Abstract
Considering the importance of drug permeation from formulations, in vitro and ex vivo drug permeation characteristics of three oral mucoadhesive suspensions of Ofloxacin were designed and compared. Three suspensions of Ofloxacin were prepared by taking two grades of Carbopol polymer such as Carbopol 934 (C934) and Carbopol 940 (C940); and Hydroxypropyl methylcellulose. The permeability study was performed by using a Franz diffusion cell through both synthetic cellulose acetate membrane and excised goat gastrointestinal membranes in acidic as well as alkaline pH. To know the permeability of the drug from control/formulations through different membranes in acidic/alkaline pH, cumulative percentage drug permeation, apparent permeability (Papp) and flux (J) were calculated. In addition, enhancement ratio (ER) of each formulation was also determined. From our results, it is evident that formulation containing C940 was the best suspension considering Papp and J values of all formulations. Moreover, it was the most beneficial formulation for improving permeation and diffusivity of Ofloxacin even after 16 h. Hence, this suspension was probably the most suitable formulation to obtain prolonged release action of the drug. The ER values of all formulations through the excised goat intestinal mucus membrane in alkaline pH were higher than those formulations through the goat stomach mucosal membrane in acidic pH. ER values of those formulations indicate that the permeability of the drug was more enhanced by the polymers in the intestinal part, leading to more bioavailability and prolonged action in that portion of the gastrointestinal tract. It may also be concluded from our results that in addition to formulation containing C940, other formulations may also show effective controlled release action.
Keywords: Ofloxacin, Permeability, C934, C940, HPMC
1. Introduction
Ofloxacin (Oflox) is a second generation fluoroquinolone antibacterial. It shows low solubility in aqueous solution and a high rate of absorption in the stomach. It is likely to be precipitated out of solution upon entry into the small intestine where the pH is alkaline. Oflox is considered to be a BCS Class III drug (highly soluble and low permeable) with permeability properties approaching the border to BCS Class I (highly soluble and high permeable). Controlled release formulations of Oflox would be effective in overcoming the dissolution/permeation limitations by slowing the drug supply from the intact matrix base so that more drug should be soluble and permeable in the small intestine. Thus, it is expected to increase controlled release action and to improve patient compliance with fewer side effects (Chavanpatil et al., 2005; Gangurde et al., 2011; Hosny, 2009; Sakore et al., 2010).
Taking into consideration of the above-mentioned factors, polymeric suspensions of Oflox were prepared by using two grades of mucoadhesive biodegradable environmentally responsive Carbopol polymer i.e., Carbopol934 (C934) and Carbopol940 (C940) (Bettini et al., 1995; Qiu and Park, 2001). Carbopol polymers have recently attracted considerable interest in the field of drug delivery (Galaev and Mattiasso, 1999; Jeong and Gutowska, 2001). The polymer can protect the drug from the physiological environment by improving its stability in vivo (Guo, 2003). Moreover, the gelling and mucoadhesive properties of Carbopol polymers, which are largely defined by their crosslinker levels, are also very important for drug permeation enhancement from different formulations. In addition to Carbopol polymers, polymeric suspension of Oflox was also prepared by using a hydrophilic polymer like Hydroxypropyl methylcellulose (HPMC). Due to nonionic, gelling and mucoadhesive properties of hydrophillic polymeric matrix systems, these are used in formulations to obtain desirable drug permeation and controlled release action (Shoaib et al., 2006).
Since the qualitative and quantitative changes in a formulation may alter drug permeation and in vivo performance, several bio-studies are usually carried out. In this regard, the use of in vitro and ex vivo drug permeation data to predict in vivo bio-performance can be considered as the rational development of controlled release formulations. As we know, in vitro permeabilities can also be used to predict in vivo bioavailability of a given drug substance. Moreover, this method is also suitable for verifying the mechanism of various penetration enhancers like polymers, or their interaction with the gastric mucosa (Singh et al., 2010). So, permeability study of the drug is having tremendous importance.
Considering the above-mentioned information, the aim of this study is to investigate Oflox permeation and diffusion (from control solution/formulations) using a Franz diffusion cell through synthetic cellulose acetate membrane, and excised goat stomach and intestinal mucosal membranes in both acidic and alkaline pH. This was done to examine the effect of different polymers on drug permeation. To know the in vitro and ex vivo permeability of the drug from control/formulations in the presence of different membranes and pH values, cumulative percentage drug permeation (%CDP), permeability coefficient or apparent permeability (Papp) and flux (J) were calculated. In addition, enhancement ratio (ER) of each formulation was also determined.
2. Materials and methods
2.1. Materials
The following materials were used: Oflox was obtained from Dr. Reddy’s Lab, Hyderabad, India, as a gift sample. C934, C940, Pluronic F 68 and Soya lecithin were purchased from Himedia Laboratories Pvt. Ltd., India. HPMC (HPMC E15 LV Premium) was supplied by Loba Chemie Pvt. Ltd., India. Glycerol, Methyl paraben sodium, Propyl paraben sodium, Sorbitol solution I.P. and Sucrose were obtained from Cosmo Chem. Laboratory, Pune, India.
2.2. Methods
2.2.1. Preparation of control
Oflox was dissolved in distilled water to prepare a control solution for the present study. The final concentration of the control solution was 10 mg/ml.
2.2.2. Preparation of formulations
Mucoadhesive formulations of Oflox were prepared as per the method described by Chakraborti et al. (2012). Moreover, mucoadhesive property of those suspensions was confirmed subsequently by Mukherjee et al. (2014).
2.2.3. Permeability study
In the present investigation, the permeability study of separately used optimized mucoadhesive formulations of Oflox was conducted using a static Franz diffusion cell (Hanson Research Corporation, USA). The Franz cell is a diffusion chamber, made of glass comprising of an upper donor compartment, which is open from above and a lower receptor (acceptor) compartment, which is closed from the bottom side. Between the compartments, the tissue was clamped with the mucosal side oriented upwards (Singh et al., 2010). The capacity of the receptor compartment was 22 ml. The area available for diffusion was about 3.90 cm2. The lower chamber, containing a sampling port, had a Teflon-coated needle at the base. The junction between the two compartments was designed to hold the mucosa in such a manner that the mucosa did not shift from its place once the dosage form was incorporated into it. The hooks were secured with rubber bands on the sides of both compartments. In this manner, the two compartments formed one single unit without leakage. The donor compartment of diffusion cell was filled separately with each formulation containing 250 μl of Oflox. The donor cell was covered with an aluminium foil to prevent evaporation of vehicle. The fluid, in the receptor compartment, was maintained at 37 ± 0.5 °C and stirred continuously at a very low speed (30 rpm), using thermostatically controlled magnetic stirrer with Teflon coated bead. The external jacket of Franz diffusion cell was connected to a water bath so as to maintain temperature in cell. The excised goat stomach mucosal membrane was mounted between the half cells, keeping contact with the receptor fluid at pH 1.2 acidic buffer. However, the excised goat intestinal mucosal membrane was mounted similarly with the receptor fluid at pH 7.2 phosphate buffer. Care was taken to make sure that no air bubbles were present inside the receptor compartment.
Aliquot (500 μl each time) was withdrawn periodically at preset time from the above mentioned receiver cell, which was 20 times diluted and filtered through 0.2 μm filter. Oflox content was determined by UV spectrophotometer at 294 nm. The diffusion fluid of same volume was prewarmed at 37 °C. The volume of withdrawn sample was replaced by prewarmed diffusion fluid into the diffusion cell to keep the volume constant so that sink condition could be maintained. Experiment was carried out up to 16 h with the excised stomach/intestinal mucosal membranes. The rates of drug permeation at different time points were calculated in each case.
2.2.3.1. Data analysis
Data, obtained from the permeability study for each formulation (such as F1 - Oflox and C934, F2 - Oflox and C940, and F3 - Oflox and HPMC; and solution of the pure drug, Fo - control) were used to calculate cumulative drug permeation (CDP), %CDP (mean ± standard deviation), Papp, J, and ER. Papp, J, and ER were calculated by following the standard formulae (Haigh and Smith, 1994; Patel et al., 2010; Singh et al., 2010).
| Permeability coefficient (apparent permeability) - |
| Papp = (VA/Area × time) × ([drug]acceptor/[drug]donor]); |
| where, VA = volume in acceptor compartment |
| Area = surface area of the intestinal membrane |
| Time = total transport time |
| Flux - |
| J = Papp × CD; |
| where, CD = concentration of donor solution. |
| Enhancement ratio - |
| ER = Papp of formulation/Papp of control |
3. Results
In vitro permeability study of different formulations of Oflox, such as F1 (Oflox and C934), F2 (Oflox and C940), and F3 (Oflox and HPMC); and solution of the pure drug, Fo (control), was done by Franz diffusion cell, using cellulose acetate membrane and acidic buffer of pH 1.2 as diffusional fluid at different time points up to 6 h. The results of this investigation have been shown in Table 1. The values of %CDP, Papp, and J were also calculated after 6 h in cases of all samples. These values were found to be least/minimum in case of the formulation F3, while F1 showed maximum values. However, in phosphate buffer, formulation F1 displayed minimum values, whereas they were maximum in case of F3 (Tables 1 and 2). The above-mentioned results have also been depicted in different graphs by taking %CDP at different time points versus time (Figs. 1 and 2). In addition, bar diagrams (by taking Papp of different samples of Oflox) also indicate the results of the present investigation (Figs. 3 and 4).
Table 1.
%CDP ± SD of Oflox from control and three suspensions of Oflox using synthetic membrane.
| Time (h) | Acidic buffer (pH 1.2) |
Phosphate buffer (pH 7.2) |
||||||
|---|---|---|---|---|---|---|---|---|
| aFo | bF1 | cF2 | dF3 | Fo | F1 | F2 | F3 | |
| 0.25 | 19.34 ± 0.01 | 16.30 ± 0.05 | 18.03 ± 0.03 | 18.47 ± 0.01 | 01.30 ± 0.02 | 1.30 ± 0.05 | 1.52 ± 0.04 | 1.73 ± 0.01 |
| 0.50 | 35.42 ± 0.03 | 20.21 ± 0.01 | 25.20 ± 0.01 | 22.38 ± 0.02 | 01.95 ± 0.03 | 2.60 ± 0.04 | 3.25 ± 0.02 | 3.04 ± 0.03 |
| 0.75 | 47.80 ± 0.01 | 28.13 ± 0.02 | 29.12 ± 0.04 | 27.16 ± 0.05 | 02.17 ± 0.01 | 4.78 ± 0.01 | 5.43 ± 0.01 | 4.12 ± 0.02 |
| 1.00 | 55.84 ± 0.04 | 44.33 ± 0.04 | 48.67 ± 0.02 | 45.41 ± 0.03 | 03.04 ± 0.01 | 6.51 ± 0.02 | 6.95 ± 0.03 | 6.30 ± 0.01 |
| 1.25 | 60.41 ± 0.05 | 50.19 ± 0.03 | 56.05 ± 0.01 | 54.97 ± 0.01 | 03.91 ± 0.04 | 8.25 ± 0.01 | 8.69 ± 0.01 | 8.69 ± 0.04 |
| 1.50 | 65.40 ± 0.02 | 53.67 ± 0.01 | 59.97 ± 0.03 | 56.49 ± 0.04 | 04.78 ± 0.01 | 9.34 ± 0.03 | 10.21 ± 0.01 | 10.42 ± 0.01 |
| 1.75 | 68.66 ± 0.01 | 55.19 ± 0.05 | 63.01 ± 0.04 | 58.23 ± 0.01 | 05.64 ± 0.02 | 10.64 ± 0.04 | 11.73 ± 0.03 | 12.60 ± 0.05 |
| 2.00 | 71.49 ± 0.04 | 60.84 ± 0.01 | 66.27 ± 0.02 | 61.49 ± 0.02 | 06.08 ± 0.01 | 12.38 ± 0.01 | 13.25 ± 0.01 | 14.34 ± 0.01 |
| 3.00 | 75.62 ± 0.03 | 62.36 ± 0.03 | 68.44 ± 0.01 | 64.75 ± 0.03 | 06.95 ± 0.03 | 13.68 ± 0.01 | 15.42 ± 0.05 | 17.60 ± 0.01 |
| 4.00 | 82.35 ± 0.01 | 68.44 ± 0.04 | 71.26 ± 0.05 | 65.18 ± 0.01 | 08.04 ± 0.04 | 14.77 ± 0.02 | 17.60 ± 0.03 | 20.42 ± 0.02 |
| 5.00 | 86.48 ± 0.05 | 74.75 ± 0.01 | 76.48 ± 0.02 | 68.66 ± 0.01 | 08.90 ± 0.01 | 14.99 ± 0.01 | 18.90 ± 0.01 | 22.81 ± 0.01 |
| 6.00 | 90.17 ± 0.04 | 81.48 ± 0.02 | 80.17 ± 0.04 | 78.87 ± 0.02 | 09.56 ± 0.01 | 16.29 ± 0.02 | 19.99 ± 0.01 | 24.98 ± 0.01 |
Pure solution of Oflox.
Oflox and C934.
Oflox and C940.
Oflox and HPMC.
Table 2.
In vitro permeability profiles of the samples of Oflox through synthetic membrane up to 6 h.
| Samples | %CDPe | fPapp (cm s−1) × 10−7 | gJ (μg cm−2 s−1) × 10−3 | hER |
|---|---|---|---|---|
| (a) Acidic buffer (pH 1.2) | ||||
| aFo | 90.17 | 26.76 | 26.76 | – |
| bF1 | 81.48 | 24.18 | 24.18 | 0.904 |
| cF2 | 80.17 | 23.79 | 23.79 | 0.889 |
| dF3 | 78.87 | 23.41 | 23.41 | |
| (b) Phosphate buffer (pH 7.2) | ||||
| Fo | 09.56 | 2.84 | 2.84 | – |
| F1 | 16.29 | 4.84 | 4.84 | 1.704 |
| F2 | 19.99 | 5.92 | 5.92 | 2.085 |
| F3 | 24.98 | 7.42 | 7.42 | 2.613 |
Pure solution of Oflox.
Oflox and C934.
Oflox and C940.
Oflox and HPMC.
Cumulative percentage drug permeation.
Apparent permeability.
Flux.
Enhancement ratio.
Figure 1.
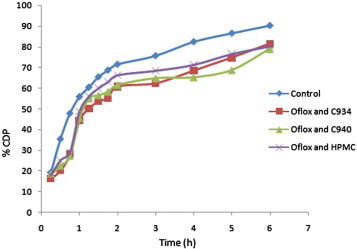
Comparative %CDP ± SD of different samples of Oflox versus time through synthetic membrane in acidic buffer up to 6 h.
Figure 2.
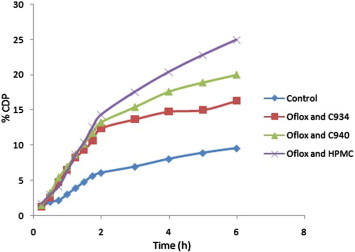
Comparative %CDP ± SD of different samples of Oflox versus time through synthetic membrane in phosphate buffer up to 6 h.
Figure 3.

Comparative Papp values of different samples of Oflox through synthetic membrane in acidic buffer up to 6 h.
Figure 4.

Comparative Papp values of different samples of Oflox through synthetic membrane in phosphate buffer up to 6 h.
The ex vivo permeability study of the above-mentioned samples was also performed in a similar manner, using the excised goat stomach mucosal membrane and acidic buffer at different time points up to 16 h. The results of this experiment have been mentioned in Table 3. The values of %CDP, Papp, and J were calculated after 16 h in cases of all samples. These values were minimum in case of the formulation F2, while F3 showed maximum values. However, by using the excised goat intestinal mucosa in presence of phosphate buffer, the formulation F1 displayed minimum values, whereas they were maximum in case of F2 after 16 h. The Papp values of different formulations of Oflox were more than the control when the goat intestinal membrane was used, while those values of these samples were less than the control in presence of the goat stomach mucosal membrane (Tables 3 and 4). The above-mentioned results have also been depicted in different graphs by taking the %CDP at different time points versus time (Figs. 5 and 6). In addition, bar diagrams of Papp values of different samples of Oflox also indicate the results of the present investigation (Figs. 7 and 8).
Table 3.
%CDP ± SD of Oflox from control and three suspensions of Oflox using biological membranes.
| Time (h) | Excised goat stomach mucosal membrane at pH 1.2 |
Excised goat intestinal mucosal membrane at pH 7.2 |
||||||
|---|---|---|---|---|---|---|---|---|
| aFo | bF1 | cF2 | dF3 | Fo | F1 | F2 | F3 | |
| 0.25 | 04.99 ± 0.02 | 03.05 ± 0.01 | 02.82 ± 0.02 | 03.04 ± 0.01 | 01.73 ± 0.02 | 03.69 ± 0.02 | 03.69 ± 0.04 | 03.04 ± 0.04 |
| 0.50 | 13.47 ± 0.01 | 10.21 ± 0.02 | 10.21 ± 0.03 | 11.08 ± 0.03 | 03.47 ± 0.03 | 11.08 ± 0.01 | 11.08 ± 0.01 | 10.21 ± 0.01 |
| 0.75 | 18.68 ± 0.01 | 13.68 ± 0.04 | 14.12 ± 0.01 | 16.29 ± 0.01 | 05.43 ± 0.01 | 14.99 ± 0.01 | 15.43 ± 0.03 | 14.99 ± 0.03 |
| 1.00 | 21.94 ± 0.03 | 17.81 ± 0.03 | 18.46 ± 0.01 | 20.20 ± 0.01 | 07.17 ± 0.02 | 19.33 ± 0.04 | 19.56 ± 0.01 | 19.34 ± 0.01 |
| 1.25 | 26.72 ± 0.04 | 22.38 ± 0.05 | 22.59 ± 0.04 | 24.98 ± 0.04 | 09.34 ± 0.04 | 23.03 ± 0.01 | 23.25 ± 0.02 | 22.16 ± 0.01 |
| 1.50 | 29.98 ± 0.01 | 25.85 ± 0.01 | 26.72 ± 0.05 | 29.11 ± 0.05 | 11.08 ± 0.01 | 26.29 ± 0.02 | 26.51 ± 0.01 | 25.20 ± 0.03 |
| 1.75 | 35.20 ± 0.01 | 31.28 ± 0.01 | 30.85 ± 0.01 | 33.46 ± 0.02 | 13.25 ± 0.02 | 29.98 ± 0.03 | 30.42 ± 0.05 | 29.55 ± 0.01 |
| 2.00 | 39.76 ± 0.02 | 34.76 ± 0.03 | 34.98 ± 0.01 | 37.59 ± 0.01 | 16.07 ± 0.03 | 35.20 ± 0.01 | 35.20 ± 0.01 | 34.11 ± 0.02 |
| 3.00 | 44.97 ± 0.05 | 40.19 ± 0.01 | 41.06 ± 0.01 | 43.23 ± 0.02 | 19.12 ± 0.04 | 39.98 ± 0.05 | 40.42 ± 0.01 | 39.76 ± 0.01 |
| 4.00 | 51.49 ± 0.01 | 47.15 ± 0.02 | 47.36 ± 0.02 | 48.88 ± 0.01 | 22.16 ± 0.01 | 46.93 ± 0.01 | 47.15 ± 0.03 | 46.06 ± 0.01 |
| 5.00 | 56.71 ± 0.03 | 53.01 ± 0.01 | 53.23 ± 0.03 | 53.88 ± 0.03 | 25.63 ± 0.02 | 53.01 ± 0.01 | 53.67 ± 0.01 | 52.37 ± 0.04 |
| 6.00 | 62.36 ± 0.02 | 58.01 ± 0.05 | 59.31 ± 0.05 | 59.97 ± 0.01 | 28.24 ± 0.01 | 58.90 ± 0.01 | 59.10 ± 0.04 | 58.45 ± 0.01 |
| 8.00 | 66.48 ± 0.01 | 62.14 ± 0.01 | 62.14 ± 0.01 | 64.09 ± 0.01 | 29.33 ± 0.01 | 62.36 ± 0.03 | 63.66 ± 0.05 | 62.40 ± 0.02 |
| 10.0 | 70.61 ± 0.03 | 64.75 ± 0.04 | 65.18 ± 0.02 | 68.00 ± 0.03 | 31.07 ± 0.03 | 65.18 ± 0.04 | 67.14 ± 0.01 | 66.05 ± 0.01 |
| 12.0 | 74.09 ± 0.01 | 68.87 ± 0.02 | 68.22 ± 0.03 | 72.35 ± 0.04 | 32.59 ± 0.01 | 68.44 ± 0.01 | 70.40 ± 0.01 | 68.80 ± 0.02 |
| 14.0 | 78.22 ± 0.02 | 72.79 ± 0.03 | 71.48 ± 0.01 | 76.26 ± 0.01 | 34.54 ± 0.04 | 71.05 ± 0.02 | 74.09 ± 0.03 | 71.27 ± 0.03 |
| 16.0 | 83.43 ± 0.01 | 76.04 ± 0.01 | 75.61 ± 0.02 | 80.82 ± 0.01 | 36.06 ± 0.01 | 74.96 ± 0.01 | 78.00 ± 0.02 | 73.44 ± 0.01 |
Pure solution of Oflox.
Oflox and C934.
Oflox and C940.
Oflox and HPMC.
Table 4.
Ex vivo permeability profiles of the samples of Oflox through biological membranes up to 16 h.
| Samples | %CDPe | fPapp (cm s−1) × 10−7 | gJ (μg cm−2 sec−1) × 10−3 | hER |
|---|---|---|---|---|
| (a) Excised goat stomach mucosal membrane at pH 1.2 | ||||
| aFo | 83.43 | 9.27 | 9.27 | – |
| bF1 | 76.04 | 8.45 | 8.45 | 0.912 |
| cF2 | 75.61 | 8.40 | 8.40 | 0.906 |
| dF3 | 80.43 | 8.98 | 8.98 | 0.969 |
| (b) Excised goat intestinal mucosal membrane at pH 7.2 | ||||
| Fo | 36.06 | 4.01 | 4.01 | – |
| F1 | 74.96 | 8.33 | 8.33 | 2.077 |
| F2 | 78.00 | 8.67 | 8.67 | 2.162 |
| F3 | 73.44 | 8.16 | 8.16 | 2.035 |
Pure solution of Oflox.
Oflox and C934.
Oflox and C940.
Oflox and HPMC
Cumulative percentage drug permeation.
Apparent permeability.
Flux.
Enhancement ratio.
Figure 5.

Comparative %CDP ± SD of different samples of Oflox versus time through excised goat stomach mucosal membrane in acidic buffer up to 16 h.
Figure 6.
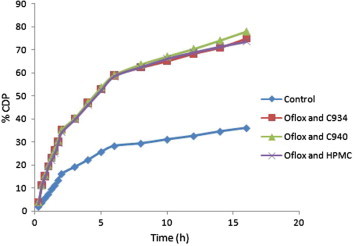
Comparative %CDP ± SD of different samples of Oflox versus time through excised goat intestinal mucosal membrane in phosphate buffer up to 16 h.
Figure 7.

Comparative Papp values of different samples of Oflox through excised goat stomach mucosal membrane in acidic buffer up to 16 h.
Figure 8.

Comparative Papp values of different samples of Oflox through excised goat intestinal mucosal membrane in phosphate buffer up to 16 h.
The Papp value of F3 was maximum, while F2 showed minimum value when the excised goat stomach mucosal membrane was used. On the other hand, in the excised goat intestinal mucosal membrane, just the opposite trend was found. However, in both cases Papp value of F1 was in between F2 and F3 (Table 4). The effect of polymers on diffusion and permeation of Oflox was evaluated in terms of the ER values of different Oflox containing formulations by separately taking both buffers and different diffusional membranes. The ER values of Oflox containing formulations were more in the intestinal mucosa and the value was found to be highest in case of F2 (Tables 2 and 4).
4. Discussion
In cases of Oflox containing mucoadhesive suspensions, F1 and F2 were found to be more permeable than F3 in acidic medium, but F3 showed the highest permeability than others at pH 7.2 when synthetic medium was used. So, in acidic medium, Carbopol polymers may show lesser gelling property and viscosity than HPMC, whereas these properties of Carbopol polymers seem to be relatively more than HPMC at pH 7.2 (Basaran and Bozakir, 2012) (Tables 1 and 2; Figs. 1–4). Moreover, the permeability of formulations containing Carbopol polymers (F1/F2) in the excised goat stomach was less than the formulation with HPMC (F3). However, in case of the goat intestine, the permeability of F1/F2 was more than F3, which may be due to more mucoadhesive property of formulations containing Carbopol polymers at the intestinal part (Barakat, 2010) (Tables 3 and 4; Figs. 5–8). On the basis of the overall results (using both synthetic/biological membranes), it might be mentioned that more controlled release action of Oflox from the formulations containing Carbopol is expected. These overcome the low absorption window of the drug at the intestinal part.
As mentioned earlier, from suspensions and aqueous solution of pure drug, the extent of drug permeated up to 16 h was significantly different. The ER values of all suspensions showed higher permeation percent than aqueous solution of Oflox in presence of the goat intestinal membrane. Moreover, the ER values of all formulations in the excised goat intestinal mucosal membrane were more than those in presence of the goat stomach mucosal membrane (Table 4). It indicates that the permeability of the drug was more enhanced by the polymers in the intestinal part, leading to more bioavailability and prolonged action in the intestine (Bregni et al., 2008). In this manner, the limitation of BCS Class III drugs (such as Oflox) could be overcome. In this connection, it may be mentioned that an electrostatic attraction force occurs between the negative charge of each formulation and positive charge of GI mucosal membrane. As a result, mucoadhesive property of the gel base might ensure an intimate contact between suspension and GI mucosal membrane, which seems to prolong the retention of the formulation at site of absorption. This is beneficial for enhancing permeation (Hosny, 2009). That is why by taking into consideration of ER values of the present study, it may be concluded that in addition to F1, and F2, other formulation (F3) is also expected to show effective controlled release action (Shojaei, 1998).
It has already been mentioned that both F1 and F2 may produce more controlled release action than F3. Out of two Carbopol containing formulations (F1 and F2), F2 was appeared to be the better formulation than F1 considering their Papp and flux values. So, it might be mentioned that F2 was probably the most beneficial for improving in vitro/ex vivo permeation and diffusivity of Oflox even after 16 h. Hence, this preparation may be considered as the best suspension to obtain effective controlled release action of the drug.
In addition, in our laboratory, particle size distribution of Oflox formulations was studied (using SEM), and their values of zeta potential were also determined. From particle size distribution study, it was found that maximum particle size ranges for formulations containing Oflox with C940, Oflox with C934, and Oflox with HPMC were from 2–4 μm, 10–15 μm and 4–6 μm, respectively. So, maximum particle sizes of all formulations were from 2 to 15 μm, i.e., they were pharmaceutically acceptable (Sahoo et al., 2012). Moreover, the values of zeta potential of Oflox formulations were found between -0.205 and -0.431 Mv. So, this study suggested that all formulations showed good flocculation and maximum/strong agglomeration properties, as the value of zeta potential of each formulation (suspension) was between -5 mV and +5 mV (Sahoo et al., 2014). Thus, all suspensions were pharmaceutically acceptable and stable suspensions.
5. Conclusions
Considering all the above mentioned information, it may be concluded that effective controlled release action seems to be better when mucoadhesive suspensions of Oflox containing Carbopol like F1 and F2 would be used. Out of these formulations, F2 was the best suspension to obtain effective controlled release action of the drug. Moreover, it was pharmaceutically acceptable and stable suspension. The present study is having tremendous importance because modelling, understanding and characterizing the penetration and permeation process of drugs through various biological membrane barriers are essential in order to predict the in vivo behaviour of formulation. Relevant in vivo studies of the formulations should be carried out in future to finally conclude their importance.
Footnotes
Peer review under responsibility of King Saud University.
References
- Barakat N.S. Evaluation of glycofurol-based gel as a new vehicle for topical application of naproxen. AAPS PharmSciTech. 2010;11:1138–1146. doi: 10.1208/s12249-010-9485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaran B., Bozakir A. Thermosensitive and pH induced in situ ophthalmic gelling system for ciprofloxacin hydrochloride: hydroxypropyl-β-cyclodextrin complex. Acta Pol. Pharm. 2012;69:1137–1147. [PubMed] [Google Scholar]
- Bettini R., Colombo P., Peppas N.A. Solubility effects on drug transport through pH-sensitive, swelling-controlled release systems: transport of theophylline and metoclopramide monohydrochloride. J. Control. Release. 1995;37:105–111. [Google Scholar]
- Bregni C., Chiappetta D., Faiden N., Carlucci A., García R., Pasquali R. Release study of diclofenac from new carbomer gels. Pak. J. Pharm. Sci. 2008;21:12–16. [PubMed] [Google Scholar]
- Chakraborti C.K., Sahoo S., Behera P.K. Antibacterial activities study of ofloxacin containing mucoadhesive suspensions. J. Pharm. Res. 2012;5:822–824. [Google Scholar]
- Chavanpatil M., Jain P., Chaudhari S., Shear R., Vavia P. Development of sustained release gastroretentive drug delivery system for ofloxacin: in vitro and in vivo evaluation. Int. J. Pharm. 2005;304:178–184. doi: 10.1016/j.ijpharm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Galaev I.Y., Mattiasso B. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999;17:335–340. doi: 10.1016/s0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- Gangurde H.H., Chordiya M.A., Tamizharasi S., Senthikumaran K., Sivakumar T. Formulation and evaluation of sustained release bioadhesive tablets of ofloxacin using 32 factorial design. Int. J. Pharm. Invest. 2011;1:148–156. doi: 10.4103/2230-973X.85964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.H. Carbopol polymers for pharmaceutical drug delivery applications. Drug Deliv. Technol. 2003;3(6) [Google Scholar]
- Haigh J.M., Smith E.W. The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur. J. Pharm. Sci. 1994;2:311–330. [Google Scholar]
- Hosny K.M. Preparation and evaluation of thermosensitive liposomal hydrogel for enhanced transcorneal permeation of ofloxacin. AAPS PharmSciTech. 2009;10:1336–1342. doi: 10.1208/s12249-009-9335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong B., Gutowska A. Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2001;20:305–311. doi: 10.1016/s0167-7799(02)01962-5. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Sahoo S., Sarma H.D., Chakraborti C.K., Samuel G. Preparation and evaluation of three mucoadhesive dosage forms using 99mTc-Ofloxacin. Appl. Radiat. Isot. 2014;89:192–198. doi: 10.1016/j.apradiso.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Patel H.J., Patel J.S., Desai B.G., Patel K.D. Permeability studies of anti hypertensive drug amlodipine besilate for transdermal delivery. Asian J. Pharm. Clin. Res. 2010;3:31–34. [Google Scholar]
- Qiu Y., Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- Sahoo S., Chakraborti C.K., Mishra S.C., Nanda U.N. Particle size distribution and aspect ratio analysis of ofloxacin mucoadhesive polymeric suspension using scanning electron microscopy. Int. J. Pharm. Res. 2012;4:56–60. [Google Scholar]
- Sahoo S., Chakraborti C.K., Behera P.K. Stability prediction of polymeric suspensions by zeta potential measurement. J. PharmaSciTech. 2014;3:92–94. [Google Scholar]
- Sakore S., Choudhari S., Chakraborty B. Biowaiver monograph for immediate release solid oral dosage forms: ofloxacin. Int. J. Pharm. Pharm. Sci. 2010;2:156–161. [Google Scholar]
- Shoaib M.H., Tazeen J., Merchant H.A., Yousuf R.I. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak. J. Pharm. Sci. 2006;19:119–124. [PubMed] [Google Scholar]
- Shojaei A.H. Buccal mucosa as a route for systemic drug delivery: a review. J. Pharm. Pharm. Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- Singh N., Gupta P., Bhattacharyya A. Enhancement of intestinal absorption of poorly absorbed Ceftriaxone Sodium by using mixed micelles of Polyoxy Ethylene (20) Cetyl Ether and Oleic Acid as peroral absorption enhancers. Arch. Appl. Sci. Res. 2010;2:131–142. [Google Scholar]


