Abstract
Adherens junctions (AJs) and tight junctions (TJs) are crucial regulators of the integrity and restitution of the intestinal epithelial barrier. The structure and function of epithelial junctions depend on their association with the cortical actin cytoskeleton that, in polarized epithelial cells, is represented by a prominent perijunctional actomyosin belt. The assembly and stability of the perijunctional cytoskeleton is controlled by constant turnover (disassembly and reassembly) of actin filaments. Actin-interacting protein (Aip) 1 is an emerging regulator of the actin cytoskeleton, playing a critical role in filament disassembly. In this study, we examined the roles of Aip1 in regulating the structure and remodeling of AJs and TJs in human intestinal epithelium. Aip1 was enriched at apical junctions in polarized human intestinal epithelial cells and normal mouse colonic mucosa. Knockdown of Aip1 by RNA interference increased the paracellular permeability of epithelial cell monolayers, decreased recruitment of AJ/TJ proteins to steady-state intercellular contacts, and attenuated junctional reassembly in a calcium-switch model. The observed defects of AJ/TJ structure and functions were accompanied by abnormal organization and dynamics of the perijunctional F-actin cytoskeleton. Moreover, loss of Aip1 impaired the apico-basal polarity of intestinal epithelial cell monolayers and inhibited formation of polarized epithelial cysts in 3-D Matrigel. Our findings demonstrate a previously unanticipated role of Aip1 in regulating the structure and remodeling of intestinal epithelial junctions and early steps of epithelial morphogenesis.
Keywords: actin cytoskeleton, actin turnover, adherens junctions, epithelial barrier, tight junctions
the assembly of intercellular junctions is a key event in epithelial morphogenesis. Junctional complexes regulate tissue integrity and transduction of forces and signals and coordinate cellular behavior within epithelial layers (15, 51). Furthermore, junctions are critical for establishing the paracelluar barrier and the acquisition of apico-basal cell polarity (53). In mammalian intestinal epithelium, the most apically located tight junctions (TJs) and adjacent adherens junctions (AJs) are major determinants of intercellular adhesions and barrier permeability. AJs mediate initial intercellular contacts driven by transmembrane adhesive proteins such as E-cadherin and nectins (22, 36, 58). TJs seal the paracellular space and separate the apical and basolateral plasma membrane domains by forming claudin-based, membrane-embedded fibrils that associate with other integral and cytoplasmic proteins (28, 34, 61).
Interaction with the cortical actin cytoskeleton represents a crucial mechanism that regulates the integrity and remodeling of epithelial junctions (10, 18, 56, 69). One of the most recognizable cytoskeletal structures in polarized epithelial cells is the circumferential F-actin belt associated with a cytosolic AJ plaque (16). Furthermore, TJs interact with a complex network of F-actin bundles assembled at the cell apex (35). A number of studies have demonstrated that pharmacological or genetic disruption of the actin cytoskeleton results in AJ/TJ disassembly and the disruption of epithelial barriers (19, 21, 26, 27, 54, 62, 63). Interestingly, the biochemical properties of affiliated actin filaments display significant effects on the dynamics of junctional complexes. For example, TJs appear to be associated with stable filaments composed of γ-cytoplasmic actin, whereas AJs selectively interact with more dynamic β-cytoplasmic actin-based filaments (3). Overall, these data suggest that the organization and dynamics of the cortical actin cytoskeleton are of paramount importance for maintaining the architecture and function of epithelial junctions.
Actin filaments are subject to elaborate regulation involving a large number of accessory, signaling, and motor proteins (12, 46). Given the intimate dependence of epithelial junctions on the underlying cytoskeleton, this imposes additional regulatory mechanisms on TJs and AJs. The turnover of actin filaments, and their interactions with nonmuscle myosin II (NM II), represents two major mechanisms that control the architecture and dynamics of the actin cytoskeleton. NM II acts as a molecular motor driving actin filament contractility and as a lateral filament cross-linker that mediates the assembly of actomyosin cables (64). The turnover of filamentous (F) actin mediates polarized filament growth and movement, which occurs by the addition of monomeric (G) actin to one (barbed) filament end and its subsequent removal from the opposite (pointed) end (49, 55). NM II is a well-established regulator of intestinal epithelial junctions that stabilizes a steady-state barrier and mediates remodeling (disassembly of reassembly) of AJs and TJs (19, 21, 30, 54). By contrast, the role of F-actin turnover in junctional regulation remains less understood.
In eukaryotic cells, F-actin dynamics are markedly accelerated by different actin-binding proteins that selectively control either polymerization or depolymerization processes. The aforementioned polymerization step is regulated by the actin-related protein (Arp) 2/3 complex and formins, driving the assembly of branched and linear actin filaments, respectively (8, 37, 68). F-actin depolymerization is mediated by several proteins, most notably by members of the actin-depolymerizing factor (ADF)/cofilin family (2, 46) and glial-maturating factors (GMF) (50, 67). A number of pharmacological and genetic studies have established a critical role of F-actin polymerization in the assembly of AJs and TJs (21, 27, 62, 63). Both Arp2/3 complex and formins have been implicated in the regulation of junctional structure and functions (21, 26, 27, 31, 66). In contrast, little is known about how depolymerization of actin filaments could regulate the structure, permeability, and remodeling of epithelial junctions. Cofilin has been shown to be an essential downstream component of the molecular cascade mediating Par-3-dependent TJ assembly (7, 65). However, the mechanisms that control the recruitment and activity of actin-depolymerizing proteins at epithelial junctions are poorly understood.
Actin-interacting protein (Aip) 1 (also known as WDR1) is an emerging regulator of F-actin dynamics in eukaryotic cells. Aip1 is a major cofactor of ADF/cofilin proteins, dramatically enhancing filament disassembly (38, 40, 45). Moreover, a recent study implicated Aip1 in assisting GMF-dependent F-actin depolymerization in Drosophilia cells (50). A growing body of evidence highlights Aip1 as an essential regulator of various actin-dependent processes in living cells, including cytokinesis, cell migration, and muscle contractility (24, 32, 38, 47, 70). Interestingly, Aip1 localizes at areas of cell-cell contact in Xenopus embryos (44) and was recently implicated in the regulation of AJ remodeling in Drosophila eye epithelium (9). Nothing is known regarding the involvement of Aip1 in the maintenance and remodeling of apical junctions in mammalian epithelia. This study provides the first evidence that Aip1 controls permeability of intestinal epithelial barrier and regulates AJ and TJ assembly in vitro.
MATERIALS AND METHODS
Antibodies and other reagents.
The following primary polyclonal (pAb) and monoclonal (mAb) antibodies were used to detect AJ/TJ and cytoskeletal proteins: anti-E-cadherin mAb (BD Biosciences, San Jose, CA); anti-zonula occludens (ZO)-1, junctional adhesion molecule (JAM)-A, and occludin pAbs (Invitrogen, Carlsbad, CA); anti-β-catenin and ADF pAbs (Sigma-Aldrich, St. Louis, MO); anti-α-catenin and EBP50 pAbs (Abcam, Cambridge, MA); anti-total actin (MAB1501) mAb and anti-Par-3 pAb (EMD-Millipore, Billerica, MA); anti-NM IIB pAb (Covance, Princeton, NJ); anti-cofilin, phospho-cofilin, poly(ADP-ribose) polymerase (PARP), caspase-3, and GAPDH pAbs (Cell Signaling, Beverly, MA); anti-PKC-ζ pAb (Santa Cruz Biotechnology, Dallas, TX), anti-PKC-ι pAb (Abgent, San Diego, CA); anti-Par-6 pAb (Bioss, Woburn, MA); and anti-phospho PKC-ζ (T410 and T560) pAbs (Assay Biotech, Sunnyvale, CA). Anti-Aip1 rat mAb (32) and anti-JAM-A mouse mAb (33) were provided by Drs. Junying Yuan (Harvard Medical School) and Charles Parkos (University of Michigan), respectively. Alexa Fluor-488- and Alexa Fluor-555-conjugated donkey anti-mouse, anti-rabbit, and anti-rat secondary antibodies and Alexa Fluor-555 phalloidin were obtained from Invitrogen. Horseradish peroxidase conjugate, goat anti-mouse, anti-rabbit, and anti-rat antibodies were purchased from Bio-Rad Laboratories (Hercules, CA) and Invitrogen. Lantrunculin B was obtained from EMD-Millipore. All other chemicals were obtained from Sigma-Aldrich.
Cell culture and calcium depletion.
SK-CO15 human colonic epithelial cells (29) were provided by Dr. Enrique Rodriguez-Boulan (Cornell University). Caco-2 and T84 cells were purchased from American Type Culture Collection (Manassas, VA). SK-CO15 and Caco-2 cells were cultured in high-glucose DMEM medium, supplemented with 10% FBS, HEPES, nonessential amino acids, and antibiotics. T84 cells were cultured in a 1:1 mixture of DMEM and Ham's F-12 medium supplements, as previously described (21). For immunolabeling experiments, epithelial cells were grown on either collagen-coated permeable polycarbonate filters (0.4-μm pore size; Costar, Cambridge, MA) or on collagen-coated coverslips. For biochemical experiments, cells were cultured on six-well plates.
To deplete extracellular calcium, SK-CO15 cell monolayers were washed twice with Eagle's Minimum Essential Medium for suspension culture, supplemented with 5 μM CaCl2, 10 mM HEPES, 14 mM NaHCO3, and 10% dialyzed FBS (designated here as S-MEM), and incubated overnight in S-MEM at 37°C. To induce junctional reassembly, the cells were returned to normal cell culture medium for the times indicated.
RNA interference.
Small-interference RNA (siRNA)-mediated knockdown of Aip1 was carried out using individual siRNA duplexes obtained either from Dharmacon-Thermo Scientific (Waltham, MA) or Qiagen (Venlo, Limburg), as previously described (41, 42). The following sequences were selected to target human Aip1: Dharmacon siRNA duplex 1, GGAAAGUGCGUCAUCCUAA and duplex 2, GGUGGGAUUUACGCAAUUA; and Qiagen siRNA duplex 3, AAAGTGCGTCATCCTAAGGAA and duplex 4, CTCCCTGTCCGGGTACATCAA. Dharmacon and Qiagen scrambled siRNAs, lacking complementarity to any human gene, were used as controls. Cells were transfected using DharmaFECT 1 reagent in Opti-MEM I medium (Invitrogen), according to the manufacturer's protocol, with a final siRNA concentration of 50 nM. Cells were utilized for experiments on days 3 and 4 posttransfection.
Epithelial barrier permeability measurements.
Transepithelial electrical resistance (TEER) was measured using an EVOMX voltohmmeter (World Precision Instruments, Sarasota, FL). The resistance of cell-free collagen-coated filters was subtracted from each experimental point. Dextran flux assay was performed, as previously described (3, 41). Briefly, on day 4 after siRNA transfection, SK-CO15 cell monolayers growing on Transwell filters were exposed to FITC dextran 4,000 Da or 40,000 Da [1 mg/ml in HEPES-buffered Hanks balanced salt solution (HBSS)] added to the upper chamber, whereas HBSS only was added to the lower chamber. After 60 min of incubation, HBSS samples were collected from the lower chamber, and FITC fluorescence intensity was measured using a Victor3 V plate reader (Perkin Elmer, Waltham, MA) with excitation and emission wavelengths at 485 and 544 nm, respectively. Relative fluorescence intensity was calculated using Prism 5 software (GraphPad, La Jolla, CA).
SDS gel electrophoresis and immunoblotting.
Cells were homogenized in RIPA buffer [20 mM Tris, 50 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% sodium deoxycholate, 1% Triton X-100 (TX-100), and 0.1% SDS, pH 7.4] containing protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). Protein concentration of total lysates was determined using a BCA protein assay kit. Samples were diluted with 2× SDS sample buffer and boiled. SDS-PAGE electrophoresis and immunoblotting were performed using a standard protocol with equal amounts of total protein (10 μg) loaded per lane and transferred onto nitrocellulose membrane. Results shown are representative immunoblots of at least three independent experiments. Protein expression was quantified by densitometry, and signal intensities were calculated using Image J software (National Institutes of Health, Bethesda, MD). Data are presented as normalized values, assuming that the expression levels of control siRNA-treated groups are 100%. Statistical analysis was performed with row densitometric data using Microsoft Excel (Microsoft, Redmond, WA).
G/F-actin fractionation.
Quantification of G- and F-actin was performed by TX-100 fractionation of cellular actin, as previously described (11). In brief, epithelial monolayers were washed with HBSS, and G-actin was extracted by gentle shaking for 7 min at room temperature with cytoskeleton stabilization buffer (10 mM MES, 140 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 280 mM sucrose, pH 6.1) supplemented with 0.5% TX-100, proteinase inhibitor cocktail, and 1 μg/ml phalloidin to prevent filament disassembly. The TX-100-soluble G-actin fraction was mixed with an equal volume of 2× SDS sample buffer and boiled. Cells were then briefly washed with HBSS, and the TX-100-insoluble F-actin fraction was collected by scraping cells in two volumes of SDS sample buffer, with subsequent homogenizing and boiling. The amount of actin in each fraction was determined by gel electrophoresis and immunoblotting, as described above.
Immunofluorescence labeling and confocal microscopy.
To label AJ/TJ and cytoskeletal proteins, epithelial cells were fixed with 100% ethanol for 20 min at −20°C. For β-actin staining, cells were fixed with prewarmed 1% paraformaldehyde in DMEM for 30 min, followed by 5-min permeabilization with 100% methanol at −20°C (13). Fixed cell monolayers were washed with HBSS, blocked with 1% bovine serum albumin in HBSS (blocking buffer) for 60 min at room temperature, and incubated for another 60 min with primary antibodies diluted in blocking buffer. Cells were then washed, incubated for 60 min with Alexa Fluor-conjugated secondary antibodies, rinsed with blocking buffer, and mounted on slides with ProLong Gold Antifade Reagent (Invitrogen). F-actin was labeled with Alexa-555 phalloidin. Fluorescently labeled cell monolayers were examined using a Zeiss LSM700 laser-scanning confocal microscope (Zeiss Microimaging, Thornwood, NY). The Alexa Fluor 488 and 555 signals were imaged sequentially, in frame-interlaced mode, to eliminate cross-talk between channels. The images were processed using Zen 2011 software and Adobe Photoshop. Signal intensity was measured using Image J software after selecting a rectangular area of cellular border signal. Images shown are representative of at least three experiments, with multiple images taken per slide. To quantify the junctional length per microscopic field, six to seven fields with similar cell density were randomly selected from each coverslip, and the total junctional length in pixels of each field was measured using Image J.
G-actin incorporation assay.
The incorporation of Alexa 488-labeled G-actin (Invitrogen) into saponin-permeabilized cells has been described previously (27, 63). In short, SK-CO15 cells were equilibrated at 25°C for 10 min and then lightly permeabilized with 0.2 mg/ml of saponin in permeabilization buffer (138 mM KCl, 4 mM MgCl2, and 20 mM HEPES, pH 7.4). Alexa-488-labeled G-actin (0.45 mM) in permeabilization buffer was added to the cells and incubated for 7 min at 25°C to allow barbed-end incorporation. The cells were fixed on ice in 4% paraformaldehyde in cytoskeleton stabilization buffer containing 0.2% TX-100 and labeled with Alexa-555 phalloidin for 1 h. Quantification analysis of fluorescence intensity at epithelial junctions was performed using Image J software after selecting a rectangular area of cellular border signal.
Wound-healing assay.
SK-CO15 cells transfected with either control or Aip1-specific siRNAs were grown to confluence. On day 3 posttransfection, cells were mechanically wounded by dragging a 200-μl pipette tip across the cell monolayer. The cells were washed twice with complete media, and images were acquired at 0 and 24 h postwounding, using an inverted bright-field microscope equipped with a camera. The relative surface area traveled by the leading edge was calculated using TScratch software (14). Three independent experiments were performed in duplicate.
Epithelial cyst formation in Matrigel.
Three-dimensional epithelial cyst assay was performed as described in detail previously (3, 20). Briefly, Caco-2 cells were transfected with either control or Aip1-specific siRNA. On the next day, cells were trypsinized, resuspended in DMEM, and mixed with a growth factor-reduced Matrigel (BD Biosciences). Matrigel-embedded cells were plated in eight-chamber glass slides (BD Biosciences). Cysts were allowed to form for 72 h at 37°C, with 1 μM forskolin to enhance cyst lumen formation. Cysts were fixed in 4% paraformaldehyde, permeabilized with 0.5% TX-100, and stained using a standard protocol, except that blocking, primary, and secondary antibody incubation steps were performed for 2 h each, as opposed to 1 h, and all washing steps were performed for 30 min. For quantitative analysis, cyst images were acquired at low resolution, and total numbers of cysts and the number of cysts with single lumen were counted manually.
Statistics.
Numerical values from individual experiments were pooled and expressed as means ± SE throughout. Obtained numbers were compared by two-tailed Student's t-test, with statistical significance assumed at P < 0.05.
RESULTS
Aip1 is localized at epithelial junctions in vitro and in vivo.
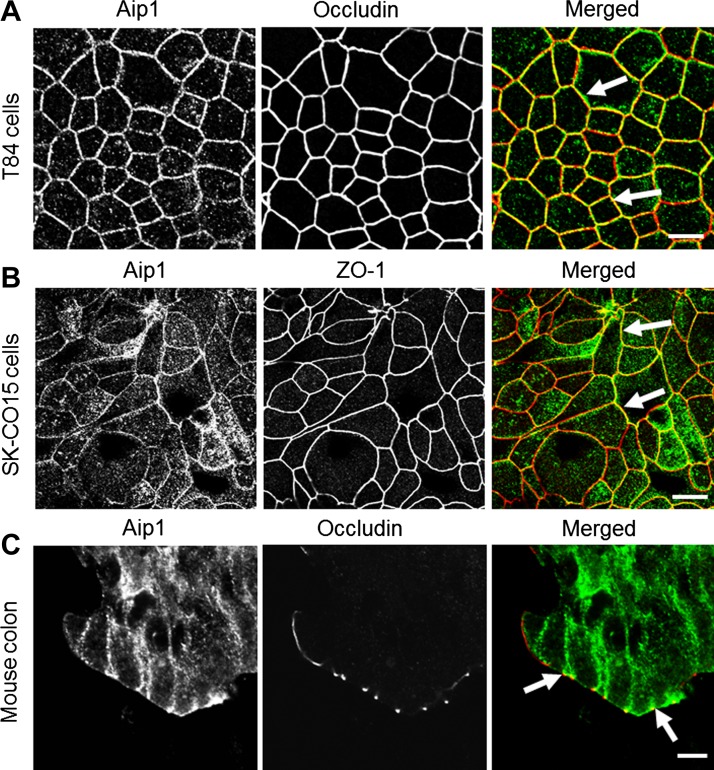
To control remodeling of the junction-associated actin cytoskeleton, Aip1 should be present at epithelial junctions. Therefore, we first sought to determine the localization of Aip1 in polarized intestinal epithelial cell monolayers, in vitro, and intestinal mucosa, in vivo. Figure 1 shows that in well-differentiated T84 and SK-CO15 human colonic epithelial cells, a significant fraction of Aip1 colocalizes with TJ proteins occludin and ZO-1 (arrows). Extracellular calcium depletion that triggers AJ/TJ disassembly resulted in Aip1 translocation from areas of cell-cell contact into intracellular compartments (data not shown). We also compared Aip1 localization in epithelial MCF7 and mesenchymal MDA-MB 435 breast cancer cell lines. In MCF7 cells, which form robust AJs, Aip1 accumulated at cell-cell contacts. In MDA-MB 435 cells, which form poor attachments, Aip1 was diffusely distributed in the cytoplasm and nucleus (data not shown). Moreover, in normal mouse colonic mucosa, a significant pool of Aip1 localized at the lateral plasma membrane, especially enriched at the apical junctional complex (Fig. 1C, arrows). Collectively, this data provides the first evidence that Aip1 is associated with apical junctions in mammalian epithelial cells in vitro and in vivo.
Fig. 1.
Actin-interacting protein (Aip) 1 accumulates at apical junctions in model colonic epithelial cell monolayers and mouse colonic mucosa. Polarized T84 (A) and SK-CO15 (B) human colonic epithelial cell monolayers and sections of normal mouse colon (C) were subjected to dual immunolabeling for Aip1 (green) and tight junction (TJ) proteins, occludin, and zonnula occludens (ZO)-1 (red). Arrows demonstrate colocalization of Aip1 with TJ proteins in vitro and in vivo. Scale bar = 20 μm.
Downregulation of Aip1 expression increased paracellular permeability and attenuated junctional recruitment of AJ and TJ proteins.
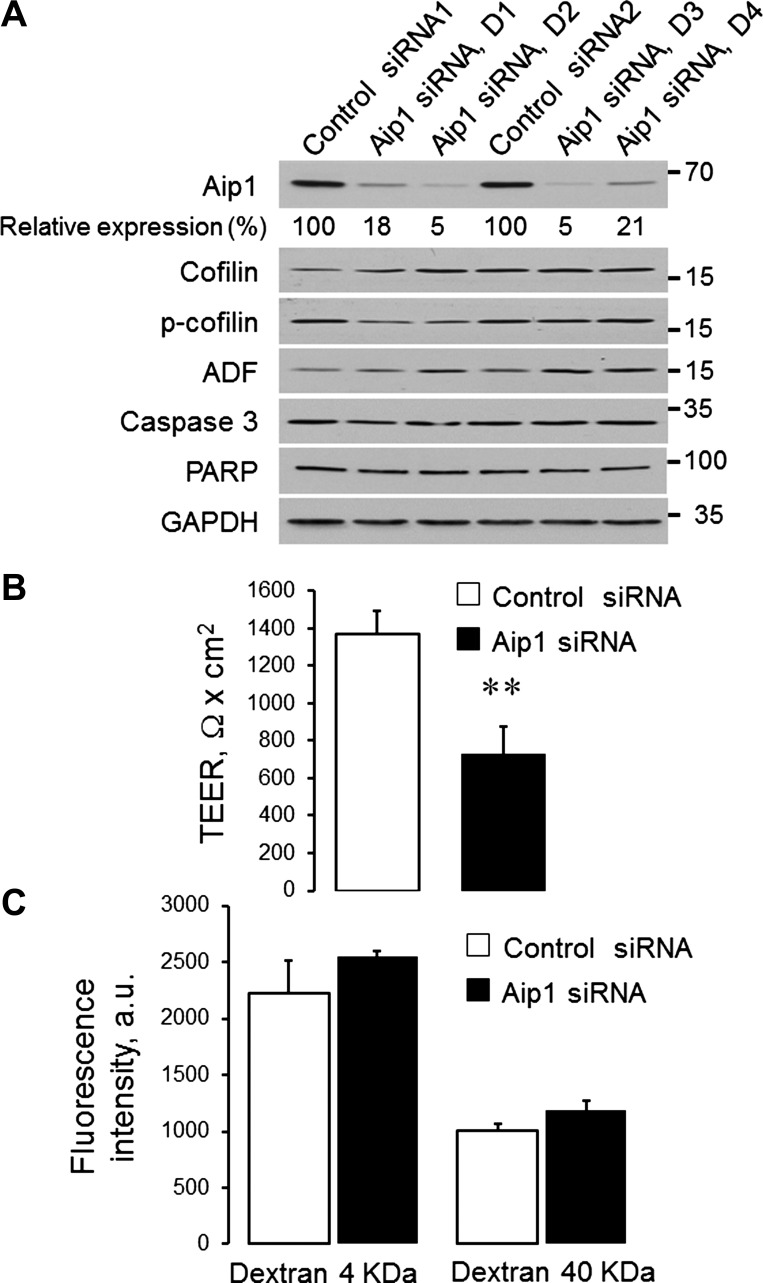
We next sought to investigate the effects of Aip1 depletion on the structure and permeability of epithelial junctions. For these experiments, we used SK-CO15 cells, which assemble robust AJs and TJs and are easily transfectable with siRNAs (3, 29, 42, 43). To minimize possible off-target effects, we used four different Aip1 siRNAs and their appropriate controls from two commercial sources (Dharmacon and Qiagen). These siRNAs efficiently (up to 95%) decreased Aip1 expression in SK-CO15 cells without affecting the levels of total and phosphorylated ADF and cofilin-1 (Fig. 2A). Importantly, the loss of Aip1 did not result in cell apoptosis, based on the lack of PARP and caspase 3 cleavage (Fig. 2A). All subsequent figures show data obtained using the most efficient Aip1 siRNA, duplex 2. However, similar results were obtained using an alternative Aip1 siRNA, duplex 3 (data not shown).
Fig. 2.
Downregulation of Aip1 expression increases paracellular permeability of colonic epithelial cell monolayers. SK-CO15 epithelial cells were transfected with different Aip1-specific siRNA duplexes and corresponding control siRNAs. On day 4 posttransfection, the efficiency of siRNA knockdown as well as the expression of Aip1-interacting cytoskeletal regulators and apoptosis markers was examined by electrophoresis and immunoblotting (A). ADF, actin-depolymerizing factor; PARP, poly(ADP-ribose) polymerase. Permeability of control and Aip1-depleted SK-CO15 cells was examined by measuring transepithelial electrical resistance (TEER) (B) and transepithelial flux of fluoresceinated dextrans (C). Data are presented as means ± SE (n = 3); **P < 0.005.
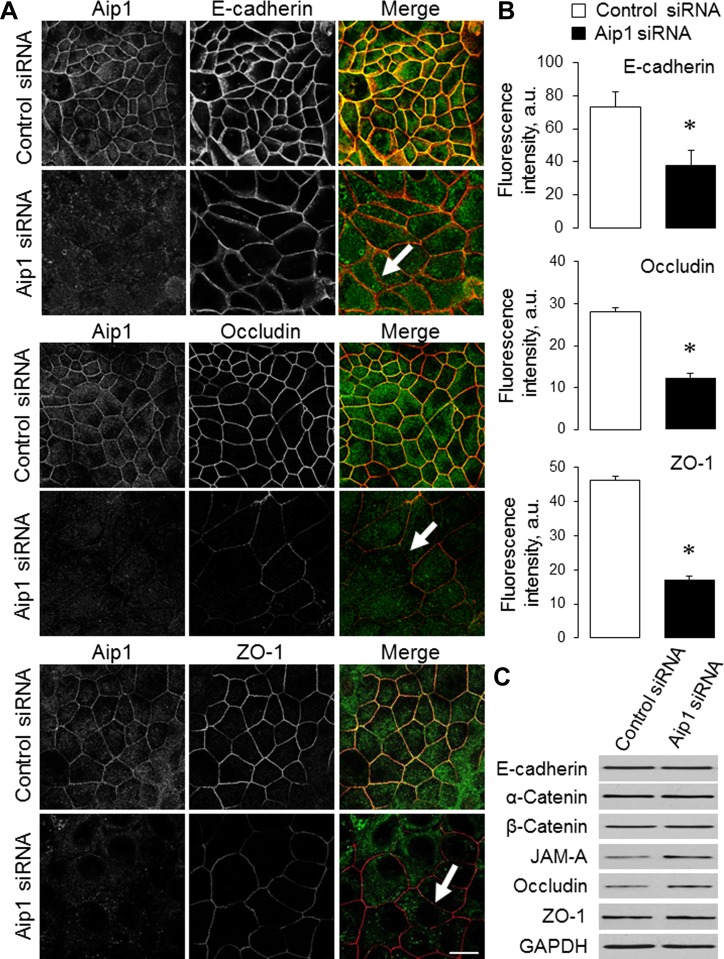
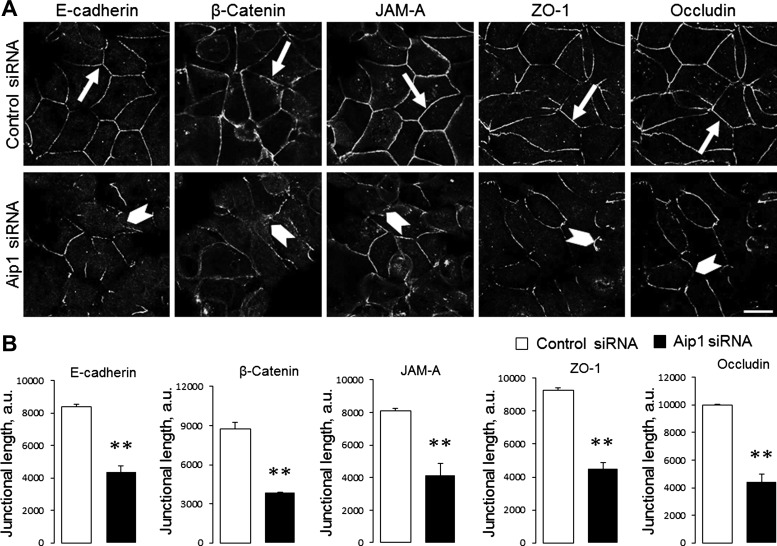
To elucidate the role of Aip1 in the regulation of paracellular permeability, TEER and transepithelial flux of FITC dextrans were measured in SK-CO15 cell monolayers on day 4 after siRNA transfection. Loss of Aip1 resulted in a significant decrease in TEER, thus indicating increased permeability to small ions. Thus TEER values were decreased from 1,367 ± 129 to 730 ± 146 Ω × cm2 (P < 0.005) by the Dharmacon Aip1 siRNA duplex 2 (Fig. 2B) and from 1,282 ± 53 to 870 ± 44 Ω × cm2 (P < 0.005) by the Qiagen Aip1 siRNA duplex 3. In contrast, transepithelial flux of 4-kDa and 40-kDa dextrans was not affected by Aip1 knockdown (Fig. 2C and data not shown), which suggests unchanged permeability to large molecules. We next sought to determine whether Aip1 is essential for the integrity of apical junctions. Depletion of Aip1 did not result in a dramatic disruption of junctional architecture, as evident by the well-preserved, characteristic, “chicken wire” labeling pattern of different AJ/TJ molecules (Fig. 3A). However, the labeling intensity of junctional E-cadherin, occludin, and ZO-1 was significantly lower in Aip1-depleted cells, compared with control cell monolayers (Fig. 3, A, arrows, and B). Because loss of Aip1 did not affect the total levels of key molecular constituents of SK-CO15 cell junctions (Fig. 3C), our data suggest that depletion of this actin regulator specifically attenuates the recruitment of AJ/TJ proteins to areas of cell-cell contact.
Fig. 3.
Downregulation of Aip1 expression attenuates the recruitment of adherens junction (AJ) and TJ proteins to steady-state intercellular contacts. A and B: SK-CO15 cells transfected with either control or Aip1-specific siRNAs were dual immunolabeled for Aip1 (green) and different AJ/TJ proteins (red) on day 4 posttransfection. Representative microscopic images (A) and quantification of fluorescence intensity (B) are shown. Arrows indicate normal general architecture but a decreased labeling intensity of AJ and TJ proteins in Aip1-depleted cells. C: effects of Aip1 knockdown on the expression of different AJ and TJ proteins were examined by immunoblotting. JAM, junctional adhesion molecule. Data are presented as means ± SE (n = 3); *P < 0.05. Scale bar = 20 μm.
Depletion of Aip1 altered the architecture and dynamics of the perijunctional actomyosin belt.
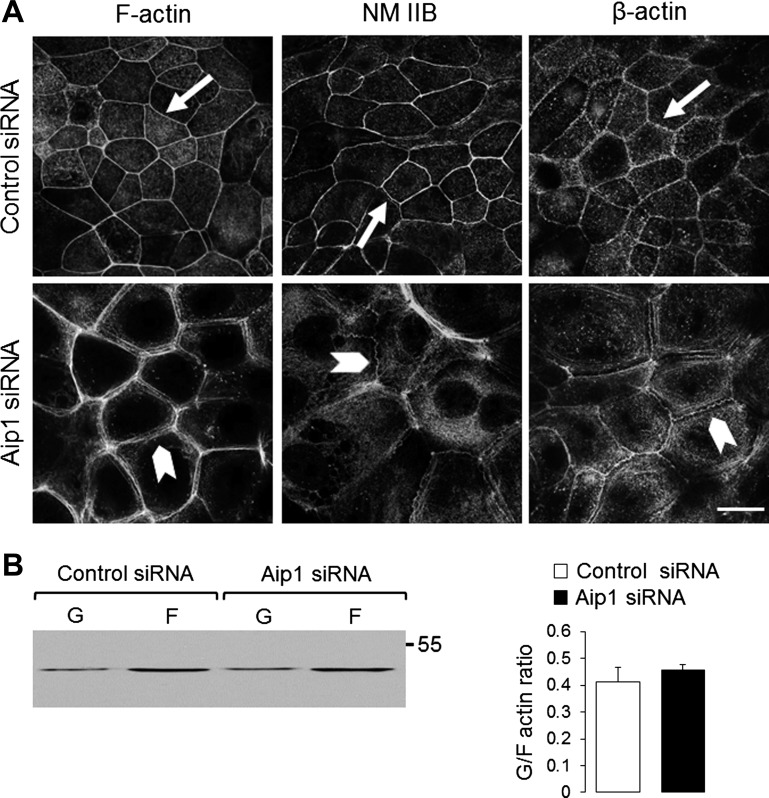
In consideration of the critical roles of the actin cytoskeleton in the formation and maintenance of epithelial barrier, it is logical to suggest that loss of Aip1 increases junctional permeability via cytoskeleton-dependent mechanisms. We tested this hypothesis by examining the organization of the perijunctional F-actin belt in control and Aip1-depleted SK-CO15 cells. The actin cytoskeleton was visualized using three different methods, including labeling of F-actin with fluorescent phalloidin and staining of β-actin and NM II using specific antibodies (Fig. 4A). Showing a smooth and narrow perijunctional actomyosin belt in control SK-CO15 cells (Fig. 4A, arrows), all these techniques yielded similar results. The morphology of this belt was markedly affected by Aip1 knockdown. Indeed, we observed significant “debundling” and loose packaging of perijunctional actin cables in Aip1-depleted SK-CO15 cells (Fig. 4A, arrowheads). Furthermore, these actomyosin cables lost their smooth appearance and became wavy or spiky (Fig. 4A). Interestingly, biochemical fractionation demonstrated that loss of Aip1 did not affect the total G/F-actin ratio (Fig. 4B), thus indicating a lack of global changes in F-actin polymerization.
Fig. 4.
Loss of Aip1 impairs the organization of the perijunctional actomyosin cytoskeleton. A: organization of perijunctional actin cytoskeleton in control and Aip1-depleted SK-CO15 cells was examined by fluorescence labeling. Arrows show smooth and narrow actomyosin cables in control cells. Arrowheads highlight loss of crosslinking and the spiky appearance of these cables, following Aip1 knockdown. Scale bar = 20 μm. NM II, nonmuscle myosin II. B: effect of Aip1 knockdown on the total amount of F-actin was determined by G/F-actin fractionation.
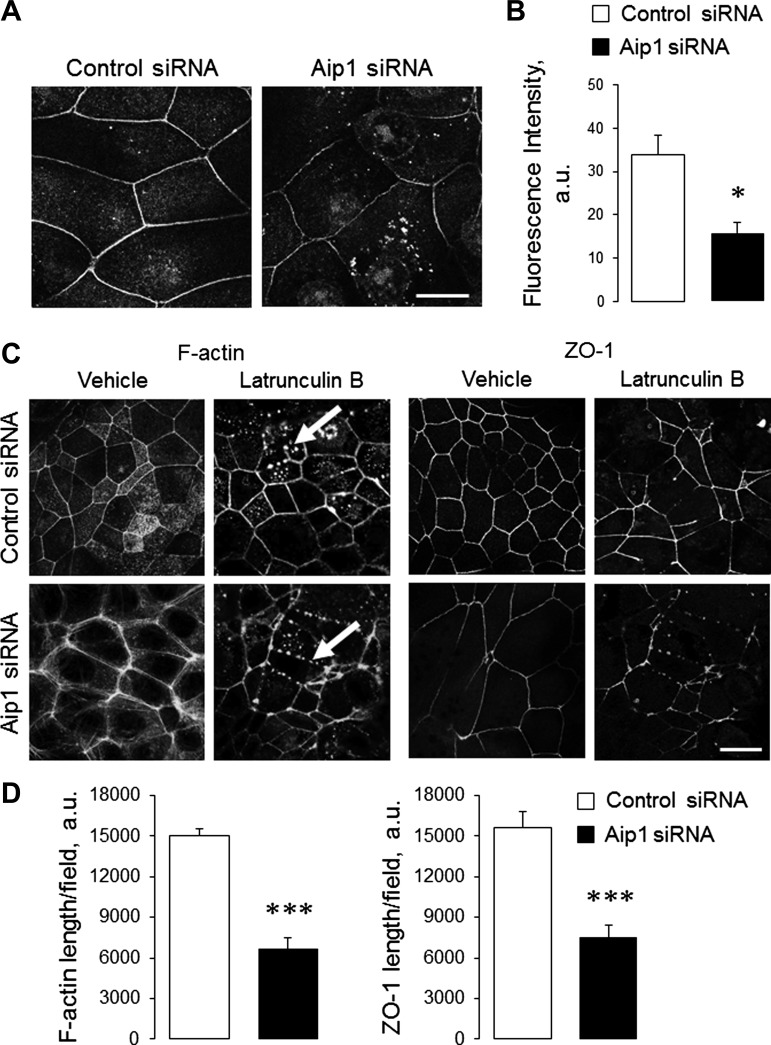
Perijunctional F-actin cables are known to undergo constant turnover, even in steady-state epithelial cell monolayers (27, 30). Consequently, we examined the effect of Aip1 depletion on the dynamics of the junction-associated cytoskeleton. De novo F-actin assembly was examined by measuring incorporation of fluorescently labeled G-actin in live, permeabilized SK-CO15 cells. Exogenously added G-actin was efficiently inserted into the perijunctional F-actin belt of control SK-CO15 cells (Fig. 5, A and B). In contrast, such G-actin incorporation was attenuated after Aip1 knockdown (Fig. 5, A and B). These data suggest a decreased rate of actin polymerization in the junction-associated cables of Aip1-depleted epithelial cells. We also evaluated the F-actin depolymerization rate in these structures by examining their disappearance following G-actin sequestration by Latrunculin B (Lat B) (39). In accordance with previous publications (3, 21), 1 h of Lat B treatment of control SK-CO15 cell monolayers caused focal disassembly of the continuous F-actin belt and its replacement with scattered F-actin puncta (Fig. 5C, arrows). This process was accompanied by fragmentation of AJs and TJs (Fig. 5C, arrows, and data not shown). Surprisingly, Lat B-induced decomposition of perijunctional F-actin and TJ disassembly was accelerated in Aip1-depleted SK-CO15 cells, compared with control cell monolayers (Fig. 5, C and D). These results indicate a decreased stability of perijunctional F-actin cables following Aip1 knockdown.
Fig. 5.
Depletion of Aip1 alters the dynamics of the perijunctional actin cytoskeleton. A and B: control and Aip1-depleted SK-CO15 cell monolayers were subjected to a G-actin incorporation assay to evaluate actin polymerization on day 4 posttransfection. Representative images (A) and quantification of incorporated G-actin intensity (B) are shown. C and D: to measure F-actin stability, the cells were incubated for 2 h with either vehicle or Latrunculin B (Lat B) (2 μM). Cells were fixed and labeled for F-actin and ZO-1. Representative images (C) and quantification of the junctional length (D) are shown. Arrows indicate replacement of the circumferential F-actin belt by F-actin puncta and TJ disassembly in Lat B-treated cells. Data are presented as means ± SE (n = 3); *P < 0.05, ***P < 0.0005. Scale bar = 20 μm.
Loss of Aip1 attenuated junctional reassembly and impaired apico-basal polarization of planar epithelial monolayers and 3-D epithelial cysts.
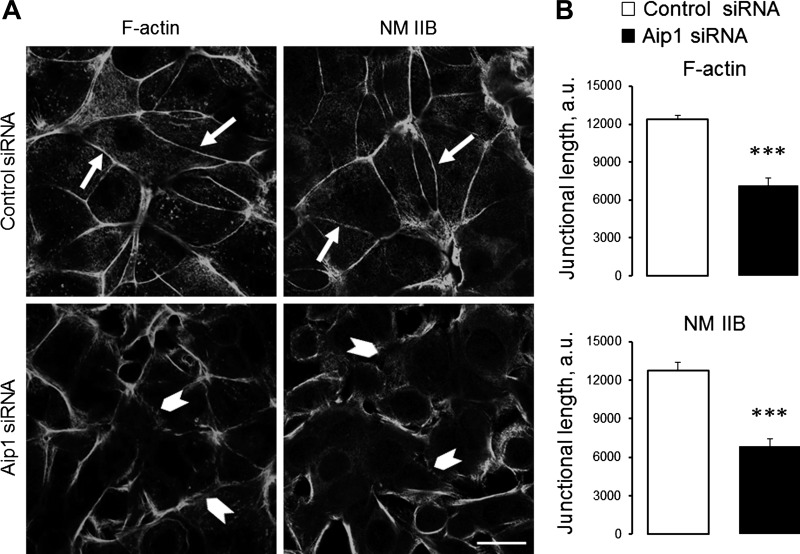
We next sought to elucidate whether Aip1 controls de novo assembly of epithelial junctions by using a classical calcium-switch assay. This assay involves a robust and orchestrated reformation of AJs and TJs in stationary epithelial cells, which allows the distinguishing of the specific effects of actin regulators on epithelial junctions from their effects on cell motility (19, 42, 43). Confluent control and Aip1-depleted epithelial cell monolayers were subjected to overnight extracellular calcium depletion to disassemble existing AJs and TJs. Junctional reassembly was triggered by restoration of normal calcium levels. Control SK-CO15 cells assembled the majority of their E-cadherin and β-catenin-based AJs after 1 h and formed well-defined TJs after 2 h of calcium repletion (Fig. 6A, arrows). Reassembly of both junctional complexes was attenuated in Aip1-depleted cells (Fig. 6, A, arrowheads, and B. Calcium-stimulated AJ/TJ reassembly was accompanied by the restoration of prominent perijunctional actomyosin cables (Fig. 7A, arrows). This process was also attenuated by Aip1 knockdown (Fig. 7, A, arrowheads, and B).
Fig. 6.
Depletion of Aip1 expression attenuates AJ and TJ reassembly. Control and Aip1-depleted SK-CO15 cells were subjected to overnight calcium depletion to disassemble cellular contacts, followed by calcium restoration for 1 and 2 h to induce AJ and TJ reassembly, respectively. Cells were fixed and immunolabeled for different AJ (E-cadherin, β-catenin) and TJ (occludin, JAM-A, ZO-1) proteins. Representative images (A) and quantification of junctional length (B) are shown. Arrows indicate AJ/TJ reassembly in control cells. Arrowheads show attenuation of junctional reassembly caused by Aip1 depletion. Data are presented as means ± SE (n = 3); **P < 0.005. Scale bar = 20 μm.
Fig. 7.
Loss of Aip1 impairs reassembly of the perijunctional actin cytoskeleton. Control and Aip1-depleted SK-CO15 cells were subjected to overnight extracellular calcium depletion followed by restoration of normal calcium for 2 h. Cells were fixed and the perijunctional cytoskeleton was visualized using F-actin and NM IIB labeling. Representative images (A) and image quantification (B) are shown. Arrows indicate reassembly of perijunctional actomyosin cables in control cells. Arrowheads show attenuation of actomyosin reassembly caused by Aip1 depletion. Data are presented as means ± SE (n = 3); ***P < 0.0005. Scale bar = 20 μm.
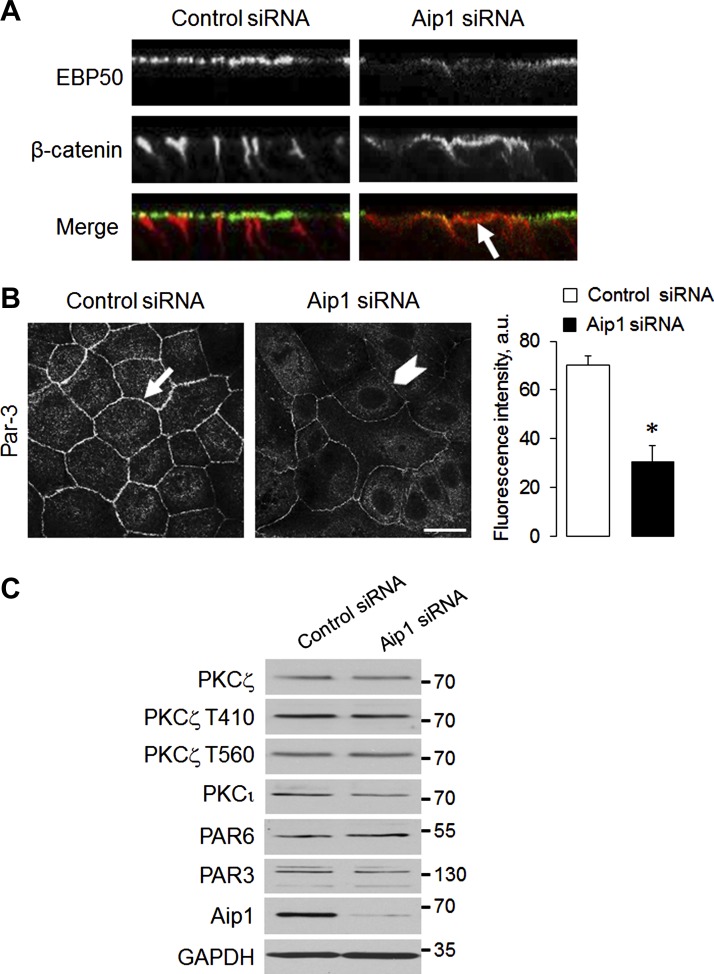
Taking into account the crucial role of the actin cytoskeleton in the formation of polarized epithelial structures, we next sought to determine whether Aip1 can play a role in the establishment of apico-basal polarity in intestinal epithelial cell monolayers and the development of polarized epithelial spheroids in 3-D hydrogel. Control SK-CO15 cell monolayers growing on permeable membrane supports become rapidly polarized, judging by the proper localization of protein markers of the apical (EBP50) and the basolateral (β-catenin) plasma membrane (Fig. 8A). Such polarization was impaired by Aip1 knockdown, which decreased apical localization of EBP50 and caused mistargeting of β-catenin to the apical plasma membrane (Fig. 8A, arrows). Loss of Aip1 also decreased accumulation of Par-3 polarity protein at intercellular junctions (Fig. 8B), without altering the expression or phosphorylation of members of the Par-3-Par-6-atypical PKC polarity complex (Fig. 8C).
Fig. 8.
Downregulation of Aip1 impairs apico-basal cell polarity. A: reconstructed xz confocal images show proper localization of apical plasma membrane marker EBP50 (green) and basolateral membrane marker β-catenin (red) in control SK-CO15 cells. Arrows indicate mistargeting of β-catenin to the apical plasma membrane in Aip1-depleted cells. B: representative immunofluorescence images and densitometric image analysis show junctional localization of Par-3 in control cells (arrows) and decreased recruitment of Par-3 to epithelial junctions following Aip1 depletion (arrowheads). C: immunoblotting analysis shows the expression of molecular constituents of the Par-3-Par-6-atypical PKC polarity complex in control and Aip1-depleted SK-CO15 cells. Data are presented as means ± SE (n = 3); *P < 0.05. Scale bar = 20 μm.
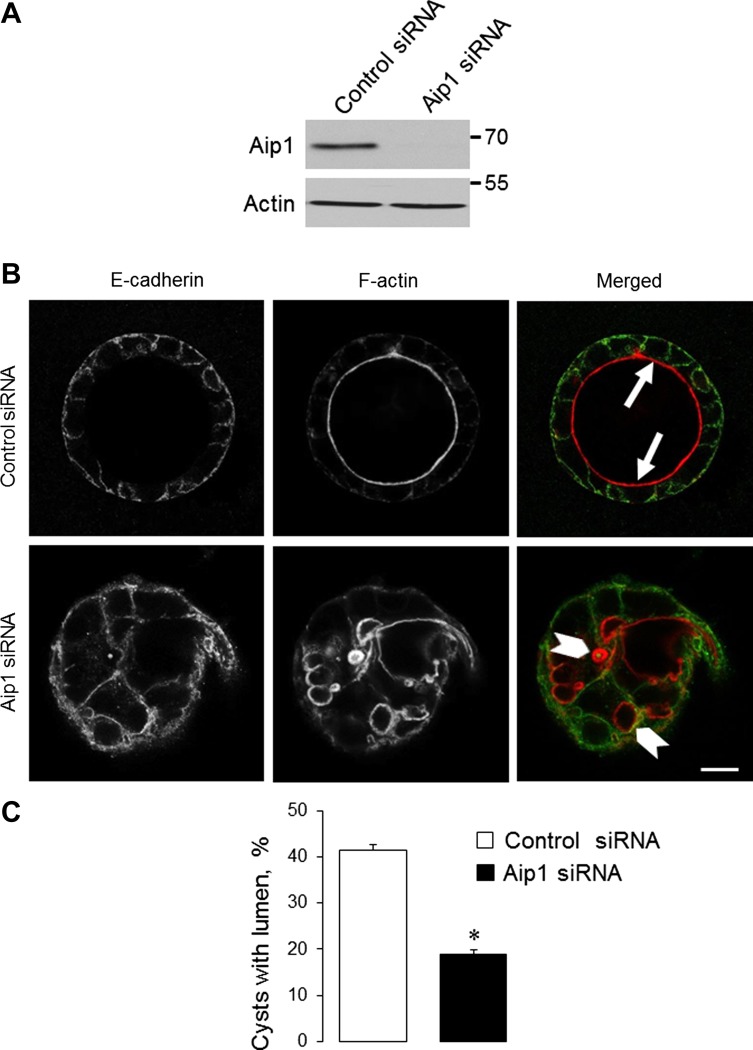
To investigate the effects of Aip1 knockdown on epithelial cyst formation, we used Caco-2 colonic epithelial cells because SK-CO15 cells do not form well-polarized 3-D structures (20). Transfection with Aip1-specific siRNA efficiently decreased the expression of this protein in Caco-2 cells (Fig. 9A). In agreement with our recent study (3), control Caco-2 cells formed spherical cysts with a well-defined central lumen after 3 days of growth in Matrigel (Fig. 9B). The lumen was lined by prominent F-actin bundles (arrows). Aip1 depletion significantly decreased the formation of well-polarized single-lumen epithelial cysts (Fig. 9, B and C). Instead, Aip1-deficient cysts either possessed multiple small lumens (arrowheads) or were devoid of lumen, thus indicating defects in 3-D epithelial morphogenesis.
Fig. 9.
Downregulation of Aip1 attenuates the formation of intestinal epithelial cysts. Caco-2 human colonic epithelial cells were transfected with either control or Aip1-specific siRNAs. A: immunoblotting analysis shows the efficiency of Aip1 knockdown on day 4 posttransfection. B and C: cells grown for 3 days in Matrigel were fixed and labeled for F-actin and E-cadherin. Arrows show the F-actin lining of well-polarized single-lumen cysts formed by control cells. Arrowheads highlight abnormal multilumen cysts formed by Aip1-depleted cells. Data are presented as means ± SE (n = 3); *P < 0.05. Scale bar = 20 μm.
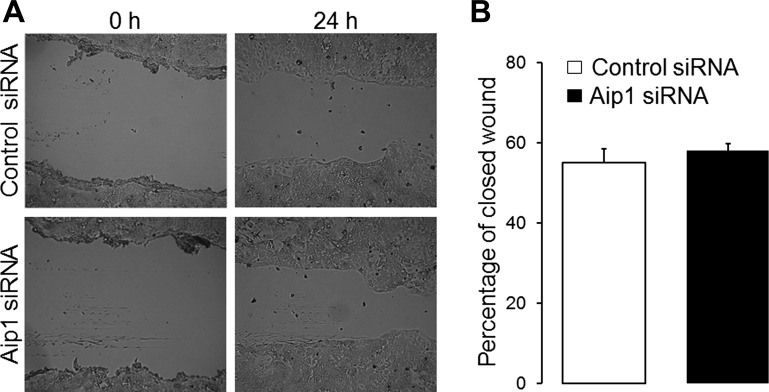
Finally, we sought to determine whether loss of Aip1 selectively affects epithelial junctions and polarity or whether it can also influence other cytoskeletal-based processes, such as cell motility. Planar migration of control and Aip1-depleted epithelial cells was examined using wound-closure assay. We observed that loss of Aip1 did not affect the velocity of wound closure in SK-CO15 (Fig. 10) and Caco-2 cells (data not shown), suggesting that Aip1 is dispensable for intestinal epithelial cell motility.
Fig. 10.
Loss of Aip1 does not affect epithelial cell migration. Control and Aip1-depleted SK-CO15 cell monolayers were mechanically wounded on day 3 posttransfection. The cell-free area was measured at 0 and 24 h after wounding, and the percentage of closed-wound area was calculated as described in the materials and methods. Original bright-field images (A) and quantification of wound closure (B) are shown.
DISCUSSION
The formation and maintenance of epithelial apical junctions depend on their interactions with the cortical actin cytoskeleton and particularly with the circumferential F-actin belt (18, 56). The positioning and architecture of the apical belt are regulated by the interplay between actin filament turnover and NM II-dependent contractility. The prevailing body of evidence indicates that inhibition of F-actin polymerization or myosin activity impairs the integrity and permeability of epithelial junctions (19, 21, 26, 27, 30, 31, 48, 54, 62). The present study demonstrates, for the first time, that F-actin depolymerization is also essential for AJ/TJ assembly and functions in polarized intestinal epithelium. This conclusion is based on the study of epithelial cells depleted of Aip1, an actin-binding protein that acts as a cofactor for ADF/cofilin, and GMF to promote actin filament disassembly. We present the first evidence that Aip1 is abundant at apical junctions in cultured human epithelial cells and mouse colonic mucosa (Fig. 1). Because confocal microscopy does not have sufficient resolution to spatially differentiate AJs from TJs, we do not yet definitively know whether Aip1 interacts with the AJ- or TJ-associated actin cytoskeleton in polarized T84 or SK-CO15 cell monolayers. However, two lines of evidence suggest that Aip1 most likely regulates AJ-associated F-actin cables. First, this protein is enriched at areas of cell-cell contact in MCF7 cells, which do not have functional TJs but form robust AJs. Second, Aip1 is translocated to newly forming junctions at the early stages of calcium repletion, together with the recruitment of AJ proteins (data not shown).
Our data indicate that loss of Aip1 affects different stages of junctional biogenesis. Specifically, it decreases recruitment of AJ/TJ proteins to steady-state intercellular contacts (Fig. 3) and attenuates calcium-induced junctional reassembly (Fig. 6). The described defects of AJs and TJs are associated with abnormal architecture of the perijunctional F-actin cytoskeleton (Figs. 4 and 7). In confluent colonic epithelial cell monolayers, depletion of Aip1 disrupts self-association of actin filaments into thin, cross-linked perijunctional cables (Fig. 4). Furthermore, depletion affects actin turnover within these cables by decreasing barbed-end polymerization under steady-state conditions and accelerating cable disassembly triggered by G-actin sequestration (Fig. 5). Decreased perijunctional actin polymerization in Aip1-depleted epithelial cells most likely indicates inhibition of ADF/cofilin-dependent filament severing. Indeed, ADF and cofilin are known to break existing actin filaments, and this process is greatly accelerated by Aip1 (40, 45, 52). Actin filament severing results in the formation of barbed ends that are predominant sites of G-actin incorporation. Hence, diminished incorporation of G-actin indicates decreased formation of barbed ends in the perijunctional F-actin cables of Aip1-depleted epithelial cells (Fig. 5, A and B).
On the other hand, the observed increase in Lat B-induced disassembly of Aip1-deficient F-actin cables is surprising, considering previous reports of increased F-actin stability following Aip1 inhibition (38, 45, 57). We believe that this paradoxical effect is unique to the perijunctional F-actin belt and reflects structural alterations caused by Aip1 depletion. Indeed, Aip1 knockdown transforms tightly packed and cross-linked apical actin cables into an array of loosely packed and poorly cross-linked filaments (Fig. 4). This transformation is in agreement with the previously reported ability of Aip1 to maintain a high density of closely aligned actin filaments suitable for cross-linking (5). Because actin turnover is known to be suppressed in tightly packed actin bundles (1, 4), loss of filament bundling in Aip1-depleted epithelial cells should lead to their destabilization and increased rate of disassembly. Molecular mechanisms underlying the debundling of perijunctional F-actin cables in Aip1-depleted epithelial cells remain to be investigated. The most plausible mechanism suggests an altered balance in the different modes of F-actin assembly. Indeed, formation of the F-actin belt is known to be regulated by the interplay of Arp2/3 and formin-dependent actin polymerization (10, 18, 56). The Arp2/3 mechanism mediates the initial steps of the process, leading to the formation of branched actin filaments that later become transformed into long linear cables. Although the molecular details of such transformation remain unclear, it most likely involves debranching of the initial F-actin network. We speculate that Aip1 accelerates this filament debranching and promotes the assembly of linear perijunctional F-actin cables. Depletion of Aip1 is likely to trigger excessive assembly of branched actin filaments, thereby impairing side-by-side filament packing and bundling.
How destabilization of apical F-actin cables could impair AJ/TJ structure and function in Aip1-depleted intestinal epithelial cells remains unclear although at least two mechanisms could be considered. Because recent live cell imaging reveals the dynamic equilibrium between apical junctional and lateral plasma membrane pools of AJ/TJ proteins (6, 17, 60, 66), destabilization of AJ/TJ-associated F-actin is likely to shift these proteins toward the lateral membrane. An alternative mechanism may involve removal of steric hindrance to perijunctional vesicle trafficking, leading to increased endocytosis of AJ/TJ proteins (23, 59). Another important consequence of disorganized perijunctional F-actin cables in Aip1-depleted cells is a decrease in their tension/contractility reflected by the loss of the linear morphology of these cytoskeletal structures (48) (Fig. 4). Such diminished myosin-dependent tension can explain the attenuated reassembly of circumferential AJs and TJs following the restoration of extracellular calcium (Fig. 6). This process is driven by perijunctional actomyosin bundles that generate the necessary forces for the extension of initial cell-cell contacts (19, 21, 30, 54, 66).
Our data suggest that Aip1 is essential for intestinal epithelial morphogenesis. This conclusion is based on the profound effects of Aip1 depletion on the establishment of apico-basal polarity in planar epithelial cell monolayers and the formation of polarized epithelial cysts in 3-D matrix (Figs. 8 and 9). Previously demonstrated morphogenic roles of Aip1 are limited to cardiac and skeletal muscle in mice and C. elegans (47, 70) and Drosophilia eye discs (9). Because total knockout of Aip1 leads to either embryonic or early postnatal lethality (25, 70), it would be important to investigate the effects of intestinal epithelial-specific knockout of Aip1 on gut development and function. Interestingly, mutations that decrease Aip1 expression in mice result in a marked autoinflammatory disease and macrothrombocytopenia (25). This finding raises the intriguing possibility that Aip1 dysfunctions could also contribute to the damage and restitution of the epithelial barrier during intestinal inflammation. We have found that decreased expression of Aip1 accompanies AJ/TJ disassembly in HT-29 colonic epithelial cells exposed to a combination of tumor necrosis factor-α and interferon-γ (data not shown). Therefore, it is tempting to speculate that loss of Aip1 expression in inflamed intestinal mucosa accelerates disruption and/or attenuates restitution of the epithelial barrier. The functional roles of Aip1 in the intestinal epithelium appear to be limited to apical junctions and do not involve other actin-dependent processes such as cell motility (Fig. 10). This contradicts the reported involvement of Aip1 in leukocyte migration (24, 25) and suggests tissue-specific functions of this cytoskeletal regulator.
GRANTS
Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from the NIH-NINDS Center Core Grant 5P30NS047463. This work was supported by National Institute of Health grants RO1 DK083968 and R01 DK084953 to A. Ivanov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.L., S.B., and A.I.I. conception and design of research; S.M.L., S.B., and A.I.I. performed experiments; S.M.L., S.B., and A.I.I. analyzed data; S.M.L., S.B., and A.I.I. interpreted results of experiments; S.M.L., S.B., and A.I.I. prepared figures; S.M.L., S.B., and A.I.I. drafted manuscript; S.M.L., S.B., and A.I.I. edited and revised manuscript; S.M.L., S.B., and A.I.I. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Junying Yuan, Enrique Rodriguez-Boulan, and Charles Parkos for sharing reagents, Dr. Shoichiro Ono for insightful discussion on this project, and Alex Feygin for editing the manuscript.
REFERENCES
- 1.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci USA 105: 13–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15: 185–230, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Ivanov AI. Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell 23: 3542–3553, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartles JR. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol 12: 72–78, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berro J, Pollard TD. Synergies between Aip1p and capping protein subunits (Acp1p and Acp2p) in clathrin-mediated endocytosis and cell polarization in fission yeast. Mol Biol Cell 25: 3515–3527, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, Fromm M, Koval M, Parkos C, Nusrat A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell 25: 2710–2719, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol 172: 671–678, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol 9: 1110–1121, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chu D, Pan H, Wan P, Wu J, Luo J, Zhu H, Chen J. AIP1 acts with cofilin to control actin dynamics during epithelial morphogenesis. Development 139: 3561–3571, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Collinet C, Lecuit T. Stability and dynamics of cell-cell junctions. Prog Mol Biol Transl Sci 116: 25–47, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Cramer LP, Briggs LJ, Dawe HR. Use of fluorescently labelled deoxyribonuclease I to spatially measure G-actin levels in migrating and non-migrating cells. Cell Motil Cytoskeleton 51: 27–38, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez R. Actin filament nucleation and elongation factors–structure-function relationships. Crit Rev Biochem Mol Biol 44: 351–366, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci 122: 2980–2988, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Geback T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 46: 265–274, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harbor Perspect Biol 2: a000125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirokawa N, Tilney LG. Interactions between actin filaments and between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J Cell Biol 95: 249–261, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indra I, Troyanovsky R, Troyanovsky SM. Afadin controls cadherin cluster stability using clathrin-independent mechanism. Tissue Barriers 2: e28687, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 13: 6662–6681, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2: e658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov AI, Hopkins AM, Brown GT, Gerner-Smidt K, Babbin BA, Parkos CA, Nusrat A. Myosin II regulates the shape of three-dimensional intestinal epithelial cysts. J Cell Sci 121: 1803–1814, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 16: 2636–2650, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol 303: 27–99, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays 27: 356–365, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kato A, Kurita S, Hayashi A, Kaji N, Ohashi K, Mizuno K. Critical roles of actin-interacting protein 1 in cytokinesis and chemotactic migration of mammalian cells. Biochem J 414: 261–270, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Kile BT, Panopoulos AD, Stirzaker RA, Hacking DF, Tahtamouni LH, Willson TA, Mielke LA, Henley KJ, Zhang JG, Wicks IP, Stevenson WS, Nurden P, Watowich SS, Justice MJ. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood 110: 2371–2380, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6: 21–30, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12: 379–382, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Koval M. Differential pathways of claudin oligomerization and integration into tight junctions. Tissue Barriers 1: e24518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA 86: 9313–9317, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol 24: 1689–1699, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol 13: 529–540, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, von Andrian UH, Mitchison T, Yuan J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol 9: 276–286, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 113: 2363–2374, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Lu Z, Ding L, Lu Q, Chen YH. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 1: e24978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 253: C171–C175, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harbor Perspect Biol 1: a002899, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelot A, Drubin DG. Building distinct actin filament networks in a common cytoplasm. Curr Biol 21: R560–R569, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohri K, Ono K, Yu R, Yamashiro S, Ono S. Enhancement of actin-depolymerizing factor/cofilin-dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the Caenorhabditis elegans body wall muscle. Mol Biol Cell 17: 2190–2199, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol 2: 376–378, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Nadkarni AV, Brieher WM. Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends. Curr Biol 24: 2749–2757, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naydenov NG, Baranwal S, Khan S, Feygin A, Gupta P, Ivanov AI. Novel mechanism of cytokine-induced disruption of epithelial barriers: Janus kinase and protein kinase D-dependent downregulation of junction protein expression. Tissue Barriers 1: e25231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle 8: 2110–2121, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell 21: 3506–3517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada K, Obinata T, Abe H. XAIP1: a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J Cell Sci 112: 1553–1565, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Okada K, Ravi H, Smith EM, Goode BL. Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol Biol Cell 17: 2855–2868, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol 258: 1–82, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Ono S, Nomura K, Hitosugi S, Tu DK, Lee JA, Baillie DL, Ono K. The two actin-interacting protein 1 genes have overlapping and essential function for embryonic development in Caenorhabditis elegans. Mol Biol Cell 22: 2258–2269, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol 175: 135–146, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct 29: 545–576, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Poukkula M, Hakala M, Pentinmikko N, Sweeney MO, Jansen S, Mattila J, Hietakangas V, Goode BL, Lappalainen P. GMF promotes leading-edge dynamics and collective cell migration in vivo. Curr Biol 24: 2533–2540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pruitt BL, Dunn AR, Weis WI, Nelson WJ. Mechano-transduction: from molecules to tissues. PLoS Biol 12: e1001996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC. Aip1p interacts with cofilin to disassemble actin filaments. J Cell Biol 145: 1251–1264, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15: 225–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12: 696–702, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stossel TP, Fenteany G, Hartwig JH. Cell surface actin remodeling. J Cell Sci 119: 3261–3264, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15: 397–410, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Talman AM, Chong R, Chia J, Svitkina T, Agaisse H. Actin network disassembly powers dissemination of Listeria monocytogenes. J Cell Sci 127: 240–249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troyanovsky S. Adherens junction assembly. Subcell Biochem 60: 89–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Troyanovsky SM. Regulation of cadherin-based epithelial cell adhesion by endocytosis. Front Biosci 1: 61–67, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Twiss F, Oldenkamp M, Hiemstra A, Zhou H, Matheron L, Mohammed S, de Rooij J. HGF signaling regulates claudin-3 dynamics through its C-terminal tyrosine residues. Tissue Barriers 1: e27425, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Itallie CM, Anderson JM. Claudin interactions in and out of the tight junction. Tissue Barriers 1: e25247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100: 209–219, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell 23: 4601–4610, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J 25: 5058–5070, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol 16: 167–178, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Ydenberg CA, Padrick SB, Sweeney MO, Gandhi M, Sokolova O, Goode BL. GMF severs actin-Arp2/3 complex branch junctions by a cofilin-like mechanism. Curr Biol 23: 1037–1045, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ydenberg CA, Smith BA, Breitsprecher D, Gelles J, Goode BL. Cease-fire at the leading edge: new perspectives on actin filament branching, debranching, and cross-linking. Cytoskeleton 68: 596–602, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol 23: 515–522, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Yuan B, Wan P, Chu D, Nie J, Cao Y, Luo W, Lu S, Chen J, Yang Z. A cardiomyocyte-specific Wdr1 knockout demonstrates essential functional roles for actin disassembly during myocardial growth and maintenance in mice. Am J Pathol 184: 1967–1980, 2014. [DOI] [PubMed] [Google Scholar]