Abstract
The status of the GP130-STAT3 signaling pathway in humans with nonalcoholic fatty liver disease (NAFLD) and its relevance to disease pathogenesis are unknown. The expression of the gp130-STAT3 axis and gp130 cytokine receptors were studied in subjects with varying phenotypes of NAFLD including nonalcoholic steatohepatitis (NASH) and compared with lean and weight-matched controls without NAFLD. Gp130 and its downstream signaling element (Tyk2 and STAT3) expression were inhibited in obese controls whereas they were increased in NAFLD. IL-6 levels were increased in NASH and correlated with gp130 expression (P < 0.01). Palmitate inhibited gp130-STAT3 expression and signaling. IL-6 and palmitate inhibited hepatic insulin signaling via STAT3-dependent and independent mechanisms, respectively. STAT3 overexpression reversed palmitate-induced lipotoxicity by increasing autophagy (ATG7) and decreasing endoplasmic reticulum stress. These data demonstrate that the STAT3 pathway is activated in NAFLD and can worsen insulin resistance while protecting against other lipotoxic mechanisms of disease pathogenesis.
Keywords: NAFLD, NASH, IL-6, palmitate, gp130, autophagy, STAT3, insulin resistance
nonalcoholic fatty liver disease (NAFLD) and obesity affect about a third of the US population (13, 26). Nonalcoholic steatohepatitis (NASH), the aggressive phenotype of NAFLD, leads to cirrhosis and is increasing as a cause of end-stage liver disease and an indication for liver transplantation (5, 18). The factors that drive disease progression are not fully understood. This gap in knowledge is a barrier toward development of targeted therapeutics to prevent liver-related outcomes in affected subjects.
Lipotoxicity as a result of excess free fatty acids (FFA) has been implicated in the pathogenesis of NAFLD by driving hepatic insulin resistance, oxidative stress, hepatocellular injury, and inflammation (7). Saturated fatty acids such as palmitate produce lipotoxicity both by its direct cellular effects and by enhancing the production of proinflammatory, insulin resistance-promoting cytokines such as TNF-α and IL-6 (1). These effects are mediated via the direct cellular effects of fatty acids as well as by cytokine-mediated signaling (1).
The gp130 cell surface receptor mediates a multitude of cellular responses to external cues and is a key element of the cellular response to metabolic and other stresses (11, 25). It is engaged by numerous cytokines often referred to as the gp130 cytokine family (27). IL-6, a cytokine known to be increased in obesity and insulin-resistant states (20, 22), is a prototypic gp130 cytokine and activates Janus-activated kinase (JAK)-signal transduction and activator of transcription 3 (JAK-STAT3), Src-Ras, and the phosphatidylinositol 3-kinase-Akt pathways via gp130 (23, 28). Gp130-mediated signaling plays a role on hematopoiesis, immune regulation, inflammation, and carcinogenesis (3, 17). The status of gp130-STAT3 axis in NAFLD is unknown. It is also not known whether this pathway is affected by lipotoxic stress. The potential cross talk between palmitate and IL-6, which are both increased in NAFLD in modulating gp130 signaling, is also unknown.
The primary objective of this work was to determine the status of this pathway in humans with NAFLD and the potential consequences of its activation. The specific aims were to 1) define the status of the gp130-STAT3 axis in subjects with fatty liver or NASH and compare them with lean healthy controls and obese subjects without NAFLD, 2) determine whether the status of gp130 expression was linked to circulating IL-6 levels, 3) define the ability of palmitate to modulate the activity of the gp-130-STAT3 axis, 4) define the effects of gp130-STAT3 signaling on selected pathways considered relevant for pathogenesis of NASH, and 5) define cross talk between IL-6 and palmitate in modulating gp130-STAT3 mediated effects on such pathways.
MATERIALS AND METHODS
Reagents.
Antibodies for Western blotting, SP600125, and IL-6 protein were purchased from Cell Signaling (Beverly, MA). β-Actin, RIPA buffer, protease inhibitor mixture, STAT3 inhibitor (S3I-201), AR-42, IKK2 inhibitor, PD98059, and FFA-free BSA were purchased from Sigma Aldrich (St. Louis, MO). NF-κB antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). AR-42 was purchased from Thermo Fisher Scientific (Waltham, MA). Horseradish peroxidase (HRP)-conjugated secondary antibody and SuperSignal chemiluminescence kits were purchased from Pierce Biotechnology (Rockford, IL). Cell culture media, insulin, and Western blot supplies were obtained from Invitrogen (Carlsbad, CA).
Human subjects.
Four groups of subjects were studied: 1) lean healthy normal controls, 2) obese controls without NAFLD, 3) nonalcoholic fatty liver, and 4) nonalcoholic steatohepatitis. Both lean and obese controls had normal alanine aminotransferase (< 19 IU/l for women and 31 IU/l for men) and normal liver histology. NAFL and NASH were confirmed by liver biopsy in all instances. Liver tissue from lean, normal subjects was obtained the Tissue and Data Acquisition and Analysis Core, Virginia Commonwealth University, whereas that from obese subjects was collected from liver biopsy obtained at the time of cholecystectomy or gastric bypass surgery. Liver histology was scored based on NASH Clinical Research Network Criteria (4). The nonalcoholic nature of the disease was established clinically by using a daily intake cutoff of <20 g/day of alcohol for female subjects and <30 g/day for male subjects within the past 5 years, as used in other studies of this disease (21). All subjects provided informed consent and the study was approved by the institutional review board (VCU IRB no. 1960). Blood samples were obtained from each patient at the time of liver biopsy, processed to plasma, and stored frozen at −80°C. The plasma was subsequently used for quantitative measurement of IL-6 by a commercially available ELISA kit (R&D Systems, Minneapolis, MN). All assays were performed in triplicate, and the absorbance was determined by a microplate reader (Infinite F50, San Jose, CA).
In vitro experiments.
The Huh-7 cell line was grown in DMEM containing 10% FBS, 100 IU/ml penicillin, and 100 mg/ml streptomycin as previously described (16). Huh-7 cells were grown in 2 ml of DMEM medium in a six-well plate at 37°C for overnight in a 0.5% CO2 incubator. On the next day, cells were treated with the indicated concentrations of 0.5 mM palmitate in the presence of 1% FFA-free BSA. For the gp130 inhibition experiments, Huh-7 cells were plated in a six-well plate in a 2.0-ml total volume at a density of 6 × 105 cells per well. Cells were treated with DMEM media containing 0, 0.01, and 1.0 μmol/l AR-42 for 1 h and then treated with 0.5 mM palmitate for 12 h. The plate was incubated with a 20 ng/ml of insulin at 37°C in a 5% CO2 incubator for 15 min. For the STAT3 inhibition experiments, cells were preincubated with STAT3 inhibitor (S31-201) (0, 10, 50, and 100 μM) for 4 h and then exposed with or without 0.5 mM palmitate in DMEM medium containing 1% FFA-free BSA for 12 h. For studies on cross talk between palmitate and IL-6 on insulin signaling pathways, Huh-7 cells were stimulated with palmitate (0.5 mM) for 12 h and then incubated with recombinant human IL-6 at 20 ng/ml for 15 min, and the cells were further exposed with insulin at 100 nM for 30 min.
Adenovirus-mediated gene transduction.
Adenoviral vectors expressing STAT3 (Ad-STAT3) and control green fluorescent protein (GFP; Ad-GFP) were purchased from Vector BioLabs. Briefly, for infection of Ad-STAT3 or Ad-GFP in Huh-7 cells, 6 × 105 cells/well were seeded to six-well plates containing DMEM medium and allowed to adhere for 1 days and then cells were infected with multiplicity of infection (MOI) (0.001, 0.01, 0.1, or 1) of Ad-STAT3 or Ad-GFP for 12 h. After infection with adenovirus, the cells were treated with 0.5 mM palmitate for 8 or 12 h.
Protein extraction and Western blot analysis.
All liver tissues were homogenized by using RIPA lysis buffer and then sonicated on ice with a sonicator cell disrupter, model 100 Sonic Dismembrator (Thermo Fisher Scientific, Waltham, MA; power 2, 6 pulses ×2). Huh-7 cells were washed with phosphate-buffered saline and lysed in RIPA buffer, containing a protease inhibitor mixture. All experiments for Western blot were performed as previously described (16). Briefly, the sample proteins were electrophoretically separated by using 4–12% NuPAGE Novex Bis-Tris Mini Gels (Invitrogen) and were transferred to a nitrocellulose membrane for 1 h at 40 V by use of a Western blot apparatus (Invitrogen). The membrane was blocked for 2 h in 5% nonfat dry milk in TBST buffer [0.1 M Tris (pH 7.5), 0.15 M NaCl, 0.1% (vol/vol) Tween-20] at room temperature. The primary antibodies were incubated overnight at 4°C or 2 h at room temperature and then removed. The membrane was washed three times (5 min each) with TBST. The membranes were then incubated with HRP-conjugated secondary and were detected by use of the SuperSignal chemiluminescence kit (Pierce). All immunoblots were scanned by using a model Fluorchem M imaging system (Proteinsimple, San Jose, CA). Densitometry analysis for the expression of proteins was performed with ImageJ software. The protein levels were normalized for β-actin or total protein as appropriate.
Statistical analysis.
For levels of gp130, Tyk2, and STAT3 expression in humans, the data were compared across groups by Kruskal-Wallis analysis of variance with a Dunn's posttest for multiple comparisons. Each cell culture-based study was performed in triplicate. For comparisons of protein expression and phosphorylation in these studies, an unpaired two-tailed t-test was used to compare groups. Significance was set at P < 0.05. There were no prior data to base power calculations before the study was started. After seven subjects were enrolled in each arm, the sample size needed to have a power of 80% for the observed differences was estimated. The decision to terminate the study was based on having achieved 80% or higher power to demonstrate the key differences in gp130.
RESULTS
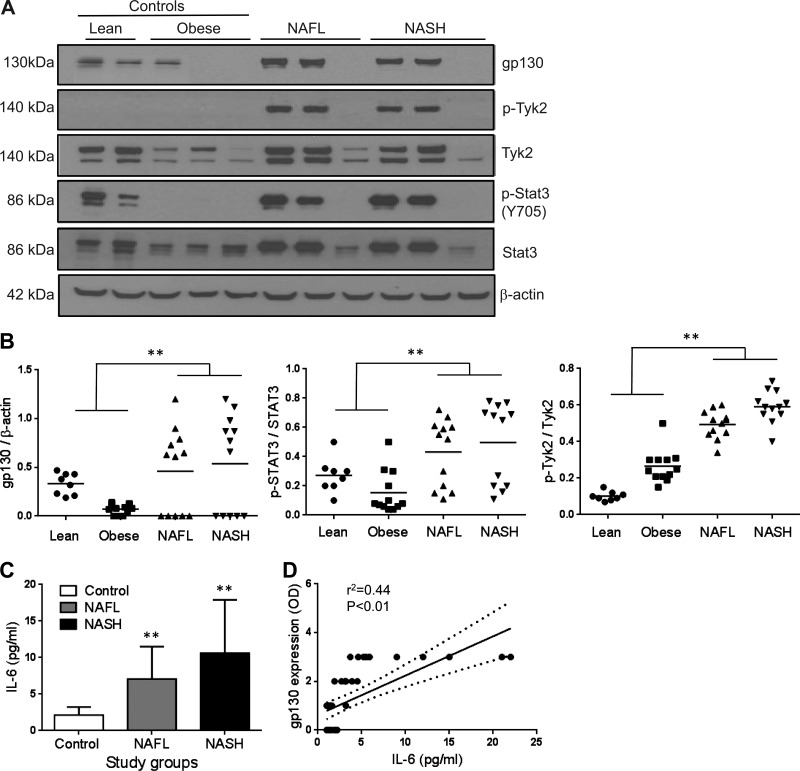
The gp130-Tyk2-STAT3 pathway is suppressed in obese controls without steatosis but enhanced in subjects with NAFL or NASH.
A total of 12 subjects each with nonalcoholic fatty liver (NAFL) and NASH without bridging fibrosis or cirrhosis were studied and compared with 8 lean normal control subjects and 12 obese weight-, age-, gender-, and race-matched controls without NAFLD. The summaries of demographic, clinical, and laboratory data are shown in Table 1. The gp130 and Tyk2 expression were significantly lower in obese controls compared with lean normal subjects (Fig. 1A). Tyk2 and STAT3 phosphorylation (p-Tyk2 and p-STAT3, respectively) was virtually undetectable in obese control subjects. In terms of gp130 expression, two distinct patterns were noted in subjects both with NAFL and with NASH. The expression was high in 7/12 subjects with NAFL and 7/12 subjects with NASH whereas it was undetectable in the rest (Fig. 1B). Overall, both NAFL and NASH still had significantly higher gp130 expression compared with obese controls as well as lean controls (Fig. 1B, P < 0.01 for both NAFL and NASH vs. either control). Similarly, there was also greater Tyk2 and STAT3 phosphorylation in NAFL and NASH compared with controls (P < 0.01 for both NAFL and NASH vs. either control, Fig. 1B).
Table 1.
Demographic, clinical, and laboratory profile
| Parameter | Lean Normal (N = 8) |
Obese Normal (N = 12) |
NAFL (N = 12) |
NASH (N = 12) |
P Value |
|---|---|---|---|---|---|
| Age, yr | 51.9 ± 6.0 | 49.8 ± 11.0 | 49.9 ± 14.1 | 57.4 ± 10.3 | n.s. |
| Men:women, n | 4:4 | 2:10 | 4:8 | 5:7 | n.s. |
| Caucasian, % | 60 | 60 | 75 | 100 | n.s. |
| BMI, kg/m2 | 24.1 ± 3.8 | 41.6 ± 6.3 | 37.1 ± 7.0 | 41.8 ± 5.4 | < 0.0001* |
| Type 2 diabetes mellitus, n | 0 | 3 | 4 | 4 | n.s.‡ |
| Hypertension, n | 0 | 5 | 6 | 6 | 0.02*‡ |
| AST, IU/l | 24.0 ± 7.3 | 28.2 ± 9.5 | 30.8 ± 14.7 | 41.7 ± 17.8 | <0.005† |
| ALT, IU/l | 25.1 ± 16.6 | 49.4 ± 31.5 | 70.1 ± 44.4 | 80.1 ± 16.8 | <0.008† |
| Alk phos, IU/l | 86 ± 21 | 90 ± 9 | 97 ± 18 | 101 ± 21 | n.s. |
| Bilirubin, mg/dl | 0.2 ± 0.08 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | n.s. |
| Albumin, g/dl | 4.2 ± 0.2 | 4 ± 0.4 | 3.9 ± 0.3 | 4 ± 0.3 | n.s. |
| Fasting blood sugar, mg/dl | 88 ± 8 | 95 ± 8 | 98 ± 11 | 99 ± 12 | n.s. |
| Fasting insulin, μIU/dl | 6.0 ± 2 | 16.2 ± 9 | 20 ± 11 | 22 ± 7 | <0.005* |
| Hemoglobin A1C, % | 5.0 ± 0.2 | 6.1 ± 1.3 | 6.2 ± 2.1 | 6.5 ± 1.4 | n.s. |
| Total cholesterol, mg/dl | 194.9 ± 20.4 | 199.0 ± 51.1 | 206.6 ± 70.7 | 216.0 ± 28.9 | n.s. |
| LDL-cholesterol, mg/dl | 108.1 ± 21.4 | 116.6 ± 38.5 | 109.8 ± 57.3 | 139.8 ± 17.8 | <0.01* |
| HDL-cholesterol, mg/dl | 55.6 ± 15.4 | 50.0 ± 7.6 | 43.6 ± 13.7 | 43.6 ± 8.8 | <0.05† |
| Triglycerides, mg/dl | 99.1 ± 40.9 | 102.8 ± 48.2 | 171.8 ± 81.6 | 211.3 ± 44.7 | <0.01* |
Values are means ± SD. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Alk phos, alkaline phosphatase.
Lean vs. other groups;
NAFL or NASH vs. either control group;
χ2 for trend; n.s., not significant.
Fig. 1.
The expression of gp130 and its downstream signaling elements Tyk2 and STAT3 was examined in subjects with nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) (n = 12 each) and compared with lean (n = 8) and weight-matched controls (n = 12) with normal liver histology. Obese controls had decreased gp130, p-Tyk2, and p-STAT3 expression whereas these were increased in both NAFL and NASH compared with lean controls (A and B; P < 0.01). Western blot data of representative samples are shown in A. B demonstrates data for individual groups with mean shown as horizontal line. Mean (± SD) IL-6 levels in subjects with NAFL and NASH were significantly higher than in lean and obese controls combined (C, P < 0.01). Gp130 expression was closely related to IL-6 levels (r = 0.44, P < 0.01) (D). **P < 0.01. OD, optical density.
gp130 expression is directly related to circulating IL-6 in NAFLD.
To further determine the potential factors driving the expression and activation of the gp130-STAT3 axis in NAFLD, the levels of circulating IL-6 was compared in controls vs. those with NAFL or NASH. Both those with NAFL and those with NASH had significantly increased levels of IL-6 compared with controls (Fig. 1C, P < 0.01). IL-6 levels were directly related to gp130 expression in these subjects (Fig. 1D, r2 = 0.44, P < 0.01).
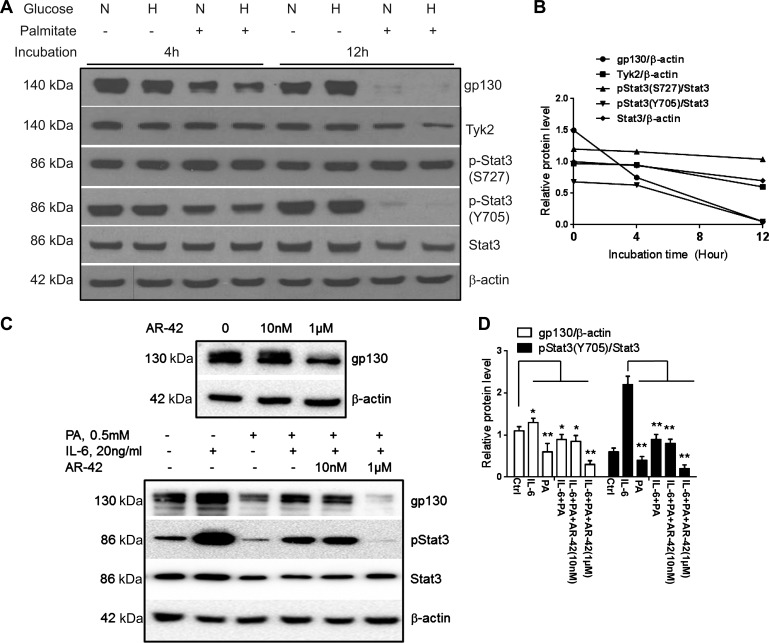
Palmitate inhibits gp130 protein and STAT3 signaling pathway in hepatocytes.
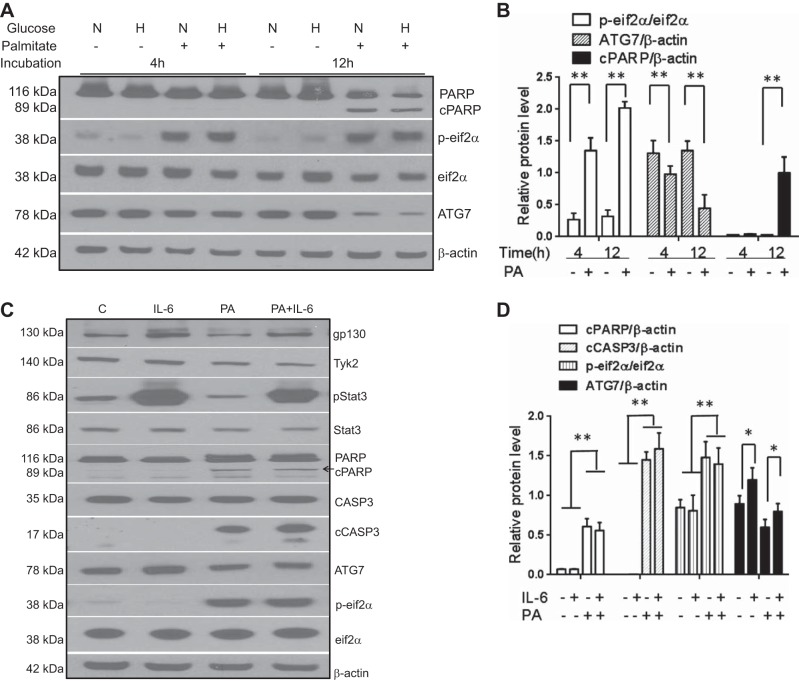
Human hepatoma cells (Huh-7) were cultured in the presence of normal (5.5 mM) or high glucose (30.0 mM) concentration with or without 0.5 mM palmitate up to 12 h. Palmitate produced a modest and nonsignificant downregulation of both gp130 and phosphorylated STAT3 (Y705) proteins at 4 h (Fig. 2, A and B) and completely suppressed both gp130 expression and STAT3 (Y705) phosphorylation at 12 h (Fig. 2, A and B; P < 0.01). Of note, serine phosphorylation of STAT3 (S727) remained unchanged at both 4 and 12 h. Total STAT3 and Tyk2 also decreased after 12 h of exposure to palmitate (Fig. 2, A and B; P < 0.01). These data demonstrate that palmitate inhibits the STAT3 signaling pathway in a time-dependent manner.
Fig. 2.
The effect of palmitate on gp130-STAT3 expression and signaling was studied in Huh-7 cells. Regardless of ambient glucose levels (N, normal: 5.5 mM glucose; H, high: 30 mM), palmitate (0.5 mM) produced a time-dependent inhibition of gp130, Tyk2, and tyrosine-phosphorylated p-STAT3 (Y705), whereas the serine-phosphorylated p-STAT3 (S727) did not change significantly (A and B; P < 0.01 baseline vs. 12 h). IL-6 increased gp130 (C and D; P < 0.05) and p-STAT3 compared with controls (C and D; P < 0.01) whereas palmitate inhibited their expression (C and D; P < 0.01). Palmitate inhibited IL-6-mediated increase in p-STAT3 (C and D; P < 0.01). AR-42, a gp130 antagonist, directly inhibited gp130 in a dose-dependent manner (C, top) and a dose-dependent blockade of IL-6 mediated STAT3 phosphorylation (C, bottom, and D; P < 0.01 for 1 μM). Means ± SD from 3 independent experiments are shown for all graphical data. *P < 0.05; **P < 0.01.
Palmitate attenuates IL-6 effects on the gp130-STAT3 axis.
Next, the interactions between IL-6, a cytokine that activates gp130 and is produced in hepatocytes, and palmitate were studied. Palmitate inhibited the gp130-STAT3 axis whereas IL-6 activated the axis as expected (Fig. 2, C and D; P < 0.01 for palmitate and P < 0.05 for IL-6). Palmitate also attenuated the IL-6-mediated activation of STAT3 (Fig. 2C, bottom, lane 4, P < 0.01). The specificity of these effects for the gp130-STAT3 pathway was confirmed by abrogation of the IL-6 effects by the gp130 antagonist AR-42 (31) (P < 0.01 IL-6 vs. IL-6 + AR42, Fig. 2, C, top, and D).
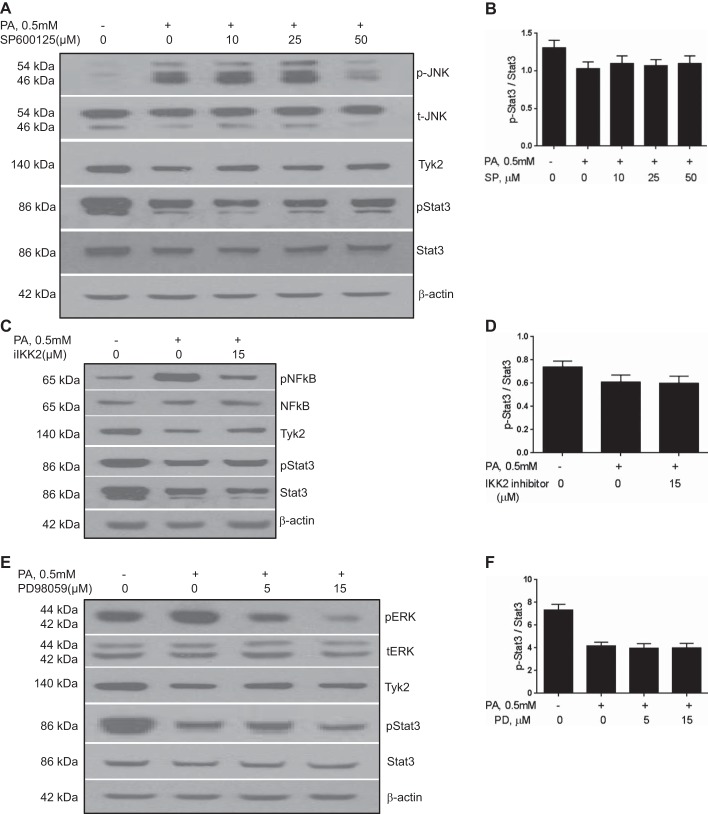
Palmitate-induced inhibition of STAT3 is independent of JNK, NF-κB, and ERK activation.
Serum-starved cells in DMEM medium were incubated with specific MAPK- and IKK2 inhibitors 1 h before treatment with 0.5 mM palmitate for 8 h. Palmitate increased the phosphorylation of JNK, NF-κB, and ERK compared with controls (Fig. 3, A–F, P < 0.01 for all three targets). As expected, pretreatment with JNK, IKK2, and ERK inhibitors before palmitate exposure significantly reduced phosphorylation of each of the corresponding proteins compared with palmitate-treated controls (Fig. 3, A–F). Neither palmitate-mediated changes in Tyk2 levels nor STAT3 phosphorylation were significantly affected by inhibition of NF-κB, JNK, or ERK. These data demonstrate that palmitate-induced inhibition of STAT3 phosphorylation is independent of these pathways.
Fig. 3.
Palmitate-induced inhibition of STAT3 is independent of JNK, NF-κB, and ERK activation. Prior treatment of cells with a JNK inhibitor SP600125 (A and B), IKK2 inhibitor (iIKK2, C and D), or ERK inhibitor PD98059 (E and F) did not affect palmitate-induced suppression of the gp130-STAT3 axis. Palmitate-induced inhibition of STAT3 phosphorylation is thus independent of these pathways. Means ± SD from 3 independent experiments are shown for all graphical data. PA, palmitate; SP, SP600125; PD, PD98059.
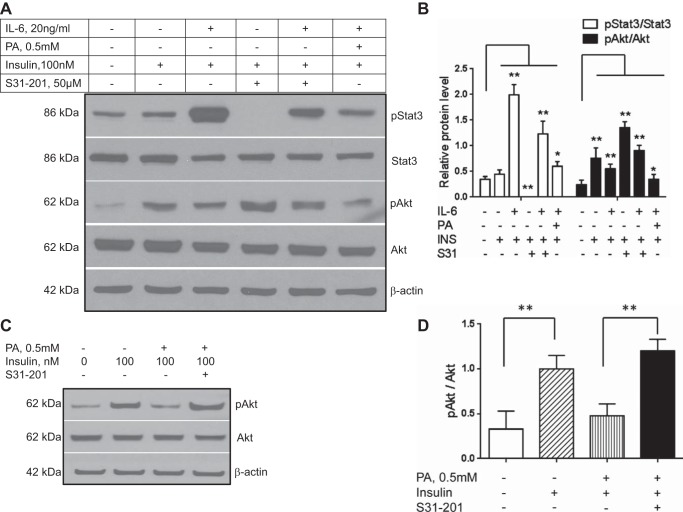
The potential role of STAT3 in IL-6 and palmitate-induced impairment in insulin signaling.
Hepatic insulin resistance is considered to be a key pathophysiological factor in the genesis of NAFLD (14, 24). IL-6 increased p-STAT3 (Y705) and also inhibited insulin-mediated p-Akt (Fig. 4, A and B; P < 0.01 for both targets). These effects were abrogated by pretreatment with the STAT3 inhibitor S31-201 (Fig. 4, A, lanes 3 vs. 5, and B; P < 0.01 for both targets). On the other hand, palmitate reduced IL-6-mediated STAT3 phosphorylation yet still had an additive effect with IL-6 suppression of insulin-mediated Akt-phosphorylation (Fig. 4, A, lane 6, and B; P < 0.01 for both targets). Interestingly, STAT3 inhibition even in the absence of IL-6 increased insulin-mediated p-Akt levels to levels greater than those seen with insulin alone (Fig. 4, A, lane 4, and B; P < 0.01). Inhibition of STAT3 prior to palmitate exposure also restored p-Akt levels to those seen with insulin alone (Fig. 4, C and D; P < 0.01).
Fig. 4.
The effects of IL-6 and palmitate on hepatic insulin signaling were evaluated in Huh-7 cells. IL-6 increased p-STAT3 and inhibited insulin-stimulated Akt phosphorylation (A and B). Administration of a STAT3 inhibitor S31-201 prior to insulin (INS) exposure significantly increased p-Akt to levels greater than those seen with insulin alone (A and B; P < 0.01). It also reduced IL-6-mediated suppression of insulin-stimulated Akt phosphorylation (A, lane 5, and data shown graphically in B). Palmitate and IL-6 had an additive suppressive effect on insulin-stimulated Akt phosphorylation (A, lane 6, and graphical representation in B). Next, the role of STAT3 signaling in palmitate-induced decrease in insulin signaling was studied (C and D). Suppression of STAT3 abrogated palmitate-induced suppression of insulin-mediated Akt phosphorylation (C, lane 4 and D; P < 0.01). Means ± SD from 3 independent experiments are shown for all graphical data. *P < 0.05; **P < 0.01.
IL-6 increases autophagy and improves ER stress via STAT3.
Impaired autophagy and induction of endoplasmic reticulum (ER) stress are important pathophysiological factors in the genesis of NAFLD (2, 19). Palmitate induced ER stress as assessed by phosphorylation of the eukaryotic inhibitory factor-2 (p-eIF-2α) and modestly suppressed ATG7 a marker of autophagy (Fig. 5, A and B; P < 0.01). It also activated poly-(ADP-ribose) polymerase (PARP) cleavage, indicating activation of apoptosis. IL-6, in concentrations seen in insulin-resistant states (20 ng/ml) (20), had no significant effect on PARP cleavage or p-eIF-2α levels while increasing ATG7 (Fig. 5, C and D; P < 0.01).
Fig. 5.
The effects of IL-6 and components of the gp130-STAT3 pathway on palmitate-induced activation of lipotoxic pathways were studied in Huh-7 cells under normoglycemic (N; glucose concentration = 5.5 mM) and hyperglycemic (H; glucose concentration = 30.0 mM) conditions. Palmitate produced a time-dependent increase in PARP cleavage (cPARP) and activation of endoplasmic reticulum stress (measured by p-eIF2α) while inhibiting autophagy (measured by ATG7 expression) (A and B; P < 0.01 for all). Compared with controls (C), IL-6 increased p-STAT3 but had no effect on cPARP, caspase 3 cleavage, or p-eIF2α but significantly induced ATG7 (C and D; P < 0.05 for ATG7). Palmitate inhibited p-STAT3 and activated PARP and caspase 3 cleavage (P < 0.01 for all) while decreasing ATG7 (P < 0.05) (C and D). IL-6 did not alter these palmitate-induced changes. Means ± SD from 3 independent experiments are shown for all graphical data. *P < 0.05; **P < 0.01.
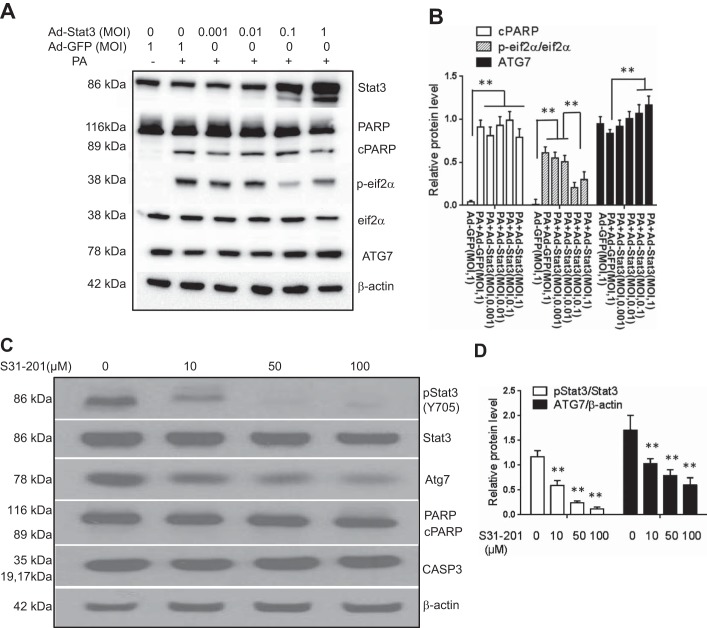
To directly assess whether STAT3 affected these pathways known to be relevant for NASH, the effects of both gain and loss of function were studied in Huh-7 cells. STAT3 overexpression had no significant effects on palmitate-induced PARP cleavage (Fig. 6, A and B). It, however, inhibited palmitate-induced increase in p-eIF-2α (Fig. 6B, P < 0.01) except at very high MOI 1 where p-eIF-2α was increased. STAT3 overexpression also produced a dose-dependent increase in ATG7 in the presence of palmitate (Fig. 6, A and B; P < 0.01) and reversed palmitate-induced ATG7 suppression. Conversely, inhibition of STAT3 with pretreatment with S31-201 produced a dose-dependent significant decrease in ATG7 (Fig. 6, C and D; P < 0.01).
Fig. 6.
The effects of STAT3 on palmitate lipotoxicity are shown. STAT3 was overexpressed by using an adenovirus vector (Ad-STAT3). Compared with controls (Ad-GFP), STAT3 overexpression did not affect palmitate-induced PARP cleavage (A and B). However, it produced a dose-dependent blockade of palmitate-induced activation of p-eIF2α (A and B; P < 0.01). It also produced a dose-dependent increase in ATG7 (A and B; P < 0.01). The effect of inhibition of STAT3 by the S31-201 was studied next (C and D). STAT3 inhibition was associated with decreased ATG7 (P < 0.01) without significant effects on cPARP or caspase 3 (C and D). Means ± SD from 3 independent experiments are shown for all graphical data; **P < 0.01. MOI, multiplicity of infection.
DISCUSSION
The present study demonstrates increased expression and activation of the gp130-STAT3 axis in the majority of subjects with either NAFL or NASH and links it to the levels of IL-6 in this population. Gp130-mediated signaling is a key component of the cellular response to metabolic stress and injury and affects cell survival and inflammation (12). These effects are largely mediated via STAT3, which has also been linked to inflammation and cancer (30). NAFLD, especially NASH, is associated with substantial metabolic stress, inflammation, and an increased risk of hepatocellular cancer (15, 29). It is therefore certainly within the realm of possibility that activation of the gp130-STAT3 axis plays a role in modulating development and progression of NASH.
It is interesting to note that weight-matched obese controls without NAFLD not only have less insulin resistance but also lower IL-6 levels and very low levels of gp130 expression. Gp-130 levels may be decreased by increased turnover due to palmitoylation; however, existing literature suggests that palmitoylation in fact protects proteins from proteosomal degradation (6). The mechanism for decreased gp130 in obese subjects without NAFLD is thus unknown and is an area of future research.
Several lines of evidence suggest that IL-6 is the principal driver of increased gp130 expression in NAFLD. These include the elevation of IL-6 in subjects with NAFL or NASH compared with either lean or obese controls (Fig. 1C), the direct relationship between IL-6 and gp130 expression (Fig. 1D), the IL-6-mediated increase in gp130 in vitro (Fig. 2C), and the well-known effect of IL-6 as a gp130 cytokine (8). It is also interesting to note that all subjects with NAFLD did not have increased expression of gp130 and in fact had levels similar to obese controls. The IL-6 levels in these subjects were lower than in those with increased gp130.
The present study also provides insights on the effects of STAT3 on insulin signaling and the cross talk between lipotoxic stress and IL-6 in mediating impairment of hepatic insulin signaling. IL-6 activated p-STAT3 and impaired insulin-mediated Akt phosphorylation, and these effects could be blocked by pretreatment with a p-STAT3 inhibitor S31-201, indicating that this effect was mediated by p-STAT3. Palmitate inhibited p-STAT3 (the tyrosine-phosphorylated form Y705) but also impaired insulin-mediated Akt phosphorylation (Fig. 4, A–D). These data are compatible with the well-known palmitate-induced activation of other pathways such as JNK, etc., that also inhibit insulin signaling (9, 10). Thus IL-6 and palmitate contribute to impaired hepatic insulin signaling via STAT3-dependent and independent pathways, respectively.
Our data further suggest that STAT3 signaling provides an inhibitory control for Akt phosphorylation because, even in the absence of a gp130 agonist, inhibition of STAT3 raises insulin-mediated Akt phosphorylation to levels greater than those seen with insulin alone (Fig. 4C). Also, the abrogation of palmitate-induced suppression of insulin-mediated Akt phosphorylation by a STAT3 inhibitor suggests that this pathway is important for palmitate effects on Akt phosphorylation. STAT3 also had several effects that are expected to improve NASH. Autophagy has been demonstrated to be an important cellular mechanism to prevent steatosis, and decreased autophagy has been associated with increased steatosis. Similarly, continued activation of the unfolded protein response has been associated with inflammation, apoptosis, and disease progression in NASH (19). STAT3 increased ATG7 and inhibited palmitate-induced eIF-2α phosphorylation (Fig. 6, A and B); both of these effects would be expected to ameliorate NASH. These data provide a rationale to target STAT3 for the treatment of NASH. One must, however, remain cognizant of the potential for unrestrained STAT3 activation to promote inflammation and cancer (3, 30).
There are, however, some limitations of the present study and other open questions that can now be raised. The nature of the study precluded assessment of the specific hepatic cell type in which gp130 expression was altered in subjects with NAFLD; our studies in Huh-7 cells suggest hepatocytes are the likely cell type involved but these data are not definitive. Also, long-term prospective studies are now needed to evaluate whether STAT3 activation drives future development of hepatocellular cancer in humans with NAFLD.
In summary, the present study describes activation of the gp130-STAT3 axis in humans with NAFLD and demonstrates that, whereas palmitate and IL-6 have opposing effects on gp130 expression and signaling, they have additive effects on hepatic insulin signaling due to differing downstream signaling mechanisms that converge on Akt phosphorylation. It also demonstrates that palmitate inhibits autophagy by inhibiting the gp130-STAT3 axis. These data open the door for future investigations on the relevance of this pathway for disease progression in NASH, which may provide a rationale for targeting this pathway to prevent disease progression and development of hepatocellular cancer.
GRANTS
This work was supported by NIDDK 5RO1DK081410-05 and NIDDK T32 DK 007150-35 to A. J. Sanyal. It was also supported by grants from the Basic Research Program (project no. PJ90693833) of the National Institute of Horticultural and Herbal Science (NIHHS), Rural Development Administration, Republic of Korea to H. K. Min. ULITR000058 CTSA was awarded to Virginia Commonwealth University.
DISCLOSURES
A. J. Sanyal has stock options in Genfit. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Nimbus, Salix, Tobira, Takeda, Fibrogen, Immuron, Exhalenz, and Genfit. He has been an unpaid consultant to Intercept and Echosens. His institution has received grant support from Gilead, Salix, Tobira, and Novartis. None of these are related to the present study.
AUTHOR CONTRIBUTIONS
H.-K.M. and A.J.S. conception and design of research; H.-K.M., F.M., A.V., T.P., V.P., C.-G.P., A.C., J.-H.L., and C.-B.P. performed experiments; H.-K.M. and A.J.S. analyzed data; H.-K.M., S.R., and A.J.S. interpreted results of experiments; H.-K.M. and A.J.S. prepared figures; H.-K.M., F.M., and A.J.S. drafted manuscript; H.-K.M., S.R., and A.J.S. edited and revised manuscript; H.-K.M. and A.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This work has been presented in partial form at the annual meeting of the American Gastroenterological Association in Chicago, 2014.
REFERENCES
- 1.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3–L1 adipocytes. J Nutr 135: 1841–1846, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroent 5: 159–166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-Mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15: 91–102, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Brunt EM. Nonalcoholic steatohepatitis (NASH): further expansion of this clinical entity? Liver 19: 263–264, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141: 1249–1253, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Dumaresq-Doiron K, Jules F, Lefrancois S. Sortilin turnover is mediated by ubiquitination. Biochem Biophys Res Commun 433: 90–95, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs M, Sanyal AJ. Lipotoxicity in NASH. J Hepatol 56: 291–293, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular-cloning and expression of an Il-6 signal transducer, Gp130. Cell 63: 1149–1157, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Hirosumi J, Tuncman G, Chang LF, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, Liedtke C, Wasmuth HE, Trautwein C, Streetz KL. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology 51: 463–473, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, Liedtke C, Wasmuth HE, Trautwein C, Streetz KL. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology 51: 463–473. [DOI] [PubMed] [Google Scholar]
- 13.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 178: 38–45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leclercq IA, Lebrun VA, Starkel P, Horsmans YJ. Intrahepatic insulin resistance in a murine model of steatohepatitis: effect of PPARgamma agonist pioglitazone. Lab Invest 87: 56–65, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 10: 656–665, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou HP, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab 15: 665–674, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity 28: 477–487, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberbach A, Schlichting N, Heinrich M, Till H, Stolzenburg JU, Neuhaus J. Free fatty acid palmitate impairs the vitality and function of cultured human bladder smooth muscle cells. PloS One 7: e41026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278: 45777–45784, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51: 3391–3399, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg GR, Watt MJ, Ernst M, Birnbaum MJ, Kemp BE, Jorgensen SB. Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity. Diabetes 58: 829–839, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Wagner MA, Siddiqui MA. The JAK-STAT pathway in hypertrophic stress signaling and genomic stress response. JAKSTAT 1: 131–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr 162: 496–500 e1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des 17: 340–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamauchi-Takihara K, Kishimoto T. Cytokines and their receptors in cardiovascular diseases — role of gp130 signalling pathway in cardiac myocyte growth and maintenance. Int J Exp Pathol 81: 1–16, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yki-Jarvinen H. Nonalcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2: 901–910, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9: 798–809, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Suvannasankha A, Crean CD, White VL, Chen CS, Farag SS. The novel histone deacetylase inhibitor, AR-42, inhibits gp130/Stat3 pathway and induces apoptosis and cell cycle arrest in multiple myeloma cells. Int J Cancer 129: 204–213, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]