Abstract
Hepatic zinc deficiency has been well documented in alcoholic patients, but the mechanisms by which zinc deficiency mediates cell death have not been well defined. The objectives of this study were to determine whether alcohol perturbs subcellular zinc homeostasis and how organelle zinc depletion may link with cell death pathways. Wistar rats were pair-fed with the Lieber-DeCarli control or ethanol diet for 5 mo. Chronic alcohol exposure significantly reduced zinc level in isolated hepatic endoplasmic reticulum (ER) and mitochondria. Among the detected zinc transporters, ER Zrt/Irt-like protein (ZIP)13 and mitochondrial ZIP8, which transport zinc from ER and mitochondria to cytosol, were significantly increased. Mitochondrial zinc transporter (ZnT) 4, which transports zinc from cytosol to mitochondria, was also increased. ER phosphorylated eukaryotic initiation factor 2α, activating transcription factor 4, and C/EBP homologous protein were significantly upregulated, and mitochondrial cytochrome c release and Bax insertion were detected in association with caspase-3 activation and apoptotic cell death. To define the role of zinc deficiency in ER and mitochondrial stress, H4IIEC3 cells were treated with 3 μM N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine for 6 h with or without supplementation with zinc or N-acetylcysteine (NAC). The results demonstrated that zinc deprivation induced caspase-3 activation and apoptosis in association with ER and mitochondria dysfunction, which were inhibited by zinc as low as 10 μM but not by 2 mM NAC. These results suggest that chronic ethanol exposure induced in ER and mitochondrial zinc deficiency might activate intrinsic cell death signaling pathway, which could not be effectively rescued by antioxidant treatment.
Keywords: alcoholic liver disease, apoptosis, zinc, endoplasmic reticulum, mitochondria
alcohol consumption is associated with increased disease burden worldwide. Alcoholic liver disease (ALD) contributes to the majority of the morbidity and mortality from alcohol abuse (20). The spectrum of ALD is from hepatic steatosis, steatohepatitis, fibrosis, and cirrhosis (27). The pathogenesis of ALD is derived from toxic metabolites of ethanol and a series of dysregulated signal transduction pathways. Ethanol is oxidized to acetaldehyde by cytosolic alcohol dehydrogenase (ADH) and microsomal cytochrome P450 2E1 (CYP2E1) (3). Chronic alcohol intake induces CYP2E1 rather than ADH (3). Acetaldehyde is converted to acetate by aldehyde dehydrogenase (ALDH) in mitochondria. In addition to the direct product of toxic acetaldehyde, ethanol metabolism also generates reactive oxygen species (ROS) (28). Thus endoplasmic reticulum (ER) and mitochondria as the major places for ethanol detoxification are the frontier organelles most easily being affected by chronic alcohol intake.
Zinc deficiency is a phenomenon consistently observed in patients with ALD, especially patients with alcoholic cirrhosis. Zinc supplementation to patients with ALD reversed impaired night vision, skin lesions, and immune dysfunction (25). Both animal and cell culture studies showed that zinc deprivation increased fat accumulation, inflammatory cell infiltration, and apoptotic hepatocytes (15, 38, 39). Accompanying these abnormalities are increased ROS and decreased antioxidant components such as glutathione. Zinc supplementation to animals chronically fed alcohol increased ADH activity, suppressed CYP2E1 activity, and prevented a decrease in glutathione (40). Because zinc plays an important role in maintenance of normal structure and functions of a large number of metalloenzymes and zinc proteins, zinc levels are tightly regulated by channels, zinc-sensing molecules, such as metallothionein, metal-responsive-element-binding transcription factor-1, and zinc transporters (5). Two families of zinc transporters are Zn transporter Zrt/Irt-like protein (ZIP) and zinc transporter (ZnT) (17). There are 14 members in the ZIP family, and they function in transporting zinc into cytosol from the extracellular side or intracellular vesicles (11). On the contrary, 10 members in the ZnT family export zinc from cytosol to either the outside of the cell or subcellular compartments (10). Mechanistic studies demonstrated that disrupted zinc transporters are linked with zinc dysregulation-associated pathogenesis (7, 30).
Alcohol-induced hepatocyte apoptosis has been well documented in patients with ALD and animal models (25). Mechanistic studies revealed that hepatic apoptosis is triggered by multiple signaling pathways, including ROS generation, cell membrane death receptor cascade, ER stress, and dysfunction of mitochondria (8, 16, 19). However, the importance of each mechanism may be different at a certain disease stage. Increasing evidence shows that ER stress plays a vital role in ALD (12). ER stress induces unfolded protein response (UPR) to restore ER homeostasis, but prolonged UPR leads to activation of inflammation, antioxidant defense, and/or insulin action signal pathways and finally apoptosis (13). The master regulator of ER stress-induced apoptosis is C/EBP homologous protein (Chop), which upregulates proapoptotic protein expression, downregulates prosurvival protein expression, and enhances oxidative stress (13, 14). The unbalanced proapoptotic and prosurvival proteins could then affect mitochondrial morphology and function and trigger mitochondrial-mediated intrinsic apoptosis (2). Therefore, we hypothesized that ethanol-induced hepatic apoptosis is ROS dependent, which is caused by decreased subcellular zinc levels. In the present study, zinc levels in hepatic subcellular compartments and hepatic apoptotic cell death signaling activation were evaluated in ethanol-fed rats. Mechanistic studies were conducted with rat hepatoma cells to establish the link between organelle zinc deficiency and apoptosis.
MATERIALS AND METHODS
Animals and alcohol-feeding experiments.
Male Wistar rats were obtained from Harlan (Indianapolis, IN). The animal protocol was approved by the Institutional Animal Care and Use Committee of the North Carolina Research Campus. Eight-week-old male rats were pair fed a modified Lieber-DeCarli alcohol or isocaloric maltose dextrin control liquid diet for 5 mo (n = 6 for each group) with a stepwise feeding procedure. The ethanol content (%, wt/vol) in the diet was 5.0 (36% of total calories) for the first 3 wk and increased by 0.25% every 2 wk to reach 6.3 (44% of total calories), which was maintained for the last 8 wk. The amount of food given to the pair-fed rats was the same that the alcohol-fed rats consumed in the previous day. At the end of 5 mo of feeding, rats were anesthetized with inhalational isoflurane. The left lobe of the liver was collected for the organelle isolation process, and the rest of the livers were fixed for pathology or stored at −80°C.
Histopathology analysis of liver.
Liver tissues were fixed in 10% formalin and processed for paraffin embedding. Paraffin sections were cut at 5 μm and stained with hematoxylin and eosin to assess the histological features of steatosis and inflammation.
ALT and AST activities.
The alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in the serum were measured with an Infinity kit (Thermo Scientific, Waltham, MA).
Subcellular compartments isolation.
Liver was perfused with buffer A (0.25 M sucrose, 10.00 mM Hepe-NaOH, and 0.25 M KCl, pH 7.8), and the left lobe was removed and weighed (around 5 g). After being minced with scissors, the left lobe of the liver was homogenized with 40 ml dounce All-Glass tissue grinder (Kimble Chase, Vineland, NJ) in 20 ml of buffer A. The homogenates were centrifuged at 700 g for 5 min at 4°C with Allegra X-22R centrifuge (Beckman Coulter, Brea, CA). The pellets were crude nuclei, which were purified by the differential centrifugation process. Briefly, 60% Opti-prep (Sigma-Aldrich, St. Louis, MO) was mixed with crude nuclei samples to reach homogenate in 25% Opti-prep. Thirty percent of Opti-prep and 35% Opti-prep were prepared by mixing with diluent solution (0.25 M sucrose and 60 mM Hepe-NaOH, pH 7.4). The solutions were carefully loaded in centrifuge tubes to three layers and centrifuged at 13,000 g for 1.5 h at 4°C with Sorvall RC6 plus centrifuge (Thermo Scientific). The supernatant from the first centrifugation was then further centrifuged at 10,000 g for 15 min at 4°C with Sorvall RC6 plus. The pellets were crude mitochondria, which was then homogenated and mixed with Opti-prep to reach 36% Opti-prep. Ten percent and 30% Opti-prep were carefully loaded on top of it. The centrifuge process was carried out at 50,000 g for 4 h at 4°C with Sorvall WX ultra series. Meanwhile, supernatants of 10,000 g centrifugation were centrifuged again at 100,000 g for 1 h at 4°C with Sorvall WX ultra series. The supernatant was cytosol, and the pellets were microsome. The microsomes were homogenized and mixed with 60% Opti-prep to prepare a homogenate in 20% Opti-prep. The differential centrifugation process was conducted with the help of 15% and 30% Opti-prep medium and centrifuge (Sorvall, WX ultra series) at 210,000 g for 2 h at 4°C.
Determination of zinc concentrations in the liver, plasma, and subcellular compartments.
Zinc concentrations in the liver, plasma, and subcellular compartments, namely ER, mitochondria, cytosol, and nuclei, were determined by inductively coupled plasma mass spectrometry (ICP-MS). Twenty micrograms of liver, 100 μl of plasma, purified ER, mitochondria, cytosol, and nuclei were frozen in liquid nitrogen and subsequently lyophilized for 1.5 days. Dried samples were removed from the microcentrifuge tubes and added to microwave digester vessels. The mass of sample added to the digesters was recorded and used to normalize the concentration. The samples in the vessels were then digested with 5 ml of concentrated (69%) nitric acid (HNO3) and dried under a gentle flow of N2 for 1 day. After being dried, samples were reconstituted in 5 ml of 3% HNO3 in Nanopure H2O. An Agilent 7500cx ICP-MS platform was used to develop a standard curve and to subsequently analyze the digested samples. The zinc concentrations in samples were calculated as micrograms per gram of dry weight.
Immunoblot analysis.
Liver tissue, ER, or mitochondrial proteins were extracted by tissue protein extraction reagent (Thermo Scientific) containing protease inhibitors (Sigma-Aldrich). Aliquots containing 30 μg protein were loaded onto a 10–15% SDS-PAGE. After electrophoresis, proteins were transferred to a PVDF membrane and probed with polyclonal antibodies against Bax, ZIP7, ZnT5, ZnT6, ZnT10 (Santa Cruz Biotechnologies, Santa Cruz, CA), phosphorylated eukaryotic initiation factor α (p-eIF-α), eIF-α, activating transcription factor 4 (ATF-4), Chop, ZIP1, ZIP4, ZIP5, ZIP14, ZnT4 (Novus Biological, Littleton, CO), ZIP8, ZnT7 (Proteintech, Chicago, IL), cytochrome c (Abcam, Cambridge, MA), and β-actin (Sigma-Aldrich), respectively. The membrane was then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, goat anti-mouse IgG, or rabbit anti-goat IgG antibody. The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Detection of hepatic and rat hepatoma cell cleaved caspase-3.
Hepatic cleaved caspase-3 levels were detected by immunohistochemical staining. Briefly, liver tissue paraffin sections were incubated with 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidases. Tissue sections were then incubated with a polyclonal rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology, Danvers, MA) at 4°C overnight, followed by incubation with EnVision+-labeled polymer-HRP-conjugated anti-rabbit IgG (DAKO, Carpinteria, CA) at room temperature for 30 min. Diaminobenzidine (DAB) was used as HRP substrate for visualization. The positive staining area was quantified with Image J, and the data were expressed as percentage of positive staining area to the total area.
Rat H4IIEC3 hepatoma cells grown on slide chambers (Lab-Tek, Hatfield, PA) were detected by immunofluorescence staining. Briefly, cells were fixed with ice-cold methanol for 10 min and then incubated with polyclonal rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology) at 4°C overnight, followed by incubation with Alexa Fluor 594-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) for 30 min.
TUNEL assay.
Apoptotic cell death in the liver and cell culture was assessed by detection of DNA fragmentation using an ApopTag peroxidase in situ apoptosis detection kit (Millipore, Billerica, MA). Briefly, cell slides were fixed with 1% paraformaldehyde for 5 min. Liver tissue slides or cell slides were then pretreated with proteinase K and H2O2 and incubated with the reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and digoxigenin-conjugated dUTP for 1 h at 37°C. The labeled DNA was visualized with either HRP-conjugated anti-digoxigenin antibody with DAB as the chromogen followed by counterstaining with methyl green or Alexa Fluor 594-conjugated IgG anti-digoxigenin antibody (Jackson ImmunoResearch) followed by DAPI counterstaining. The TdT dUTP-mediated nick-end labeling (TUNEL)-positive cells were counted under ×20 objective, and the data were expressed as the average number of TUNEL-positive cells per view.
Cell culture and treatment.
Rat H4IIEC3 hepatoma cells obtained from the American Type Culture Collection (Manassas, VA) were grown in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and penicillin (100 U/ml) streptomycin sulfate (100 μg/ml) (Invitrogen). H4IIEC3 hepatoma cells were seeded at 8 × 105 cells per well for 6-well plates, 3 × 105 cells per well for 12-well plates, or 1 × 105 cells per well for 8-well chamber slides overnight. Then cells were treated with 3.0 μM N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) (Sigma-Aldrich), 3.0 μM TPEN plus 10.0 μM zinc, 3.0 μM TPEN plus 25.0 μM zinc, 0.5 mM N-acetylcysteine (NAC) (Sigma-Aldrich), 1.0 mM NAC, 2.0 mM NAC, 3.0 mM TPEN plus 0.5 mM NAC, 3.0 μM TPEN plus 1.0 mM NAC, or 3.0 μM TPEN plus 2.0 mM NAC for 6 h in absence of FBS.
Fluorometric analysis of ROS.
Dihydroethidium (DHE; Life Technologies, Carlsbad, CA) is a superoxide indicator. After being uptaken by cells, DHE was oxidized to ethidium, which then intercalates into DNA and generates a bright red fluorescence. Live cells grown on 12-well plates were incubated with 5 μM DHE at 37°C for 30 min in the dark. The cells were trypsinized and washed twice with 1% BSA in PBS. The ROS generation was measured with microplate readers using excitation wavelength of 535 nm and an emission wavelength of 610 nm. The ROS production was expressed as the fluorescence ratio of the treated sample over the control.
Fluorescence microscopy.
Mitochondrial membrane potential was assessed in live cells by tetramethylrhodamine, ethyl ester (TMRE) mitochondrial kit (Abcam). TMRE is positively charged red-orange dye, which easily accumulates in active mitochondria. Depolarized or inactive mitochondria fail to sequester TMRE because of decreased membrane potential. Rat H4IIEC3 hepatoma cells were stained with 200 nM TMRE in culture medium at 37°C for 20 min. The strength and the distribution pattern of red fluorescence reflect the alteration of mitochondrial membrane potential among different treatments.
Cellular ROS was detected by CellROX Deep Red oxidative stress reagent (Life Technologies) in live cells. The reagent is nonfluorescent while in a reduced state and upon oxidation showed strong fluorogenic signals in cytoplasm. ER was detected by ER-Tracker green dye (Life Technologies), which is the drug that conjugates glibenclamide BODIPY FL. Glibenclamide binds to the sulphonylurea receptor of ATP-sensitive K+ channels, which are mainly on ER. Live cells were stained with 5 μM CellROX Deep Red at 37°C for 30 min, and then cells were either counterstained with 1 μM ER-tracker at 37°C for 20 min, or fixed with 3.7% formaldehyde for 15 min and counterstained with DAPI (Life Technologies).
Statistical analysis.
Results are expressed as means ± SD. Differences among multiple groups were analyzed by ANOVA followed by Tukey's test. Differences between two groups were analyzed by two-tailed Student's t-test. The significance between groups was defined as P < 0.05.
RESULTS
Body weight change and liver injury.
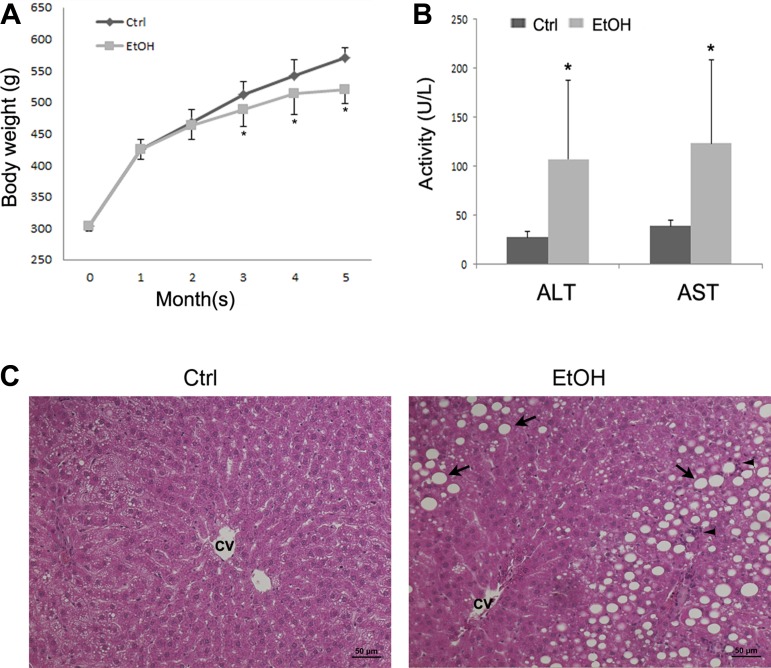
The average body weight of rats increased from 303.36 ± 8.21 g to 571.17 ± 15.38 g in control groups and 303.42 ± 8.52 g to 520.15 ± 23.12 g in ethanol group, respectively, after 5 mo of feeding (Fig. 1A). The body weight did not show significant difference between control and ethanol groups at 1 and 2 mo, whereas significant difference was found at and after 3 mo. Alcohol feeding also significantly increased the serum ALT activity (control 27.4 ± 6.3 U/l vs. ethanol 106.9 ± 80.6 U/l, P < 0.05) and AST activity (control 38.9 ± 6.4 U/l vs. ethanol 123.5 ± 85.3 U/l, P < 0.05) (Fig. 1B). Light microscopy revealed that alcohol feeding caused formation of lipid droplets in hepatocytes and increased the number of neutrophil cells in the rat liver (Fig. 1C).
Fig. 1.
Body weight change, plasma markers of liver injury, and liver histopathology in rats chronically fed ethanol (EtOH) or control (Ctrl) liquid diet for 5 mo. A: body weight change. B: plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. Plasma ALT and AST activities were measured using Infinity ALT and AST reagents. C: light microscopy with hematoxylin and eosin staining shows accumulation of lipid droplet (arrows) and neutrophil infiltration (arrow heads) in the liver of ethanol-fed rat. CV: central vein. Scale bar = 50 μM. Results are means ± SD (n = 6). Significant differences (*P < 0.05) between control and ethanol-fed rats were determined by Student's t-test.
Effect of alcohol feeding on subcellular zinc levels and zinc transporters.
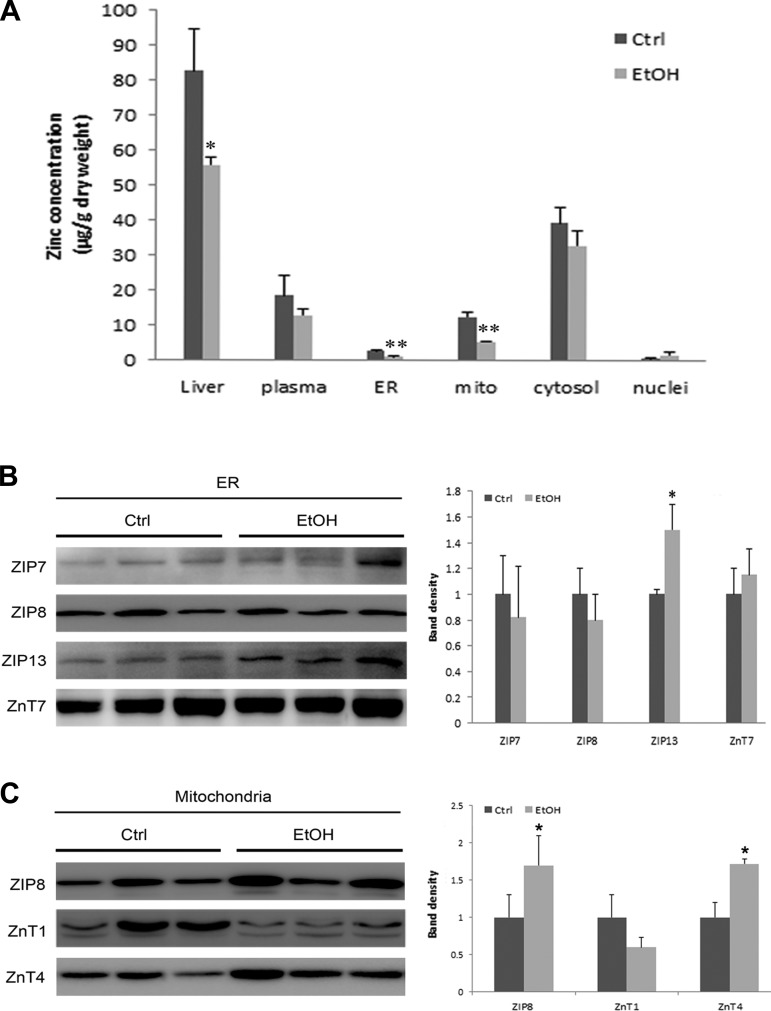
Chronic alcohol exposure significantly decreased total zinc levels in liver but differentially affected zinc levels in subcellular compartments. As shown in Fig. 2A, the zinc levels in liver decreased from 82.6 ± 11.8 μg/g to 55.37 ± 2.08 μg/g dry weight after 5 mo of alcohol feeding. Alcohol feeding also reduced the zinc level in isolated ER and mitochondria from 2.66 ± 0.3 μg/g to 0.9 ± 0.08 μg/g and from 12.4 ± 1.4 μg/g to 5.3 ± 0.3 μg/g dry weights, respectively. However, alcohol feeding did not significantly affect the zinc level in cytosol or nuclei. In addition, plasma zinc level was not affected by alcohol feeding.
Fig. 2.
Subcellular zinc levels and protein levels of zinc transporters in rats chronically fed ethanol (EtOH) or control (Ctrl) liquid diet for 5 mo. A: zinc levels in the whole liver, plasma, and isolated endoplasmic reticulum (ER), mitochondria (mito), cytosol, and nuclei of hepatocytes were measured by inductively coupled plasma mass spectrometry. Results are expressed as means ± SD (n = 6). B: immunoblot of ER zinc transporter proteins. ZnT, zinc transporter; ZIP, ER Zrt/Irt-like protein. C: immunoblot of mitochondrial zinc transporter proteins. The bands were quantified by densitometry analysis. The ratio to the total ER or mitochondrial proteins was calculated by setting the value of control as 1. Significant differences (*P < 0.05, **P < 0.01) between control and ethanol-fed rats were determined by Student's t-test.
To determine which zinc transporters are localized in ER and mitochondria and which ones are responsible for alcohol feeding-induced zinc dyshomeostasis, 12 zinc transporters (ZIP1, 4, 5, 7, 8, 13, and 14 and ZnT1, 4, 6, 7, and 10) were examined by immunoblot. As shown in Fig. 2B, ZIP7, ZIP8, ZIP13, and ZnT7 were detected in ER, and only ZIP13 showed a significant increase after chronic alcohol feeding. Meanwhile, three zinc transporters, ZIP8, ZnT1, and ZnT4, were detected in mitochondria (Fig. 2C). ZIP8 and ZnT4 were significantly upregulated at protein levels after alcohol exposure.
Alcohol feeding induced ER stress, mitochondria dysfunction, and hepatocyte apoptosis.
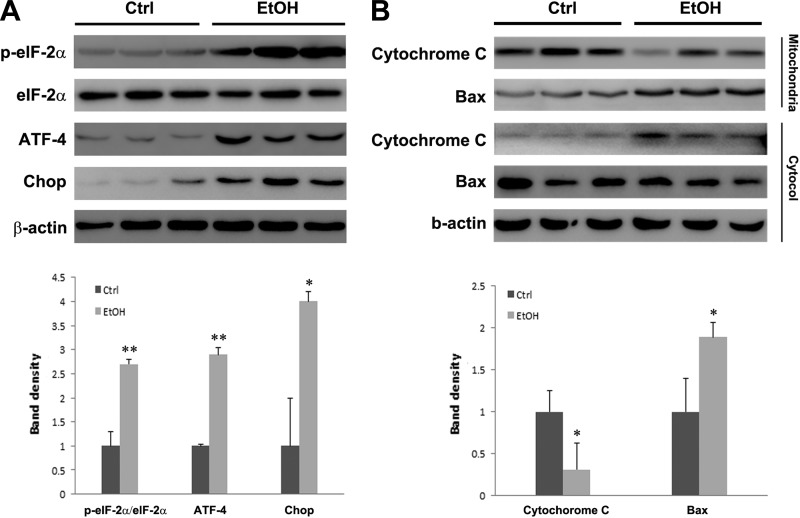
To determine whether cell death pathways were activated in association with zinc deficiency, ER and mitochondrial cell death pathway markers were examined. As shown in Fig. 3A, alcohol feeding significantly increased p-eIF-2α-to-eIF-2α ratio and protein levels of ATF-4 and Chop. Cytochrome c and Bcl-2-associated X protein (Bax) levels were determined in both mitochondria and cytosol. Figure 3B shows that alcohol feeding remarkably increased mitochondrial Bax insertion and cytosolic cytochrome c release, which indicates activation of intrinsic apoptotic pathway.
Fig. 3.
Protein levels of ER stress and mitochondrial apoptosis markers. Rats were chronically fed ethanol (EtOH) or control (Ctrl) liquid diet for 5 mo. A: immunoblot of ER stress makers. p-eIF-2α, phosphorylated eukaryotic initiation factor 2α; ATF-4, activating transcription factor 4; Chop, C/EBP homologous protein. B: immunoblot of mitochondrial and cytosolic cytochrome c and Bcl-2-associated X protein (Bax). The bands were quantified by densitometry analysis. The ratio to β-actin (A) or the ratio to mitochondrial proteins to cytosolic proteins (B) was calculated by setting the value of control as 1. Significant differences (*P < 0.05, **P < 0.01) between control and ethanol-fed rats were determined by Student's t-test.
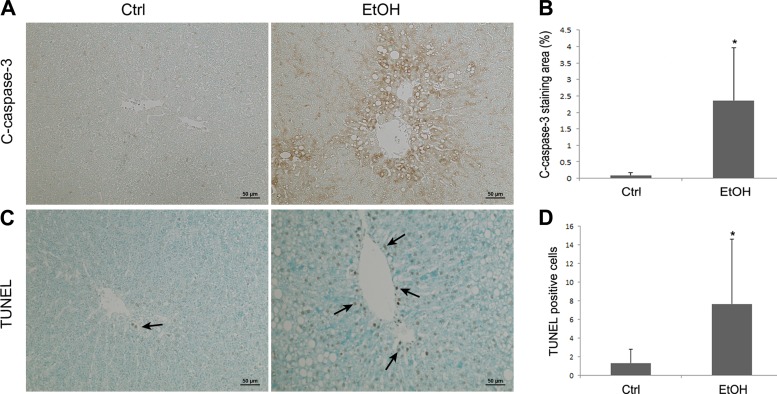
Apoptosis was then evaluated by measuring cleaved caspase-3 and DNA fragmentation. Hepatic cleaved caspase-3 was determined by immunohistochemistry. As shown in Fig. 4A, the cleaved caspase-3 staining was faint in the liver of controls, but alcohol feeding enhanced the staining, especially around the vein areas. The image quantification analysis (Fig. 4B) shows that alcohol feeding significantly increased expression of cleaved caspase-3 in the liver compared with controls. The number of hepatic apoptotic cells was evaluated by TUNEL assay. As shown in Fig. 4, C and D, alcohol exposure remarkably increased the number of TUNEL-positive cells (dark brown color) in the liver.
Fig. 4.
Immunohistochemical staining of cleaved caspase-3 and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay in the rat liver. Rats were chronically fed with ethanol (EtOH) or control (Ctrl) liquid diet for 5 mo. A: representative image of immunostaining of cleaved caspase-3 (arrows). B: quantitative measurements of cleaved-caspase-3 in the liver. C: representative image of TUNEL assay. Arrows indicate TUNEL-positive cells. D: quantitative measurements of TUNEL-positive cells in liver. Scale bar = 50 μm. Results are means ± SD (n = 6). Significant differences (*P < 0.05) between control and ethanol-fed rats were determined by Student's t-test.
Experimental zinc deprivation induced apoptosis, mitochondrial depolarization, and ER stress in rat hepatoma cells.
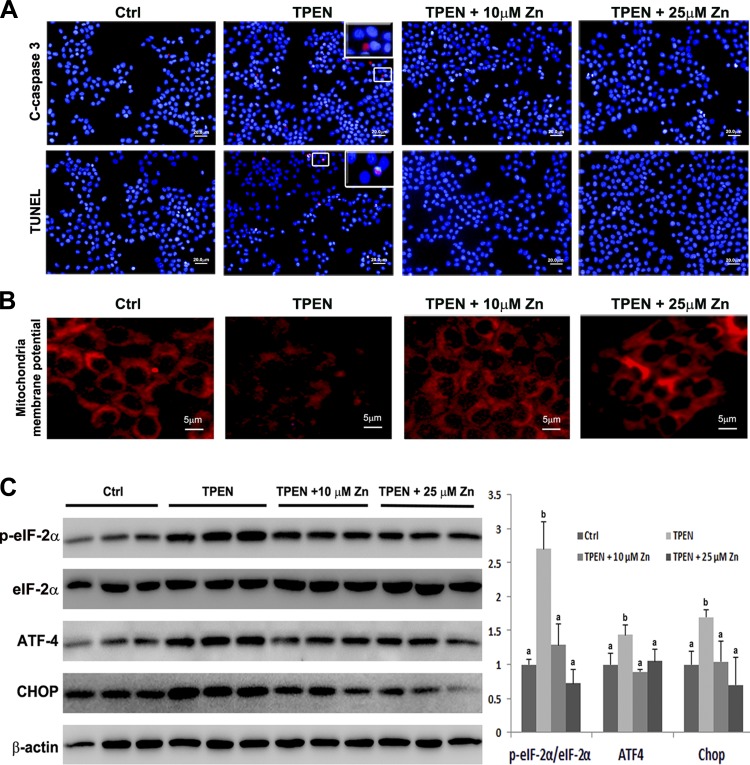
To determine the link of zinc deprivation and hepatocyte apoptosis, rat H4IIEC3 hepatoma cells were treated with zinc chelator, TPEN, for 6 h. Activation of cleaved caspase-3 and an increased number of TUNEL-positive cells were observed by fluorescence microscopy (Fig. 5A). Meanwhile, both 10 μM and 25 μM zinc treatment along with TPEN challenge inactivated cleaved caspase-3 and reduced the number of TUNEL-positive cells (Fig. 5A), indicating a specific chelation of zinc by TPEN.
Fig. 5.
Effects of N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) and zinc supplementation on ER and mitochondrial cell death signaling in rat hepatoma cells. H4IIEC3 cells were treated with 3 μM TPEN with or without 10 μM or 25 μM Zn for 6 h. A: representative images of immunofluorescence staining of cleaved caspase-3 and TUNEL assay of apoptosis. Insets: enlarged image of the framed area. B: representative images of fluorescence staining of mitochondrial membrane potential. C: immunoblot of ER stress markers. The band density was quantified by densitometry analysis. The ratio to β-actin was calculated by setting the value of Ctrl as 1. Results for bars that do not share a letter differed significantly among groups (P < 0.05). Significant differences among groups are determined by ANOVA followed by Tukey's test. Scale bar = 20 μm (A); scale bar = 5 μm (B).
Mitochondrial membrane potential and ER stress were then evaluated to determine whether zinc deprivation activates ER- and mitochondria-mediated intrinsic apoptotic pathway. As shown in Fig. 5B, 6 h of 3 μM TPEN treatment reduced mitochondrial membrane potentials as indicated by decreased red fluorescence intensity, which was prevented by zinc supplementation. TPEN treatment significantly increased p-eIF-2α-to-eIF-2α ratio and the protein levels of ATF-4 and Chop, and zinc supplementation reversed the elevation of the ER stress makers (Fig. 5C).
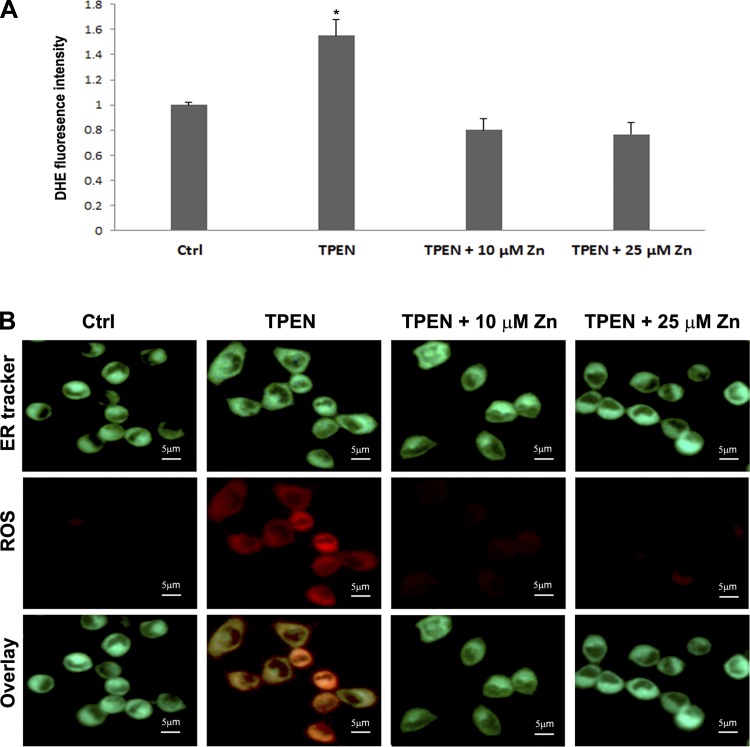
Fig. 6.
Effects of TPEN and zinc supplementation on generation of reactive oxygen species (ROS). H4IIEC3 cells were treated with 3 μM TPEN with or without 10 μM or 25 μM Zn for 6 h. A: ROS were quantified by dihydroethidium (DHE) fluorescence intensity. Results are expressed as means ± SD (n = 4). Significant differences (*P < 0.05) among groups are determined by ANOVA followed by Tukey's test. B: representative images of double fluorescence staining of ROS and ER. ROS were detected by CellROX Deep Red oxidative stress reagent; ER was detected by ER Tracker. Scale bar = 5 μm.
Zinc deprivation induced ROS accumulation in hepatoma cells.
To examine whether zinc deprivation induces oxidative stress, ROS were detected by DHE fluorescence staining. Figure 6A shows that TPEN treatment significantly increased ROS production compared with control, and zinc supplementation at either 10 μM or 25 μM prevented ROS accumulation. Localization of ROS in ER was determined by double staining of ROS and ER marker. Figure 6B shows that TPEN induced oxidative stress in ER, which was inhibited by zinc supplementation.
ROS only partially mediated the proapoptotic effect of zinc deprivation in hepatoma cells.
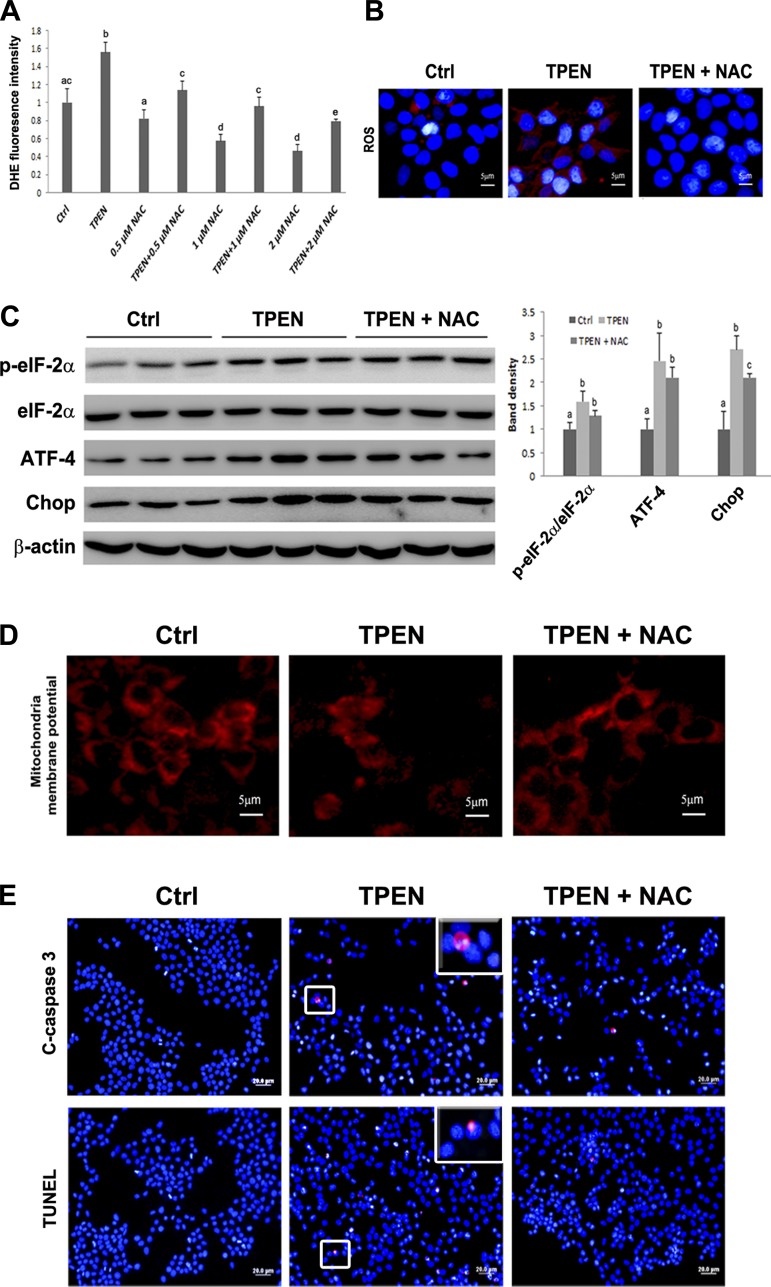
To determine whether ROS mediates zinc deprivation-activated ER and mitochondrial cell death signal pathways, rat hepatoma cells were treated with TPEN with or without antioxidant, NAC, at 0.5, 1.0, and 2.0 mM. ROS were measured by fluorescence spectrometry (Fig. 7A) and microscopy (Fig. 7B). NAC at 2 mM completely blocked TPEN-induced ROS accumulation.
Fig. 7.
Effects of N-acetylcysteine (NAC) on TPEN induced ER and mitochondrial cell death signaling. A: H4IIEC3 cells were treated with 0.0, 0.5, 1.0, or 2.0 mM NAC with or without 3.0 μM TPEN for 6 h. ROS production was measured by DHE fluorescence intensity. B: H4IIEC3 cells were treated with 3 μM TPEN with or without 2 mM NAC. Representative images of ROS staining. ROS was detected by CellROX Deep Red oxidative stress reagent. C: immunoblot of ER stress markers. The bands were quantified by densitometry analysis. The ratio to β-actin was calculated by setting the value of control (Ctrl) as 1. Results for bars that do not share a letter differed significantly among groups (P < 0.05). Significant differences among groups are determined by ANOVA followed by Tukey's test. D: representative images of fluorescence staining of mitochondrial membrane potential. E: representative images of immunofluorescence staining of cleaved caspase-3 and TUNEL assay. Scale bar = 5 μm (B and C); scale bar = 20 μm (E).
The ER stress and mitochondrial membrane potential were then assessed. As illustrated in Fig. 7C, among the ER markers tested, NAC at 2 mM only partially inhibited TPEN-increased Chop protein level. NAC treatment also partially reversed TPEN-induced loss of mitochondrial membrane potential (Fig. 7D).
Lastly, caspase-3 activation and apoptotic cell death were measured to determine whether NAC treatment could inhibit TPEN-induced apoptosis. As shown in Fig. 7E, 2 mM NAC treatment partially decreased TPEN-induced activation of caspase-3 and the number of TUNEL-positive cells. The results indicated that NAC could not completely rescue TPEN-induced ER- and mitochondria-mediated apoptosis in H4IIEC3 hepatoma cells.
DISCUSSION
The present study, not only presented the phenomenon that chronic alcohol exposure decreased hepatic zinc levels, but also showed for the first time that the subcellular, ER and mitochondria, zinc levels were disturbed. The results indicate that, compared with nuclei and cytosol, ER and mitochondria are the two organelles most likely responsible for alcohol consumption-induced hepatic zinc deficiency. It is known that chronic alcohol drinking activates the microsomal ethanol oxidizing system (MEOS) to accelerate ethanol clearance. In addition, mitochondria are the main site for acetaldehyde oxidation. As a result, it is not surprising that ER and mitochondria are the organelles most vulnerable to prolonged alcohol consumption.
Along with activation of MEOS and increased oxidation burden of mitochondria is the elevation of ROS production. It has been reported that alcohol drinking disturbed hepatic zinc transporters in mice, which might result from increased ROS (30). Consistent with that observation, we found alteration of zinc transporters in hepatic ER and mitochondrial membranes in rats after alcohol consumption. Four zinc transporters (ZIP7, ZIP8, ZIP13, and ZnT7) were detected in ER membrane, and ZIP13 was significantly increased by alcohol consumption. Three zinc transporters (ZIP8, ZnT1, and ZnT4) were detected in mitochondrial membrane, and ZIP8 and ZnT4 significantly increased with alcohol consumption. The altered expression of ZIP13 and ZIP8 may be caused by increased oxidative stress attributable to alcohol metabolism (30). However, the upregulation of ZnT4 may be the adaptive response to reverse the reduction of zinc level in mitochondria. However, we did not find upregulation of any ZnT proteins in ER to correct the zinc level. Other mechanisms may also be involved in decreasing zinc levels in ER and mitochondria, such as altered activity of zinc transporters and/or decreased zinc absorption from intestine. The alteration of spatial structure could decrease zinc-binding activity. It has been shown that alcohol-induced ROS inactivate zinc proteins, such as hepatocyte nuclear factor-4 and peroxisome proliferator-activated receptor-α (15). Therefore, further studies are required to confirm whether the spatial structure and activity of zinc transporters are affected by chronic alcohol exposure. Besides, our previous study has shown that chronic alcohol exposure induced intestinal barrier dysfunction and reduced zinc level of the ileum (36). Thus impaired intestinal zinc absorption may be another cause of hepatic zinc reduction and subsequent hepatic subcellular zinc dyshomeostasis. Although hepatic zinc deficiency has been well documented in patients with ALD (1, 6), mechanisms of how alcohol interferes with hepatic zinc homeostasis are poorly understood. The present study, not only revealed a differential effect of alcohol on the zinc level in subcellular compartments, but also demonstrated dysregulation of subcellular zinc transporters after chronic alcohol exposure. To the best of our knowledge, this is the first report on subcellular distribution of zinc transporters in the liver regardless of alcohol exposure. Although ZIP4 has been detected in human liver at the tissue level (31, 32), information on subcellular distribution of zinc transporters in human liver is not available. Therefore, one limitation of the study is that the data of hepatic zinc transporters lack support by human data. Our research group is very interested in obtaining the human data on subcellular distribution of zinc transporters and subcellular zinc concentrations at normal and ALD conditions in a future study.
Increased apoptosis is one of the major mechanisms involved in the progression of ALD. The present study showed that alcohol exposure increased the number of apoptotic cells in rat liver, and the in vitro study demonstrated that zinc deprivation activates apoptotic cell death signaling. It is known that extrinsic and intrinsic signals can both initiate apoptotic mechanisms. Extrinsic apoptotic pathway is activated by external death signals, namely through TNF-R1/TNF-α and Fas/FasL system; however, intrinsic apoptotic pathway activation depends on morphology change of mitochondria, such as increased permeability and loss of membrane potential (2, 22). Moreover, studies showed that ER stress-induced upregulation of Chop is one way to initiate mitochondria-mediated apoptotic pathway (14). Therefore, ER could be the site in triggering intrinsic apoptosis. It has been reported that chronic alcohol drinking activated the extrinsic apoptotic pathway (37, 39). However, whether zinc deprivation plays a role in activating ER- and mitochondria-mediated intrinsic apoptotic pathway is still unclear. In the present study, ER stress and mitochondrial cytochrome c release were evaluated. The results indicated that the protein expression of Chop was increased and mitochondrial cytochrome c was released into cytosol; meanwhile the caspase-3 was activated after 5 mo of alcohol feeding. The in vitro zinc deprivation study showed Chop upregulation, loss of mitochondrial membrane potential, and increased cleaved caspase-3. Although, in the present study, zinc supplementation was not given to rats to evaluate the protective effect of zinc on the expression of ER stress markers and mitochondrial dysfunction protein, a previous study from our group has shown that zinc supplementation attenuates hepatic apoptosis through inhibition of cell death receptor (TNFR1 and Fas)-mediated apoptotic pathway in mice chronically fed alcohol (39). In contrast, feeding mice a zinc-deficient diet, in our previous study, exaggerated chronic alcohol exposure-induced liver injury in association with upregulation of hepatic cell death receptors, TNFR1 and CD95 (37). Another report from our group further showed that zinc supplementation attenuates hepatic oxidative stress, leading to significant improvement of the ultrastructure of ER and mitochondria of hepatocytes in mice chronically fed alcohol (40). These studies indicate that hepatic zinc homeostasis plays a critical role in the regulation of alcohol-induced pathogenesis of hepatocellular apoptosis. Therefore, it is predictable that protection of the integrity of mitochondrial membrane by zinc supplementation may lead to inhibition of alcohol-induced Bax insertion to mitochondria and suppression of alcohol-induced cytochrome c release from mitochondria. Besides, ROS are important inducers of ER stress (13), and inhibition of oxidative stress by zinc supplementation would ameliorate alcohol-induced ER stress and consequent cell death signaling. Further investigations are required to confirm the protective effects of zinc supplementation on mitochondria- and ER-mediated cell death pathways. The results suggest that zinc deprivation-induced ER stress could trigger mitochondria-mediated intrinsic apoptotic pathway. However, we do not rule out the possibility that zinc deprivation directly affects mitochondrial function, and the enhanced apoptotic process is the synergistic effect of ER and mitochondrial dysfunction.
Decreased zinc level is associated with increased oxidative stress. The present study demonstrates that zinc deprivation increased the production of ROS; however, antioxidant, NAC, treatment did not completely reverse zinc withdrawal-caused hepatoma cell apoptosis. It has been found that TPEN challenge decreased the glutathione level in rat primary hepatocytes (26). In addition, Pelicci et al. (24) reported that increased oxidative stress induced apoptosis through p66Shc stress adaptor protein. Therefore, in the present study, we tested whether zinc deprivation-induced ROS is a key mechanism in apoptosis. The results indicate that zinc deprivation-induced apoptosis is partially dependent on oxidative stress. There are two possible explanations for the partial failure of antioxidant treatment in rescuing zinc deficiency-induced hepatic cell apoptosis. First, that zinc deprivation caused activation of caspase-3 is probably the key factor in apoptosis. Kaufmann et al. (23) reported that, in a cell-free system, the presence of cytochrome c and ATP activates caspase-3, -6, and -7, and adding zinc to the system inhibited the activation of the above caspase species (23). Besides, studies (18, 26, 29) measured cleaved caspase-3 activity after TPEN challenge and NAC treatment, and they found that NAC treatment did not reverse TPEN-induced activation of caspase-3. Second, zinc deprivation-induced ER stress does not completely result from ROS. It is well known that zinc plays a role in maintenance of catalytic and structural activity of proteins, signal transduction, and antioxidant defense (5, 21, 33). Therefore, it is most likely that zinc deprivation-induced ER stress is caused by a series of events instead of a single factor. It has been reported that zinc deficiency conditions affect the structure of a zinc-requiring enzyme, superoxide dismutase 1, which induces ER stress response (9). In addition, Younce et al. (35) demonstrated that monocyte chemotactic protein-1 (MCP-1)-induced protein, a zinc finger protein caused ER stress in cardiomyoblasts (35). Therefore, zinc proteins may be involved in zinc deprivation-induced ER stress and the consequent apoptosis. The results suggest that other mechanisms may be involved in zinc deprivation-induced ER stress and mitochondrial dysfunction besides increased oxidative stress.
In summary, we demonstrated, for the first time, that alcohol-induced hepatic zinc deficiency might be from ER and mitochondrial zinc reduction, and the reduced zinc levels in ER and mitochondria caused organelle dysfunction, including ER stress, loss of mitochondrial membrane potential, and increase of mitochondria permeability. The interruption of normal subcellular compartment function led to activation of caspase-3 and excessive hepatocyte apoptosis. The pathogenic process was partially dependent on ROS.
GRANTS
This research was supported by the National Institutes of Health (R01AA020212 and R01AA018844).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.S. and Z.Z. conception and design of research; Q.S., W. Zhong, W. Zhang, Q.L., Xiuhua Sun, X.T., Xinguo Sun, and D.D. performed experiments; Q.S. analyzed data; Q.S. and Z.Z. interpreted results of experiments; Q.S. prepared figures; Q.S. drafted manuscript; Q.S., W. Zhong, W. Zhang, Q.L., Xiuhua Sun, X.T., and Z.Z. edited and revised manuscript; Q.S., W. Zhong, W. Zhang, Q.L., Xiuhua Sun, X.T., Xinguo Sun, D.D., and Z.Z. approved final version of manuscript.
REFERENCES
- 1.Bartholomay AF, Robin ED, Vallee RL, Wacker WE. Zinc metabolism in hepatic dysfunction. I. Serum zinc concentrations in Laennec's cirrhosis and their validation by sequential analysis. N Engl J Med 255: 403–408, 1956. [DOI] [PubMed] [Google Scholar]
- 2.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol 21: 871–877, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Cederbaum AI. Alcohol metabolism. Clin Liver Dis 16: 667–685, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA 102: 3401–3406, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem 16: 1123–1134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goode HF, Kelleher J, Walker BE. Relation between zinc status and hepatic functional reserve in patients with liver disease. Gut 31: 694–697, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groth C, Sasamura T, Khanna MR, Whitley M, Fortini ME. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development 140: 3018–3027, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hentze H, Kunstle G, Volbracht C, Ertel W, Wendel A. CD95-mediated murine hepatic apoptosis requires an intact glutathione status. Hepatology 30: 177–185, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Homma K, Fujisawa T, Tsuburaya N, Yamaguchi N, Kadowaki H, Takeda K, Nishitoh H, Matsuzawa A, Naguro I, Ichijo H. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell 52: 75–86, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Tepaamorndech S. The SLC30 family of zinc transporters—a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 34: 548–560, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med 34: 612–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int 2012: 216450, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol 2014: 513787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 29: 1496–1503, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology 50: 1241–1250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, Usukura J, Soji T, Wakabayashi T. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med 26: 396–409, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Lopez JJ, Redondo PC, Salido GM, Pariente JA, Rosado JA. N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine induces apoptosis through the activation of caspases-3 and -8 in human platelets. A role for endoplasmic reticulum stress. J Thromb Haemost 7: 992–999, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 54: 795–809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health 27: 209–219, 2003. [PMC free article] [PubMed] [Google Scholar]
- 21.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev 109: 4682–4707, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21: 92–101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesner PW Jr, Bible KC, Martins LM, Kottke TJ, Srinivasula SM, Svingen PA, Chilcote TJ, Basi GS, Tung JS, Krajewski S, Reed JC, Alnemri ES, Earnshaw WC, and Kaufmann SH. Characterization of caspase processing and activation in HL-60 cell cytosol under cell-free conditions. Nucleotide requirement and inhibitor profile. J Biol Chem 274: 22635–22645, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio E, Giorgio M, Pelicci PG. Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal 8: 600–608, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract 27: 8–20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatani T, Tawaramoto M, Opare Kennedy D, Kojima A, Matsui-Yuasa I. Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular reduced glutathione level in rat hepatocytes. Chem Biol Interact 125: 151–163, 2000. [DOI] [PubMed] [Google Scholar]
- 27.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol 105: 14–32; quiz 33, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res 33: 191–205, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolf E, Cervinka M. Depletion of endogenous zinc stores induces oxidative stress and cell death in human melanoma cells. Acta Medica (Hradec Kralove) 47: 91–96, 2004. [PubMed] [Google Scholar]
- 30.Sun Q, Li Q, Zhong W, Zhang J, Sun X, Tan X, Yin X, Sun X, Zhang X, Zhou Z. Dysregulation of hepatic zinc transporters in a mouse model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 307: G313–G322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS One 5: e13158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Guo HJ, Xie HY, Li J, Zhuang RZ, Ling Q, Zhou L, Wei XY, Liu ZK, Ding SM, Chen KJ, Xu ZY, Zheng SS. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J Biol Sci 10: 245–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol 177: 637–645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz Y, Alahdab YO, Ozdogan O, Dolar E. Non-alcoholic fatty liver disease and microalbuminuria in non-diabetic patients: role of insulin resistance. Intern Med J 39: 709–710, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Younce CW, Kolattukudy PE. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem J 426: 43–53, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 298: G625–G633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One 8: e76522, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Kang X, Jiang Y, Song Z, Feng W, McClain CJ, Kang YJ. Preservation of hepatocyte nuclear factor-4alpha is associated with zinc protection against TNF-alpha hepatotoxicity in mice. Exp Biol Med (Maywood) 232: 622–628, 2007. [PubMed] [Google Scholar]
- 39.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 233: 540–548, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 166: 1681–1690, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]