Abstract
Adenosine plays an important role in regulation of renal microcirculation. All receptors of adenosine, A1, A2A, A2B, and A3, have been found in the kidney. However, little is known about the location and function of the A3 receptor in the kidney. The present study determined the expression and role of A3 receptors in mediating the afferent arteriole (Af-Art) response and studied the interaction of A3 receptors with angiotensin II (ANG II), A1 and A2 receptors on the Af-Art. We found that the A3 receptor expressed in microdissected isolated Af-Art and the mRNA levels of A3 receptor were 59% of A1. In the isolated microperfused Af-Art, A3 receptor agonist IB-MECA did not have a constrictive effect. Activation of A3 receptor dilated the preconstricted Af-Art by norepinephrine and blunted the vasoconstrictive effect of both adenosine A1 receptor activation and ANG II on the Af-Art, respectively. Selective A2 receptor antagonist (both A2A and A2B) had no effect on A3 receptor agonist-induced vasodilation, indicating that the dilatory effect of A3 receptor activation is not mediated by activation of A2 receptor. We conclude that the A3 receptor is expressed in the Af-Art, and activation of the A3 receptor dilates the Af-Art.
Keywords: adenosine, A3 receptor, Af-Art, tubuloglomerular feedback
adenosine is an endogenous adenine nucleoside that is formed by the hydrolysis of ATP. Cellular signaling by adenosine occurs through following receptor subtypes: A1, A2, and A3. The adenosine A2 receptor (A2R) family consists of two subtypes, A2A and A2B (36). All receptors of adenosine have been found in the kidney (1, 16, 20, 37, 41). Adenosine has been suggested as a mediator (via A1 receptor signaling) and a modulator (via A2 receptor) of renal autoregulation (2, 4, 5, 15, 28, 32, 34, 36). In the renal vasculature, adenosine elicits biphasic effects with vasoconstriction via A1 receptors (A1R) at a lower concentration and vasodilation via A2R as the concentration of adenosine increases (14, 27, 33).
Adenosine A3 receptor (A3R) is first found from the rat striatum (40), and it is highly expressed in the lung and liver in humans (22, 31). In kidneys, A3R has been detected both on mRNA and protein levels in various species (7, 22, 23, 26, 30). However, the location and function of the A3R in the kidney are poorly characterized. The objective of the present study is to identify whether A3R is expressed in the afferent arterioles (Af-Art), which are the most important resistant vessels in the kidney (6), and its role in regulating the tone of the Af-Art.
METHODS
Animals.
Experiments were conducted on male 8- to 12-wk-old C57BL/6J mice. All experiments were preapproved by the Animal Care and Use Committees of University of Mississippi Medical Center and University of South Florida.
Expression of adenosine receptors in microdissected Af-Art using RT-PCR and real-time PCR.
An individual Af-Art with attached glomerulus was dissected as previously described (24), and then cut between interlobular artery and glomerulus to get a clean Af-Art. Fifteen to twenty arterioles were microdissected in ice-cold MEM within 40 min from a mouse and transferred into RLT buffer (RNeasy Micro Kit, Qiagen) for total RNA extraction.
For RT-PCR measurement, total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions. Sequences of A3R primers were 5́-CGGGATCCCGTTCCGTGGTCAGTTTG-3́; 5́-GGAATTCGCAGGCGTAGACAATAGG-3́. β-Actin was used to serve as a housekeeping gene. After qualification of the cDNA templates and primers, PCR was performed in a thermal cycler (Bio-Rad, Hercules, CA). Negative controls were performed by omitting cDNA template from the PCR amplification. The mixed samples were heated to 95°C for 5 min and then cycled for 40 cycles at 94°C for 30 s, 60°C for 20 s, 72°C for 30 s. Final extension was 8 min at 72°C. PCR products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide. Images were captured using a VersaDoc image analysis system (Bio-Rad).
For real-time PCR measurement, the first-strand cDNA was synthesized with reverse transcription system (Promega). The specific primers were the same as published by Vitzthum et al. (37): A1: 5′-CGGG ATCC TACA TCTC GGCC TTCC AGG-3′; 5′-GGAA TTCA GTAG GTCT GTGG CCCA ATG-3′, A2A: 5′-CGGG ATCC GTCC CTGG CCAT CATC GT-3′; 5′-GGAA TTCG ATCC TGTA GGCG TAGAT-3′, A2B: 5′-CGGG ATCC TTTC ACGG CTGC CTCT TC-3′; 5′-GGAATTCCATCCCCCAGTTCTGTGC-3′, A3: 5′-CGGG ATCC CGTT CCGT GGTC AGTT TG-3′; 5′-GGAA TTCG CAGG CGTA GACA ATAGG-3′. β-Actin was used as a housekeeping gene. Quantitative PCR analysis was performed using iQ SYBR Green Supermix (Bio-Rad) and CFX96 Real-Time Detection System (Bio-Rad).
A3R agonist-induced vascular response of Af-Art.
The microisolation and perfusion of the Af-Art were similar as described previously (24). Briefly, the mice were anesthetized with ketamine and xylazine. Kidneys were removed and sliced along the corticomedullary axis immediately after euthanization and placed in 4°C minimum essential medium (MEM; Gibco, Grand Island, NY) containing 5% bovine serum albumin (BSA; Sigma, St. Louis, MO). Af-Art with attached glomerulus was microdissected under a stereomicroscope (SMZ 1500, Nikon) and then transferred to a temperature-controlled chamber on the stage of an inverted microscope (Eclipse Ti, Nikon), and perfused using a micromanipulator system with concentric holding and perfusion pipettes. Microdissections and perfusions were completed within 40 min at 4°C. The bath consisted of MEM and was exchanged continuously at a rate of 1 ml/min at 37°C. The perfusion pressure of the Af-Art was maintained at 60 mmHg during experiment. Once the temperature was stable, a 30-min equilibration period was allowed before any measurements were taken. The imaging system consisted of a microscope (Eclipse Ti, Nikon), digital CCD camera (CoolSnap, Photometrics), xenon light (LB-LS/30, Shutter Instruments), and optical filter changer (Lambda 10-3, Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
To study the potential role of A3R in modifying vascular responsiveness, we first examined the vasoconstrictor response of Af-Art in response to A3R agonists. The diameter of Af-Art was measured in the MEM perfusion solution. Then, A3R agonist was added and the diameter of the Af-Art was measured again 15 min later. To test the vasodilatory response, the Af-Art was preconstricted with norepinephrine (NE) and two different A3R agonists, N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (IB-MECA) or 1-[2-chloro-6-{[(3-iodophenyl)methyl]amino}-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (2-CL-IB-MECA), were added to the bath, respectively, in the presence of NE and 15 min later the diameter of the Af-Art was again recorded. The 2-CL-IB-MECA compound is a high-affinity and selective A3R agonist (Ki = 0.33 nmol/l). It displays 2,500-fold selectivity over A1 and 1,400-fold selectivity over A2A receptors, respectively.
Interaction of A1R and A2R with A3R.
To study the interaction of A3R and A1R, the dose-response curve of A1R mimetic N6-cyclohexyladenosine (CHA) was first measured and then compared with the presence of A3R agonist IB-MECA.
To test whether the A3R agonist is also stimulating A2R, the Af-Art was preconstricted with NE, and then an A2 antagonist, 4-{-2-[7-amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5)triazin-5-yl-amino]ethyl}phenol (ZM241385), was added to the bath for 15 min, followed by adding A3R agonist IB-MECA. The concentration used for ZM241385 (10−7 mol/l) is considered to inhibit both A2A and A2B receptors (18). To ensure that the vascular dilation effect of A3R agonist was through activation of A3R and not through A2R, additional series of experiments were performed. A selective A3R antagonist, 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS 1191), was added to the bath for 15 min in the presence of NE, followed by adding A3R agonist IB-MECA.
Interaction of ANG II with A3R.
To determine the effect of A3R agonist on ANG II-induced renal vasoconstriction, we compared the vasoconstrictor response of Af-Art to ANG II with and without the presence of an A3R agonist IB-MECA.
Statistics.
Data were analyzed as repeated measures when multiple samples were compared with common control. Data are presented as means ± SE. The significance of differences in control and experimental values within the same animal was determined by a paired t-test. The significance of differences in corresponding mean values between groups was determined by ANOVA followed by Holm-Sidak test. P < 0.05 was considered as statistically significant changes.
RESULTS
Adenosine A3 receptors are expressed in Af-Art.
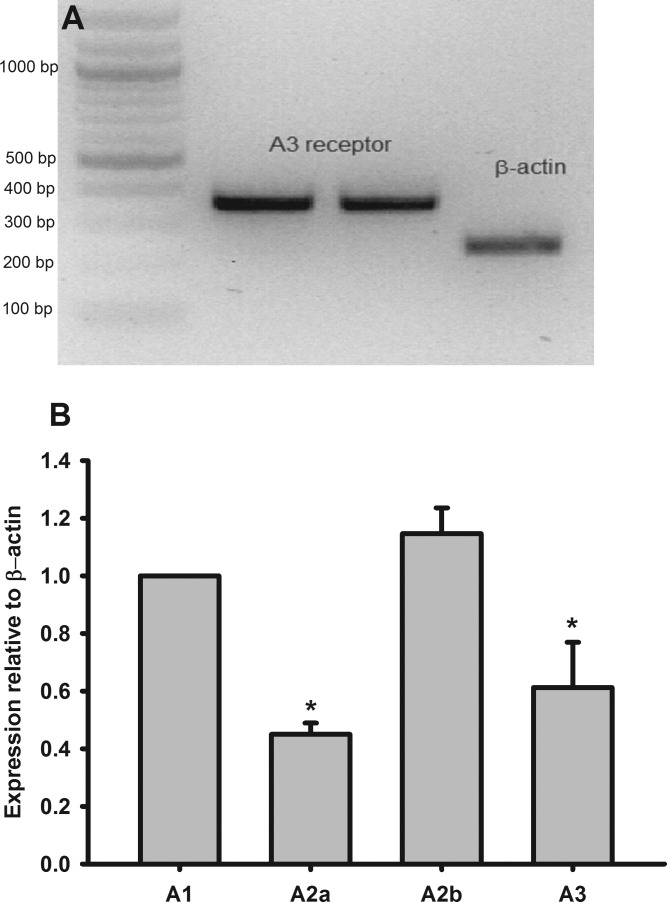
We first tested whether adenosine A3R was expressed in the Af-Art by RT-PCR. As shown in Fig. 1A, A3R was expressed in Af-Art. Then, we compared the expression level of A3R with other adenosine receptors using real-time PCR. All adenosine subtype receptors were present in Af-Art as shown in Fig. 1B, where mRNA levels of A2A, A2B, and A3 were 44, 113, and 59%, respectively, compared with A1R (n = 5 mice).
Fig. 1.
Adenosine receptor mRNA expression in afferent arteriole (Af-Art). A: adenosine A3 receptor (A3R) mRNA expressed in microdissected Af-Art. B: relative mRNA levels of adenosine receptors expressed in the Af-Art measured with real-time PCR (n = 5). *P < 0.05 compared with A1 receptor expression.
Activation of A3R dilates Af-Art.
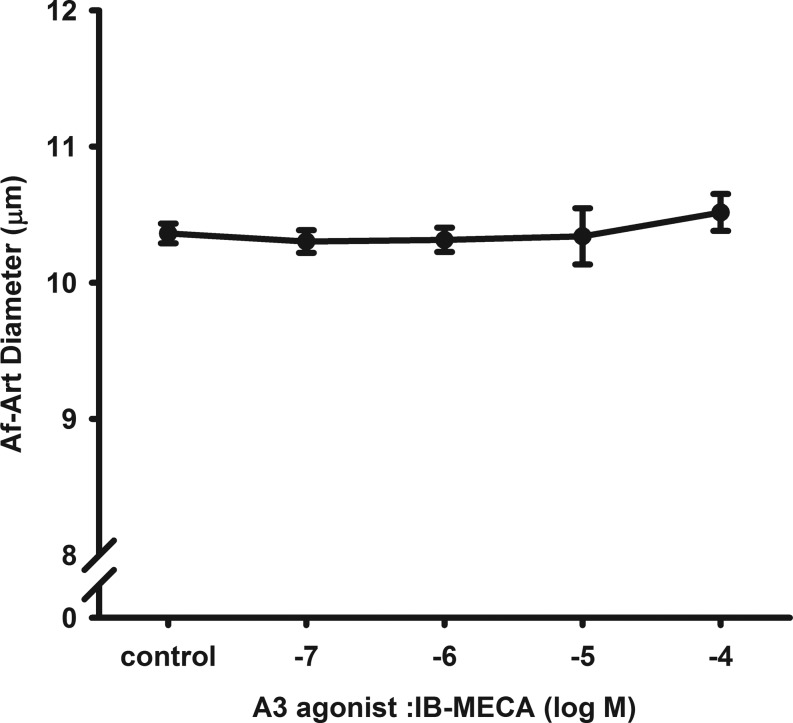
To test whether activation of the A3R constricts the Af-Art, we used a selective A3R agonist IB-MECA. At basal condition when the Af-Art was perfused with MEM, the diameter of the Af-Art was 10.4 ± 0.1 μm. Then, we added different doses of IB-MECA to the bath for 15 min, respectively. As shown in Fig. 2, IB-MECA from 10−7 to 10−4 mol/l did not significantly change the diameter of the Af-Arts, indicating that activation of the A3R did not constrict the Af-Art (n = 9).
Fig. 2.
Effect of A3R agonist on isolated perfused Af-Art. Dose-response curve of A3R agonist N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (IB-MECA) on the perfused Af-Art and showed no constrictive effect (n = 9).
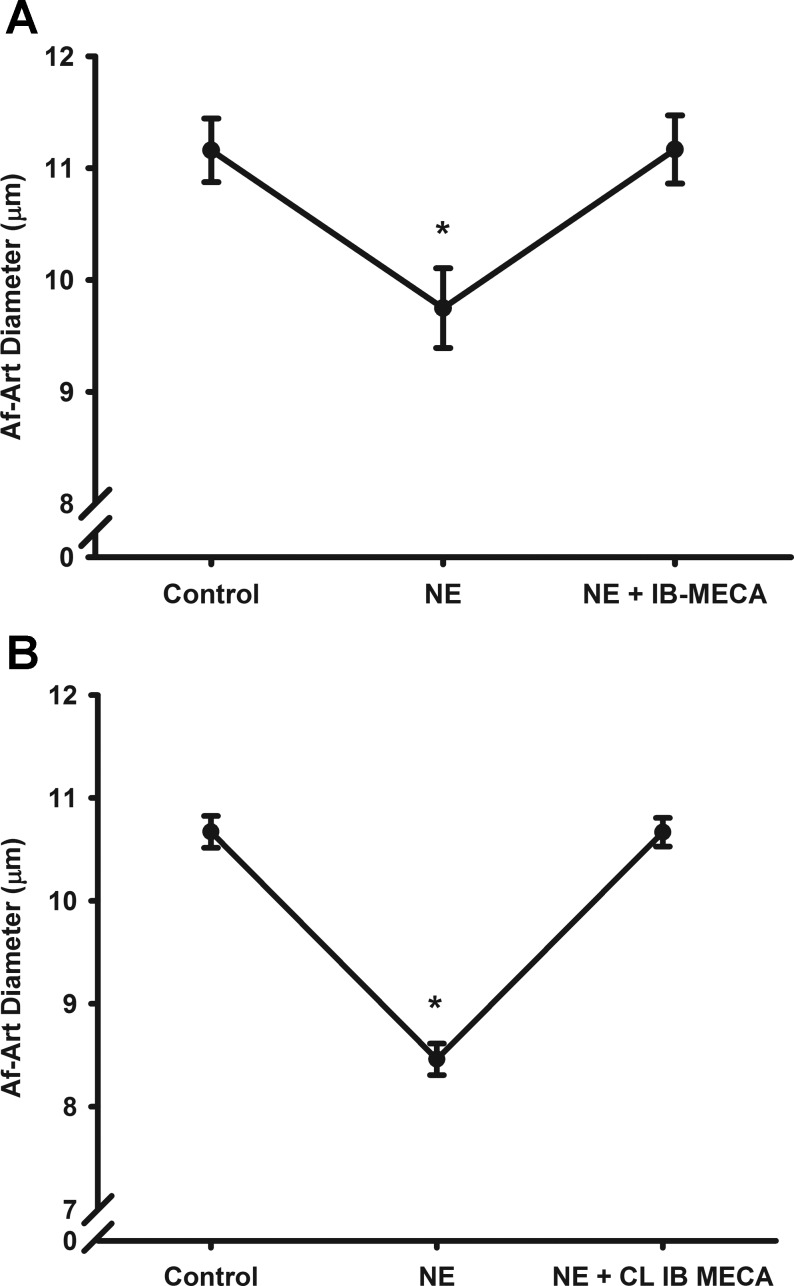
To test whether activation of the A3R dilates the Af-Art, we examined the effect of A3R agonist on preconstricted Af-Art. We preconstricted the Af-Art by ∼20% with NE in the bath (10−6 to 10−7 mol/l; depends on the response of Af-Art). As shown in Fig. 3A, the Af-Arts were constricted from 11.1 ± 0.2 to 9.5 ± 0.2 μm following application of NE. Then, we added A3R agonist IB-MECA at the concentration of 10−4 mol/l in the bath in the presence of NE. The Af-Art dilated to 11.1 ± 0.6 μm (n = 8). All the effects occurred within 5 min after IB-MECA administration.
Fig. 3.
Effect of A3R agonist on preconstricted Af-Art. The Af-Art was preconstricted by norepinephrine (NE). A3R agonist IB-MECA (n = 8; A) or 1-[2-chloro-6-{[(3-iodophenyl)methyl]amino}-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (CL-IB-MECA; n = 6; B) dilated the preconstricted Af-Art. *P < 0.05 vs. basal control.
To further confirm this effect, we used another more selective A3R agonist 2-CL-IB-MECA and repeated the above experiments as shown in Fig. 3B. The Af-Arts were preconstricted from 11.16 ± 0.3 to 9.75 ± 0.4 μm with NE. Similar to IB-MECA, 2-CL-IB-MECA (10−7 mol/l) also dilated the preconstricted Af-Art from 8.46 ± 0.1 to 10.67 ± 0.1 μm (n = 6). Vehicle alone had no constriction or dilatory effect on preconstricted Af-Art. These data indicate that activating the A3R causes acute vasodilation of the Af-Art.
Activation of the A3R counteracts ANG II- and A1R-mediated contraction of the Af-Art.
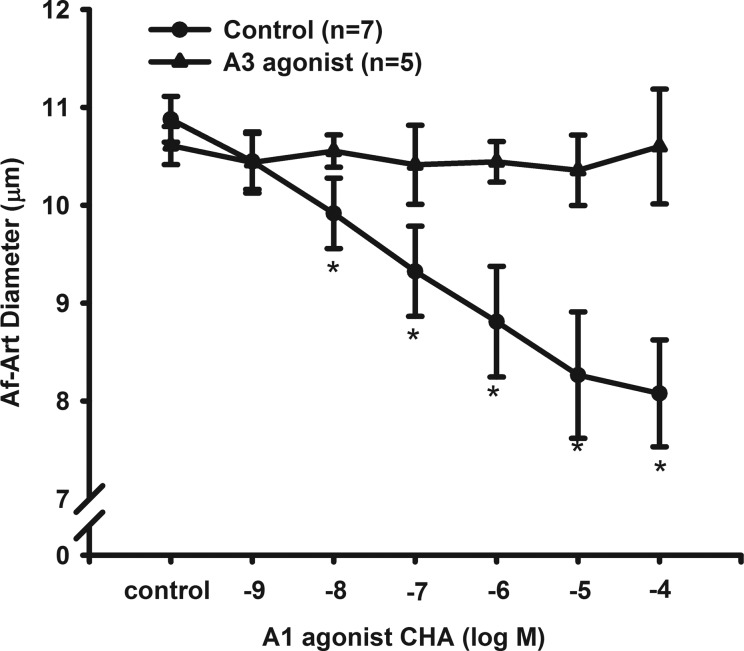
Activation of A1R is known to constrict Af-Art. We tested whether simultaneous activation of A3R influenced the constrictive effect of A1R activation. We used the selective A1R agonist CHA and found that CHA constricted Af-Arts in a dose-dependent manner. The diameters of the Af-Arts were 10.9 ± 0.2 μm at control and constricted to 10.5 ± 0.3, 9.9 ± 0.4, 9.3 ± 0.4, 8.8 ± 0.6, 8.3 ± 0.7, and 8.1 ± 0.5 μm in response to CHA at concentrations from 10−9 to 10−4 mol/l (n = 7).
In separate experiments, we first pretreated the Af-Art with A3R agonist IB-MECA for 15 min and repeated the CHA dose-response experiments in the presence of the IB-MECA. As shown in Fig. 4, activation of A3R with IB-MECA inhibited the constrictive effect of A1R on the Af-Art (n = 5). These data further supported that activation of A3R dilates the Af-Art.
Fig. 4.
Interaction between activation of A3R and A1R. A dose-response curve of a A1 agonist N6-cyclohexyladenosine (CHA) on the isolated perfused Af-Art. In the presence of a A3R agonist IB-MECA, the constriction of the Af-Art induced by CHA was inhibited. *P < 0.05 vs. corresponding control without IB-MECA.
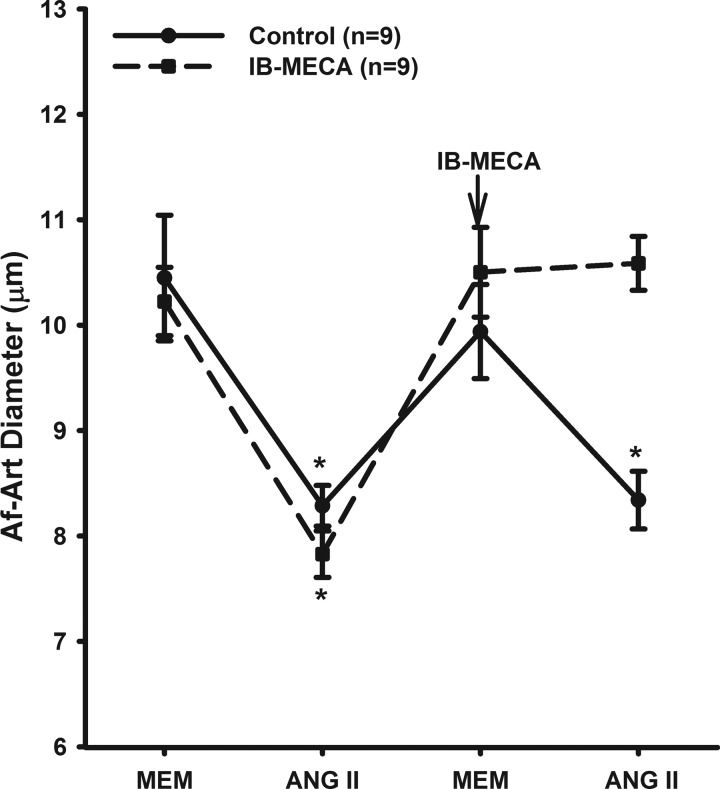
We then studied whether A3R-induced dilation counteracts the ANG II-induced constriction. As shown in Fig. 5, administration of ANG II (10−8 M) reduced the diameter of the Af-Art from 10.22 ± 0.33 to 7.83 ± 0.22 μm. Then, we switched the bath solution to MEM for 15 min. The diameter of the Af-Arts returned to basal 9.9 ± 0.4 μm. ANG II was added again and the Af-Arts were constricted to 8.3 ± 0.3 μm (n = 9, P < 0.05). In separate experiments, we first constricted the Af-Arts with ANG II (10−8 M) from 10.2 ± 0.3 to 7.8 ± 0.2 μm. Then, we switched the bath solution to MEM with A3R agonist IB-MECA for 15 min. The diameter of the Af-Art returned to basal (10.5 ± 0.4 μm). ANG II was added again and the diameter of Af-Arts was 10.5 ± 0.2 μm, indicating activating A3R counteracts the constrictive effect of ANG II.
Fig. 5.
Interaction between A3R activation and ANG II. ANG II constricted the isolated perfused Af-Art. In the presence of a A3R agonist IB-MECA, the constriction of the Af-Art induced by ANG II was blocked. MEM, minimum essential medium. *P < 0.05 vs. control without IB-MECA.
Dilatory effect of A3R activation is mediated through the A3R, not the A2R.
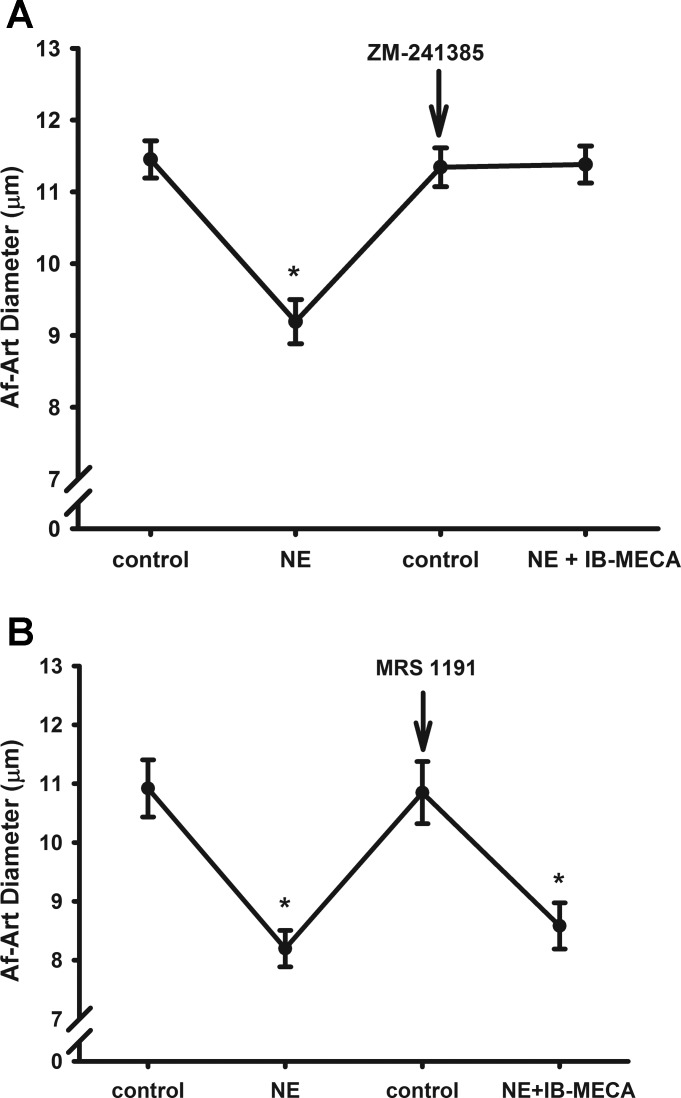
Since the activation of adenosine A2R also causes vasodilation, we tested whether the A3R agonist was activating the A2 receptor and dilated the Af-Art, rather than activating the A3R. For this, we examined the effect of the A3R agonist in the presence of either the selective A2 receptor antagonist ZM241385 or the selective A3 receptor antagonist MRS 1191. As shown in the Fig. 6A, we first preconstricted the Af-Arts from 11.4 ± 0.3 to 9.2 ± 0.3 μm by adding NE. Then, we switched the bath solution to MEM with an A2 receptor antagonist ZM241385 (10−7 mol/l) in the bath for 15 min. The diameter of Af-Art returned to basal at 11.3 ± 0.3 μm. We added the same concentration of NE and A3R agonist IB-MECA in the bath. The diameter of the Af-Art was 11.4 ± 0.3 μm (Fig. 6A, n = 6), indicating that the dilatory effect of A3R agonist was not mediated by activation of A2A or A2B receptors.
Fig. 6.
Interaction between activation of A3R and A2R. A A3R agonist IB-MECA still dilated the preconstricted Af-Art in the presence of a A2R antagonist ZM241385 (A), but did not dilate the preconstricted Af-Art in the presence of a A3R antagonist MRS 1191 (B). *P < 0.05 vs. corresponding control.
To further test the role of A3R activation on the tone of the Af-Art, we repeated the protocol and used a selective A3R antagonist MRS 1191. As shown in Fig. 6B, we first preconstricted the Af-Arts from 10.9 ± 0.5 to 8.2 ± 0.3 μm by adding NE. Then, we switched the bath solution to MEM with an A3R antagonist MRS 1191 (5 × 10−5 mol/l) in the bath for 15 min. The diameter of Af-Art returned to basal at 10.8 ± 0.5 μm. Then, we added the same concentration of NE with A3R agonist IB-MECA (10−4 mol/l) in the bath. The diameter of the Af-Art was 8.6 ± 0.4 μm (Fig. 6B, n = 5). These data further demonstrated that the dilatory effect of A3R agonist on the Af-Art is mediated by activation of A3R.
DISCUSSION
In the present study, we found that adenosine A3R is expressed in mouse Af-Arts, while the expressions of A1 and A2B receptor mRNAs are predominant. Activation of the A3 receptor dilates the Af-Art.
Adenosine is an endogenous purine nucleoside that modulates many physiological processes. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2A, A2B, and A3) (10, 11, 25). In the renal vasculature, A1, A2A, and A2B receptors have been found in the preglomerular microvessels (16). However, A3R expression failed to detect from several tubular segments including the following: proximal tubule, outer medullary descending thin limb of Henle's loop, inner medullary descending and ascending thin limb, thick ascending limb of Henle's loop, distal convoluted tubule, connecting tubule, cortical collecting duct, and outer medullary and inner medullary collecting duct (12, 16, 37–39). As we know, there is no study yet to examine the expression of adenosine receptors specifically in the Af-Arts. In the present study, we dissected ∼20 Af-Arts from each mouse to extract RNA for a RT-PCR and real-time PCR measurement. We found a clear band of A3R mRNA expression. The A3R mRNA level was ∼59% of A1R, while A2BR mRNA level was highest at ∼114%, and A2AR mRNA level was the lowest at ∼45% of A1R in the Af-Arts. In isolated preglomerular microvessels isolated with an iron oxide-sieving technique (3, 16, 19), high expressions of A1R and A2BR were found, with lower expressions of A2AR, which were similar to our findings; however, A3R was very low or not detectable both at mRNA and protein levels (16). These preglomerular microvessels were mainly interlobular arteries and Af-Arts. Therefore, it is possible that A3R are exclusively expressed in the Af-Arts, while A1R and A2BR are widely expressed along the preglomerular vessels. Further studies need to be done by comparing the expression levels of adenosine receptors between Af-Arts and interlobular arteries. The microdissection and isolation technique are very specific for Af-Art, but the amount of sample is very limited. Therefore, it is difficult to measure protein levels using this method.
Adenosine induces biphasic effects with vasoconstriction via A1R and vasodilation via A2R on the Af-Arts (8, 14). However, the function of A3R in regulation of the Af-Art vasculature tone is unknown. In this study, we approached this question by using selective adenosine receptor agonists and antagonists.
We first examined whether A3R activation constricts the Af-Art. We used a selective A3R agonist IB-MECA and did not find alterations in the diameter of perfused Af-Art, suggesting no vasoconstrictive effect of A3R activation. Next, we measured whether activation of the A3R dilates the Af-Art. Since the basal vascular tone in isolated perfused Af-Art is very weak, the vessels need to be preconstricted to examine the dilatory effect. We found that activation of A3R dilated the preconstricted Af-Art, indicating that A3R has a vasodilatory effect. The studies regarding the functions of A3R are limited. Even though activation of A3R has been reported to have an anti-inflammatory effect (13, 17, 35), there is very little information regarding the functional role of A3R in the vasculature. Stimulation of A3R has been reported to decrease blood pressure in ANG II-treated rats (9) and A3R activation has been found to be protective for renal ischemia injury (21). However, whether and how the vasodilatory effect of A3R on the Af-Art that was demonstrated in this study may contribute to these situations need to be ascertained in future studies.
To study the interaction between adenosine A1 and A3 receptor activation on the Af-Art, we examined the effect of A3R agonist IB-MECA on the Af-Art constricted by selective A1R agonist CHA. We found that the A3R agonist IB-MECA inhibited the constrictive effect induced by A1R agonist CHA. Then, we examined the effect of activation of A3R on the Af-Art constricted by ANG II. We found A3R agonist IB-MECA blocked the effect of ANG II on the Af-Art. These data also supported that activation of A3R dilates the Af-Art, thereby counteracting the constrictive effect of A1R agonist and ANG II. However, we cannot exclude the possibility whether A3R has any direct interaction with A1R or ANG II AT1 receptor. We are aware that we used a high concentration of A3R agonist IB-MECA to see the maximum effect of A3R activation. However, the observed effect in the present study that A3R activation blocked the constrictive effect of A1R activation and ANG II may not be a physiological phenomenon. In addition, high concentration of IB-MECA may not be selective anymore and may activate adenosine A2 receptors.
To determine whether A3R agonist dilated the Af-Arts by stimulating A2 receptors, we examined the effect of the A3R agonist in the presence of either the selective A2 receptor antagonist or the selective A3R antagonist. ZM241385 is a potent and highly selective adenosine A2 antagonist and is active in vivo. It has binding affinities of 540, 1.4, 31, and 270 nM for human A1, A2A, A2B, A3 receptors, respectively, and shows selectivity of 1,000, 91, and 500,000 over A1, A2B, and A3 sites (18). Thus, the dose of ZM241385 used in the present study (i.e., 10−7 mol/l) should efficiently block both A2A and A2B receptors, and not affect the activity of A1 and A3 receptors. We found that inhibition of A2R had no effect on the dilatory effect on the Af-Art induced by A3R agonist. However, A3R antagonist totally blocked dilatory effect on the Af-Art induced by A3R agonist. Our data indicated that the vasodilatory effect of the A3R agonist was mediated by activation of A3R, not A2R.
Adenosine has been proposed to mediate tubuloglomerular feedback (TGF) via activation of the adenosine A1R. At the same time, activation of adenosine A2R has been demonstrated to attenuate the TGF response (29, 33). The dilatation effect of A3R may also contribute to the regulation of TGF responsiveness. The significance of A3R in the Af-Arts in control of TGF and renal hemodynamics needs to be further investigated.
In conclusion, we found that adenosine A3R is expressed in the Af-Art and activation of A3R is associated with a vasodilatory effect. The conclusion regarding the functional role of A3 receptor in this study is limited by its pharmacological approach and future studies using transgenic mice are warranted. Future studies are also aimed to determine the in vivo effects of A3 receptor signaling on renal and cardiovascular function in health and diseases.
GRANTS
This work was supported by National Institutes of Health Grants R01DK098582, R01DK099276, and American Heart Association Postdoctoral Fellowship Award 13POST1422006 (to Y. Lu).
Present address of Rui Zhang: Shandong Medical College, 5460 Erhuan Nanlu, Jinan, China.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., M.C., Y.F., and R.L. conception and design of research; Y.L., Y.G., S.W., Y.F., L.C., J.W., L.W., and X.G. performed experiments; Y.L., Y.G., S.W., and L.W. analyzed data; Y.L., M.C., and R.L. interpreted results of experiments; Y.L. and Y.G. prepared figures; Y.L. and R.L. drafted manuscript; Y.L., Y.G., M.C., R.J.R., and R.L. edited and revised manuscript; Y.L., R.Z., R.J.R., and R.L. approved final version of manuscript.
REFERENCES
- 1.Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A(2) adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Carlstrom M, Lai EY, Steege A, Sendeski M, Ma Z, Zabihi S, Eriksson UJ, Patzak A, Persson AE. Nitric oxide deficiency and increased adenosine response of afferent arterioles in hydronephrotic mice with hypertension. Hypertension 51: 1386–1392, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 300: F457–F464, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2 receptors modulate tubuloglomerular feedback. Am J Physiol Renal Physiol 299: F412–F417, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmines PK, Ohishi K, Ikenaga H. Functional impairment of renal afferent arteriolar voltage-gated calcium channels in rats with diabetes mellitus. J Clin Invest 98: 2564–2571, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118: 1461–1468, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol 299: F310–F315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fozard JR, Carruthers AM. Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat. Br J Pharmacol 109: 3–5, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev 46: 143–156, 1994. [PMC free article] [PubMed] [Google Scholar]
- 11.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci 18: 79–82, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sallstrom J, Fredholm BB, Persson AE, Carlstrom M. Adenosine A(1)-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol 301: R1669–R1681, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 102: 4472–4478, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther 315: 1150–1157, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol 290: F888–F891, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest 100: 2849–2857, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol 362: 382–391, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp Nephrol 10: 383–392, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci 15: 298–306, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44: 524–532, 1993. [PubMed] [Google Scholar]
- 24.Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend Med 9: 103–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens 12: 497–502, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Morton MJ, Sivaprasadarao A, Bowmer CJ, Yates MS. Adenosine receptor mRNA levels during postnatal renal maturation in the rat. J Pharm Pharmacol 50: 649–654, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol 280: F406–F414, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R273–R279, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Osswald H, Hermes HH, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int Suppl 12: S136–S142, 1982. [PubMed] [Google Scholar]
- 30.Sajjadi FG, Firestein GS. cDNA cloning and sequence analysis of the human A3 adenosine receptor. Biochim Biophys Acta 1179: 105–107, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA 90: 10365–10369, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65: 501–529, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Spielman WS, Thompson CI. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol Renal Fluid Electrolyte Physiol 242: F423–F435, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J Clin Invest 105: 361–367, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int 65: 1180–1190, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 263: F991–F995, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi S, Umemura S, Tamura K, Iwamoto T, Nyui N, Ishigami T, Ishii M. Adenosine A1 receptor mRNA in microdissected rat nephron segments. Hypertension 26: 1181–1185, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA 89: 7432–7436, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou AP, Wu F, Li PL, Cowley AW Jr. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension 33: 511–516, 1999. [DOI] [PubMed] [Google Scholar]