetiology and incidence remain largely unknown. Since sialoliths are comprised mainly of calcium phosphate salts, we hypothesise that drinking water calcium levels and other elements in drinking water could play a role in sialolithiasis. Owing to substantial intermunicipality differences in drinking water composition, Denmark constitutes a unique environment for testing such relations.
Design
An epidemiological study based on patient data extracted from the National Patient Registry and drinking water data from the Geological Survey of Denmark and Greenland retrieved as weighted data on all major drinking water constituents for each of the 3364 waterworks in Denmark.
All patient cases with International Statistical Classification of Diseases 10th Revision (ICD-10) codes for sialolithiasis registered between the years 2000 and 2010 were included in the study (n=3014) and related to the drinking water composition on a municipality level (n=98).
Primary and secondary outcome measures
Multiple regression analysis using iterative search and testing among all demographic and drinking water variables with sialolithiasis incidence as the outcome in search of possible relations among the variables tested.
Results
The nationwide incidence of hospital-admitted sialolithiasis was 5.5 cases per 100 000 citizens per year in Denmark. Strong relations were found between the incidence of sialolithiasis and the drinking water concentration of calcium, magnesium and hydrogen carbonate, however, in separate models (p<0.001). Analyses also confirmed correlations between drinking water calcium and magnesium and their concentration in saliva whereas this was not the case for hydrogen carbonate.
Conclusions
Differences in drinking water calcium and magnesium may play a role in the incidence of sialolithiasis. These findings are of interest because many countries have started large-scale desalination programmes of drinking water.
Keywords: ORAL MEDICINE, ORAL & MAXILLOFACIAL SURGERY, OTOLARYNGOLOGY
Strengths and limitations of this study.
All patient cases in Denmark with International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes for sialolithiasis registered between the years 2000 and 2010 were included (n=3014) and related to the drinking water composition on a municipality level (n=98).
The drinking water in Denmark displays substantial intermunicipality compositional differences in a country covering only a limited area of land and with a relatively genetically homogeneous population.
The validity of the diagnoses was tested in a subsample (n=200) and showed no differences between low-incidence and high-incidence regions, indicating that systematic misclassification was not present in the data analysed.
On a global scale, all information about the possible medical effects of changes to drinking water, including desalination and other modifying measures, will be valuable for high-level decision-making.
The study is epidemiological, and therefore patient intrinsic factors, such as saliva secretion and saliva composition, and parameters regarding calcium metabolism and duct anatomy are unknown. Thus, the contributing role of such factors in addition to drinking water composition was not analysed.
Introduction
Sialolithiasis is not a rare disease of the major salivary glands, although little is known about the aetiology and the incidence on nationwide scales.1–3 Since sialolithiasis occurs mainly unilaterally, patient intrinsic factors more than likely contribute to this disease.4 The symptoms are swelling and pain, caused by obstruction of the physiological salivary flow and commonly meal related.5 6 Although sialolithiasis is a benign condition that occurs mostly in the ducts of glandula submandibularis,7 sialolithiasis can lead to painful inflammation of the salivary glands, sialadenitis,8 which, if not spontaneously resolved, requires surgical treatment either by direct incision (sialodochotomy) or endoscopic removal of the stone (sialoendoscopy) or finally by excision of the gland.9
In other exocrine glands and the kidneys, the concentration of specific constituents can influence stone formation,10 and it therefore seems reasonable that similar relations could apply for sialolithiasis. Since sialoliths predominantly consist of calcium phosphate salts,11 and as calcium in drinking water is associated with other diseases such as atopic dermatitis in childhood, colon cancer and dental caries,12–15 it is tempting to speculate on an association between drinking water calcium levels and salivary stone formation. In some areas of Denmark, the drinking water contains considerable amounts of calcium,15 and in these areas a substantial part of the daily calcium intake may arise from the intake of drinking water.
The main determinant of calcium in Danish drinking water, which is almost solely derived from groundwater, is the geochemical difference among aquifers (figure 1A). Major differences among the country municipalities have previously allowed for analyses of strong associations with other diseases and drinking water composition, especially calcium and fluoride.15 From this perspective, it is also possible that drinking water calcium is linked to sialolithiasis. In a pilot study, the incidence of sialolithiasis differed among the five main Danish regions (each comprising several municipalities) and the incidence was higher in the regions with high-calcium levels in the water than in regions with lower calcium levels (p<0.001). Consequently, we here aimed to test whether drinking water calcium and other ions may explain part of the variation of sialolithiasis on a municipality level (98 municipalities vs the 5 main regions) in Denmark.
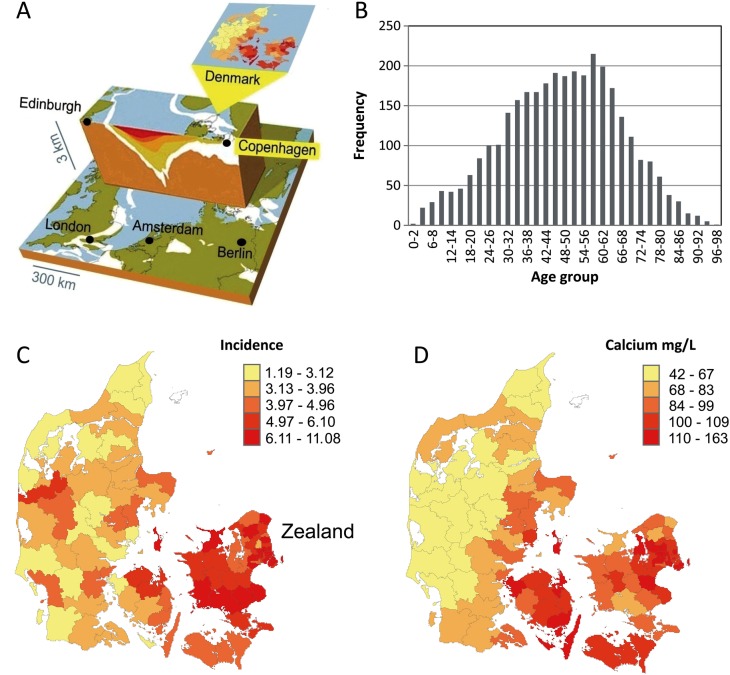
Figure 1.
(A) The proximity of the limestone layer to the surface of the earth in Northern Europe with special focus on Denmark. (B) The frequency (2000–2010) of hospital-admitted sialolithiasis with respect to the age of the patient at the time of diagnosis. (C) The incidence (number of cases per 100 000 citizens per year) of hospital-admitted sialolithiasis in the 98 Danish municipalities and (D) the drinking water calcium levels (mg/L) in each of the municipalities. (C and D) Mean values (2000–2010) for each of the municipalities. For magnesium and hydrogen carbonate, the map covering the land of Denmark would be very similar to (D) for calcium.
Materials and methods
Data collection and validity
Data were extracted from the Danish National Patient Registry as all patient cases with International Statistical Classification of Diseases 10th Revision (ICD-10) codes for sialolithiasis registered between the years 2000 and 2010. Care was taken to avoid the same sialolith being counted more than once, such as for a patient diagnosed on more than one occasion (thus, duplicates were excluded). For each case, we recorded the age of the patient at the time of diagnosis, gender, hospital of treatment and municipality of residence during the past 3 years. To evaluate whether the validity of the sialolithiasis diagnosis was comparable among regions, we conducted a test sample on 100 patient records from a low-incidence region and 100 patient records from a high-incidence region. Only direct stone visualisation by the treating physician, or stones confirmed through imaging techniques, such as ultrasound, MRI, CT or X-ray, were accepted as an accurate diagnosis. Population size data were gathered from Statistics Denmark, which is the national governmental institute for statistics.
Drinking water composition and chemistry
Within the observation period of study, data on the average drinking water composition (20 variables) were retrieved from the Geological Survey of Denmark and Greenland (table 1). All chemical data were obtained as average concentrations (mg/L) from the year 2000 to 2010 for each of the waterworks in the 98 Danish municipalities. In total, data from 3364 waterworks were obtained, and a weighted average was calculated based on the total drinking water volume from each waterworks in the municipality in question and the total drinking water consumption for the same municipality. Hydrogen carbonate was by far the most abundant ion, constituting more than half the constituents in the water by weight, followed by calcium, chloride, sulfate, sodium and magnesium (table 1). The term aggressive carbon dioxide, mentioned in table 1, is used by water suppliers and is a measure of the amount of carbon dioxide that will react with calcium carbonate to achieve calcium carbonate equilibrium.
Table 1.
Standard chemical characteristics of Danish drinking water from 2000 to 2010 (mean and range)
| Mean | Range | Per cent of total | |
|---|---|---|---|
| Physicochemical characteristics | |||
| Evaporation residue (mg/L) | 434 | 177–886 | – |
| Ionic strength (mol/L) | 0.010 | 0.004–0.023 | – |
| pH | 7.57 | 7.14–7.92 | – |
| Water hardness (odH) | 16.0 | 7.4–30.0 | – |
| Gases (mg/L) | |||
| Aggressive carbon dioxide | 4.6 | 0.0–77.2 | 0.8 |
| Oxygen | 8.8 | 6.0–10.6 | 1.6 |
| Cations (mg/L) | |||
| Ammonia and ammonium | 0.06 | 0.01–0.39 | <0.1 |
| Calcium | 91.2 | 42.0–163.0 | 16.4 |
| Iron | 0.13 | 0.02–2.91 | <0.1 |
| Magnesium | 14.5 | 3.9–41.2 | 2.6 |
| Manganese | 0.06 | 0.01–1.82 | <0.1 |
| Potassium | 3.6 | 1.3–10.5 | 0.6 |
| Sodium | 33.7 | 11.0–103.0 | 6.1 |
| Anions (mg/L) | |||
| Chloride | 54.7 | 14.5–164.1 | 9.8 |
| Fluoride | 0.38 | 0.09–1.35 | 0.1 |
| Hydrogen carbonate | 287 | 112–427 | 51.6 |
| Nitrate | 4.15 | 0.63–23.97 | 0.7 |
| Nitrite | 0.02 | 0.00–0.10 | <0.1 |
| Inorganic phosphorus | 0.03 | 0.01–0.09 | <0.1 |
| Sulfate | 50.7 | 4.0–237.0 | 9.1 |
| Organic compounds (mg/L) | |||
| Organic carbon | 1.86 | 0.62–9.43 | 0.3 |
| Saturation indices as log10 (IAP/Ksp) | |||
| Hydroxyapatite* | 2.66 | −1.82 to 6.05 | – |
| Brushite† | −2.68 | −3.45 to −2.12 | – |
| Mg-whitlockite* | 4.87 | −4.04 to 11.58 | – |
| Calcium carbonate* | 0.26 | −0.64 to 0.87 | – |
| Octacalcium phosphate† | −5.35 | −8.74 to −2.81 | – |
| Fluorapatite* | 7.91 | 3.25 to 11.47 | – |
Weighted average values for 20 standard chemical characteristics, as well as ionic strength and saturation indices, of Danish drinking water from 2000 to 2010 in the 98 Danish municipalities. The part of each ion and compound relative to the total amount of ions and compounds is given in the right hand column in per cent of total by weight. Saturation indices were determined by calculation.
*Denotes general (mean) supersaturation.
†General undersaturation. It should be noticed that the result for Mg-whitlockite is based on a temperature of 25°C. In addition to the total concentrations of all species, pH is known as well. For this reason, the hydrogen carbonate concentration was treated as variable, which seems justified as the value may easily change by exchange with the atmosphere.
Saturation indices were determined by computer programmes for an assumed drinking water temperature of 10°C.16 17 Solubility data for calcium phosphates at 10°C were retrieved from the National Bureau of Standards (NIST),18–20 data on fluorapatite (Ca5(PO4)3F) were retrieved at temperatures from 25°C to 50°C,21 22 and data for calcium carbonate (CaCO3) were taken from Plummer and Busenberg.23 The first two dissociation constants of the phosphate system were also retrieved from NIST,24 whereas the third was obtained by non-linear extrapolation from additional data.25 When not given at 10°C directly, values were obtained from 25°C and the standard enthalpy increment. We also considered magnesium whitlockite (Mg-whitlockite). Crystal structure determination has indicated it to be Ca9MgH(PO4)7,26 and its solubility product was determined under this assumption.27 The following solubility products (pKsp) were found from the sources quoted: hydroxyapatite (Ca5(PO4)3OH) 58.50, brushite (CaHPO4.2H2O) 6.61, Mg-whitlockite 122.33, calcium carbonate (calcite) 8.41, octacalcium phosphate (Ca4H(PO4)3.2.5H2O) 48.26, and fluorapatite 61.54. Nitrite was ignored due to its low concentration and iron was assumed to be Fe(III) due to the presence of oxygen and high drinking water pH (table 1).
Statistical analyses
Primary statistical models were based on multiple regressions using iterative search and testing among the main 20 drinking water variables against the incidence of sialolithiasis in the 98 Danish municipalities as the outcome. One-way analyses of variance were used to study associations among incidence of sialolithiasis and each of the characteristics of the municipalities. Adjusted multiple linear regression models were fitted with incidence as the explained variable and water characteristics as explanatory variables. Models were adjusted for mean age, region and percentage of females in the population. One-to-one correlation analyses were conducted among all water characteristics to avoid misleading effects in the models and thus in order to rule out strong inter-relationships. Similar analyses were performed to test the effect of drinking water on human saliva composition. When assessing relations between drinking water composition and sialolithiasis incidence, patients who had not resided in the same municipality 3 years prior to diagnosis (n=390) were excluded in order to avoid bias from patients being exposed to drinking water in more than one municipality. The main statistical analyses were performed with SAS V.9.3 and additional analyses with R.28 29 The level of significance was set at p<0.05.
Results
Incidence, epidemiology and data validity
A total of 3014 cases were recorded with a mean of 298 annual cases. For the obtained population size of 5 475 791 citizens (average size of the Danish population between years 2000 and 2010), the mean yearly incidence was 5.5 cases per 100 000 citizens nationwide (95% CI 5.3 to 5.5). Incidence was also calculated for men and women separately being 5.4 (CI 5.3 to 5.5) and 5.5/100 000/year (CI 5.4 to 5.6), respectively, with no significant difference between genders. The mean age at the time of diagnosis for patients with sialolithiasis was 48.2±18.3 years nationwide with the gender-specific values being males 46.9±17.3 and females 49.6±17.6 years, respectively, and with 75% of patients being between 36 and 61 years of age (IQR 26 years) at the time of diagnosis (figure 1B). Although some variation was present, the mean age among municipalities did not differ. Among all age groups, the lowest incidence of sialolithiasis was among children and adolescents. In the test sample, there was no difference in the validity of the sialolithiasis diagnosis between areas with low and high incidence (p=0.880).
The incidence differed considerably among municipalities in Denmark (p<0.001), ranging from 1.2 cases to as much as 11.1 cases per 100 000 citizens per year (figure 1C). The incidence was higher in the Zealand municipalities in eastern Denmark, where the calcium levels in drinking water are considerably higher compared with the western municipalities characterised by much lower levels of drinking water calcium (figure 1C). There were neither significant correlations between municipality size and incidence, nor between age and incidence. However, municipalities with higher incidence tended to comprise more females than males, although the incidence among females was not significantly higher than among males.
Drinking water and incidence
To evaluate associations between sialolithiasis incidence and drinking water composition, all 20 drinking water constituents were tested against incidence. Several constituents of drinking water were correlated with the incidence of sialolithiasis (table 2). Specifically, this was the case for calcium (p<0.001), magnesium (p<0.001), hydrogen carbonate (p<0.001), fluoride (p<0.001) and pH (p<0.001). However, strong positive inter-relationships were also identified among most drinking variables, especially among calcium, magnesium and hydrogen carbonate (p<0.001).
Table 2.
Statistical models of Danish drinking water composition and sialolithiasis from 2000 to 2010
| Estimate | SD | T-Value | p Value | |
|---|---|---|---|---|
| Calcium model | ||||
| Copenhagen region (east Zealand) | 12.71 | 5.07 | −1.40 | 0.165 |
| Western Zealand region | 16.09 | 4.84 | 3.32 | 0.001 |
| North West Denmark region | −5.77 | 5.40 | −1.07 | 0.289 |
| South West Denmark region | 0.11 | 4.12 | 0.03 | 0.979 |
| Mean age | −1.43 | 0.73 | 1.96 | 0.054 |
| Per cent females | 475.77 | 261.49 | 1.82 | 0.072 |
| Drinking water calcium | 0.22 | 0.08 | 2.69 | 0.009 |
| Drinking water pH | 23.12 | 13.63 | 1.70 | 0.094 |
| Adjusted R2 0.50 | ||||
| Magnesium model | ||||
| Copenhagen region (East Zealand) | 11.24 | 5.57 | 2.02 | 0.047 |
| Western Zealand region | 11.96 | 5.89 | 2.03 | 0.045 |
| North West Denmark region | −4.41 | 5.41 | −0.82 | 0.417 |
| South West Denmark region | 1.54 | 4.08 | 0.38 | 0.706 |
| Mean age | −1.63 | 0.76 | −2.14 | 0.035 |
| Per cent females | 492.66 | 263.31 | 1.87 | 0.065 |
| Drinking water magnesium | 1.84 | 0.80 | 2.29 | 0.024 |
| Drinking water pH | 8.76 | 11.63 | 0.75 | 0.454 |
| Adjusted R2 0.49 | ||||
| Hydrogen carbonate model | ||||
| Copenhagen region (East Zealand) | 9.66 | 5.39 | −1.79 | 0.077 |
| Western Zealand region | 9.16 | 5.98 | 1.53 | 0.130 |
| North West Denmark region | −3.93 | 5.28 | −0.74 | 0.459 |
| South West Denmark region | −0.55 | 4.10 | −0.13 | 0.893 |
| Mean age | −1.65 | 0.74 | −2.24 | 0.028 |
| Per cent females | 419.62 | 261.11 | 1.61 | 0.112 |
| Drinking water hydrogen carbonate | 0.09 | 0.03 | 3.06 | 0.003 |
| Drinking water pH | 17.33 | 12.08 | 1.43 | 0.155 |
| Adjusted R2 0.51 | ||||
Estimates, t values, p values and adjusted R2 values for three models relating drinking water calcium, magnesium and hydrogen carbonate levels to the incidence of hospital-admitted sialolithiasis in the years from 2000 to 2010 (ie, number of cases per 100 000 citizens per year) on a municipality level (n=98).
When fitting multiple regression analyses to adjust for mean age, female proportion, regions and drinking water pH, a model containing calcium could be developed that explains 50% (p<0.001) of the variation in the incidence of sialolithiasis among municipalities (table 2 and figure 1C,D). The incidence of sialolithiasis was high in municipalities with high-calcium levels and vice versa. Adding pH to the analyses had some effect and pH was also positively related to the incidence of sialolithiasis. Fitting a similar model for magnesium, this ion was also positively related to the incidence of sialolithiasis with an explanatory power of 49% (p<0.001). A model containing hydrogen carbonate also explained 51% of the variation in the incidence of sialolithiasis with a positive relation (p<0.001). Finally, a model with calcium and magnesium, although neither could be significant at the same time, also returned an explanatory power of 51% for the incidence of sialolithiasis (p<0.001).
Drinking water and human saliva
The saturation indices for Danish drinking water are given in table 1. As shown, the drinking water was indeed supersaturated with respect to some of the main elements of sialoliths, namely hydroxyapatite and Mg-whitlockite. We further speculated that for a correlation to signify causality, it would require that the saliva levels of the ions in question were affected by the drinking water levels of the same ions. So in order to determine which of the ions in drinking water could indicate such a causative relation, we reanalysed saliva data from patients with known drinking water composition from a previous study, performed on 255 healthy subjects distributed among 12 municipalities in Denmark.30 We found saliva calcium levels to be positively and significantly correlated to drinking water calcium levels (r=0.19; p<0.004, unpublished). Also, saliva magnesium was significantly and positively correlated with drinking water magnesium levels (r=0.23; p<0.001; unpublished), whereas no relations were present with hydrogen carbonate, sodium, chloride, pH and the saliva levels of the same ions.
Discussion
Sialolithiasis is painful and in recurrent and complicated cases, requiring surgical removal, a condition that will lead to loss of gland function with troublesome sequelae such as xerostomia. The surgical treatment of this condition by sialoendoscopy requires painstakingly devised procedures that are complicated, expensive and time-consuming. Thus, from a quality-of-life as well as a socioeconomic perspective, it is desirable to understand aetiological factors for future treatment and prevention of sialolithiasis.
In the current study, the incidence of hospital-admitted sialolithiasis in Denmark was 5.5 cases per 100 000 citizens, although with significant variation among municipalities. The variation in incidence in sialolithiasis among municipalities could not be explained by population size in the municipality, nor the age and gender distribution. However, by adjusting for influence from the region (the five main regions in Denmark), female gender and age, strong relations between incidence and several drinking constituents were obtained, including calcium, magnesium and hydrogen carbonate. After mathematical and statistical evaluation, all three models (calcium, magnesium and hydrogen carbonate; table 2) showed strong and statistically significant relations with the incidence of sialolithiasis. However, it cannot be determined which of the three models is the most important as strong inter-relationships among the ions in question exist. Therefore, analyses were performed on data from a previous study, showing that saliva levels of calcium and magnesium were affected by the drinking water levels of the same ions, whereas no relations were obtained with hydrogen carbonate.30 We therefore excluded hydrogen carbonate as being related to the incidence of sialolithiasis.
With calcium comprising the major component of all sialoliths,31 in the form of precipitated hydroxyapatite, subsequent to heterogeneous nucleation in ducts and glands, it seems to be the most likely ion for this relation to occur. Thus, in the municipalities with the highest drinking water calcium levels, the contribution of calcium from drinking water may be as much as 40% of the average calcium intake among Danes, although this value is much less in the western municipalities. As saliva is always supersaturated with respect to hydroxyapatite,32 saliva calcium concentrations are higher in stone formers versus non-stone formers,33 and saliva calcium levels are positively related to drinking water calcium levels,30 the relation with this ion seems to be the most plausible despite calcium metabolism being a very well-regulated metabolic system in humans,34 and the fact that not even in a study with experimental induced hypercalcaemia in a rat had any influence on the salivary glands in terms of formation of calcified deposits.35 An uncontrolled factor, however, is the fraction of daily calcium intake derived from dairy products which could not be assessed in this study. For instance, an explanation for the effect of including the regions in the analyses may be that dairy products available for retail are normally derived from cows from within the same region. Thus, with the mammalian glands being exocrine, as are the salivary glands, an additional ‘halo-effect’ with calcium may arise from the intake of such products, adding further to the effect of drinking water calcium. Furthermore, the calcium-containing mineral brushite is also part of most sialoliths,31 and saturation indices of this mineral will also increase with increased drinking water calcium levels, adding more evidence to calcium being the most important correlating factor.
Magnesium has often been identified as an inhibitor of hydroxyapatite formation,36 37 but the magnesium concentrations in human saliva (0.3 mmol/L on average) are not sufficiently high to inhibit hydroxyapatite precipitation.38 It therefore seems unlikely that there should be a negative interaction between calcium and magnesium. In contrast, magnesium constitutes parts of many sialoliths in the form of Mg-whitlockite,31 and therefore this correlation coupled with the significant correlation between drinking water and saliva magnesium levels also seems causative. Thus, with normal saliva magnesium levels, precipitation of Mg-whitlockite is indeed likely.39 The combination of high saliva calcium and magnesium concentrations will increase the pressure on precipitation of Mg-whitlockite even further, indicating a positive relation between the two ions. Thus, magnesium undoubtedly also influences sialolithiasis in some individuals, but not all, because Mg-whitlockite is not found in all sialoliths.31
It should be emphasised that the correlations, despite high-statistical significance, does not infer causality. This said, on the basis of a pilot study our a priori assumption was that calcium would be linked to sialolithiasis, which was confirmed in this study, given the strong relation between sialolithiasis and drinking water calcium, and also observed for magnesium, explaining half the variation among municipalities. Taken together, with these ions being prominent in sialoliths and that the drinking water calcium and magnesium content in a separate study is linked to the saliva ions content, we suggest that drinking water calcium and magnesium may play a role in sialolithiasis. Since external factors and patient intrinsic factors are also important, the drinking water calcium and magnesium content may be viewed as preconditions for sialolithiasis.
When comparing our observations with those of other studies, the incidence and municipality variation of hospital-admitted sialolithiasis was higher in Denmark than in the UK and Germany, despite Denmark being a much smaller country.2 3 In western Denmark, most aquifers are situated within deep layers of sand as opposed to superficial layers of limestone in Zealand, giving rise to considerable differences in drinking water calcium (figure 1A,D) and magnesium levels (table 1). Although somewhat comparable, national patterns regarding calcium have also been found in the UK and Germany,3 11 40 even though they showed much weaker or even no relations.1 The more pronounced variation in Denmark may be related to major variations in drinking water composition in a country covering only a limited area of land and with a relatively genetically homogeneous population. The effect of the region could be due to socioeconomic differences or a representation of different genes in the populations among the regions, as well as exposure to dairy products available at a retail level in the region in question. Furthermore, socioeconomic differences may contribute to the likelihood of seeking medical help as well as the fraction of people being treated in private ear, nose, and throat practice, which may also differ due to socioeconomic differences.
Even if assuming that drinking water constituents as calcium and magnesium served as a preconditioning to sialolithiasis, these could explain only half of the variation, indicating that the aetiology of sialolithiasis is indeed multifactorial. The fact that salivary stones are rarely bilateral (BLATT) argues against a systemic aetiology and rather points to local factors, such as saliva viscosity and duct anatomy. Furthermore, the rate of stone formation increases with decreased flow of saliva as seen after parasympathectomy,41 and finally it is also observed that sialolithiasis is associated with the presence of bacteria.42 43
It is important to stress that since this study is epidemiological, patient-related parameters, for example, saliva secretion, saliva contents and duct anatomy, have not been registered. Therefore, the contributing role of such factors in addition to drinking water composition is unknown. Finally, the validity of the hospital diagnosis is characterised by some degree of uncertainty. Differences among hospital practices in the five regions may influence the results. Nonetheless, the validity of the diagnoses in the test sample (n=200) was comparable between low-incidence and high-incidence regions, so overall there is no reason to believe that systematic misclassification is present in the data analysed for this study.
Conclusions
The mean Danish incidence of hospital-admitted sialolithiasis was 5.5 cases and most frequent at 36–61 years of age. Strong correlations were found between incidence of sialolithiasis and drinking water calcium and magnesium. About half the variation among municipalities could be explained by drinking water calcium and magnesium (p<0.001), although in separate models. We therefore conclude that drinking water calcium and magnesium may play a significant role in sialolithiasis and may serve as a precondition for the disease. As the remaining half of the variation was not explained by drinking water constituents, this supports the notion of sialolithiasis as a disease with a multifactorial aetiology. For future perspectives, it is interesting that many countries have started large-scale desalination programmes, for example, in the Mediterranean area, Singapore and Australia, not because of health-related issues, but because of shortage of drinking water and because high drinking water calcium levels have a negative impact on water distribution supply systems, increasing the maintenance costs significantly.44 45 Also in Zealand, similar programmes are now planned. It will therefore be interesting to see whether desalination brings changes to the incidence of sialolithiasis in the Zealand municipalities.
Acknowledgments
The authors would like to thank professors Colin Dawes (Canada), Henrik Spliid and Erik Arvin (Denmark), Bob ten Cate (the Netherlands), Peter Werness (USA), Berit Heitmann and Arne Astrup (Denmark) as well as Arla Foods (largest dairy products company, Denmark) for many useful and helpful advices on linguistics, mathematics, drinking water chemistry, precipitation chemistry and dynamics as well as nutritional issues.
Footnotes
Contributors: All authors contributed to the interpretation of the results and writing of this manuscript. SS wrote and prepared the first draft of the manuscript with input from PH and NW within the field of otolaryngology. A-LV performed the main statistical analyses. HELM and AB performed all chemical analyses and AB did the final corrections throughout the manuscript, tables and figure.
Funding: The financial support was by the research foundation at the Hospital of Northern Zealand.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Sherman JA, McGurk M. Lack of correlation between water hardness and salivary calculi in England. Br J Oral Maxillofac Surg 2000;38:50–5. 10.1054/bjom.1999.0074 [DOI] [PubMed] [Google Scholar]
- 2.Escudier MP, McGurk M. Symptomatic sialoadenitis and sialolithiasis in the English population, an estimate of the cost of hospital treatment. Br Dent J 1999;186:463–6. [DOI] [PubMed] [Google Scholar]
- 3.Zenk J, Constantinidis J, Kydles S et al. Clinical and diagnostic findings of sialolithiasis [German]. HNO 1999;47:963–9. 10.1007/s001060050476 [DOI] [PubMed] [Google Scholar]
- 4.Blatt IM. Studies in sialolithiasis. III. Pathogenesis, diagnosis and treatment. South Med J 1964;57:723–9. 10.1097/00007611-196406000-00022 [DOI] [PubMed] [Google Scholar]
- 5.Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician 2014;89:882–8. [PubMed] [Google Scholar]
- 6.Levi DM, Remine WH, Devine KD. Salivary gland calculi. JAMA 1962;181:1115–19. 10.1001/jama.1962.03050390017005 [DOI] [PubMed] [Google Scholar]
- 7.Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of the literature. Int J Oral Maxillofac Surg 1990;19:135–8. 10.1016/S0901-5027(05)80127-4 [DOI] [PubMed] [Google Scholar]
- 8.Epker BN. Obstructive and inflammatory diseases of the major salivary glands. Oral Surg Oral Med Oral Pathol 1972;33:2–27. 10.1016/0030-4220(72)90203-4 [DOI] [PubMed] [Google Scholar]
- 9.Koch M, Zenk J, Iro H. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin North Am 2009;42:1173–92. 10.1016/j.otc.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 10.Reddy SV, Shaik AB, Bokkisam S. Effect of potassium magnesium citrate and vitamin B-6 prophylaxis for recurrent and multiple calcium oxalate and phosphate urolithiasis. Korean J Urol 2014;55:411–16. 10.4111/kju.2014.55.6.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison JD. Causes, natural history, and incidence of salivary stones and obstructions. Otolaryngol Clin North Am 2009;42:927–47. 10.1016/j.otc.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 12.McNally NJ, Williams HC, Phillips DR et al. Atopic eczema and domestic water hardness. Lancet 1998;352:527–31. 10.1016/S0140-6736(98)01402-0 [DOI] [PubMed] [Google Scholar]
- 13.Chang CC, Chen CC, Wu DC et al. Nitrates in drinking water and the risk of death from rectal cancer: does hardness in drinking water matter? J Toxicol Environ Health A 2010;73:1337–47. 10.1080/15287394.2010.490178 [DOI] [PubMed] [Google Scholar]
- 14.Chiu HF, Tsai SS, Chen PS et al. Does calcium in drinking water modify the association between nitrate in drinking water and risk of death from colon cancer? J Water Health 2011;9:498–506. 10.2166/wh.2011.006 [DOI] [PubMed] [Google Scholar]
- 15.Bruvo M, Ekstrand K, Arvin E et al. Optimal drinking water composition for caries control in populations. J Dent Res 2008;87:340–3. 10.1177/154405910808700407 [DOI] [PubMed] [Google Scholar]
- 16.Berland Y, Olmer M, Grandvuillemin M et al. In vitro and clinical study of oxalate influence on calcium oxalate crystal formation. J Cryst Growth 1988;87:494–506. 10.1016/0022-0248(88)90097-8 [DOI] [Google Scholar]
- 17.Parkhurst DL, Appelo CAJ. User's guide to PHREEQC (version 2). A computer program for speciation, batch reaction, one dimensional transport, and inverse geochemical calculations. Water-Resources Investigations Report 99–4259. US Geological Survey 1999. http://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc
- 18.Gregory TM, Moreno EC, Brown WE. Solubility of CaHPO4 2H2O in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37.5°C. J Res Natl Bur Stand 1970;74A:461–75. 10.6028/jres.074A.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDowell H, Gregory TM, Brown WE. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37°C. J Res Natl Bur Stand 1977;81A:273–81. [Google Scholar]
- 20.Tung MS, Eidelman N, Brown WE et al. Octacalcium phosphate solubility product from 4 to 37°C. J Res Natl Bur Stand 1988;93:613–24. 10.6028/jres.093.153 [DOI] [Google Scholar]
- 21.McCann HG. The solubility of fluorapatite and its relationship to that of calcium fluoride. Arch Oral Biol 1968;13:987–1001. 10.1016/0003-9969(68)90014-9 [DOI] [PubMed] [Google Scholar]
- 22.Ben Ayed F, Bouaziz J, Khattech I et al. Produit de solubilitéapparent de la fluorapatitefrittée [French]. Ann Chim Sci Mat 2001;26:75–86. 10.1016/S0151-9107(01)80101-X [DOI] [Google Scholar]
- 23.Plummer LN, Busenberg E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim Cosmochim Acta 1982;46:1011–40. 10.1016/0016-7037(82)90056-4 [DOI] [Google Scholar]
- 24.Bates RG. First dissociation constant of phosphoric acid from 0° to 60°C; limitations of the electromotive force method for moderately strong acids. J Res Natl Bur Stand 1951;47:127–34. 10.6028/jres.047.017 [DOI] [Google Scholar]
- 25.Martell AE, Smith RM, Motekaitis RJ. Critically selected stability constants of metal complexes. NIST Stand Ref Database 46; Version 8.0, 2004.
- 26.Gopal R, Calvo C, Ito J et al. Crystal structure of synthetic Mg-whitlockite, Ca18Mg2H2(PO4)14. Can J Chem 1974;52:1155–64. 10.1139/v74-181 [DOI] [Google Scholar]
- 27.Li X, Ito A, Sogo Y et al. Solubility of Mg-containing β-tricalcium phosphate at 25°C. Acta Biomater 2009;5:508–17. 10.1016/j.actbio.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Statistic Software. 2014. http://www.sas.com/en_us/home.html
- 29.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. http://www.r-project.org [Google Scholar]
- 30.Bardow A, Lykkeaa J, Qvist V et al. Saliva composition in three selected groups with normal stimulated salivary flow rates, but yet major differences in caries experience and dental erosion. Acta Odontol Scand 2014;72:466–73. 10.3109/00016357.2013.860621 [DOI] [PubMed] [Google Scholar]
- 31.Teymoortash A, Buck P, Jepsen H et al. Sialolith crystals localized intraglandularly and in the Wharton's duct of the human submandibular gland: an X-ray diffraction analysis. Arch Oral Biol 2003;48:233–6. 10.1016/S0003-9969(02)00211-X [DOI] [PubMed] [Google Scholar]
- 32.Larsen MJ, Pearce EI. Saturation of human saliva with respect to calcium salts. Arch Oral Biol 2003;48:317–22. 10.1016/S0003-9969(03)00007-4 [DOI] [PubMed] [Google Scholar]
- 33.Grases F, Santiago C, Simonet BM et al. Sialolithiasis: mechanism of calculi formation and etiologic factors. Clin Chim Acta 2003;334:131–6. 10.1016/S0009-8981(03)00227-4 [DOI] [PubMed] [Google Scholar]
- 34.Emkey RD, Emkey GR. Calcium metabolism and correcting calcium deficiencies. Endocrinol Metab Clin North Am 2012;41:527–56. 10.1016/j.ecl.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 35.Epivatianos A, Tsougas M. The effect of the hypercalcaemia on the major salivary glands of the rat [in Modern Greek]. Stomatologia (Athenai) 1991;47:306–13. [PubMed] [Google Scholar]
- 36.Wilson JW, Werness PG, Smith LH. Inhibitors of crystal growth of hydroxyapatite: a constant composition approach. J Urol 1985;134:1255–8. [DOI] [PubMed] [Google Scholar]
- 37.Lagier R, Baud CA. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol Res Pract 2003;199:329–35. 10.1078/0344-0338-00425 [DOI] [PubMed] [Google Scholar]
- 38.Lundager Madsen HE. Influence of foreign metal ions on crystal growth and morphology of brushite (CaHPO4, 2H2O) and its transformation to octacalcium phosphate and apatite. J Cryst Growth 2008;310:2602–12. 10.1016/j.jcrysgro.2008.01.047 [DOI] [Google Scholar]
- 39.Rowles SL. The precipitation of whitlockite from aqueous solutions. Bull Soc Chim Fr 1968;2:1797. [Google Scholar]
- 40.Escudier MP, Brown JE, Putcha V et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope 2010;120:1545–9. 10.1002/lary.21000 [DOI] [PubMed] [Google Scholar]
- 41.Triantafyllou A, Harrison JD, Garrett JR. Production of salivary microlithiasis in cats by parasympathectomy: light and electron microscopy. Int J Exp Pathol 1993;74:103–12. [PMC free article] [PubMed] [Google Scholar]
- 42.Teymoortash A, Wollstein AC, Lippert BM et al. Identification of uncultured bacteria in human salivary calculus. Res Commun Mol Pathol Pharmacol 2000;108:437–40. [PubMed] [Google Scholar]
- 43.Teymoortash A, Wollstein AC, Lippert BM et al. Bacteria and pathogenesis of human salivary calculus. Acta Otolaryngol 2002;122:210–14. 10.1080/00016480252814252 [DOI] [PubMed] [Google Scholar]
- 44.Rygaard M, Arvin E, Binning PJ. The valuation of water quality: effects of mixing different drinking water qualities. Water Res 2009;43:1207–18. 10.1016/j.watres.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 45.Rygaard M, Arvin E, Bath A et al. Designing water supplies: optimizing drinking water composition for maximum economic benefit. Water Res 2011;45:3712–22. 10.1016/j.watres.2011.04.025 [DOI] [PubMed] [Google Scholar]