Abstract
Adverse Drug Reactions (ADRs) are scantly reported with poor contribution by healthcare professionals worldwide and in particular in developing countries. The aim of this study was to assess the knowledge and awareness of adverse drug reactions (ADRs) reporting and pharmacovigilance system among healthcare professionals in Al-Madinah Al-Munawwarah hospitals, Kingdom of Saudi Arabia. A questionnaire was designed addressing; awareness of ADRs, knowledge of pharmacovigilance system, availability of ADRs reporting system, patient counseling about ADRs and documentation of ADRs. The questionnaire was distributed to randomly selected healthcare professionals (n = 585) such as physicians, pharmacists, nurses and pharmacists’ technicians of hospitals. Completed questionnaires were collected and data were analyzed. Data were expressed in number as well as percentage. Of the 585 questionnaires circulated, a total of 384 healthcare professionals responded. Healthcare professional categories involved in the study were 148 physicians, 37 pharmacists, 158 nurses and 41 pharmacist technicians. The percent of the respondents who accepted to enroll in the study was 65.64%. Most of the respondents were unable to correctly define the pharmacovigilance term, but they were aware of ADRs. The awareness among healthcare professionals of the national pharmacovigilance system was 39.6%. Pharmacists had a good knowledge of pharmacovigilance and ADRs terminology and showed a more positive attitude to report ADRs. A greater number of the healthcare professionals were aware of ADRs reporting, but practically it is not implemented in hospitals. Most hospitals had follow-up documentation systems, but did not include ADRs reporting. There was no distinct pharmacovigilance system in place. Our study has demonstrated a lack of knowledge and awareness of pharmacovigilance and ADRs reporting among healthcare professionals in hospitals. The poor knowledge of ADRs reporting emphasized the urgent need to implement the appropriate strategies to improve the awareness of pharmacovigilance practices and ADRs reporting in Al-Madinah Al-Munawwarah hospitals.
Keywords: Adverse drug reactions, Pharmacovigilance, Knowledge, Awareness, Healthcare professionals, Hospitals
1. Introduction
Medications have caused and will continue to cause harm to a number of people’s lives alongside many benefits. Adverse Drug Reactions (ADRs) are a major problem and are one of the leading causes of mortality and morbidity (Lazarou et al., 1998; Classen et al., 1997). Adverse drug reaction (ADR) is defined according to WHO (2002a) as any response to a drug which is noxious and unintended and occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease or the modification of physiological function.
ADRs are associated with prolonged length of hospital stay, increased economic burden and increased death. Many studies have reported that ADRs were responsible for large numbers of hospital admissions (Lazarou et al., 1998; Jick, 1984; Pirmohamed et al., 2004; Ahmed, 1997). In the United States, more than 100,000 deaths are attributed annually to serious adverse drug reactions (Lazarou et al., 1998). In the UK, about 6.5% of all admissions to hospitals are due to an ADR, and the overall fatality was 0.15% (Pirmohamed et al., 2004). A prospective study by Ahmed (1997) demonstrated that drug-related problems report in Saudi Arabia showed that the mortality rate associated with ADRs in provincial hospitals was found to be 3.8% from the overall deaths in the general practice. There are differences among countries and regions within countries in the occurrence of drug-related problems. This may be due to differences in diseases, prescribing practices, genetics, diet habits and use of herbal remedies which may pose specific toxic problems. Under-reporting of adverse drug reactions (ADRs) is very common. It has been estimated that only 6–10% of all the ADRs are reported (Feely et al., 1990).
There is a considerable increase in awareness about the issues related to drug safety among healthcare providers, healthcare institutions and the public. The term pharmacovigilance has evolved to recognize the importance for monitoring and improving the safe use of medicines.
World Health Organization (WHO, 2002b) defines pharmacovigilance as “the science and activities relating to the detection, evaluation, understanding, and prevention of adverse reactions to medicines or any other medicine-related problems”. Uppsala Monitoring Centre (UMC), located in Uppsala, Sweden, is the field name for the World Health Organization Collaborating Centre for International Drug Monitoring. The UMC works by collecting, assessing and communicating information from member countries’ national programs in regard to the benefits, harm, effectiveness and risks of drugs.
A national pharmacovigilance center was established by Saudi Food and Drug Authority (SFDA) in March 2009 and it is one member of the WHO Collaborating Centre in Uppsala, Sweden. The national pharmacovigilance system is the main way to collect information about ADRs occurrences in both the hospital and community settings. The effectiveness and success of any pharmacovigilance system depend highly on the participation of all healthcare professionals and the degree of co-operation and communication between the practitioners and pharmacovigilance center.
There is a lack of studies that address the knowledge, attitudes and perception of healthcare professionals toward the pharmacovigilance system and ADRs reporting, which is carried out in this country. In a country like Saudi Arabia with multiethnic groups and a high rate of use of herbal and complementary medicine, practitioners can play a major role in detecting and reporting ADRs associated with the use of such products. It is important to conduct comprehensive studies to explore and evaluate the roles of healthcare professionals and their contributions in the pharmacovigilance activities. The aim of the present study was to assess the knowledge and awareness of ADRs reporting and pharmacovigilance system among the healthcare professionals in Al-Madinah Al-Munawwarah Hospitals, Kingdom of Saudi Arabia.
2. Materials and methods
2.1. Design of the questionnaire
A questionnaire was developed to obtain information on the knowledge and awareness of pharmacovigilance and adverse drug reactions reporting, availability of ADRs reporting systems, patient counseling about ADRs and documentation of ADRs.
2.2. Hospitals visits
The healthcare professionals (physicians, pharmacists, nurses and pharmacist’ technicians) of some 9 hospitals were provided with a copy of the questionnaire after explanation of the objectives of the study. The healthcare professionals were provided with enough time to fill the questionnaire. The questionnaire was explained to each practitioner in order to preclude any potential misunderstanding, and also the name of the participant was made optional.
2.3. Healthcare professionals participation
A total 585 questionnaires were circulated, and a total of 384 healthcare professionals responded to fill the questionnaire (Table 1). Healthcare professional categories involved were 148 physicians, 37 pharmacists, 41 pharmacist technicians and 158 nurses. A total of 201 of the healthcare professionals refused to participate in the study. Table 2 shows the respondents’ nationalities from different hospitals visited. The questionnaire consisted of information about familiarity with the ADRs reporting and pharmacovigilance system, attitude toward ADRs reporting and patient counseling about ADRs and documentation of ADRs.
Table 1.
Total number of healthcare professional respondents in hospitals.
| Healthcare professional Respondents | Total (585) | Response |
|
|---|---|---|---|
| Accepted (384) | Rejected (201) | ||
| Pharmacists | 52 | 37 | 15 |
| Pharmacists’ Technicians | 68 | 41 | 27 |
| Physicians | 209 | 148 | 61 |
| Nurses | 253 | 158 | 95 |
Table 2.
Total number of healthcare professional respondents’ nationalities in hospitals.
| Nationality | Total (585) | Accepted (384) | Rejected (201) |
|---|---|---|---|
| Egypt | 159 | 105 | 54 |
| Saudi | 181 | 107 | 74 |
| Sudan | 39 | 32 | 7 |
| Syrian | 27 | 17 | 10 |
| Philippine | 173 | 117 | 56 |
| Jordon | 5 | 5 | 0 |
| Pakistan | 1 | 1 | 0 |
2.4. Collection of questionnaires and analysis of data
Complete questionnaires were collected from each participant in each hospital to evaluate the awareness of pharmacovigilance knowledge and ADRs reporting among healthcare professionals. Data were analyzed and presented as a percent (%) of the respondents. In case of unanswered questions, a participant was excluded from the study.
3. Results
A total 384 healthcare professionals participated in the study and the overall percent of the respondents who accepted to enroll in the study was 65.64%. The major reasons for non-participation were being too busy and/or unwilling to participate, too busy or fear. Healthcare professionals to participate in the study were 148 physicians, 37 pharmacists, 41 pharmacist technicians and 158 nurses. A total of 201 of the healthcare professionals refused to participate in the study.
3.1. Awareness of the healthcare professionals of pharmacovigilance system
The healthcare professionals (physicians, pharmacists, nurses and pharmacist technicians) were asked about their knowledge of the pharmacovigilance in KSA. Most of the practitioners were unfamiliar with the availability of national pharmacovigilance system in KSA. The total percent of the respondent’s knowledge of national pharmacovigilance system in KSA was 39.6% (Fig. 1). The highest percent of the awareness of pharmacovigilance system was among the pharmacists (70.27%), pharmacists’ technicians (61%), physicians (39.2%), and nurses were 27.2% (Fig. 2). The awareness of the healthcare professionals of pharmacovigilance by nationality distribution is presented in Fig. 3. This figure shows that the number of Saudi nationality was the highest, followed by Sudanese, Egyptian, Syrian and Philippines. One of the limits of this interpretation was the small number of the Saudi and Sudanese participated in the study.
Figure 1.
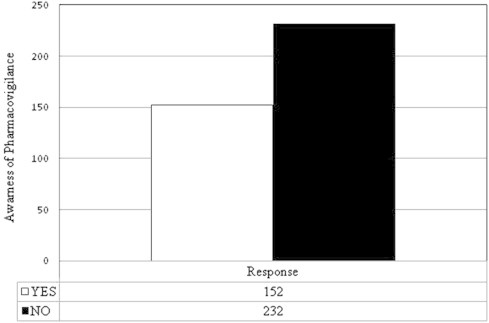
Awareness of the healthcare professionals of national pharmacovigilance system.
Figure 2.
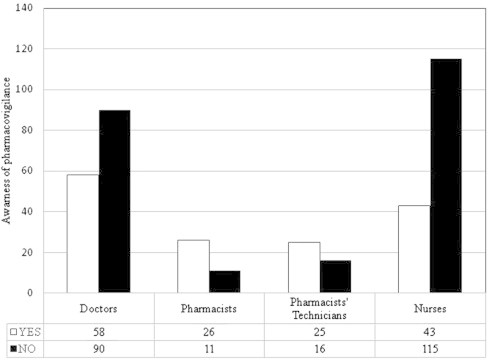
Awareness of the different groups of healthcare professionals of national pharmacovigilance system.
Figure 3.
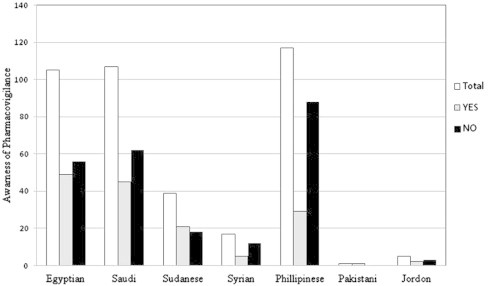
Awareness of respondents of the healthcare professionals of pharmacovigilance system by nationalities.
3.2. Availability of a pharmacovigilance system in hospitals
The healthcare professionals were then asked about the availability of pharmacovigilance system in their hospitals, the doctors had a high score followed by nurses, and then pharmacists. The response of the participants about the availability of pharmacovigilance system at their hospitals is shown in Fig. 4. It was observed that there was no electronic reporting system in all hospitals visited, which affects the availability and sharing of pharmacovigilance information among the different hospital departments (Fig. 5).
Figure 4.
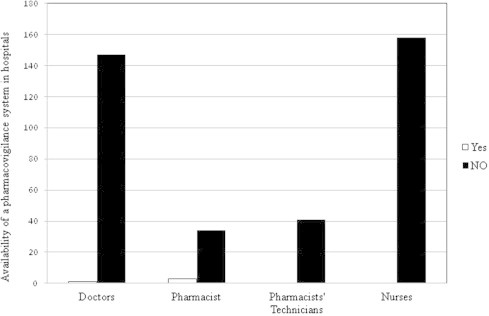
Availability of a pharmacovigilance system in hospitals.
Figure 5.
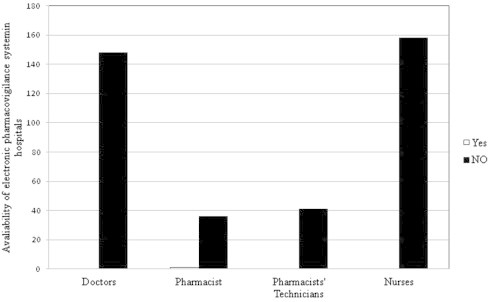
Availability of an electronic pharmacovigilance reporting system in hospitals.
3.3. Awareness of adverse drug reactions (ADRs) reporting
All respondents of healthcare professionals had a positive awareness for adverse drug reactions (ADRs) reporting. Fig. 6 shows that a higher number of the healthcare professionals were aware of ADRs reporting, but practically it is not applied in the visited hospitals.
Figure 6.
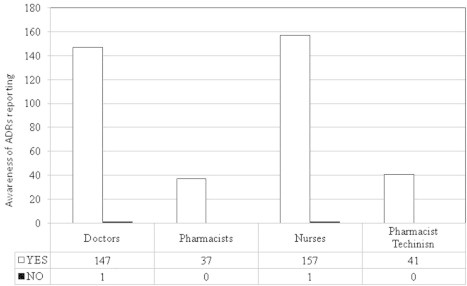
Awareness of the healthcare professionals of adverse drug reactions (ADRs) reporting.
3.4. Frequency of encountering ADRs
The healthcare professionals were asked about the frequency of encountering ADRs during their daily work in hospitals. Fig. 7 shows that physicians encountered ADRs occasionally, while pharmacists’ technicians and nurses rarely encountered ADRs. Some pharmacists answered that they encountered ADRs occasionally, while others they encountered rarely depending on the hospital and the environment of practice.
Figure 7.
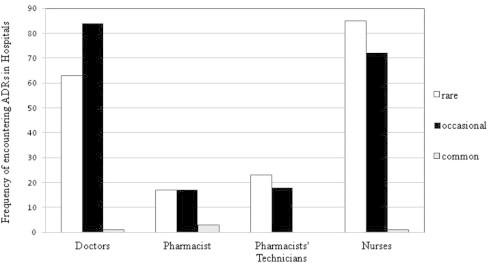
Frequency of encountering of ADRs in Hospitals.
3.5. Patient counseling about ADRs by healthcare professionals
Effective communication between healthcare professionals and patients is one of the most important elements for reducing ADRS and improving health outcomes. Therefore, healthcare professionals were asked about their role in counseling patients about ADRs. Fig. 8 demonstrates that doctors had the highest percent (82.4%) among the other healthcare professional groups in counseling patients about ADRs. Around 75.7% of pharmacists and 73.4% of nurses were willing to counsel patients about ADRS.
Figure 8.
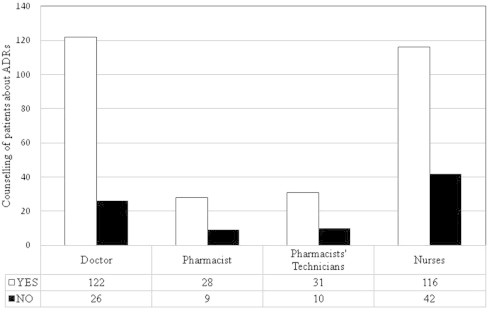
Counseling of patients by healthcare professionals about ADRs.
3.6. Follow-up and documentation of ADRs in hospitals
Follow-up and documentation is the cornerstone of the pharmacovigilance system in any hospital. A percent of 47.7% of physicians and 50.6% of nurses were aware of follow-up and documentation of ADRs in the visited hospitals (Fig. 9).
Figure 9.
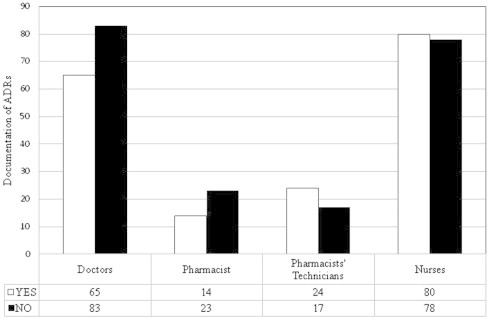
Follow-up and documentation of ADRs.
4. Discussion
Adverse drug reactions (ADRs) are one of the most serious health problems, and are one of the leading causes of mortality and morbidity (Pirmohamed et al., 2004; Ernst and Grizzle, 2001). There are differences among countries in the occurrence of drug-related problems; this may be due to differences in diseases, prescribing practices, diet, traditions of the people and the use of herbal remedies which may pose specific toxicological problems. Pharmacovigilance has grown in importance in recent years, and good pharmacovigilance systems will identify the risks of drug-related problems so that harm can be avoided. Pharmacovigilance deals with detection, assessment, understanding and prevention of adverse effects or any other drug related problems.
In a country like Saudi Arabia with multiethnic groups and a high rate of use of herbal and complementary medicine, practitioners can play a major role in detecting and reporting ADRs associated with the use of such products. It is important to conduct comprehensive studies to explore and evaluate the roles of healthcare professionals and contributions in the pharmacovigilance system activities. Despite the Saudi national pharmacovigilance center that regularly publishes an ADR bulletin, and information pertaining to ADRs is stored in a national ADRs database, there is a lack of studies that address the knowledge and awareness of healthcare professionals about the pharmacovigilance system and ADRs reporting in this country. The present study aimed to evaluate the knowledge, awareness of the healthcare professionals regarding pharmacovigilance and ADRs reporting. It was observed that the awareness of the availability of national pharmacovigilance system among healthcare professionals was 39.6%. Interestingly, pharmacists were the most knowledgeable group (70.27%) about pharmacovigilance and ADRs reporting, despite the absence of distinct pharmacovigilance systems or reporting systems in most hospitals. The findings in our study were reasonably similar to the observations made in both developing and developed countries. In China 71% of the healthcare professionals did not have knowledge of the reporting procedure (Aziz et al., 2007). In Malaysia, about 40% of the respondents were not aware of the existence of the national reporting system (Li et al., 2004). In the European Union (EU), many healthcare professionals did not know how to report an ADR (Belton, 1997).
The healthcare professionals were asked about the availability of pharmacovigilance system at their hospitals, the majority of participants answered that there was no pharmacovigilance system in hospitals. It was noted that healthcare professionals (physicians, pharmacists, nurses and pharmacist technicians) working in hospitals have insufficient knowledge of pharmacovigilance practices. The main reasons for under-reporting of ADRs are lack of time, poor knowledge on the reporting mechanisms, unfamiliarity with the existence of national pharmacovigilance system, belief that the ADR was already well known, doubt about the importance of the ADRs reporting and fear to report ADRs (Lee and Thomas, 2003; Herdeiro et al., 2005). Al-Hazmi and Naylor (2013) reported that only 18% of community pharmacists were aware of the ADR system and 56% of the respondents were not aware of the existence of the Saudi National Pharmacovigilance center. Bawazir (2006) also reported that the majority of community pharmacists surveyed (86.8%) were not aware of the ADRs reporting program in Saudi Arabia and only twenty-nine percent of pharmacists were aware that pharmacists in Saudi Arabia could report an ADR to the Ministry of Health (MOH). A survey study in France showed that the majority of medical residents had minimal knowledge regarding pharmacovigilance (Graille et al., 1994). Another study from Italy reported that doctors had little information regarding ADRs and ADR reporting systems (Cosentino et al., 1997). In the UK, where pharmacovigilance programs are well established, there is a high level of under-reporting is documented (Belton et al., 1995). Healthcare professionals should consider adverse drug reactions (ADRs) reporting as an obligation and should be aware of the existing pharmacovigilance mechanisms. The re-enforcement of pharmacovigilance systems in hospitals should be a priority basis for the success of the pharmacovigilance programs and the better clinical management of the patients.
5. Conclusions
The present study pointed out to the lack of the knowledge and awareness among healthcare professionals of pharmacovigilance system and ADRs reporting systems in hospitals. The doctors, pharmacists and nurses have a great responsibility in reporting ADRs and strengthening the pharmacovigilance mechanisms. Our findings also provide the healthcare policy makers and health authorities with base line data that can be used in the future evaluation or re-enforcement plans and educational initiatives for improving the pharmacovigilance practices and ADRs reporting in hospitals.
Acknowledgments
I would like to thank the student Hossam A. Al-Nakhli, Department of Clinical and Hospital Pharmacy, College of Pharmacy, Taibah University, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia, who helped in the distribution and collection of the questionnaire.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed M.el-B. Drug-associated admissions to a district hospital in Saudi Arabia. J. Clin. Pharm. Ther. 1997;22:61–66. doi: 10.1046/j.1365-2710.1997.8375083.x. [DOI] [PubMed] [Google Scholar]
- Al-Hazmi N.N., Naylor I.L. A study of community pharmacists’ awareness and contributions to adverse drug reactions (ADRs) reporting systems in the Makkah, Kingdom of Saudi Arabia (KSA) J. Clin. Trials. 2013;3:127–132. [Google Scholar]
- Aziz Z., Siang T.C., Badarudin N.S. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol. Drug Saf. 2007;16:223–228. doi: 10.1002/pds.1313. [DOI] [PubMed] [Google Scholar]
- Bawazir S.A. Attitude of community pharmacists in Saudi Arabia towards adverse drug reaction reporting. Saudi Pharm. J. 2006;14:75–83. [Google Scholar]
- Belton K.J. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. The European Pharmacovigilance Research Group. Eur. J. Clin. Pharmacol. 1997;52:423–427. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- Belton K.J., Lewis S.C., Payne S., Rawlins M.D., Wood S.M. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br. J. Clin. Pharmacol. 1995;39:223–326. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen D.C., Pestonik S.L., Evans R.S., Lioyd J.F., Burke J.P. Adverse drug events in hospitalized patient- excess length of stay, extra cost and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- Cosentino M., Leoni O., Banfi F., Leechini S., Frigo G. Attitudes to adverse drug reaction reporting by medical practitioners in a Northern Italian district. Pharmacol. Res. 1997;35:85–88. doi: 10.1006/phrs.1996.0138. [DOI] [PubMed] [Google Scholar]
- Ernst F.R., Grizzle A.J. Drug-related morbidity and mortality: updating the cost-of-illness model. J. Am. Pharm. Assoc. 2001;41:192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- Feely J., Moriarty S., O’Connor P. Stimulating the reporting of an adverse drug reaction by using a fee. Br. Med. J. 1990;300:22–23. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graille V., Lapeyre-Mestre M., Montastruc J.L. Drug vigilance: opinion survey among residents of a university hospital. Therapie. 1994;49:451–454. [PubMed] [Google Scholar]
- Herdeiro M.T., Figueiras A., Polónia J., Gestal-Otero J.J. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28:825–833. doi: 10.2165/00002018-200528090-00007. [DOI] [PubMed] [Google Scholar]
- Jick H. Adverse drug reactions: the magnitude of the problem. J. Allergy Clin. Immunol. 1984;74:555–557. doi: 10.1016/0091-6749(84)90106-4. [DOI] [PubMed] [Google Scholar]
- Lazarou J., Pomeranz B.H., Corey P.N. The incidence of adverse drug reactions in hospitalized patients-a meta- analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- Lee A., Thomas S.H.L. Adverse drug reactions. In: Walker R., Edward C., editors. Clinical Pharmacy and Therapeutics. 3rd ed. Churchill Livingstone; 2003. pp. 33–46. [Google Scholar]
- Li Q., Zhang S.M., Chen H.T., Fang S.P., Yu X., Liu D., Shi L.Y., Zeng F.D. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin. Med. J. (Engl.) 2004;117:856–861. [PubMed] [Google Scholar]
- Pirmohamed M., James S., Meakin S., Green C., Scott A.K., Walley T.J., Keith Farrar K., Park B.K., Breckenridge A.M. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudi Food & Drug Authority (SFDA). www.sfda.gov.sa/En/Drug.
- The World Health Organization, 2002a. Safety of medicines: A guide to detecting and reporting adverse drug reactions, Geneva.
- The World Health Organization, 2002b. The importance of Pharmacovigilance: Safety Monitoring of Medicinal Products, Geneva.
- WHO Programme Members-Uppsala Monitoring Centre, Sweden: www.who-umc.org.


