Abstract
G protein coupled receptors (GPCRs) represent the most important targets in modern pharmacology because of the different functions they mediate, especially within brain and peripheral nervous system, and also because of their functional and stereochemical properties. In this paper, we illustrate, via a variety of examples, novel advances about the GPCR-related molecules that have been shown to play diverse roles in GPCR pathways and in pathophysiological phenomena. We have exemplified how those GPCRs’ pathways are, or might constitute, potential targets for different drugs either to stimulate, modify, regulate or inhibit the cellular mechanisms that are hypothesized to govern some pathologic, physiologic, biologic and cellular or molecular aspects both in vivo and in vitro. Therefore, influencing such pathways will, undoubtedly, lead to different therapeutical applications based on the related pharmacological implications. Furthermore, such new properties can be applied in different fields. In addition to offering fruitful directions for future researches, we hope the reviewed data, together with the elements found within the cited references, will inspire clinicians and researchers devoted to the studies on GPCR’s properties.
Keywords: G protein coupled receptors, Signaling pathway, Molecular therapy, Potential target
1. Overview
Among cell surface receptors, Heptahelical GPCRs constitute the largest single family (Lagerstrom and Schioth, 2008). Whereas, this family is estimated to comprise at least 800 members in the human genome (Gloriam et al., 2007), it constitutes the largest protein family in vertebrates (Schulein et al., 2012). This family was recognized in 1986 after a work that has shown a sequence similarity between rhodopsin and the β2AR (Dixon et al., 1986). In addition to cell surface, GPCRs are suggested to exist in the endoplasmic reticulum, Golgi apparatus, nuclear membrane and even inside the nucleus itself (Boivin et al., 2008; Calebiro et al., 2010; Cheng et al., 2011; Re et al., 2010). Diverse array of endogenous ligands (stimuli) activate GPCRs including biogenic amines, neuropeptides, amino acids, ions, hormones, chemokines, lipid-derived mediators, proteases peptides, proteins, photons (Hazell et al., 2012; Marinissen and Gutkind, 2001), protons (H+) and ions (Ca2+) (Lagerstrom and Schioth, 2008). In contrast, we have orphan GPCRs with non identified endogenous ligand (Chung et al., 2008). It is estimated that between 120 and 130 receptors are non-chemosensory orphan GPCRs (Gloriam et al., 2007; Harmar et al., 2009). Genetic statistics showed that olfactory receptors constitute about 50% of all GPCRs in the human genome (Gloriam et al., 2007) and at least 22 genes code for the class C of GPCRs in humans (Rondard et al., 2011).
The combination of novel advances and innovative research methods such as recent cloning of some mammalian GPCRs (Heberlein et al., 2009) and comparative studies of gene expression profiles of human postmortem brains coming from both patients with neurological disorders and healthy individuals(Horvath et al., 2011; Iwamoto and Kato, 2006; Mehta et al., 2010; Sequeira and Turecki, 2006), with the applications of biochemical, mutagenesis and spectroscopic techniques (Gether, 2000), help to further the understanding of GPCRs’ properties and provide more data to develop new therapeutic strategies via targeting GPCRs.
2. GPCRs: from physiology to pharmacology
The pharmacological properties, we emphasize herein, lie basically on different functions of either GPCRs or the molecules involved in the GPCR-related mechanisms. Different receptors illustrate this concept. Indeed, whereas, glutamatergic and GABAergic neurotransmitters within amygdala have been linked with emotions, fear regulations, anxiety, learning and memory processes (LeDoux, 2000; Roozendaal et al., 2009), serotonin (5-HT) which is found throughout the central and the peripheral nervous system (Parkel et al., 2011) plays a regulatory role in the cerebral anxiety behaviors (Li et al., 2012) thermoregulation (Balcells-Olivero et al., 1998) (Seletti et al., 1995) , sexual behavior (Maswood et al., 1998), memory (Edagawa et al., 1998) immune function(Iken et al., 1995; Rauly-Lestienne et al., 2011), sleep, appetite and pain perception processes (Werry et al., 2008). Furthermore, 5-HT1A receptor has been linked with depression (Blier et al., 1997; Savitz et al., 2009) memory and cognitive function (Bert et al., 2008). GPCRs have also even been shown to play a role in sweet taste perception (Adler et al., 2000; Assadi-Porter et al., 2010; Hoon et al., 1999; Koizumi et al., 2007). Importantly, the key molecules of the neural network, which are the metabotropic glutamate receptors (mGlu), are GPCRs (Nicoletti et al., 2011) as well. Consequently different GPCRs and GPCR pathway-related molecules are already or may turn out to be targets for different drugs. As illustration, aminergic receptors (Insel et al., 2007) and serotonin1A receptor constitute the target for neuropsychiatric disorder treatment (Ganguly et al., 2011).
GPCRs, which are metabotropic receptors(Serebryany et al., 2012), constitute the most important class of pharmacological targets in all clinical areas (Lagerstrom and Schioth, 2008) and different estimations about the portion of drugs interacting with GPCRs exist. Indeed, several papers have indicated that this portion is between 30% and 60% (Hopkins and Groom, 2002; Lundstrom, 2006), whereas authors have estimated that nearly half of the pharmakons interact directly or indirectly with these 7 transmembrane receptors (7TMRs) (Heilker et al., 2009; Lagerstrom and Schioth, 2008).
In addition, to controlling a large number of physiological processes, such as signal transduction (Davies et al., 2007; Lagerstrom and Schioth, 2008) ligand diversity and unique tissue expression(Ma and Zemmel, 2002), GPCRs exist in a high number and have the property of “ligand binding specificity” (Lagerstrom and Schioth, 2008). More importantly, among all hormones and neurotransmitters we estimate that 80% of them exert their effects via interacting with GPCRs (Birnbaumer et al., 1990). All these properties increase the pharmacological importance of GPCRs and make such receptors ideal therapeutic targets for different diseases. GPCR ligands can be classified into antagonists and agonists (which include full agonists and partial agonists) in addition to a relatively new class, inverse agonists (Ambrosio et al., 2011). Many pharmaceutical companies are working on the identification of agents that target GPCRs (Lagerstrom and Schioth, 2008; Overington et al., 2006) and advances about factors such as those influencing GPCRs’ activity (Ghanemi et al., 2013) can be further investigated in drug development. Until now we know many drugs with an activity based on the interaction with GPCR-related system including blockbusters such as opiates, antihistamines, α- and β-blockers, β-agonists, dopamine receptor blockers, angiotensin receptor blockers, angiotensin – converting enzyme inhibitors and selective serotonin reuptake inhibitors (Whalen et al., 2011). Furthermore, sweet-taste synergisms, mediated by GPCRs, have been linked with some molecules used as sweeteners like Neohesperidin dihydrochalcone (NHDC) and cyclamate that synergistically potentiated cell response to sucrose (Birch, 1999; Schiffman et al., 1995) Such findings could allow us to reduce sugar contents in some beverage and food and thus, provide a new form of healthy diet products or a starting point to develop novel substitute for diabetics and other kind of metabolic diseases-related diet. This illustrates a non-therapeutical application of GPCRs’ advances as well.
3. Signal transduction and pathways
The main common mechanism of GPCRs’ function is to receive an external signal (bind to ligands which constitute the first messenger) and convert that stimulus into a cellular response within the intracellular medium via a chain of biochemical reactions and molecular interactions. After GPCR activation by the ligand(s) the receptor rearranges, which induces signal transduction to the cytoplasmic side (Serebryany et al., 2012). According to the existence or the non existence of bound ligands to it, GPCRs have two states, high and low affinity states, which correspond to G protein-coupled and uncoupled states (Ma et al., 2011). The physical description of ligand-receptor interactions involves intermolecular forces including ionic bonds, hydrogen bonds and van der Waals forces that, all together, induce a spatial conformational change in the tertiary structure of the GPCR (Ma et al., 2002). Then it activates downstream signaling cascades within the cell (Park et al., 2008; Pierce et al., 2002; Rosenbaum et al., 2009).
GPCRs are suggested to have similar topology and activation mechanisms (Hofmann et al., 2009; Rosenbaum et al., 2009) but with different G proteins’ subtypes involved within the diverse signal-transduction pathways (Rosenbaum et al., 2009). In fact, G protein has three subunits: Gα, Gβ and Gγ (Hazell et al., 2012) with at least 16 Gα, 5 Gβ, and 12 Gγ subunits in the human genome (Robishaw and Berlot, 2004). To achieve their cellular functions, GPCRs interact with multiple associated proteins which constitute intracellular networks (Hazell et al., 2012). These networks involve a variety of protein molecules such as GPCR kinase (GRK), which can phosphorylate and regulate nuclear proteins including class II histone deacetylases (HDACs) (Martini et al., 2008), and arrestin (Krupnick et al., 1997; Wilden, 1995). These network-related protein molecules govern different mechanisms and biochemical reactions that lead, according to the receptor and the cell types, to the intracellular increase in protein kinase C (PKC), protein kinase A (PKA) activity, intracellular calcium (Ca2+), cyclic AMP (Camps et al., 1992; Hazell et al., 2012) and a phosphorylation activity too (Wilden, 1995), which is very important within the downstream activity of some GPCRs. Furthermore, it is suggested that GPCR signaling can affect the dynamic reorganization of the actin cytoskeleton (Ganguly et al., 2011). GPCRs’ activation implicates, depending on the receptor type or subtype, the activation of intracellular signals that are mediated by an arsenal of biomolecules including Gβγ, guanosine diphosphate (GDP), adenylyl cyclases (AC), phosphodiesterases, phospholipases, tyrosine kinases and ion channels (Cabrera-Vera et al., 2003) resulting in the regulation of numerous biological functions (Cabrera-Vera et al., 2003). Although GPCRs have a similar mechanism of activation, the molecules involved in signal transduction may not be the same .For example, serotonin 5-HT4 receptors involve Gs, Src, cAMP and MAP kinase (Barthet et al., 2007), whereas β2-adrenoceptors(β2-AR) involve both Gs-coupled and G α-independent/tyrosine kinase Src-dependent pathway (Sun et al., 2007).
GPCRs have a functional selectivity and can activate, depending on the ligand nature, more than one class of G proteins (Kenakin and Miller, 2010; Maudsley et al., 2005; Woehler and Ponimaskin, 2009; Zheng et al., 2010) making this area of pharmacodynamics more interesting. Moreover, due to the efficacies’ differences, distinct ligands may generate different active states of GPCRs (Ambrosio et al., 2011) resulting in a pharmacological diversity. More importantly, protein G-independent interactions have been shown for GPCRs as well, mainly with β-arrestins (Beaulieu et al., 2005; Lefkowitz and Shenoy, 2005; Luttrell et al., 1999). Although G protein dependant regulation remains the principal mechanism in GPCRs’ activation, β-arrestin dependant regulation is an emerging pathway (Whalen et al., 2011). The β Arrestin binding to a receptor initiates the internalization of that receptor (Daaka, 2011). Importantly, some GPCRs are able to activate both G protein-dependent and G protein-independent pathways (Feng et al., 2005).
GPCRs’ signals transductions can lead to a variety of cellular responses including growth, death, movement, transcription and excitation (Gloriam et al., 2007; Marinissen and Gutkind, 2001). Importantly, the existent signal amplification and modulation within the pathway through downstream signaling cascades (Serebryany et al., 2012) makes this pathway more important in both cell physiology and pharmacology.
4. Modifying GPCR-related pathways: not less important that targeting GPCRs
By “GPCR-related system” we refer, herein, to GPCRs, their endogenous ligands and both the enzymes and molecules within both the cells and the synaptic gaps, that are related to the activation, the regulation or the inactivation of GPCR related pathways. This novel area is fueled by new discoveries and targeting GPCR-related pathways, by diverse compounds, constitutes one of the most important approaches among the emerging therapies. Thus, the diverse novel molecules targeting GPCR systems, that might provide new therapeutics, remain the most interesting topic of the researches on GPCRs which focus on their high pharmacological importance.
GPCR pathways involve multiple cell signaling cascades and networks within the cells some of which are beneficial or compensatory and others deleterious. The balance between these pathways, which in a large part is dictated by the cellular environment, determines the outcome as a diseased or non-diseased state. Therefore, understanding the signaling mechanisms activated by, basically, various neurohormonal stimuli has led to novel ideas about potential agents that target those cell signaling cascades. Within this paper we focus on the importance of targeting or modifying molecules that are implicated in both signal transduction and pathway downstream signaling of GPCRs rather than the concept of directly targeting receptor by agonists or antagonists.
We have noticed that this promising field of non-targeting receptor therapies is underappreciated, for that matter we put more light on how understanding, than modifying signal-transduction pathways of the GPCR-related mechanisms, by different methodologies including genetic approaches or different natural or chemical compounds, can provide potential therapies and novel starting points to develop active agents against a diversity of diseases and disorders.
4.1. β-Arrestins
Since GPCRs’, or seven transmembrane receptors’ (7TMR), functions have been shown to involve β-arrestins in a diversity of physiological and pathogenesis phenomena, influencing the β-arrestin pathway might provide novel therapies. Functions such as the activities of bronchial β2Adrenoreceptors’ (β2AR) desensitization (Deshpande et al., 2008; Wang et al., 2009) D1 dopamine-receptor – related striatal neuron apoptosis (Chen et al., 2009), angiotensin II type 1A receptor (AT1R) mediated protein-cell synthesis and antiapoptosis in vascular smooth-muscle (Ahn et al., 2009; DeWire et al., 2008) are linked to β-arrestins. Furthermore, β-arrestins are implicated in a number of toxicological processes including alcohol exposure, consumption and reward (Bjork et al., 2008; Rimondini et al., 2002) and limiting opioid-induced reward (Bohn et al., 2003). Moreover, β-arrestins have been shown to play roles not only in many physiological aspects but also in the pathogenesis of some diseases (Luttrell and Gesty-Palmer, 2010; Schmid and Bohn, 2009). The β-Arrestin-related pathways involve a variety of mechanisms (Christensen et al., 2010; DeWire et al., 2007; Xiao et al., 2007; Xiao et al., 2010) including even functions that are independent from those modulating GPCR pathways (Kim et al., 2008). Molecular pharmacology has shown that β-arrestin2 desensitizes GPCR via two mechanisms, (1) recruiting phosphodiesterases to degrade the cAMP generated by G protein signaling (Perry et al., 2002) and (2) internalizing receptor (Ahn et al., 2003), both mechanisms will result in the sterical prevention of the interaction between the GPCR and its G protein (Lohse et al., 1990). More importantly, evidence about β-arrestin is pointing out the concept of “biased agonism” (Kenakin, 2005; Kenakin and Miller, 2010; Rajagopal et al., 2010; Violin and Lefkowitz, 2007), a concept reviewed recently by Whalen et al. (2011). “Biased agonism” is described as the ability of a ligand, which after binding to a GPCR, promotes either the pathway involving only G protein signaling or only β-arrestin mediated signaling (Fig. 1). Two other publications have described the same concept of “biased agonism” as a “functional selectivity” (Mailman, 2007; Urban et al., 2007a) of the pathway that the interaction ligand-GPCR preferentially activates (Kilts et al., 2002; Urban et al., 2007b). As an example, both alprenolol and carvedilol represent β arrestin-biased ligands of the β 1-adrenergic receptor (Kim et al., 2008) and so are Carvedilol, ICI118551, propranolol, cyclopenylbutanephrine and norepinephrine for β2 adrenergic receptor (Wisler et al., 2007; Drake et al., 2008; Azzi et al., 2003). On the other hand, β-arrestin-related pharmacovigilance is strongly highlighted. Indeed, several pharmacological phenomena including tachyphylaxis and tolerance have been suggested by Erin et al. (Whalen et al., 2011) to be the results of β-arrestin-dependent receptor desensitization and downregulation. The same authors have pointed out the possibility of avoiding such undesirable consequences using biased ligands, thus improving the pharmacological properties. As illustrations, studies have shown that cardiac β 1-ARs can stimulate β arrestin1- and -2-dependant signaling in the heart resulting in transactivation of the epidermal growth factor receptor (EGFR), which is cardioprotective (Noma et al., 2007) whereas, cardiac β-AR desensitization has been proposed to implicate β-arrestin1 (Conner et al., 1997). Therefore, the discovery of β-arrestin biased ligands for β-ARs (Drake et al., 2008; Kim et al., 2008; Wisler et al., 2007) might provide better therapies in cardiology especially that chronic treatment with β agonist-stimulated bronchodilator may result in tachyphylaxis (Haney and Hancox, 2006) ,moreover, several publications (Lohse et al., 1990; Ahn et al., 2003; Deshpande et al., 2008; Perry et al., 2002; Wang et al., 2009) suggested that G protein-biased ligand could result in a non decreased bronchodilation that accompanies such therapy as results of the chronic treatment. Other examples illustrate the role biased ligand can play. Indeed, opioid analgesia and tolerance related to μ opioid receptor (OR) have also been linked to β-arrestins (Bohn et al., 1999, 2000, 2004), thus, the influence of β-arrestins on opioid analgesia and tolerance has been the topic of several studies (Bohn et al., 1999, 2000, 2004; Jiang et al., 2006). On the other hand, other properties of β-arrestin signaling might provide starting points to develop new therapies. For cerebral ischemia perspectives, we point that in vascular smooth muscle cells, activating β-arrestin signaling, in addition to the antiapoptotic effect it has (Ahn et al., 2009), enhances protein synthesis (DeWire et al., 2008) via the angiotensin II type 1A receptor (AT1R) mediated pathway. Following this line, because M3-receptor-dependent learning and memory functions have been linked to β-arrestin recruitment (Poulin et al., 2010) M3 muscarinic receptor may be a potential target of biased ligands in some pathologies in which cognitive disorders are affected. In neurological disorders, whereas aripiprazole, which is an FDA approved atypical antipsychotic agent, is considered as a functional selective D2 receptor (D2R or D2) ligands (Lawler et al., 1999; Mailman and Murthy, 2010; Urban et al., 2007b), UNC9975, UNC0006, and UNC9994 ,which are analogs of aripiprazole, were recently shown to be unprecedented β-arrestin-biased D2R ligands (Allen et al., 2011) which will have a positive impact on both antipsychotic efficacy and side effects reduction (Allen et al., 2011).
Figure 1.
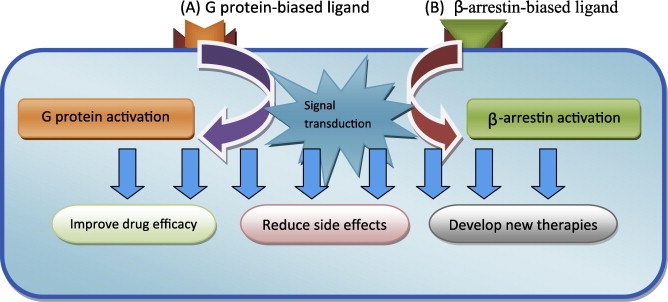
Signaling of biased ligands which have the ability to stimulate specifically either G protein (A) or β-arrestin (B) functions via the selectivity they have at the receptor level and the therapeutic implications it might have. (The biased ligands binding will stimulate either β-arrestin activation or G protein activation).
This new concept comes to add new elucidated properties about GPCRs that could be beneficial both to improve therapeutic efficiency and reduce side effects. Continued efforts in this area will lead to the development of new generations of ligands which have the ability to stimulate specifically either β-arrestin or G protein functions via the selectivity they have at the receptor level, thus controlling the intracellular pathways. We might also predict drugs that directly target either β-arrestin or G protein and thus stimulate or inhibit the related mechanisms which will result in providing a synergic effects or reducing undesirable side effects. On the other hand, such new data will surely help to better understand the diverse cellular and molecular mechanisms involved in GPCRs’ functions. In spite of this, many efforts remain to be provided because existent functionally selective GPCRs’ ligand number still is limited (Kilts et al., 2002; Mailman, 2007; Urban et al., 2007a,b; Violin and Lefkowitz, 2007) which constitute a struggle facing the development of this field.
4.2. G protein subunits
Among the two G protein subunits, Gα subunit and Gβγ subunit (Gilman, 1987; Seneviratne et al., 2011), Gβγ subunit, due to the mechanisms it mediates, is emerging as a pharmacological target. As for instance, Gβγ-dependent pathway has been involved in μ-opioid potency (Xie et al., 1999), drug addiction (Yao et al., 2003) and cancer metastasis (Bookout et al., 2003). In fact, Gβγ subunits mediate different functions via a huge amount of intracellular effectors and interactions including adenylate cyclase (AC); G protein coupled receptor kinase 2 (GRK2) (Pitcher et al., 1992);phospholipase Cβ1, β2 and β3 isoforms (Camps et al., 1992; Park et al., 1993; Smrcka and Sternweis, 1993); potassium channels (GIRK), phosphoinositide 3 kinase γ (PI3Kγ) (Stephens et al., 1994; Stephens et al., 1997); phosphatidylinositol-3-kinase beta (PI3Kβ) (Guillermet-Guibert et al., 2008) and N-type calcium channels (Ikeda and Dunlap, 1999). Therefore, modifying Gβγ Subunits’ activity might be of a therapeutical potential. Indeed, it has been suggested that blocking Gβγ signaling can stop the development of heart failures (Koch et al., 1995; Rockman et al., 1998) and blocking Gβγ-dependent regulation of PI3Kγ may inhibit neutrophil dependent inflammation. Moreover, targeting Gβγ might constitute therapeutical approaches (Casey et al., 2010; Mathews et al., 2008) for opioid-dependent antinociception (Bonacci et al., 2006; Mathews et al., 2008); inflammation (Lehmann et al., 2008) and cardiac hypertrophy(Casey et al., 2010) thus, new chemical entities that, mainly inhibit Gβγ activity, unveil novel opportunities for pharmacotherapies (Casey et al., 2010; Mathews et al., 2008). Importantly, a screening of small molecule identified compounds that may bind to Gβγ (Seneviratne et al., 2011), thus, stop Gβγ subunit-related signal transduction. The small molecules identified in this screening modify the signal transduction between Gβγ and the related effectors, confirming evidence brought out by several previous publications (Irannejad and Wedegaertner, 2010; Kirui et al., 2010; Zhao et al., 2007). Pharmacodynamically, we notice that the interaction between those small molecules and Gβγ subunits was reversible, specific, stoichiometric selective in both in vitro and in cells trials (Seneviratne et al., 2011). Those potential pharmakons have the advantages to effect even after the receptor has been activated, showing key properties about therapeutic strategy, which differentiate GPCRs from ionotropic receptors.
4.3. G protein coupled receptor kinases (GRKs)
GPCRs are mainly regulated by G protein coupled receptor kinases (GRKs) that are involved is homologous desensitization of GPCRs (Gurevich et al., 2012). Numerous signaling pathway abnormalities involve GRK dysfunctions (Gurevich et al., 2012) which highlight the potentials targeting GRKs may have in therapeutics. Indeed, changes in GRK expression and function appear to be critical in the regulatory aspects of vasoconstriction and vasorelaxation homeostasis with advancing age (Schutzer et al., 2011) and certain neurological and psychiatric disorders can be treated by therapies that influence GRKs activity (Ahmed et al., 2010) such as gene therapy that elevates the GRK function (Gurevich et al., 2012) via enhancing GRK expressions .
Observations have shown a link between Alzheimer’s disease (AD) and some GRKs. In AD patients, GRK2 was upregulated in endothelial cells (Obrenovich et al., 2006), in addition, high expression of GRK2 protein and mRNA was reported as well (Leosco et al., 2007). Furthermore, whereas, GRK5 activity decrease causes β amyloid accumulation (Cheng et al., 2010) with the known consequences that are related to the role that β-amyloid plays in the pathophysiology of AD, neurofibrillary tangles that are observed in AD patients have been shown to be linked with GRK2 (Takahashi et al., 2006) . On the other hand, L-DOPA-induced dyskinesia (LID) is a side effect observed after a long term treatment with L-DOPA, which is used in Parkinson’s disease (PD) (Fahn, 2008), moreover, the chronic treatment with L-DOPA has been linked to GRK2 and GRK6 level reduction in the striatal regions(Bezard et al., 2005). Recently a study (Ahmed et al., 2010) has pointed that an increased level of GRK6 can suppress (LID) and has suggested that dyskenesia involves GRK6-mediated regulation of the dopamine receptor pathway. Within the brain also, numerous papers have linked GRKs with both depression phenomena (Garcia-Sevilla et al., 1999; Grange-Midroit et al., 2003; Taneja et al., 2011; Vollmayr and Henn, 2001) and antidepressant drugs action (Gurevich et al., 2012). In fact, whereas, increased level of GRK3 in select brain regions can play a role in depression (Barrett et al., 2003, 2007; Zhou et al., 2008), depressive patients have a high expression of GRK2/3 in their brains (Garcia-Sevilla et al., 1999; Grange-Midroit et al., 2003).
Because inflammatory mediators, that constitute key molecules in immune system signaling, modulate GRKs signaling (Fan and Malik, 2003; Lombardi et al., 2007), transcription (Fan and Malik, 2003; Ramos-Ruiz et al., 2000) or degradation (Cobelens et al., 2007; Lombardi et al., 2002), we might target GRKs to modify the effects produced by inflammatory mediators but we still need to clarify which mediator is related to which GRK(s). Furthermore, multiple sclerosis (Poulin et al., 2010) or secondary progressive MS involves inflammatory phenomena that have been linked to GRK and patients with active relapsing-remitting MS or with secondary progressive MS have a reduced GRK2 contents in the peripheral blood mononuclear cells(Giorelli et al., 2004; Vroon et al., 2005). On the other hand, pain, which is in many phenomena associated with inflammation, has GRK2 as a mediator in its pathway. Thus, we can suppose a possible targeting of GRK to deal with pain. Indeed, in allodynia, GRK2 expression was shown to be modified (Eijkelkamp et al., 2010; Kleibeuker et al., 2007). Furthermore, it has been shown that induced chronic paw inflammation decreases GRK2 in the dorsal root ganglia (Eijkelkamp et al., 2010) and in microglia/macrophages (Willemen et al., 2010). Following the same way whereas, chronic hyperalgesia involves selective knockdown of GRK2 in microglia/macrophages (Eijkelkamp et al., 2010; Willemen et al., 2010), inflammatory mediator (including cytokines, chemokines, peptides, and neurotransmitters) effects result in hyperalgesia and allodynia (Linley et al., 2010).
GRKs have also been linked to several cardiovascular diseases involving in their pathophysiology autonomous nervous system’s receptors like catecholamines which activate βARs that regulate heart function (Gurevich et al., 2012). As illustration of the therapeutical perspectives, treatment of mice with cardiac-specific ablation of GRK2 resulted in cardiomyopathy (Matkovich et al., 2006). Moreover, cardiac hypertrophy and early heart failure have been pointed as promoted by the accumulation of GRK5 in the nuclei of cardiac myocytes (Martini et al., 2008). Importantly, GRKs 2 and 5 are considered as targets in some cardiac diseases (Penela et al., 2006). Indeed, in addition to polyanionic compounds heparin and dextran sulfate are examples of inhibitors of GRK2(Benovic et al., 1989), selective potent inhibitors for GRK2/3 subfamily have been developed by Takeda Pharmaceuticals (Gurevich et al., 2012; Thal et al., 2011) which adds novel pharmakons to the cardiovascular diseases’ arsenal.
Although insufficient or excessive GRK activity is involved in a variety of pathologies, the pharmacological value of GRKs as potential targets remains under-appreciated (Gurevich et al., 2012). Compared with ion channel linked receptors the existence of GRKs constitutes an advantage of GPCRs as it allows a control of the effects regardless of the ligand–receptor binding status. Moreover, as GRKs are not only involved in GPCR control and can also react according to a phosphorylation-independent pathway (Gurevich et al., 2012), targeting them may constitute therapies for a higher number of disease or cellular dysfunctions. In addition, among about 500 protein kinases coded by the human genome (Manning et al., 2002), many have been linked to cardiovascular diseases (CVDs) such as Rho Kinase (see the next subtitle: 4.4.), Protein kinase C, Glycogen synthase kinase-3β,G-protein-coupled receptor kinases, Phosphoinositide 3-kinase, Mitogen-activated protein kinase, and Ca2+/calmodulin dependent protein kinase II (Kumar et al., 2007). Furthermore, protein kinase signaling pathways have been described in both acute and chronic CVDs (Dorn and Force, 2005; Touyz and Schiffrin, 2000).Thus, targeting the related kinase implicated in the pathophysiological pathway rather than targeting the GPCRs provides important pharmacological options for CVDs. For example, cardiac β-adrenergic systems involve protein kinases, using protein kinase inhibitors could be more advantageous than receptor antagonists (Kumar et al., 2007). Protein kinase inhibitor does not inhibit the receptor stimulation but it blocks signal transduction.
4.4. G protein signaling (RGS) and Rho/Rho kinase
Another aspect of the β-adrenergic receptor (βAR) of the sympathetic nervous system draws attention to novel concepts about targeting GPCRs’ pathway system. Regulator of G protein signaling (RGS) proteins are very interesting molecules in both understanding pathways and therapeutics related to GPCRs’ system. RGS proteins include GTPase-activating proteins (GAPs) and thus, terminate indirectly G protein signaling after the GPCR activation (Hollinger and Hepler, 2002; Ross and Wilkie, 2000). On the other hand, βAR, a GPCR that involves RGS in its pathway, is implicated in cardiac function (Rockman et al., 2002). Furthermore, pathogenesis of cardiac hypertrophy and hypertension involves dysfunctions of RGS2 (Wieland et al., 2007). In addition, to RGS 2, the mammalian cardiac myocytes include also RGS3–5 (Riddle et al., 2005). Importantly, a recent research (Chakir et al., 2011) has demonstrated that RGS2 constitutes a novel negative regulator of the β2AR-Gi signaling in human species. The same study pointed that the therapeutic implications of the finding are promising and a potential novel target has come out to treat chronic heart failure (Chakir et al., 2011). Such findings may bring new ideas about targeting GPCRs’ system for a pharmacological purpose.
Within a similar thinking way, another therapeutic possibility has been highlighted. Small guanosine triphosphatase (GTPase) Rho and its target Rho kinase (Rho/Rho kinase)-related signal transduction pathway constitute a key element in vasoconstriction (Johns et al., 2000). Thus, agents that inhibit this signaling pathway may have benefits within the cerebral vascular system via two mechanisms, the first is (1): Preventing cerebrovascular accidents, indeed, as it has been shown that Rho and Rho kinase may have an important role in regulating arterial blood pressure thus, targeting this signaling pathway may provide an important antihypertensive treatment (Uehata et al., 1997). We note that statistics has indicated that treating hypertension result in a 48% reduction in the incidence of cerebrovascular accidents (Collins and MacMahon, 1994). The second mechanism is (2): The inhibition of Rho and its target-related pathway may provide positive results in cerebral spasm treatment (Jalil et al., 2005) and thus, makes this area more promising, especially that Rho kinase was linked to several endocellular functions including cytokinesis, gene expression, the organization of the actin cytoskeleton (Uehata et al., 1997) and reactive oxygen species (ROS) production (Noma et al., 2006).
Some GPCRs’ ligands, including angiotensin II and phenylephrine, activate GTPase Rho which activates Rho kinase then, the activated Rho kinase phosphorylates myosin light chain phosphatase (Jalil et al., 2005), this leads to myosin light chain phosphatase inhibition (Jalil et al., 2005).Thus, the idea of targeting the enzymes involved in this pathway may lead to the development of novel agents, mainly inhibitors, which will provide new therapies for some cerebral angiopathies such as cerebral ischemia and other cardiovascular diseases that have incidences on certain cerebral and neurological diseases, especially if targeting such molecules will also modulate the other functions that have been linked to Rho/Rho kinase such as some cholesterol-independent cardioprotective effects of statin therapy (Noma et al., 2006) which may expand more the therapeutic use. For example, fasudil, a Rho-dependent kinase inhibitor illustrates well the cerebral implications of targeting this pathway. In fact, Fasudil size in mice, for which the cerebral artery was occluded, has two actions (1) increased cerebral blood flow and (2) reduced infarct size (Kumar et al., 2007) showing how elucidating, then exploiting the physiological function of Rho/Rho kinase, and by extrapolation RGS, can lead to novel pharmacological applications.
4.5. Cyclic adenosine monophosphate (cAMP) and lipoic acid (LA)
Several GPCRs have been related to cAMP-dependent signaling pathway. Because cAMP has different important roles, the related pathways remain a promising pharmacological target. Indeed, cAMP is involved in proliferation, migration, apoptosis and gene expression processes (Brosens and Gellersen, 2006; Chen et al., 2000; Schillace and Carr, 2006). In addition, whereas, cAMP plays an important regulatory role during the inflammatory process (Schillace and Carr, 2006), numerous papers have pointed that cAMP elevating agents were able to inhibit lymphocyte proliferation, activation and function, cAMP elevating agents can also decrease histamine, leukotrienes, reactive oxygen species, cytokines, chemokines secretion, monocyte and neutrophil mobility as well (Joshi et al., 2001; Kuklina and Shirshev, 2000; Moore and Willoughby, 1995; Roder et al., 1980; Salinthone et al., 2008).
On the other hand, previous studies have shown that lipoic acid (LA), induces cAMP synthesis (Salinthone et al., 2008; Schillace et al., 2007) showing a common pathway for both cAMP and LA. The LA is an endogenous molecule (Morikawa et al., 2001) with antioxidant properties (Maitra et al., 1995; Nickander et al., 1996; Whiteman et al., 1996) allowing it to have therapeutic usages in Alzheimer’s disease, diabetic polyneuropathy and atherosclerosis (Salinthone et al., 2011). In addition, the LA was also pointed as neuroprotective against brain ischemic damage (Wolz and Krieglstein, 1996). Furthermore, the LA has been shown to be involved in immune process with anti-inflammatory properties. The LA decreases immune cells migration ability by inhibiting the expression of adhesion molecules (Chaudhary et al., 2006; Kunt et al., 1999).It inhibits also both cell activation and cytolytic function of Natural killer cells (NK cell) (Salinthone et al., 2008). Within some immune cells, cAMP synthesis, considered herein as an immunomodulator, is mediated by the LA (Salinthone et al., 2008; Schillace et al., 2007).
Two kinds of enzymes, phosphodiesterases (PDEs) and adenylyl cyclases (including transmembrane ACs (tmACs) and soluble ACs (sACs)) (Xiao et al., 2007, 2010) regulate cAMP cellular content (Buck et al., 1999; Neves et al., 2002). On the other hand, many GPCRs including prostanoid EP2 and EP4 receptors, histamine, adenosine and β adrenergic receptors have been shown to regulate tmACs (Neves et al., 2002). Moreover, data from many papers, together, lead us to suppose a relationship between LA activation of mechanisms, other than those linked with EP receptor’s, and the synergistic effects on cAMP production of LA and PGE2 (Han et al., 2005; Schmid et al., 2007; Sinclair et al., 2000; Stessin et al., 2006; Young et al., 2008; Zippin et al., 2003, 2004). Importantly, a recently published study (Salinthone et al., 2011), highlighted more new evidence and has confirmed that, histamine receptors and adenosine receptors are also mediators of LA stimulated cAMP production, in addition, LA stimulates soluble ACs and, more importantly, LA induces cAMP synthesis. The same paper has also pointed that LA does not activate β-adrenergic receptors (Salinthone et al., 2011). Moreover, tmACs activation can result from LA prostanoid and EP2/EP4 receptors’ binding (Salinthone et al., 2008; Schillace et al., 2007). These new findings are consistent with the data we have about the role histamine receptors and adenosine receptors play during inflammation. Whereas, histamine H2 receptors on peripheral monocytes stops interleukin (IL)-12 production (Elenkov et al., 1998), adenosine inhibits cytokine production in NK cells (Lokshin et al., 2006; Raskovalova et al., 2006) .
Several researches are highlighting the effects of LA treatment, indeed the LA reduces or inhibits several immune processes that are involved in the inflammatory phenomena (Chaudhary et al., 2006; Kunt et al., 1999; Marracci et al., 2006; Salinthone et al., 2008). Following this line of thought, anti-inflammatory effects of LA may involve adenosine receptors (Salinthone et al., 2011).Therefore, for Alzheimer’s disease (AD) and some other disease, due to their inflammatory component, cAMP has been targeted by therapies (McPhee et al., 2005; Osadchii, 2007).These novel elements about the described anti-inflammatory and the anti-oxidant properties of cAMP, cAMP pathway-related molecules and different receptors, mainly histamine and adenosine receptors, make them potential targets in several diseases that have inflammatory component or pathologies that may be improved with anti-oxidant agents as protective molecules such as multiple sclerosis, diabetic polyneuropathy, brain ischemia damages, in addition to neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and sclerosis.
The fact that LP and cAMP pathways are not together in all the pathways makes the chances of discovering new drugs with fewer side effects higher as we may target cAMP pathway independently from the LP or we may target the LP independently from the cAMP (or its pathway) thus, decreasing the impact on the other bio-functions of the cells via drugs’ selectivity.
5. Implications and perspectives
In addition to the previous illustrative examples which indicate how modifying the GPCR-related pathways can provide novel therapies, we suppose that the compounds that have the ability to interact with the cytoplasmic GPCR-related mechanisms may be very helpful for the study and the characterization of different pathways when a new drug is being investigated during laboratory researches. For example, the persistence of drug effects while a certain pathway is inhibited can indicate that that drug does not involve that inhibited pathway. It can also allow us to illustrate the molecular and the cellular mechanisms that govern some toxicological and pathophysiological phenomena. Indeed, some toxic compounds and even therapeutic agents are organophosphorus (OP) (RamaRao and Bhattacharya, 2012). OP represents irreversible inhibitors of the enzyme acetylcholinesterase (AchE) causing acetylcholine (Ach) accumulation in both peripheral and central nervous systems, which mainly explains the acute OP toxicity (Duysen et al., 2001) during which we observe hypersecretions, respiratory distress, tremor, seizures/convulsions, coma and induced chronic neurotoxicity (Damodaran et al., 2006; Miyaki et al., 2005). In addition to cholinergic-related signs, other nerve agent poisoning effects have been linked to glutamate and GABAergic receptors with diacylglycerol and calcium as second messengers (RamaRao and Bhattacharya, 2012). Moreover, this toxicity activates neuronal structure protein (Chebabo et al., 1999; Ward et al., 1993) and has been also related to mutagenic, stressogenic, immunologic, hepatotoxic, membrane and hematotoxic effects (Kassa et al., 2001). GPCRs and receptors with intrinsic tyrosine kinase activity have been shown to play a role in those processes (Costa, 1998). Furthermore; several other toxic effects have also been pointed. In fact, it modifies gene expression of key-cholinergic proteins (Bansal et al., 2009; Kaufer et al., 1998; Meshorer and Soreq, 2006), in addition, it has exitotoxic damage in the piriform cortex, entorhinal cortex, amygdala, and hippocampus (Lallement et al., 1991; McDonough and Shih, 1997) (Lallement et al., 1992; Solberg and Belkin, 1997). Whereas, a recent study has shown that after nerve agent exposure of the rat brain, cerebral cortex and cerebellum regions have high μ-calpain contents (RamaRao et al., 2011), in both Alzheimer’s and Parkinson’s diseases, increased calpain was shown to play a role (Camins et al., 2006; Saito et al., 1993) which is likely to contribute to our understanding of these neurodegenerative diseases. On the other hand, during nerve agent exposure, alterations of diverse neural processes including purinergic, NMDA-glutamatergic, GABAergic, catecholaminergic, serotogenic and calcium pathways in addition to neurodegeneration, dementia process, learning and memory alterations, are reported phenomena (Damodaran et al., 2006; Dillman et al., 2009; Pachiappan et al., 2009). Such novel description of the nerve agent toxicity will provide, if combined with further researches in pharmacology and molecular biology, new data to both understand the pathophysiology and find out therapeutics that may target the molecular elements implicated in the nerve agent toxicity pathway (GPCR and its second messengers, DNA and enzymes). On the other hand, the nerve agent has been linked to Alzheimer’s and Parkinson’s diseases (RamaRao and Bhattacharya, 2012). Therefore, such data allow us to learn more about the etiology of both neurodegenerative diseases and even to develop therapies through clarifying their molecular mechanisms and considering the implicated molecules (mainly enzymes and DNA) as novel targets to improve pathologies prognostics or slow down of the disease evolution. Investigations on laboratory regents or solvents, that are supposed to be bio-neuter but for which biological or pharmacological properties have been reported can also constitute starting points to drug development (Ghanemi, 2013a). Importantly, such novel drugs could treat the pathologies or the disorders in which acetylcholine signaling has been shown to play roles, in both the CNS and peripheral nervous system.
6. Conclusion
Although we pointed selected examples about targeting GPCRs’ pathways, the existent structural and functional similarities between the different GPCRs suppose that, a property shown for a GPCR in certain diseases or disorders may be, by extrapolation, applicable for other GPCRs within related pathophysiological cases.
The description of molecular mechanisms of diseases that will improve our understanding of pathophysiological phenomena, together with the data provided within this review about – mainly but not only – molecular aspects of GPCR-related pathways will have important implications in both in vivo and in vitro researches. Furthermore, the possibilities of designing new research protocols and eventually providing data to other research areas including molecular biology and physiology based on the above concepts constitute interesting topics.
Importantly, the advances provide elements and concepts that may lead to further drug development by giving starting points for new molecular therapeutics’ fields especially if it considers the other factors that have been shown to influence the pharmacodynamics of diverse GPCRs (Ghanemi et al., 2013) and the increased discoveries about different enzymes and molecules that control the downstream of the GPCR pathways with the description of the cellular, enzymatic and molecular interactions that govern the related pathways.
In contrast, within different systems, such as the central nervous system, the GPCRs interact with each other and with other receptors to form complex networks, such as in some psychiatric diseases (Ghanemi, 2013b). Thus, a drug that interacts with an element from the GPCR system might influence such networks and result in complexes’ side effects. Therefore, such approach must apply a severe pharmacovigilance, especially if the corresponding pathways control important in vivo functions of drugs that target GRCR related pathways.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adler E., Hoon M.A., Mueller K.L., Chandrashekar J., Ryba N.J., Zuker C.S. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Ahmed M.R., Berthet A., Bychkov E., Porras G., Li Q., Bioulac B.H., Carl Y.T., Bloch B., Kook S., Aubert I., Dovero S., Doudnikoff E., Gurevich V.V., Gurevich E.V., Bezard E. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson’s disease. Sci. Transl. Med. 2010;2(28) doi: 10.1126/scitranslmed.3000664. 28ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Kim J., Hara M.R., Ren X.R., Lefkowitz R.J. {beta}-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J. Biol. Chem. 2009;284(13):8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Nelson C.D., Garrison T.R., Miller W.E., Lefkowitz R.J. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. USA. 2003;100(4):1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.A., Yost J.M., Setola V., Chen X., Sassano M.F., Chen M., Peterson S., Yadav P.N., Huang X.P., Feng B., Jensen N.H., Che X., Bai X., Frye S.V., Wetsel W.C., Caron M.G., Javitch J.A., Roth B.L., Jin J. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio M., Zurn A., Lohse M.J. Sensing G protein-coupled receptor activation. Neuropharmacology. 2011;60(1):45–51. doi: 10.1016/j.neuropharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Assadi-Porter F.M., Tonelli M., Maillet E.L., Markley J.L., Max M. Interactions between the human sweet-sensing T1R2-T1R3 receptor and sweeteners detected by saturation transfer difference NMR spectroscopy. Biochim. Biophys. Acta. 2010;1798(2):82–86. doi: 10.1016/j.bbamem.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi M., Charest P.G., Angers S., Rousseau G., Kohout T., Bouvier M., Pineyro G. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2003;100(20):11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells-Olivero M., Cousins M.S., Seiden L.S. Holtzman and Harlan Sprague-Dawley rats: differences in DRL 72-sec performance and 8-hydroxy-di-propylamino tetralin-induced hypothermia. J. Pharmacol. Exp. Ther. 1998;286(2):742–752. [PubMed] [Google Scholar]
- Bansal I., Waghmare C.K., Anand T., Gupta A.K., Bhattacharya B.K. Differential mRNA expression of acetylcholinesterase in the central nervous system of rats with acute and chronic exposure of sarin & physostigmine. J. Appl. Toxicol. 2009;29(5):386–394. doi: 10.1002/jat.1424. [DOI] [PubMed] [Google Scholar]
- Barrett T.B., Emberton J.E., Nievergelt C.M., Liang S.G., Hauger R.L., Eskin E., Schork N.J., Kelsoe J.R. Further evidence for association of GRK3 to bipolar disorder suggests a second disease mutation. Psychiatry Genet. 2007;17(6):315–322. doi: 10.1097/YPG.0b013e3282efeeb4. [DOI] [PubMed] [Google Scholar]
- Barrett T.B., Hauger R.L., Kennedy J.L., Sadovnick A.D., Remick R.A., Keck P.E., McElroy S.L., Alexander M., Shaw S.H., Kelsoe J.R. Evidence that a single nucleotide polymorphism in the promoter of the G protein receptor kinase 3 gene is associated with bipolar disorder. Mol. Psychiatry. 2003;8(5):546–557. doi: 10.1038/sj.mp.4001268. [DOI] [PubMed] [Google Scholar]
- Barthet G., Framery B., Gaven F., Pellissier L., Reiter E., Claeysen S., Bockaert J., Dumuis A. 5-Hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol. Biol. Cell. 2007;18(6):1979–1991. doi: 10.1091/mbc.E06-12-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.M., Sotnikova T.D., Marion S., Lefkowitz R.J., Gainetdinov R.R., Caron M.G. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Benovic J.L., DeBlasi A., Stone W.C., Caron M.G., Lefkowitz R.J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Bert B., Fink H., Rothe J., Walstab J., Bonisch H. Learning and memory in 5-HT(1A)-receptor mutant mice. Behav. Brain Res. 2008;195(1):78–85. doi: 10.1016/j.bbr.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Bezard E., Gross C.E., Qin L., Gurevich V.V., Benovic J.L., Gurevich E.V. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol. Dis. 2005;18(2):323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Birch G.G. Modulation of sweet taste. Biofactors. 1999;9(1):73–80. doi: 10.1002/biof.5520090109. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A.M. Receptor-effector coupling by G proteins. Biochim. Biophys. Acta. 1990;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bjork K., Rimondini R., Hansson A.C., Terasmaa A., Hyytia P., Heilig M., Sommer W.H. Modulation of voluntary ethanol consumption by beta-arrestin 2. FASEB J. 2008;22(7):2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- Blier P., Bergeron R., de Montigny C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology. 1997;16(5):333–338. doi: 10.1016/S0893-133X(96)00242-4. [DOI] [PubMed] [Google Scholar]
- Bohn L.M., Dykstra L.A., Lefkowitz R.J., Caron M.G., Barak L.S. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol. Pharmacol. 2004;66(1):106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Bohn L.M., Gainetdinov R.R., Lin F.T., Lefkowitz R.J., Caron M.G. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn L.M., Gainetdinov R.R., Sotnikova T.D., Medvedev I.O., Lefkowitz R.J., Dykstra L.A., Caron M.G. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J. Neurosci. 2003;23(32):10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn L.M., Lefkowitz R.J., Gainetdinov R.R., Peppel K., Caron M.G., Lin F.T. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Boivin B., Vaniotis G., Allen B.G., Hebert T.E. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J. Recept. Signal Transduct. Res. 2008;28(1–2):15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- Bonacci T.M., Mathews J.L., Yuan C., Lehmann D.M., Malik S., Wu D., Font J.L., Bidlack J.M., Smrcka A.V. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312(5772):443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- Bookout A.L., Finney A.E., Guo R., Peppel K., Koch W.J., Daaka Y. Targeting Gbetagamma signaling to inhibit prostate tumor formation and growth. J. Biol. Chem. 2003;278(39):37569–37573. doi: 10.1074/jbc.M306276200. [DOI] [PubMed] [Google Scholar]
- Brosens J.J., Gellersen B. Death or survival–progesterone-dependent cell fate decisions in the human endometrial stroma. J. Mol. Endocrinol. 2006;36(3):389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- Buck J., Sinclair M.L., Schapal L., Cann M.J., Levin L.R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA. 1999;96(1):79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera T.M., Vanhauwe J., Thomas T.O., Medkova M., Preininger A., Mazzoni M.R., Hamm H.E. Insights into G protein structure, function, and regulation. Endocr. Rev. 2003;24(6):765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Calebiro D., Nikolaev V.O., Persani L., Lohse M.J. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci. 2010;31(5):221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Camins A., Verdaguer E., Folch J., Pallas M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006;12(2):135–148. doi: 10.1111/j.1527-3458.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Hou C., Sidiropoulos D., Stock J.B., Jakobs K.H., Gierschik P. Stimulation of phospholipase C by guanine–nucleotide-binding protein beta gamma subunits. Eur. J. Biochem. 1992;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Casey L.M., Pistner A.R., Belmonte S.L., Migdalovich D., Stolpnik O., Nwakanma F.E., Vorobiof G., Dunaevsky O., Matavel A., Lopes C.M., Smrcka A.V., Blaxall B.C. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ. Res. 2010;107(4):532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir K., Zhu W., Tsang S., Woo A.Y., Yang D., Wang X., Zeng X., Rhee M.H., Mende U., Koitabashi N., Takimoto E., Blumer K.J., Lakatta E.G., Kass D.A., Xiao R.P. RGS2 is a primary terminator of beta-adrenergic receptor-mediated G(i) signaling. J. Mol. Cell. Cardiol. 2011;50(6):1000–1007. doi: 10.1016/j.yjmcc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P., Marracci G.H., Bourdette D.N. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006;175(1–2):87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Chebabo S.R., Santos M.D., Albuquerque E.X. The organophosphate sarin, at low concentrations, inhibits the evoked release of GABA in rat hippocampal slices. Neurotoxicology. 1999;20(6):871–882. [PubMed] [Google Scholar]
- Chen C.H., Zhang D.H., LaPorte J.M., Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J. Immunol. 2000;165(10):5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- Chen J., Rusnak M., Lombroso P.J., Sidhu A. Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. Eur. J. Neurosci. 2009;29(2):287–306. doi: 10.1111/j.1460-9568.2008.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Maier D., Neubueser D., Hipfner D.R. Regulation of smoothened by Drosophila G-protein-coupled receptor kinases. Dev. Biol. 2010;337(1):99–109. doi: 10.1016/j.ydbio.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.B., Graeber C.T., Quinn J.A., Filardo E.J. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011;76(9):892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Christensen G.L., Kelstrup C.D., Lyngso C., Sarwar U., Bogebo R., Sheikh S.P., Gammeltoft S., Olsen J.V., Hansen J.L. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol. Cell. Proteomics. 2010;9(7):1540–1553. doi: 10.1074/mcp.M900550-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Funakoshi T., Civelli O. Orphan GPCR research. Br. J. Pharmacol. 2008;153(Suppl. 1):S339–S346. doi: 10.1038/sj.bjp.0707606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelens P.M., Kavelaars A., Heijnen C.J., Ribas C., Mayor F., Jr., Penela P. Hydrogen peroxide impairs GRK2 translation via a calpain-dependent and cdk1-mediated pathway. Cell Signal. 2007;19(2):269–277. doi: 10.1016/j.cellsig.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Collins R., MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Br. Med. Bull. 1994;50(2):272–298. doi: 10.1093/oxfordjournals.bmb.a072892. [DOI] [PubMed] [Google Scholar]
- Conner D.A., Mathier M.A., Mortensen R.M., Christe M., Vatner S.F., Seidman C.E., Seidman J.G. Beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ. Res. 1997;81(6):1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- Costa L.G. Signal transduction in environmental neurotoxicity. Annu. Rev. Pharmacol. Toxicol. 1998;38:21–43. doi: 10.1146/annurev.pharmtox.38.1.21. [DOI] [PubMed] [Google Scholar]
- Daaka Y. S-nitrosylation-regulated GPCR signaling. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbagen.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran T.V., Patel A.G., Greenfield S.T., Dressman H.K., Lin S.M., Abou-Donia M.B. Gene expression profiles of the rat brain both immediately and 3 months following acute sarin exposure. Biochem. Pharmacol. 2006;71(4):497–520. doi: 10.1016/j.bcp.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Davies M.N., Secker A., Freitas A.A., Mendao M., Timmis J., Flower D.R. On the hierarchical classification of G protein-coupled receptors. Bioinformatics. 2007;23(23):3113–3118. doi: 10.1093/bioinformatics/btm506. [DOI] [PubMed] [Google Scholar]
- Deshpande D.A., Theriot B.S., Penn R.B., Walker J.K. Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22(7):2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- DeWire S.M., Kim J., Whalen E.J., Ahn S., Chen M., Lefkowitz R.J. Beta-arrestin-mediated signaling regulates protein synthesis. J. Biol. Chem. 2008;283(16):10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman J.F., 3rd, Phillips C.S., Kniffin D.M., Tompkins C.P., Hamilton T.A., Kan R.K. Gene expression profiling of rat hippocampus following exposure to the acetylcholinesterase inhibitor soman. Chem. Res. Toxicol. 2009;22(4):633–638. doi: 10.1021/tx800466v. [DOI] [PubMed] [Google Scholar]
- Dixon R.A., Kobilka B.K., Strader D.J., Benovic J.L., Dohlman H.G., Frielle T., Bolanowski M.A., Bennett C.D., Rands E., Diehl R.E., Mumford R.A., Slater E.E., Sigal I.S., Caron M.G., Lefkowitz R.J., Strader C.D. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dorn G.W., 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005;115(3):527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.T., Violin J.D., Whalen E.J., Wisler J.W., Shenoy S.K., Lefkowitz R.J. Beta-arrestin-biased agonism at the beta2-adrenergic receptor. J. Biol. Chem. 2008;283(9):5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- Duysen E.G., Li B., Xie W., Schopfer L.M., Anderson R.S., Broomfield C.A., Lockridge O. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J. Pharmacol. Exp. Ther. 2001;299(2):528–535. [PubMed] [Google Scholar]
- Edagawa Y., Saito H., Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur. J. Pharmacol. 1998;349(2–3):221–224. doi: 10.1016/s0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N., Heijnen C.J., Willemen H.L., Deumens R., Joosten E.A., Kleibeuker W., den Hartog I.J., van Velthoven C.T., Nijboer C., Nassar M.A., Dorn G.W., 2nd, Wood J.N., Kavelaars A. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J. Neurosci. 2010;30(6):2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I.J., Webster E., Papanicolaou D.A., Fleisher T.A., Chrousos G.P., Wilder R.L. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 1998;161(5):2586–2593. [PubMed] [Google Scholar]
- Fahn S. How do you treat motor complications in Parkinson’s disease: Medicine, surgery, or both? Ann. Neurol. 2008;64(Suppl 2):S56–64. doi: 10.1002/ana.21453. [DOI] [PubMed] [Google Scholar]
- Fan J., Malik A.B. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat. Med. 2003;9(3):315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- Feng Y.H., Ding Y., Ren S., Zhou L., Xu C., Karnik S.S. Unconventional homologous internalization of the angiotensin II type-1 receptor induced by G-protein-independent signals. Hypertension. 2005;46(2):419–425. doi: 10.1161/01.HYP.0000172621.68061.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Saxena R., Chattopadhyay A. Reorganization of the actin cytoskeleton upon G-protein coupled receptor signaling. Biochim. Biophys. Acta. 2011;1808(7):1921–1929. doi: 10.1016/j.bbamem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla J.A., Escriba P.V., Ozaita A., La Harpe R., Walzer C., Eytan A., Guimon J. Up-regulation of immunolabeled alpha2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J. Neurochem. 1999;72(1):282–291. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 2000;21(1):90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Ghanemi, A., 2013a. Biological properties and perspective applications of “Bio-neuter” chemicals? Saudi Pharmaceutical J. http://dx.doi.org/10.1016/j.jsps.2013.01.006. [DOI] [PMC free article] [PubMed]
- Ghanemi, A., 2013b. Psychiatric neural networks and neuropharmacology: Selected advances and novel implications. Saudi Pharmaceutical J. http://dx.doi.org/10.1016/j.jsps.2013.01.008. [DOI] [PMC free article] [PubMed]
- Ghanemi A., He L., Yan M. New factors influencing G protein coupled receptors’ system functions. Alexandria J. Med. 2013;49(1):1–5. [Google Scholar]
- Gilman A.G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Giorelli M., Livrea P., Trojano M. Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. J. Neuroimmunol. 2004;155(1–2):143–149. doi: 10.1016/j.jneuroim.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Gloriam D.E., Fredriksson R., Schioth H.B. The G protein-coupled receptor subset of the rat genome. BMC Genomics. 2007;8:338. doi: 10.1186/1471-2164-8-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange-Midroit M., Garcia-Sevilla J.A., Ferrer-Alcon M., La Harpe R., Huguelet P., Guimon J. Regulation of GRK 2 and 6, beta-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res. Mol. Brain Res. 2003;111(1–2):31–41. doi: 10.1016/s0169-328x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A.J., Okkenhaug K., Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. USA. 2008;105(24):8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich E.V., Tesmer J.J., Mushegian A., Gurevich V.V. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol. Ther. 2012;133(1):40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Stessin A., Roberts J., Hess K., Gautam N., Kamenetsky M., Lou O., Hyde E., Nathan N., Muller W.A., Buck J., Levin L.R., Nathan C. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J. Exp. Med. 2005;202(3):353–361. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney S., Hancox R.J. Recovery from bronchoconstriction and bronchodilator tolerance. Clin. Rev. Allergy Immunol. 2006;31(2–3):181–196. doi: 10.1385/CRIAI:31:2:181. [DOI] [PubMed] [Google Scholar]
- Harmar A.J., Hills R.A., Rosser E.M., Jones M., Buneman O.P., Dunbar D.R., Greenhill S.D., Hale V.A., Sharman J.L., Bonner T.I., Catterall W.A., Davenport A.P., Delagrange P., Dollery C.T., Foord S.M., Gutman G.A., Laudet V., Neubig R.R., Ohlstein E.H., Olsen R.W., Peters J., Pin J.P., Ruffolo R.R., Searls D.B., Wright M.W., Spedding M. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 2009:37. doi: 10.1093/nar/gkn728. (Database issue): D680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell G.G., Hindmarch C.C., Pope G.R., Roper J.A., Lightman S.L., Murphy D., O’Carroll A.M., Lolait S.J. G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei – serpentine gateways to neuroendocrine homeostasis. Front. Neuroendocrinol. 2012;33(1):45–66. doi: 10.1016/j.yfrne.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U., Tsai L.T., Kapfhamer D., Lasek A.W. Drosophila, a genetic model system to study cocaine-related behaviors: a review with focus on LIM-only proteins. Neuropharmacology. 2009;56(Suppl. 1):97–106. doi: 10.1016/j.neuropharm.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilker R., Wolff M., Tautermann C.S., Bieler M. G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov. Today. 2009;14(5–6):231–240. doi: 10.1016/j.drudis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Hofmann K.P., Scheerer P., Hildebrand P.W., Choe H.W., Park J.H., Heck M., Ernst O.P. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem. Sci. 2009;34(11):540–552. doi: 10.1016/j.tibs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hollinger S., Hepler J.R. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 2002;54(3):527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hoon M.A., Adler E., Lindemeier J., Battey J.F., Ryba N.J., Zuker C.S. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96(4):541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L., Groom C.R. The druggable genome. Nat. Rev. Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Horvath S., Janka Z., Mirnics K. Analyzing schizophrenia by DNA microarrays. Biol. Psychiatry. 2011;69(2):157–162. doi: 10.1016/j.biopsych.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S.R., Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv. Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- Iken K., Chheng S., Fargin A., Goulet A.C., Kouassi E. Serotonin upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell Immunol. 1995;163(1):1–9. doi: 10.1006/cimm.1995.1092. [DOI] [PubMed] [Google Scholar]
- Insel P.A., Tang C.M., Hahntow I., Michel M.C. Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim. Biophys. Acta. 2007;1768(4):994–1005. doi: 10.1016/j.bbamem.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R., Wedegaertner P.B. Regulation of constitutive cargo transport from the trans-Golgi network to plasma membrane by Golgi-localized G protein betagamma subunits. J. Biol. Chem. 2010;285(42):32393–32404. doi: 10.1074/jbc.M110.154963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K., Kato T. Gene expression profiling in schizophrenia and related mental disorders. Neuroscientist. 2006;12(4):349–361. doi: 10.1177/1073858406287536. [DOI] [PubMed] [Google Scholar]
- Jalil J., Lavandero S., Chiong M., Ocaranza M.P. Rho/Rho kinase signal transduction pathway in cardiovascular disease and cardiovascular remodeling. Rev. Esp. Cardiol. 2005;58(8):951–961. [PubMed] [Google Scholar]
- Jiang B., Shi Y., Li H., Kang L., Ma L. Decreased morphine analgesia in rat overexpressing beta-arrestin 2 at periaqueductal gray. Neurosci. Lett. 2006;400(1–2):150–153. doi: 10.1016/j.neulet.2006.02.071. [DOI] [PubMed] [Google Scholar]
- Johns D.G., Dorrance A.M., Leite R., Weber D.S., Webb R.C. Novel signaling pathways contributing to vascular changes in hypertension. J. Biomed. Sci. 2000;7(6):431–443. doi: 10.1007/BF02253359. [DOI] [PubMed] [Google Scholar]
- Joshi P.C., Zhou X., Cuchens M., Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J. Immunol. 2001;166(2):885–891. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- Kassa J., Pecka M., Tichy M., Bajgar J., Koupilova M., Herink J., Krocova Z. Toxic effects of sarin in rats at three months following single or repeated low-level inhalation exposure. Pharmacol. Toxicol. 2001;88(4):209–212. doi: 10.1034/j.1600-0773.2001.d01-106.x. [DOI] [PubMed] [Google Scholar]
- Kaufer D., Friedman A., Seidman S., Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393(6683):373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat. Rev. Drug Discov. 2005;4(11):919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kenakin T., Miller L.J. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62(2):265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts J.D., Connery H.S., Arrington E.G., Lewis M.M., Lawler C.P., Oxford G.S., O’Malley K.L., Todd R.D., Blake B.L., Nichols D.E., Mailman R.B. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J. Pharmacol. Exp. Ther. 2002;301(3):1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- Kim I.M., Tilley D.G., Chen J., Salazar N.C., Whalen E.J., Violin J.D., Rockman H.A. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. USA. 2008;105(38):14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirui J.K., Xie Y., Wolff D.W., Jiang H., Abel P.W., Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. J. Pharmacol. Exp. Ther. 2010;333(2):393–403. doi: 10.1124/jpet.109.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleibeuker W., Ledeboer A., Eijkelkamp N., Watkins L.R., Maier S.F., Zijlstra J., Heijnen C.J., Kavelaars A. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur. J. Neurosci. 2007;25(6):1696–1704. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- Koch W.J., Rockman H.A., Samama P., Hamilton R.A., Bond R.A., Milano C.A., Lefkowitz R.J. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268(5215):1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Koizumi A., Nakajima K., Asakura T., Morita Y., Ito K., Shmizu-Ibuka A., Misaka T., Abe K. Taste-modifying sweet protein, neoculin, is received at human T1R3 amino terminal domain. Biochem. Biophys. Res. Commun. 2007;358(2):585–589. doi: 10.1016/j.bbrc.2007.04.171. [DOI] [PubMed] [Google Scholar]
- Krupnick J.G., Gurevich V.V., Benovic J.L. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J. Biol. Chem. 1997;272(29):18125–18131. doi: 10.1074/jbc.272.29.18125. [DOI] [PubMed] [Google Scholar]
- Kuklina E.M., Shirshev S.V. Role of cAMP-dependent signal transduction in the control of T lymphocyte activation. Biochemistry (Mosc.) 2000;65(6):629–639. [PubMed] [Google Scholar]
- Kumar R., Singh V.P., Baker K.M. Kinase inhibitors for cardiovascular disease. J. Mol. Cell. Cardiol. 2007;42(1):1–11. doi: 10.1016/j.yjmcc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kunt T., Forst T., Wilhelm A., Tritschler H., Pfuetzner A., Harzer O., Engelbach M., Zschaebitz A., Stofft E., Beyer J. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin. Sci. (Lond.) 1999;96(1):75–82. [PubMed] [Google Scholar]
- Lagerstrom M.C., Schioth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7(4):339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Lallement G., Carpentier P., Collet A., Baubichon D., Pernot-Marino I., Blanchet G. Extracellular acetylcholine changes in rat limbic structures during soman-induced seizures. Neurotoxicology. 1992;13(3):557–567. [PubMed] [Google Scholar]
- Lallement G., Carpentier P., Collet A., Pernot-Marino I., Baubichon D., Blanchet G. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res. 1991;563(1–2):234–240. doi: 10.1016/0006-8993(91)91539-d. [DOI] [PubMed] [Google Scholar]
- Lawler C.P., Prioleau C., Lewis M.M., Mak C., Jiang D., Schetz J.A., Gonzalez A.M., Sibley D.R., Mailman R.B. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20(6):612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lehmann D.M., Seneviratne A.M., Smrcka A.V. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol. 2008;73(2):410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leosco D., Fortunato F., Rengo G., Iaccarino G., Sanzari E., Golino L., Zincarelli C., Canonico V., Marchese M., Koch W.J., Rengo F. Lymphocyte G-protein-coupled receptor kinase-2 is upregulated in patients with Alzheimer’s disease. Neurosci. Lett. 2007;415(3):279–282. doi: 10.1016/j.neulet.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Li Q., Luo T., Jiang X., Wang J. Anxiolytic effects of 5-HTA receptors and anxiogenic effects of 5-HTC receptors in the amygdala of mice. Neuropharmacology. 2012;62(1):474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley J.E., Rose K., Ooi L., Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch. 2010;459(5):657–669. doi: 10.1007/s00424-010-0784-6. [DOI] [PubMed] [Google Scholar]
- Lohse M.J., Benovic J.L., Codina J., Caron M.G., Lefkowitz R.J. Beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lokshin A., Raskovalova T., Huang X., Zacharia L.C., Jackson E.K., Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66(15):7758–7765. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- Lombardi M.S., Kavelaars A., Penela P., Scholtens E.J., Roccio M., Schmidt R.E., Schedlowski M., Mayor F., Jr., Heijnen C.J. Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. Mol. Pharmacol. 2002;62(2):379–388. doi: 10.1124/mol.62.2.379. [DOI] [PubMed] [Google Scholar]
- Lombardi M.S., Vroon A., Sodaar P., van Muiswinkel F.L., Heijnen C.J., Kavelaars A. Down-regulation of GRK2 after oxygen and glucose deprivation in rat hippocampal slices: role of the PI3-kinase pathway. J. Neurochem. 2007;102(3):731–740. doi: 10.1111/j.1471-4159.2007.04576.x. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Latest development in drug discovery on G protein-coupled receptors. Curr. Protein Pept. Sci. 2006;7(5):465–470. doi: 10.2174/138920306778559403. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Ferguson S.S., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F., Kawakatsu H., Owada K., Luttrell D.K., Caron M.G., Lefkowitz R.J. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 2010;62(2):305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A.W., Dong J.Y., Ma D., Wells J.W. Cleavage-resistant fusion proteins of the M(2) muscarinic receptor and Galpha(i1). Homotropic and heterotropic effects in the binding of ligands. Biochim. Biophys. Acta. 2011;1810(6):592–602. doi: 10.1016/j.bbagen.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Ma B., Shatsky M., Wolfson H.J., Nussinov R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 2002;11(2):184–197. doi: 10.1110/ps.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P., Zemmel R. Value of novelty? Nat. Rev. Drug Discov. 2002;1(8):571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- Mailman R.B. GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 2007;28(8):390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman R.B., Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010;16(5):488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra I., Serbinova E., Trischler H., Packer L. Alpha-lipoic acid prevents buthionine sulfoximine-induced cataract formation in newborn rats. Free Radic. Biol. Med. 1995;18(4):823–829. doi: 10.1016/0891-5849(94)00195-p. [DOI] [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Marinissen M.J., Gutkind J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22(7):368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Marracci G.H., Marquardt W.E., Strehlow A., McKeon G.P., Gross J., Buck D.C., Kozell L.B., Bourdette D.N. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck) Biochem. Biophys. Res. Commun. 2006;344(3):963–971. doi: 10.1016/j.bbrc.2006.03.172. [DOI] [PubMed] [Google Scholar]
- Martini J.S., Raake P., Vinge L.E., DeGeorge B.R., Jr., Chuprun J.K., Harris D.M., Gao E., Eckhart A.D., Pitcher J.A., Koch W.J. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Natl. Acad. Sci. USA. 2008;105(34):12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N., Caldarola-Pastuszka M., Uphouse L. Functional integration among 5-hydroxytryptamine receptor families in the control of female rat sexual behavior. Brain Res. 1998;802(1–2):98–103. doi: 10.1016/s0006-8993(98)00554-x. [DOI] [PubMed] [Google Scholar]
- Mathews J.L., Smrcka A.V., Bidlack J.M. A novel Gbetagamma-subunit inhibitor selectively modulates mu-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J. Neurosci. 2008;28(47):12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovich S.J., Diwan A., Klanke J.L., Hammer D.J., Marreez Y., Odley A.M., Brunskill E.W., Koch W.J., Schwartz R.J., Dorn G.W., 2nd. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ. Res. 2006;99(9):996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- Maudsley S., Martin B., Luttrell L.M. The origins of diversity and specificity in g protein-coupled receptor signaling. J. Pharmacol. Exp. Ther. 2005;314(2):485–494. doi: 10.1124/jpet.105.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough J.H., Jr., Shih T.M. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci. Biobehav. Rev. 1997;21(5):559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McPhee I., Gibson L.C., Kewney J., Darroch C., Stevens P.A., Spinks D., Cooreman A., MacKenzie S.J. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem. Soc. Trans. 2005;33(Pt. 6):1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- Mehta D., Menke A., Binder E.B. Gene expression studies in major depression. Curr. Psychiatry Rep. 2010;12(2):135–144. doi: 10.1007/s11920-010-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E., Soreq H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci. 2006;29(4):216–224. doi: 10.1016/j.tins.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Miyaki K., Nishiwaki Y., Maekawa K., Ogawa Y., Asukai N., Yoshimura K., Etoh N., Matsumoto Y., Kikuchi Y., Kumagai N., Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J. Occup. Health. 2005;47(4):299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Moore A.R., Willoughby D.A. The role of cAMP regulation in controlling inflammation. Clin. Exp. Immunol. 1995;101(3):387–389. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa T., Yasuno R., Wada H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001;498(1):16–21. doi: 10.1016/s0014-5793(01)02469-3. [DOI] [PubMed] [Google Scholar]
- Neves S.R., Ram P.T., Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Nickander K.K., McPhee B.R., Low P.A., Tritschler H. Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radic. Biol. Med. 1996;21(5):631–639. doi: 10.1016/0891-5849(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Bockaert J., Collingridge G.L., Conn P.J., Ferraguti F., Schoepp D.D., Wroblewski J.T., Pin J.P. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60(7–8):1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K., Oyama N., Liao J.K. Physiological role of ROCKs in the cardiovascular system. Am. J. Physiol. Cell. Physiol. 2006;290(3):C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T., Lemaire A., Naga Prasad S.V., Barki-Harrington L., Tilley D.G., Chen J., Le Corvoisier P., Violin J.D., Wei H., Lefkowitz R.J., Rockman H.A. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 2007;117(9):2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovich M.E., Smith M.A., Siedlak S.L., Chen S.G., de la Torre J.C., Perry G., Aliev G. Overexpression of GRK2 in Alzheimer disease and in a chronic hypoperfusion rat model is an early marker of brain mitochondrial lesions. Neurotox. Res. 2006;10(1):43–56. doi: 10.1007/BF03033333. [DOI] [PubMed] [Google Scholar]
- Osadchii O.E. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc. Drugs Ther. 2007;21(3):171–194. doi: 10.1007/s10557-007-6014-6. [DOI] [PubMed] [Google Scholar]
- Overington J.P., Al-Lazikani B., Hopkins A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pachiappan A., Thwin M.M., Weng Keong L., Lee F.K., Manikandan J., Sivakumar V., Gopalakrishnakone P. ETS2 regulating neurodegenerative signaling pathway of human neuronal (SH-SY5Y) cells exposed to single and repeated low-dose sarin (GB) Chem. Res. Toxicol. 2009;22(6):990–996. doi: 10.1021/tx8003467. [DOI] [PubMed] [Google Scholar]
- Park D., Jhon D.Y., Lee C.W., Lee K.H., Rhee S.G. Activation of phospholipase C isozymes by G protein beta gamma subunits. J. Biol. Chem. 1993;268(7):4573–4576. [PubMed] [Google Scholar]
- Park P.S., Lodowski D.T., Palczewski K. Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu. Rev. Pharmacol. Toxicol. 2008;48:107–141. doi: 10.1146/annurev.pharmtox.48.113006.094630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkel S., Tontson L., Rinken A. Millimolar Mn2+ influences agonist binding to 5-HT1A receptors by inhibiting guanosine nucleotide binding to receptor-coupled G-proteins. Neurotoxicology. 2011;32(1):25–30. doi: 10.1016/j.neuro.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Penela P., Murga C., Ribas C., Tutor A.S., Peregrin S., Mayor F., Jr. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc. Res. 2006;69(1):46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Perry S.J., Baillie G.S., Kohout T.A., McPhee I., Magiera M.M., Ang K.L., Miller W.E., McLean A.J., Conti M., Houslay M.D., Lefkowitz R.J. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298(5594):834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pitcher J.A., Inglese J., Higgins J.B., Arriza J.L., Casey P.J., Kim C., Benovic J.L., Kwatra M.M., Caron M.G., Lefkowitz R.J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Poulin B., Butcher A., McWilliams P., Bourgognon J.M., Pawlak R., Kong K.C., Bottrill A., Mistry S., Wess J., Rosethorne E.M., Charlton S.J., Tobin A.B. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. USA. 2010;107(20):9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S., Rajagopal K., Lefkowitz R.J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamaRao G., Acharya J.N., Bhattacharya B.K. Changes of protein oxidation, calpain and cytoskeletal proteins (alpha tubulin and pNF-H) levels in rat brain after nerve agent poisoning. Toxicol. Lett. 2011;203(3):227–236. doi: 10.1016/j.toxlet.2011.03.020. [DOI] [PubMed] [Google Scholar]
- RamaRao G., Bhattacharya B.K. Multiple signal transduction pathways alterations during nerve agent toxicity. Toxicol. Lett. 2012;208(1):16–22. doi: 10.1016/j.toxlet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Ramos-Ruiz R., Penela P., Penn R.B., Mayor F., Jr. Analysis of the human G protein-coupled receptor kinase 2 (GRK2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation. 2000;101(17):2083–2089. doi: 10.1161/01.cir.101.17.2083. [DOI] [PubMed] [Google Scholar]
- Raskovalova T., Lokshin A., Huang X., Jackson E.K., Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol. Res. 2006;36(1–3):91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- Rauly-Lestienne I., Lestienne F., Ailhaud M.C., Binesse J., Newman-Tancredi A., Cussac D. Competitive interaction of 5-HT(1A) receptors with G-protein subtypes in CHO cells demonstrated by RNA interference. Cell Signal. 2011;23(1):58–64. doi: 10.1016/j.cellsig.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Re M., Pampillo M., Savard M., Dubuc C., McArdle C.A., Millar R.P., Conn P.M., Gobeil F., Jr., Bhattacharya M., Babwah A.V. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5(7):e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle E.L., Schwartzman R.A., Bond M., Insel P.A. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ. Res. 2005;96(4):401–411. doi: 10.1161/01.RES.0000158287.49872.4e. [DOI] [PubMed] [Google Scholar]
- Rimondini R., Arlinde C., Sommer W., Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16(1):27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Robishaw J.D., Berlot C.H. Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell. Biol. 2004;16(2):206–209. doi: 10.1016/j.ceb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Rockman H.A., Chien K.R., Choi D.J., Iaccarino G., Hunter J.J., Ross J., Jr., Lefkowitz R.J., Koch W.J. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl. Acad. Sci. USA. 1998;95(12):7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415(6868):206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Roder J.C., Argov S., Klein M., Petersson C., Kiessling R., Andersson K., Hansson M. Target-effector cell interaction in the natural killer cell system. V. Energy requirements, membrane integrity, and the possible involvement of lysosomal enzymes. Immunology. 1980;40(1):: 107–116. [PMC free article] [PubMed] [Google Scholar]
- Rondard P., Goudet C., Kniazeff J., Pin J.P., Prezeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60(1):82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.M., Wilkie T.M. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Saito K., Elce J.S., Hamos J.E., Nixon R.A. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. USA. 1993;90(7):2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinthone S., Schillace R.V., Marracci G.H., Bourdette D.N., Carr D.W. Lipoic acid stimulates cAMP production via the EP2 and EP4 prostanoid receptors and inhibits IFN gamma synthesis and cellular cytotoxicity in NK cells. J. Neuroimmunol. 2008;199(1–2):46–55. doi: 10.1016/j.jneuroim.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinthone S., Schillace R.V., Tsang C., Regan J.W., Bourdette D.N., Carr D.W. Lipoic acid stimulates cAMP production via G protein-coupled receptor-dependent and -independent mechanisms. J. Nutr. Biochem. 2011;22(7):681–690. doi: 10.1016/j.jnutbio.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J., Lucki I., Drevets W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S.S., Booth B.J., Carr B.T., Losee M.L., Sattely-Miller E.A., Graham B.G. Investigation of synergism in binary mixtures of sweeteners. Brain Res. Bull. 1995;38(2):105–120. doi: 10.1016/0361-9230(95)00062-j. [DOI] [PubMed] [Google Scholar]
- Schillace R.V., Carr D.W. The role of protein kinase A and A-kinase anchoring proteins in modulating T-cell activation: progress and future directions. Crit. Rev. Immunol. 2006;26(2):113–131. doi: 10.1615/critrevimmunol.v26.i2.20. [DOI] [PubMed] [Google Scholar]
- Schillace R.V., Pisenti N., Pattamanuch N., Galligan S., Marracci G.H., Bourdette D.N., Carr D.W. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem. Biophys. Res. Commun. 2007;354(1):259–264. doi: 10.1016/j.bbrc.2006.12.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Sutto Z., Nlend M.C., Horvath G., Schmid N., Buck J., Levin L.R., Conner G.E., Fregien N., Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol. 2007;130(1):99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C.L., Bohn L.M. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol. Ther. 2009;121(3):285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein R., Westendorf C., Krause G., Rosenthal W. Functional significance of cleavable signal peptides of G protein-coupled receptors. Eur. J. Cell. Biol. 2012;91(4):294–299. doi: 10.1016/j.ejcb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Schutzer W.E., Xue H., Reed J., Oyama T., Beard D.R., Anderson S., Mader S.L. Age-related beta-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vascul. Pharmacol. 2011;55(5–6):178–188. doi: 10.1016/j.vph.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Seletti B., Benkelfat C., Blier P., Annable L., Gilbert F., de Montigny C. Serotonin1A receptor activation by flesinoxan in humans body temperature and neuroendocrine responses. Neuropsychopharmacology. 1995;13(2):93–104. doi: 10.1016/0893-133X(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Seneviratne A.M., Burroughs M., Giralt E., Smrcka A.V. Direct-reversible binding of small molecules to G protein betagamma subunits. Biochim. Biophys. Acta. 2011;1814(9):1210–1218. doi: 10.1016/j.bbapap.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A., Turecki G. Genome wide gene expression studies in mood disorders. OMICS. 2006;10(4):444–454. doi: 10.1089/omi.2006.10.444. [DOI] [PubMed] [Google Scholar]
- Serebryany E., Zhu G.A., Yan E.C. Artificial membrane-like environments for in vitro studies of purified G-protein coupled receptors. Biochim. Biophys. Acta. 2012;1818(2):225–233. doi: 10.1016/j.bbamem.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Sinclair M.L., Wang X.Y., Mattia M., Conti M., Buck J., Wolgemuth D.J., Levin L.R. Specific expression of soluble adenylyl cyclase in male germ cells. Mol. Reprod. Dev. 2000;56(1):6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Smrcka A.V., Sternweis P.C. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C beta by G protein alpha and beta gamma subunits. J. Biol. Chem. 1993;268(13):9667–9674. [PubMed] [Google Scholar]
- Solberg Y., Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. Trends Pharmacol. Sci. 1997;18(6):183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F.T., Jackson T.R., Sternweis P.C., Hawkins P.T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Stephens L.R., Eguinoa A., Erdjument-Bromage H., Lui M., Cooke F., Coadwell J., Smrcka A.S., Thelen M., Cadwallader K., Tempst P., Hawkins P.T. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89(1):105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Stessin A.M., Zippin J.H., Kamenetsky M., Hess K.C., Buck J., Levin L.R. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J. Biol. Chem. 2006;281(25):17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Huang J., Xiang Y., Bastepe M., Juppner H., Kobilka B.K., Zhang J.J., Huang X.Y. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007;26(1):53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Uchikado H., Caprotti D., Weidenheim K.M., Dickson D.W., Ksiezak-Reding H., Pasinetti G.M. Identification of G-protein coupled receptor kinase 2 in paired helical filaments and neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 2006;65(12):1157–1169. doi: 10.1097/01.jnen.0000248542.82681.12. [DOI] [PubMed] [Google Scholar]
- Taneja M., Salim S., Saha K., Happe H.K., Qutna N., Petty F., Bylund D.B., Eikenburg D.C. Differential effects of inescapable stress on locus coeruleus GRK3, alpha2-adrenoceptor and CRF1 receptor levels in learned helpless and non-helpless rats: a potential link to stress resilience. Behav. Brain Res. 2011;221(1):25–33. doi: 10.1016/j.bbr.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D.M., Yeow R.Y., Schoenau C., Huber J., Tesmer J.J. Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol. Pharmacol. 2011;80(2):294–303. doi: 10.1124/mol.111.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M., Schiffrin E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52(4):639–672. [PubMed] [Google Scholar]
- Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Urban J.D., Clarke W.P., von Zastrow M., Nichols D.E., Kobilka B., Weinstein H., Javitch J.A., Roth B.L., Christopoulos A., Sexton P.M., Miller K.J., Spedding M., Mailman R.B. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Urban J.D., Vargas G.A., von Zastrow M., Mailman R.B. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32(1):67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- Violin J.D., Lefkowitz R.J. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Vollmayr B., Henn F.A. Learned helplessness in the rat: improvements in validity and reliability. Brain Res. Brain Res. Protoc. 2001;8(1):1–7. doi: 10.1016/s1385-299x(01)00067-8. [DOI] [PubMed] [Google Scholar]
- Vroon A., Kavelaars A., Limmroth V., Lombardi M.S., Goebel M.U., Van Dam A.M., Caron M.G., Schedlowski M., Heijnen C.J. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Immunol. 2005;174(7):4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Mihlbachler K.A., Brunnett A.C., Liggett S.B. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway beta2-adrenergic receptor physiologic signaling. Proc. Natl. Acad. Sci. USA. 2009;106(35):15007–15012. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T.R., Ferris D.J., Tilson H.A., Mundy W.R. Correlation of the anticholinesterase activity of a series of organophosphates with their ability to compete with agonist binding to muscarinic receptors. Toxicol. Appl. Pharmacol. 1993;122(2):300–307. doi: 10.1006/taap.1993.1199. [DOI] [PubMed] [Google Scholar]
- Werry T.D., Loiacono R., Sexton P.M., Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol. Ther. 2008;119(1):7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Whalen E.J., Rajagopal S., Lefkowitz R.J. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol. Med. 2011;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M., Tritschler H., Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by oxidized and reduced lipoic acid. FEBS Lett. 1996;379(1):74–76. doi: 10.1016/0014-5793(95)01489-6. [DOI] [PubMed] [Google Scholar]
- Wieland T., Lutz S., Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr. Opin. Pharmacol. 2007;7(2):201–207. doi: 10.1016/j.coph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Wilden U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry. 1995;34(4):1446–1454. doi: 10.1021/bi00004a040. [DOI] [PubMed] [Google Scholar]
- Willemen H.L., Eijkelkamp N., Wang H., Dantzer R., Dorn G.W., 2nd, Kelley K.W., Heijnen C.J., Kavelaars A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150(3):550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler J.W., DeWire S.M., Whalen E.J., Violin J.D., Drake M.T., Ahn S., Shenoy S.K., Lefkowitz R.J. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. USA. 2007;104(42):16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehler A., Ponimaskin E.G. G protein–mediated signaling: same receptor, multiple effectors. Curr. Mol. Pharmacol. 2009;2(3):237–248. doi: 10.2174/1874467210902030237. [DOI] [PubMed] [Google Scholar]
- Wolz P., Krieglstein J. Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology. 1996;35(3):369–375. doi: 10.1016/0028-3908(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Xiao K., McClatchy D.B., Shukla A.K., Zhao Y., Chen M., Shenoy S.K., Yates J.R., 3rd, Lefkowitz R.J. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA. 2007;104(29):12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Sun J., Kim J., Rajagopal S., Zhai B., Villen J., Haas W., Kovacs J.J., Shukla A.K., Hara M.R., Hernandez M., Lachmann A., Zhao S., Lin Y., Cheng Y., Mizuno K., Ma’ayan A., Gygi S.P., Lefkowitz R.J. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc. Natl. Acad. Sci. USA. 2010;107(34):15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Samoriski G.M., McLaughlin J.P., Romoser V.A., Smrcka A., Hinkle P.M., Bidlack J.M., Gross R.A., Jiang H., Wu D. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proc. Natl. Acad. Sci. USA. 1999;96(18):10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Fan P., Jiang Z., Mailliard W.S., Gordon A.S., Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc. Natl. Acad. Sci. USA. 2003;100(24):14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.J., Mehdi A., Stohl L.L., Levin L.R., Buck J., Wagner J.A., Stessin A.M. “Soluble” adenylyl cyclase-generated cyclic adenosine monophosphate promotes fast migration in PC12 cells. J. Neurosci. Res. 2008;86(1):118–124. doi: 10.1002/jnr.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Nalbant P., Hoshino M., Dong X., Wu D., Bokoch G.M. Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J. Leukoc. Biol. 2007;81(4):1127–1136. doi: 10.1189/jlb.0406251. [DOI] [PubMed] [Google Scholar]
- Zheng H., Loh H.H., Law P.Y. Agonist-selective signaling of G protein-coupled receptor: mechanisms and implications. IUBMB Life. 2010;62(2):112–119. doi: 10.1002/iub.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Barrett T.B., Kelsoe J.R. Promoter variant in the GRK3 gene associated with bipolar disorder alters gene expression. Biol. Psychiatry. 2008;64(2):104–110. doi: 10.1016/j.biopsych.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin J.H., Chen Y., Nahirney P., Kamenetsky M., Wuttke M.S., Fischman D.A., Levin L.R., Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17(1):82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- Zippin J.H., Farrell J., Huron D., Kamenetsky M., Hess K.C., Fischman D.A., Levin L.R., Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell. Biol. 2004;164(4):527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]



