Abstract
Minimal food-processing methods are not effective against foodborne viruses, such as human norovirus (NV). It is important, therefore, to explore novel nonthermal technologies for decontamination of foods eaten fresh, minimally processed and ready-to-eat foods, and food contact surfaces. We studied the in vitro virucidal activity of cold atmospheric gaseous plasma (CGP) against feline calicivirus (FCV), a surrogate of NV. Factors affecting the virucidal activity of CGP (a so-called radio frequency atmospheric pressure plasma jet) were the plasma generation power, the exposure time and distance, the plasma feed gas mixture, and the virus suspension medium. Exposure to 2.5-W argon (Ar) plasma caused a 5.55 log10 unit reduction in the FCV titer within 120 s. The reduction in the virus titer increased with increasing exposure time and decreasing exposure distance. Of the four plasma gas mixtures studied (Ar, Ar plus 1% O2, Ar plus 1% dry air, and Ar plus 0.27% water), Ar plus 1% O2 plasma treatment had the highest virucidal effect: more than 6.0 log10 units of the virus after 15 s of exposure. The lowest virus reduction was observed with Ar plus 0.27% water plasma treatment (5 log10 unit reduction after 120 s). The highest reduction in titer was observed when the virus was suspended in distilled water. Changes in temperature and pH and formation of H2O2 were not responsible for the virucidal effect of plasma. The oxidation of viral capsid proteins by plasma-produced reactive oxygen and nitrogen species in the solution was thought to be responsible for the virucidal effect. In conclusion, CGP exhibits virucidal activity in vitro and has the potential to combat viral contamination in foods and on food preparation surfaces.
INTRODUCTION
Foodborne illnesses continue to plague public health, as well as world economies, costing approximately $152 billion in the United States alone (1). Enteric viruses, particularly human norovirus (NV) and hepatitis A virus (HAV), are the leading causes of viral foodborne illnesses (2). Human NV, one of the top five pathogens with respect to the total cost of foodborne illnesses in the United States, belongs to the family Caliciviridae and is a well-known cause of “winter vomiting disease” or “stomach flu” (3). NV causes 19 to 21 million cases of acute gastroenteritis annually in the United States and leads to 1.7 to 1.9 million outpatient visits, 400,000 emergency room visits, 56,000 to 71,000 hospitalizations, and 570 to 800 deaths, mostly among young children (4). More than half of all foodborne disease outbreaks due to a known cause reported to the CDC from 2006 to 2010 were attributed to NV. In the European Union in 2007, caliciviruses (primarily NV) were responsible for 507 of 675 foodborne viral disease outbreaks (5).
Multiple issues related to the quality of thermally processed foods, e.g., nutritional losses and adverse effects on organoleptic quality, have led to the emergence of so-called nonthermal technologies, which consist of preservation treatments that are effective at ambient or sublethal temperatures, thereby minimizing the negative effects of thermal processing on nutritional and quality attributes of food (6). In addition, consumers have increasing interest in non-thermally processed and ready-to-eat food commodities with fresh-like and healthy attributes, leading to popularization of minimally processed foods. However, such foods carry a high risk of surface cross-contamination through cutting boards, knives, working surfaces, equipment, or the processing environment. In addition, the low inactivation efficiency of most minimal food-processing methods against foodborne viruses is also a concern (7). Recent experiments with NV in a variety of foods revealed that minimal food processing (freezing, cooling, and mild heat treatment) was not significantly effective in reducing titers of NV or its surrogates (8). Consequently, the development of novel efficient and safe nonthermal technology for viral decontamination of foods is of great interest to food scientists, food producers, and ultimately customers. A few recent investigations have been aimed at studying the virucidal effects of some novel nonthermal technologies, such as ozone treatment (9), gamma irradiation treatment (10), and lactic acid bacteria as an antiviral biopreservative (11), in combating foodborne viral contamination in food.
Cold atmospheric gaseous plasma (CGP) refers to a partially ionized gas composed essentially of photons, ions, free electrons, and reactive species, including atoms (O and N), molecules (e.g., O3, H2O2, and HNO2), and radicals {OH, NO, and singlet oxygen [O2(a1Δg)]}, all of which are important in biological reactions (12). Due to its unique properties, plasma is often referred to as the fourth state of matter and is normally generated in nature or artificially by subjecting a gas to a strong electric field, which leads to ionization; dissociation of molecules; and production of photons, radicals, and reactive species (13). As the electric field transfers energy to the mobile electrons and the significant mass difference between electrons and neutral gas molecules (heavy particles) leads to inefficient collisional energy transfer between electrons and heavy particles, the energy of the electrons can be significantly larger than the energy of the neutral gas molecules. Various sources of plasma exploiting this physical property allow the production of highly reactive cold atmospheric pressure plasmas for which the reactivity is induced by energetic electrons while the gas temperature remains close to room temperature (14). These conditions are easily obtained at low pressure and require specific plasma generation strategies at atmospheric pressure. Typical approaches for cold atmospheric pressure plasma generation at atmospheric pressure include corona discharge, dielectric barrier discharges (DBD), radio frequency (RF) plasma, and gliding-arc discharge (15).
CGP is attracting the attention of food researchers due to its biocidal effects against various spoilage microorganisms and food pathogens, because it can be applied at ambient air temperature without the use of heat. The use of CGP appears to be especially advantageous for decontaminating the surfaces of fresh foods, such as vegetables, fruits, meats, nuts, poultry, and eggs, and also for disinfection of food preparation surfaces (16–19).
Several studies on the antibacterial effects of cold plasma against bacteria—Escherichia coli (20), Salmonella spp. (16), Staphylococcus aureus (18), and others (6)—are available. However, the antiviral activity of CGP has not been properly explored. Only three studies have reported antiviral activity of CGP. Terrier et al. (21) recorded reductions in virus titers of 6.5, 3.8, and 4.0 log10 units for influenza virus type A, human parainfluenza virus type 3, and respiratory syncytial virus (RSV), respectively, using a newly designed system based on physical decontamination of substrates and air by cold atmospheric plasma. Zimmermann et al. (22) achieved up to 6.0 log10 unit inactivation of adenovirus using a CGP system. Alekseev et al. (23) suppressed corneal herpes simplex virus 1 infection in vitro using a nonthermal DBD plasma system. To date, no published study is available on the efficacy of CGP against foodborne viruses, such as NV and HAV. The present study was undertaken to determine the in vitro virucidal activity of cold plasma using four different gas mixtures against feline calicivirus (FCV), a surrogate for human NV, and to study factors that might determine the virucidal effects of CGP.
MATERIALS AND METHODS
Description of the plasma generation system.
The plasma jet used in this study is identical to the one described by van Gils et al. (24). The plasma is ignited in Ar, which is blown through a 1.9-mm quartz tube at 1.5 standard liters per minute (slm) by RF power (13.56 MHz). The RF power is modulated at a frequency of 20 kHz with a duty cycle of 20%. Unless otherwise stated, the work was performed at a time-averaged plasma dissipated power of 2.5 W. The power level was measured as explained by Hofmann et al. (25). In this study, 4 different gases were used: Ar, Ar plus 1% O2, Ar plus 1% dry air, and Ar plus 0.27% water. The amounts of admixtures were controlled by mass flow controllers (MKS GE50; MKS Instruments Inc., USA). In the case of Ar plus 0.27% water, part of the Ar flow was sent through a bubbler containing sterile distilled water (DW) to humidify part of the Ar gas feed.
The plasma jet is known to produce significant amounts of NO (26), O3 (27), OH, HNO2, H2O2, and O2(a1Δg) (28). These species are all biologically active and are produced in large quantities. Different gas admixtures favor the production of certain species over others. For example, mixing O2, air, and water favors the production of O3, NO, and OH/H2O2, respectively (29), although even in the cases of Ar, Ar plus water, and Ar plus O2, diffusion of the surrounding air in the jet effluent leads to the production of NO. The reactive species produced by the plasma in the gas phase are transferred to the solution through complex gas-liquid interactions (30).
For the virucidal experiments in this study, we placed a sterile 96-well microtiter plate (containing 100 μl of virus suspension) below the plasma jet. The tip of the plasma plume was allowed to approach the top of the well. A diagram of the plasma setup, including a schematic of the 96-well plate, can be seen in Fig. 1. After the desired treatment times, the samples were removed and kept at room temperature for 2 h to allow the plasma-produced reactive species in solution to react with the virus. Then, FCV titration of treated and nontreated (control) samples was carried out (as described below). The antiviral effects were identified by comparing the titers of treated and nontreated (control) viruses.
FIG 1.
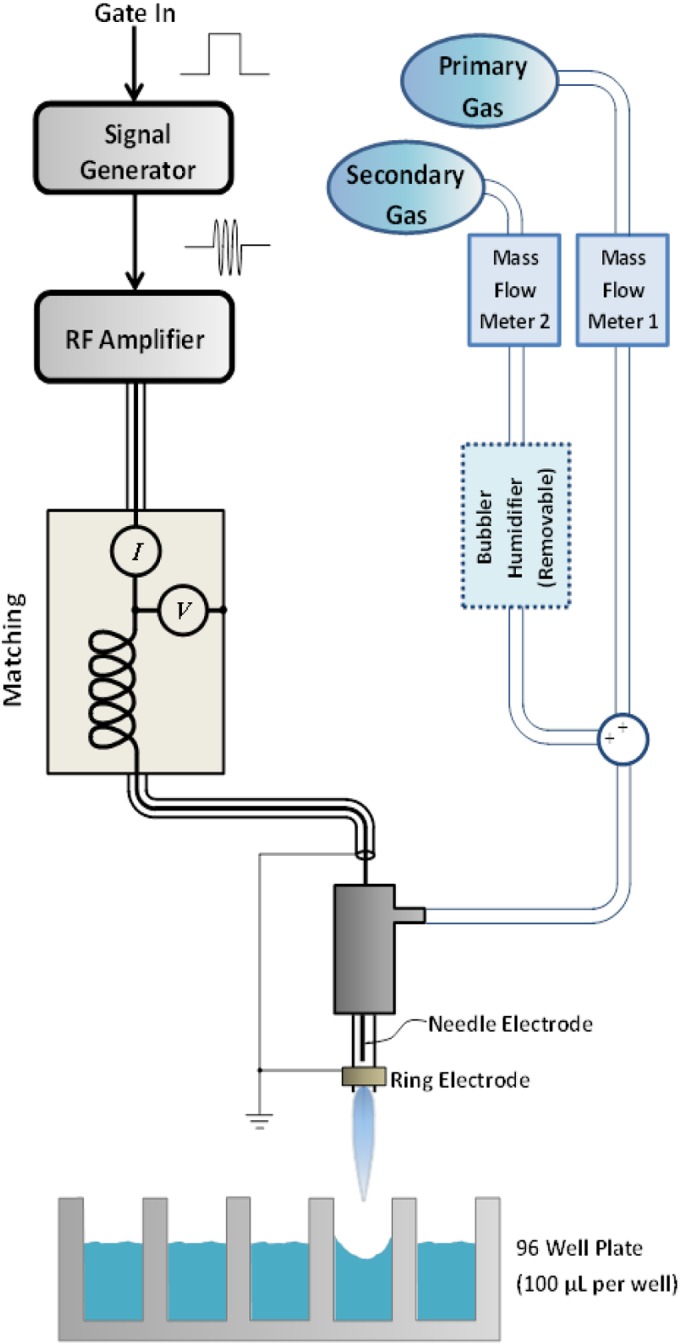
Schematic diagram of the CGP system, including the plasma jet, treatment of samples, and electrical and gas inputs.
Virus propagation.
Strain 255 of FCV was used as a surrogate for NV in this investigation. The virus was grown and titrated in Crandell-Reese feline kidney (CRFK) cells, which were grown in minimum essential medium (MEM) with Earle's salts and l-glutamine (Mediatech Inc., Manassas, VA, USA) supplemented with 8% fetal bovine serum (FBS) and antibiotics (neomycin, 90 U/ml; gentamicin, 50 μg/ml; penicillin, 455 IU/ml; streptomycin, 455 μg/ml; and amphotericin B [Fungizone], 1.5 μg/ml). The cells were incubated at 37°C under 5% CO2. The incubated cells were examined daily under an inverted microscope for appearance of cytopathic effects (CPE), which usually appeared 2 to 3 days after infection. The infected cells were frozen and thawed three times, followed by centrifugation at 2,000 × g for 30 min at 4°C. The virus-containing supernatant was decanted and distributed in 1- by 3.25-in. ultracentrifuge tubes (Beckman Instruments Inc., USA). One milliliter of sterile 30% sucrose in distilled water was underlaid in each tube. The balanced, capped tubes were then ultracentrifuged at 111,857 × g for 2.5 h at 4°C (Optima L-90K ultracentrifuge; Beckman Instruments) using an SW32 Ti swinging-bucket rotor (Beckman Instruments Inc., USA). The supernatant was carefully removed and discarded, and the virus pellet was resuspended in 1 ml of sterile DW. The purified virus was aliquoted and stored at −80°C until it was used.
Virus titration.
Serial 10-fold dilutions of samples were prepared in MEM containing 4% FBS. The dilutions were inoculated into CRFK cell monolayers prepared in 96-well microtiter plates using 3 wells per dilution. The plates were incubated at 37°C under 5% CO2 and examined daily for the development of CPE for up to 5 days. The endpoint was taken to be the highest dilution of the virus that produced CPE in 50% of the inoculated cells. Viral titers were calculated by the Kärber method (31) and are expressed as 50% tissue culture infective doses (TCID50)/0.1 ml.
Effect of plasma generation power.
Aliquots (100 μl) of FCV suspended in sterile distilled water (DW-FCV) were exposed to Ar atmospheric plasma generated at five different power levels (1 ± 0.1, 1.5 ± 0.1, 2 ± 0.1, 2.5 ± 0.1, and 3 ± 0.1 W). The exposure distance was 18.13 mm from the plasma nozzle to the surface of the treated viral suspension. The exposure times were 0 (control), 15, 30, 60, 120, and 180 s.
Effect of plasma exposure distance and type of plasma feeding gas mixture.
In a factorial experiment, four plasma feeding gas mixtures (Ar, Ar plus 1% O2, Ar plus 1% dry air, and Ar plus 0.27% water) were used separately for the generation of CGP. The plasma dissipated power was kept constant at 2.5 ± 0.1 W. Aliquots (100 μl) of DW-FCV were exposed to the four types of plasma for 0 (control), 15, 30, 60, and 120 s. To study the effect of exposure distance on the virucidal effects of each plasma type, three different exposure distances (the distance from the plasma jet nozzle to the surface of the virus suspension) were used (low exposure level, 11.25 mm; intermediate, 14.69 mm; and high, 18.13 mm [the distance from the bottom of the well plate to the surface of the virus suspension was 6.49 mm]). The length of the plasma plumes used depended on the type of feed gas mixture. The longest visible plasma plume was for Ar plasma, followed by Ar plus 1% air plasma and Ar plus 0.27% water plasma, while the shortest plume corresponded to Ar plus 1% O2. To accommodate these differences, three exposure levels were chosen to represent (i) low level, corresponding to the shortest distance between the nozzle and the surface of the viral suspension (the visible plasma tip of the shortest plasma plume was at the position of the top of the well); (ii) high level, corresponding to the greatest distance between the nozzle and the surface of the viral suspension (the tip of the longest plume was at the top of the well); and (iii) intermediate level, corresponding to the middle distance between the low and high levels. This experiment was repeated with the feed gas mixture flows with the power off (gas-phase treatment) to determine if the gas flow on the suspension medium contributed to the virucidal effect.
Effect of the virus suspension solution.
The purified FCV was suspended separately in three different types of suspension medium (sterilized double-distilled water, MEM, and NaCl-Tris-EDTA [NTE] buffer). Aliquots (100 μl) of each viral suspension were distributed in 96-well plates and exposed to the four plasma conditions for 0 (control), 15, 30, 60, and 120 s. The exposure to plasma was performed at the lower level (11.25-mm exposure distance). The plasma power was kept constant at 2.5 ± 0.1 W.
Measurement of solution temperature.
In separate experiments, 100-μl aliquots of distilled water were exposed to the four types of plasma for 15, 30, 60, and 120 s. The temperature of the liquid was measured immediately after each exposure time, using an RTD Omega thermocouple (Omega Engineering Inc., Stamford, CT, USA).
Measurement of pH changes.
In separate experiments, 100-μl aliquots of distilled water, MEM, and NTE buffer were exposed to the four types of plasma for 15, 30, 60, and 120 s at two distances (low and high exposure levels). After treatment, the pH values were measured using a Thermo Fisher Scientific (Waltham, MA, USA) Orion pH probe (model 8220BNWP).
Determination of H2O2 formed during plasma exposure.
The concentration of formed hydrogen peroxide in plasma-exposed distilled water was determined colorimetrically using the titanium sulfate method of Satterfield and Bonnell (32) and Eisenberg (33). Briefly, 100-μl aliquots of distilled water were exposed separately to the four types of plasma for 15, 30, 60, and 120 s at the low exposure level and 2.5 W power. Sodium azide solution (4 mM) was added immediately after the plasma treatment to quench nitrites, which may react with the formed H2O2. Then, 100 μl of titanium sulfate reagent was added. The development of yellow color was measured colorimetrically at 407 nm, and a standard curve of H2O2 was generated.
FCV virucidal effect of liquid hydrogen peroxide.
Aliquots (100 μl) of DW-FCV suspension were mixed with equal volumes of different H2O2 solutions. The H2O2 concentrations after mixing were 5, 10, 20, 30, 40, 50, 80, and 100 mM. The FCV-plus-H2O2 mixtures were incubated at room temperature for 30, 60, and 120 min. The surviving virus was titrated in all mixtures after each incubation time and compared with nontreated virus as a control.
The effective FCV lethal time of plasma exposure.
Aliquots (100 μl) of DW-FCV were distributed in 96-well plates. Samples were exposed separately to the four types of plasma for 15, 30, 60, 120, 180, 240, and 300 s. Immediately after each exposure time, 10 μl of 0.1 M (the final concentration after mixing with the sample) filter-sterilized sodium thiosulfate solution was added, followed immediately by FCV titration. The sodium thiosulfate solution acts as a scavenger for the oxidizing (long-lived) species in solution generated by the plasma. The amount of surviving FCV was measured as the difference between the titers of treated and nontreated (control) samples. Virus inactivation was calculated as the decimal reduction time (D value), which is defined as the time needed to achieve a 1-log-unit reduction in infectious virus titer with a given plasma treatment.
Statistical analysis.
Each titration was carried out in triplicate, and each experiment was performed three times. The results are the means ± standard deviations (SD) of triplicate experiments. The analysis of variance (ANOVA) was generated by the F test. The statistical analysis was carried out using STATISTICA software, v. 10 (Statsoft Inc., Tulsa, OK, USA). The D value of each type of plasma was determined from the negative reciprocal of the slope of the regression line, using the linear portions of the survival curves (log10 TCID50/0.1 ml versus time of exposure to the plasma under constant conditions). The exposure times needed for a 4 log10 TCID50/0.1 ml reduction and complete reduction of FCV were determined from the following equation: t = D × (log No − log Nf) = D × n, where D is the D value (in minutes) under the specified conditions; No is the initial titer of the virus; Nf is the surviving titer after an exposure time, t (in minutes), to the selected plasma type; and n is (log No − log Nf), which is equal to the log10 reduction of the initial titer of the virus.
RESULTS
Effect of Ar plasma generation power.
The exposure of DW-FCV to an Ar plasma jet at all tested power values showed time-dependent decreases in the FCV titer (Fig. 2). Exposure of FCV to 1.0, 1.5, 2.0, 2.5, and 3 W Ar plasma for 15 to 180 s led to gradual reductions in the FCV titer ranging from 0.33 to 2.66, 0.66 to 4.00, 0.88 to 4.66, 0.99 to 5.55, and 1.11 to 5.55 log10 TCID50/0.1 ml, respectively. After exposure to 2.5 and 3 W plasma for 120 s, more than 99.999% of FCV was inactivated (more than 5 log10 TCID50/0.1 ml reduction). There was no significant difference (P ≥ 0.05) between the reductions of the FCV titer after exposure to 2.5 W and 3 W Ar plasma at each tested exposure time.
FIG 2.
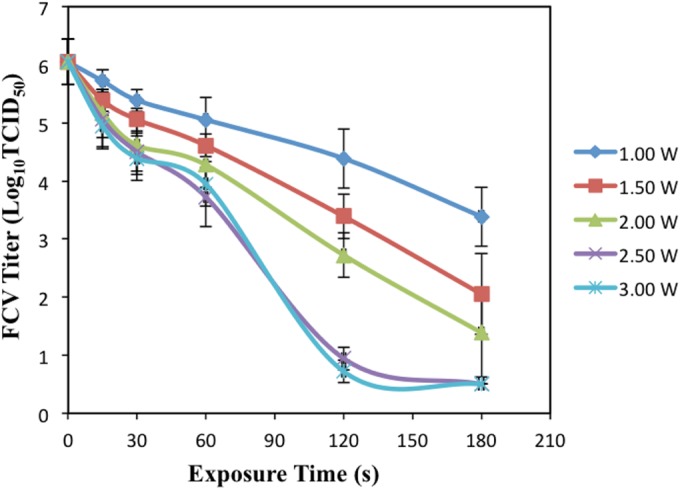
Effects of changes in Ar-based plasma generation power on virucidal activity. Aliquots (100 μM) of DW-FCV suspension were exposed to Ar plasma. The exposure distance from the nozzle to the surface of the virus suspension was 18.13 mm. Five different powers (1 ± 0.1, 1.5 ± 0.1, 2 ± 0.1, 2.5 ± 0.1, and 3 ± 0.1 W) and six exposure times (0, 15, 30, 60, 120, and 180 s) were used, followed by immediate titration. The results are averages of triplicate experiments, and the error bars indicate the standard deviations.
Effects of plasma exposure distances and plasma feeding gas mixture types.
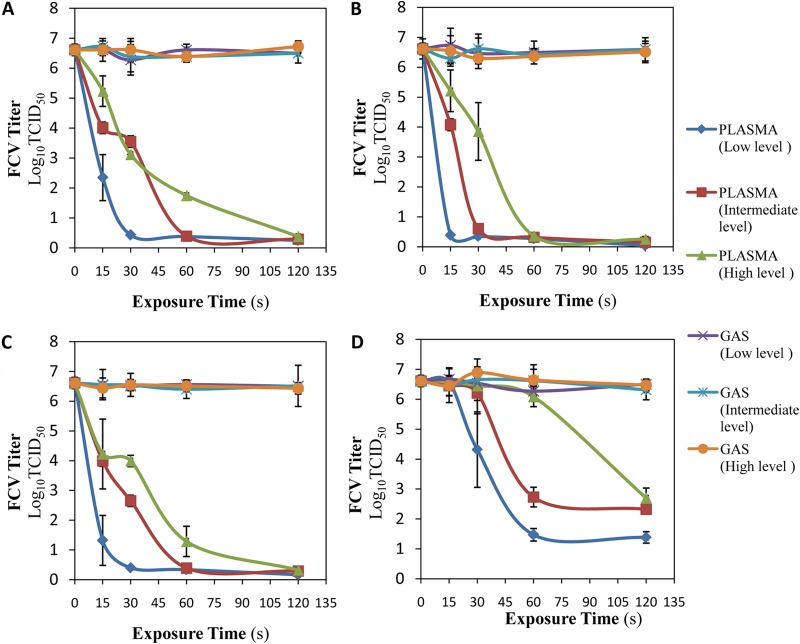
Figure 3 shows that treating FCV with gas flow only (without plasma) for all gas mixtures (Ar, Ar plus 1% O2, Ar plus 1% dry air, and Ar plus 0.27% water) resulted in no significant changes (P ≥ 0.05) in FCV titers. For all gas mixtures, the plasma exposure showed reductions in the FCV titer. The reductions increased with increasing exposure time and decreased with increasing distance from the plasma plume.
FIG 3.
Effects of plasma exposure distance and gas mixture type on virucidal activity. Aliquots (100 μM) of DW-FCV suspension were exposed to Ar plasma (A), Ar plus 1% O2 plasma (B), Ar plus 1% air plasma (C), and Ar plus 0.27% water plasma (D). Three different exposure distances (low level [11.25 mm], intermediate level [14.69 mm], and high level [18.13 mm]) from the plasma nozzle to the surface of the treated viral suspension were used. The plasma dissipated power was kept constant at 2.5 ± 0.1 W. The exposure times were 0, 15, 30, 60, and 120 s, followed by 2 h of incubation at room temperature before titration. The experiment was repeated with the feed gas mixture flows with the power off (gas-phase treatment). The results are the averages of triplicate experiments, and the error bars indicate the standard deviations.
For Ar plasma, more than 99.9999% of FCV (6.18, 6.22, and 6.23 log10 TCID50/0.1 ml) was inactivated after exposure times of 30 s, 60 s, and 120 s at low, intermediate, and high exposure levels, respectively (Fig. 3A). As shown in Fig. 3B, more than 99.9999% (6.22, 6.00, and 6.25 log10 TCID50/0.1 ml) of FCV was inactivated after exposure to Ar plus 1% O2 plasma for 15 s, 30 s, and 60 s at low, intermediate, and high exposure levels, respectively. For Ar plus 1% air plasma, more than 99.9999% of FCV (6.21, 6.22, and 6.29 log10 TCID50/0.1 ml) was inactivated after exposure times of 30 s, 60 s, and 120 s at low, intermediate and high exposure levels, respectively (Fig. 3C). As shown in Fig. 3D, the FCV titer was decreased only 99.9993% (more than 5.00 log10 TCID50/0.1 ml) after exposure to Ar plus 0.27% water plasma for 60 s at the low exposure level, whereas the intermediate and high exposure levels showed only 99.995% (4.29 log10 TCID50/0.1 ml) and 99.988% (3.91 log10 TCID50/0.1 ml) reductions, respectively, after the 120-s exposure.
Among the four tested gas mixtures, the greatest anti-FCV effect was achieved with exposure to Ar plus 1% O2 plasma, since approximately 100% (more than 6 log10 TCID50/0.1 ml) of the virus was inactivated after only 15 s at the low level, while a smaller anti-FCV effect occurred with Ar plus 0.27% water plasma treatment, resulting in a reduction of only 5 log10 TCID50/0.1 ml (99.9993) after 120-s plasma exposure.
Effect of virus suspension medium.
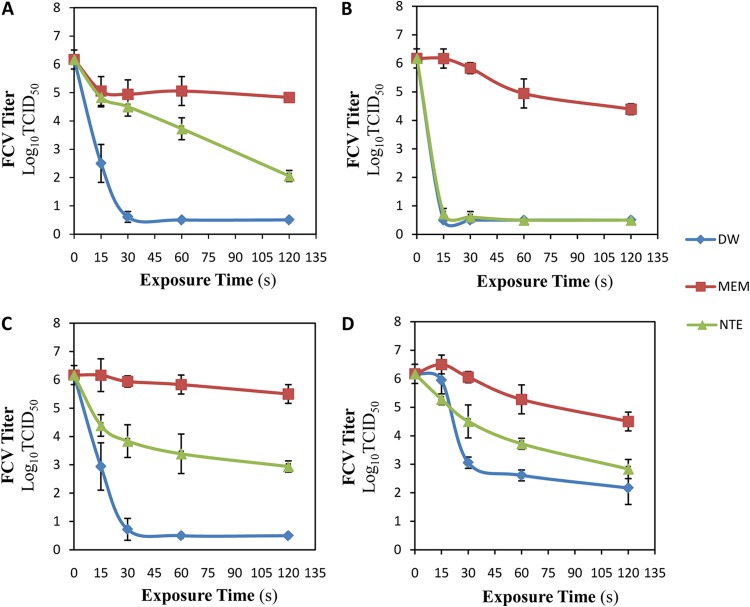
The results presented in Fig. 4 show that the type of virus suspension solution significantly (P < 0.05) affected the virucidal activities of the four tested gas mixture plasmas. For Ar plasma exposure (Fig. 4A), when DW was used to suspend FCV, 99.9997% (5.56 log10 TCID50/0.1 ml) of the FCV was inactivated after 30 s of exposure. However, 99.992% (4.11 log10 TCID50/0.1 ml) and 95.43% (1.34 log10 TCID50/0.1 ml) reductions were attained after the longest exposure time (120 s) in the cases of NTE- and MEM-suspended FCV, respectively. FCV exposed to Ar plus 1% O2 plasma using DW and NTE buffer as the suspending solutions led to almost complete inactivation (99.9998% [5.67 log10 TCID50/0.1 ml] and 99.9996% [5.45 log10 TCID50/0.1 ml] inactivation) of FCV after only 15 s, while the use of MEM led to a very small reduction (1.78 log10 TCID50/0.1 ml) in the FCV titer even after the longest exposure time (120 s). When the virus was suspended in distilled water and exposed to Ar plus 1% air plasma (Fig. 4C), 99.9996% (5.45 log10 TCID50/0.1 ml) of the FCV was inactivated after 30 s of exposure, while 99.94% (3.23 log10 TCID50/0.1 ml) and only 78.62% (0.67 log10 TCID50/0.1 ml) reductions were attained after the longest exposure time (120 s) in the cases of NTE- and MEM-suspended FCV, respectively. Eventually, as presented in Fig. 4D, FCV exposed to Ar plus 0.27% water plasma for the longest exposure time (120 s) using DW, NTE buffer, and MEM as the suspension solutions led to only 99.990% (4.00 log10 TCID50/0.1 ml), 99.950% (3.34 log10 TCID50/0.1 ml), and 97.860% (1.67 log10 TCID50/0.1 ml) reductions in FCV, respectively.
FIG 4.
Effects of virus suspension media on virucidal activity of CGP. Aliquots (100 μM) of DW-FCV, MEM-FCV, and NTE-FCV suspensions were exposed to Ar plasma (A), Ar plus 1% O2 plasma (B), Ar plus 1% air plasma (C), and Ar plus 0.27% water plasma (D). The plasma dissipated power was kept constant at 2.5 ± 0.1 W. The exposure distance from the nozzle to the surface of the virus suspension was 11.25 mm. The exposure times were 0, 15, 30, 60, and 120 s, followed by 2 h of incubation at room temperature before titration. The results are the averages of triplicate experiments, and the error bars indicate the standard deviations.
Temperature and pH value changes.
As shown in Table 1, the temperature of the DW exposed to all tested plasma types increased slightly with an increase in treatment time; the maximum increase was 2.5°C after the longest treatment time (120 s). There were minor differences (P ≥ 0.05) in the temperature changes among the four types of plasma; the highest solution temperature never exceeded 27°C.
TABLE 1.
Changes in the temperature of distilled water after exposure to various types of atmospheric gaseous plasma jets for various exposure times
| Exposure time (s) | Tempa (°C) |
|||
|---|---|---|---|---|
| Ar | Ar + 1% O2 | Ar + 1% air | Ar + 0.27% water | |
| 0 | 24.20 ± 0.21A/A | 24.20 ± 0.21A/A | 24.20 ± 0.21A/A | 24.20 ± 0.21A/A |
| 15 | 24.55 ± 0.07AB/A | 25.25 ± 0.21AB/AB | 25.60 ± 0.71B/B | 25.95 ± 0.07B/B |
| 30 | 25.60 ± 0.71ABC/A | 25.85 ± 1.34AB/A | 25.90 ± 0.14B/A | 25.90 ± 0.42B/A |
| 60 | 25.90 ± 0.57BC/A | 26.30 ± 0.28AB/A | 25.95 ± 0.21B/A | 25.95 ± 0.21B/A |
| 120 | 26.70 ± 0.85C/A | 26.91 ± 0.14B/A | 26.30 ± 0.42B/A | 26.78 ± 0.14B/A |
The values shown are averages of triplicate measurements ± standard deviations. Values followed by the same letters indicate no significant difference in the temperatures (P ≥ 0.05) across the treatment times within the same plasma treatment group (letters before slashes) or across the plasma treatment group within the same treatment time group (letters after slashes). The distance from the plasma jet nozzle to the surface of the virus suspension (low level) was 11.25 mm.
A decrease in pH values with treatment time was observed (Table 2).The amount of change depended on the treated solution and the plasma conditions. In the case of DW, at the two exposure levels, there were gradual decreases, but not exceeding pH 4.1 (−1.35) at all exposure levels and with all plasma types except Ar plus 1% air plasma, for which the pH decreased to 3.1 and 3.87 at lower and higher levels, respectively. In treated MEM, the lowest value attained (pH 8.39) was with Ar plus 1% air plasma at the lower exposure level. In NTE buffer, the pH decreased slightly but remained close to the neutral value (pH 7).
TABLE 2.
Changes in pHs of distilled water, MEM, and NTE buffer after exposure to the four types of plasma at low and high exposure levels
| Exposure level | Treatment time (s) | pHa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure Ar plasma |
Ar + 1% O2 plasma |
Ar + 1% air plasma |
Ar + 0.27% water plasma |
||||||||||
| DW | MEM | NTE | DW | MEM | NTE | DW | MEM | NTE | DW | MEM | NTE | ||
| Low | 0 (control) | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A |
| 15 | 4.64 ± 0.12B | 8.73 ± 0.07AB | 7.06 ± 0.12A | 4.75 ± 0.06B | 8.68 ± 0.03B | 7.14 ± 0.11A | 4.21 ± 0.08B | 8.50 ± 0.05B | 7.08 ± 0.08A | 4.74 ± 0.09B | 8.74 ± 0.05AB | 7.29 ± 0.11A | |
| 30 | 4.45 ± 0.08B | 8.70 ± 0.08B | 6.98 ± 0.08A | 4.68 ± 0.02B | 8.62 ± 0.04BC | 7.12 ± 0.09A | 3.84 ± 0.07C | 8.47 ± 0.13BC | 7.03 ± 0.11A | 4.67 ± 0.04B | 8.73 ± 0.06AB | 7.24 ± 0.07B | |
| 60 | 4.18 ± 0.05C | 8.69 ± 0.10B | 6.65 ± 0.15B | 4.67 ± 0.08BC | 8.51 ± 0.09C | 7.04 ± 0.13AB | 3.49 ± 0.09D | 8.45 ± 0.03BC | 6.83 ± 0.06B | 4.35 ± 0.08C | 8.70 ± 0.08B | 7.21 ± 0.06A | |
| 120 | 4.10 ± 0.02D | 8.69 ± 0.06B | 6.31 ± 0.03C | 4.59 ± 0.05C | 8.48 ± 0.05C | 6.85 ± 0.05B | 3.10 ± 0.14E | 8.39 ± 0.03C | 4.90 ± 0.08C | 4.07 ± 0.05D | 8.70 ± 0.05B | 7.20 ± 0.08A | |
| High | 0 (control) | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05A | 5.45 ± 0.00A | 8.81 ± 0.02A | 7.16 ± 0.05B |
| 15 | 4.99 ± 0.06B | 8.75 ± 0.05AB | 7.08 ± 0.07AB | 5.08 ± 0.07B | 8.65 ± 0.06B | 7.16 ± 0.05A | 4.73 ± 0.08B | 8.57 ± 0.10B | 7.20 ± 0.06A | 5.40 ± 0.06A | 8.78 ± 0.12A | 7.30 ± 0.05A | |
| 30 | 4.88 ± 0.05B | 8.75 ± 0.09AB | 7.01 ± 0.04B | 5.10 ± 0.10B | 8.63 ± 0.03B | 7.15 ± 0.07A | 4.47 ± 0.05C | 8.43 ± 0.07BC | 7.10 ± 0.10AB | 5.39 ± 0.06A | 8.77 ± 0.05A | 7.26 ± 0.06AB | |
| 60 | 4.67 ± 0.02C | 8.74 ± 0.06AB | 7.00 ± 0.05B | 5.04 ± 0.05B | 8.56 ± 0.08B | 7.12 ± 0.09A | 4.05 ± 0.07D | 8.42 ± 0.05BC | 7.06 ± 0.09AB | 5.16 ± 0.08B | 8.75 ± 0.04AB | 7.18 ± 0.05B | |
| 120 | 4.41 ± 0.11D | 8.72 ± 0.06B | 6.90 ± 0.21AB | 4.85 ± 0.03C | 8.56 ± 0.05B | 7.13 ± 0.05A | 3.87 ± 0.06E | 8.40 ± 0.03C | 6.99 ± 0.05B | 4.74 ± 0.03C | 8.72 ± 0.02B | 7.15 ± 0.08B | |
The values are averages of triplicate measurements ± SD. Values followed by the same letters indicate no significant difference (P ≥ 0.05) in the pH across the exposure times within the same type of plasma.
Determination of the H2O2 formed during plasma exposure.
As shown in Table 3, hydrogen peroxide was formed in distilled water when it was exposed to all types of plasma. The measured H2O2 concentrations were time dependent: the longer the exposure, the higher the concentration. Higher concentrations of H2O2 were detected when distilled water was exposed to Ar plus 0.27% H2O plasma (251.7 to 2,785 μM H2O2), followed by Ar plasma (36.6 to 120 μM H2O2), whereas the lowest concentrations were found with exposure to Ar plus 1% air plasma and Ar plus 1% O2 plasma (11.6 to 105 and 18.3 to 48.3 μM H2O2, respectively).
TABLE 3.
Concentrations of H2O2 formed in distilled water after exposure to various types of plasma
| Exposure time (s) | H2O2 concna (μM) |
|||
|---|---|---|---|---|
| Pure Ar plasma | Ar + 1% O2 plasma | Ar + 1% air plasma | Ar + 0.27% H2O plasma | |
| 15 | 36.6 ± 6.7C/B | 18.3 ± 1.7C/C | 11.6 ± 1.7D/C | 251.7 ± 5.0D/A |
| 30 | 70.0 ± 6.7B/B | 31.7 ± 5.0B/C | 21.7 ± 1.7C/C | 458.3 ± 31.7C/A |
| 60 | 116.7 ± 15A/B | 48.3 ± 1.7A/C | 56.6 ± 10.0B/C | 975.0 ± 8.3B/A |
| 120 | 120.0 ± 3.3A/B | 48.3 ± 5.0A/C | 105.0 ± 1.7A/C | 2785.0 ± 61.7A/A |
The values are the averages of triplicate measurements. Values followed by the same letters indicate that there was no significant difference in the H2O2 concentration (P ≥ 0.05) across the treatment times within the same plasma treatment group (letters before slashes) or across the plasma treatment within the same treatment time group (letters after slashes). The distance from the plasma jet nozzle to the surface of the virus suspension (low level) was 11:25 mm.
FCV virucidal effect of liquid hydrogen peroxide.
The virucidal effect of liquid H2O2 was concentration and time dependent, as shown in Fig. 5. In the 30-min treatment group, all tested H2O2 concentrations resulted in nonsignificant changes (P ≥ 0.05) in the FCV titer, except that the 80 and 100 mM treatments showed ∼1.1 and ∼1.5 log10 TCID50 reductions, respectively. In the 1-h treatment group, significant reductions (P < 0.05) ranged from ∼0.8 to ∼1.5 log10 TCID50 at all concentrations, except that the 80 and 100 mM treatment groups showed ∼2.2 and ∼2.6 log10 TCID50 reductions in the FCV titer, respectively. After 2 h treatment, all concentrations led to significant reductions (P < 0.05) in the FCV titer; the smallest reductions (∼1 log10 TCID50) were observed in the 5, 10, and 20 mM treatment groups, while 30, 40, 50, 60, and 80 mM H2O2 led to greater reductions (∼2.3 to 2.5 log10 TCID50). The greatest virucidal effect (∼2.7 log10 TCID50) was seen in the 100 mM H2O2 treatment group.
FIG 5.
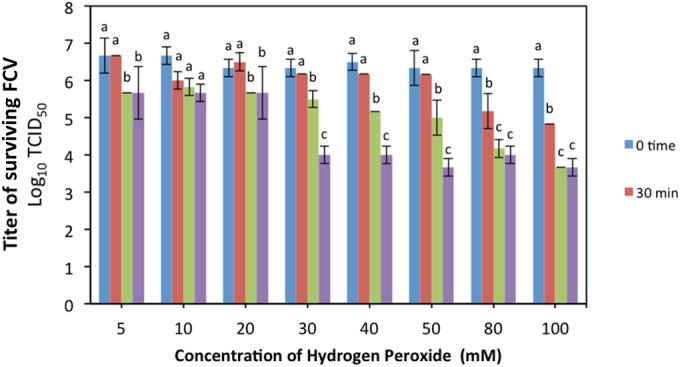
Virucidal effect of liquid hydrogen peroxide against FCV. Aliquots (100 μl) of DW-FCV suspension were mixed with equal volumes of different H2O2 solutions (5, 10, 20, 30, 40, 50, 80, and 100 mM final H2O2 concentrations). The samples were incubated for 30, 60, and 120 min at room temperature. The results are the averages of triplicate experiments, and the error bars indicate the standard deviations. The letters on the bars represent comparisons between the means of the treatment times within the same H2O2 concentration group; there is no significant difference (P ≥ 0.05) between bars labeled with the same letter.
The effective FCV lethal time of plasma exposure.
The virucidal times of the four plasma types are shown in Fig. 6. More than 5 log10 TCID50 (∼100%) of FCV was inactivated after 2, 3, and 5 min of exposure to Ar plus 1% O2, Ar plus 1% dry air, and Ar plasmas, respectively, whereas exposure of FCV to Ar plus 0.27% plasma did not reach the virucidal effect of the other types of plasma (∼3.3 log10 TCID50 after 5-min exposure). The exposure times needed for 1 log10 reductions (D values) of Ar, Ar plus 1%O2, Ar plus 1% air, and Ar plus 0.27% water were 0.94, 0.38, 0.56, and 1.49 min, respectively.
FIG 6.
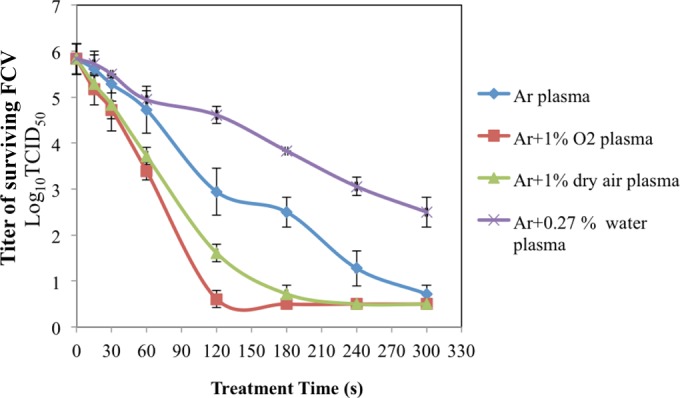
Effective FCV lethal time of plasma exposure. Aliquots (100 μM) of DW-FCV suspensions were exposed to Ar, Ar plus 1% O2, Ar plus 1% air, and Ar plus 0.27% water plasmas for 15, 30, 60, 120, 180, 240, and 300 s. Immediately after plasma exposure, 0.1 M sodium thiosulfate was added, followed immediately by FCV titration. The exposure distance from the nozzle to the surface of the virus suspension was 11.25 mm. The results are the averages of triplicate experiments, and the error bars indicate the standard deviations.
DISCUSSION
Feline calicivirus was chosen in this study as a surrogate for human NV because NV cannot be grown in vitro, although several attempts have been made (27, 34). FCV belongs to the same family (Caliciviridae) as human NV, and therefore, it has similar characteristics (35). The chemical interaction between the chemical species of plasma and the virus was the supposed mode of action of the plasma; therefore, we selected FCV in our study as a worst-case scenario, since it is known to have high resistance to chemical disinfectants. Many investigators recommend the use of murine norovirus (MNV) instead of FCV, although FCV is more resistant to chemical disinfectants and high temperature than MNV. Cannon et al. (36) found that FCV was more stable than MNV type 1 (MNV-1) at 56°C. Sattar et al. (37) compared the effects of ethanol-based hand rubs to eliminate FCV and MNV. They showed that over a short contact time (20 s), FCV was 100 times more resistant to inactivation than MNV. Park et al. (38) reported that MNV was more readily inactivated by alcohols than FCV. Thus, we preferred to use it in our study instead of MNV. In addition, FCV has been used previously as a surrogate to evaluate the efficacies of common preservation processes used in the food industry (39).
In a preliminary test, we found that FCV is susceptible to cold Ar plasma. Since previous studies reported that the biological activity of CGP is multifactorial and highly dependent on plasma type and exposure parameters (6, 23, 40), we studied the effects of several operational and exposure parameters affecting the reactivity of the resultant cold plasma and its virucidal activity in order to reach the objective of complete inactivation of FCV in solution. The effects of the plasma generation power, feed gas mixtures, exposure time, exposure distance, and virus suspension medium were studied.
To determine if changes in temperature might be responsible for the virucidal activity, we measured the changes in temperature during several treatments. It has been reported that FCV has D values of 4 days and 6.7 min at 25°C in tap water (41) and 56°C in MEM (36), respectively. None of our treatments produced temperatures higher than 27°C (Table 1), indicating that the observed virucidal effects (>5 log10 TCID50/0.1 ml after 15 s of plasma exposure) were not due to an increase in temperature.
Also, the observed reductions in FCV titers induced by plasma cannot be attributed to changes in pH, either, since only ∼2.5 log10 and ∼2 log10 TCID50/0.1 ml decreases in FCV titers were seen when wet virus was exposed for 30 min to pH 4 and pH 9, respectively, at 37°C (36). We believe that the effect of the plasma is caused by the production of active chemical species in solution.
To study the effect of the plasma generation power (Fig. 2), we used Ar plasma, which was generated at various power values, as a reference plasma type. The results showed an increase in the virucidal effect with increasing plasma generation power up to 2.5 W. This is consistent with the observed increase in reactive species, such as O3 and NO, with increasing power (26, 29). In the present study, a plasma generation power of 2.5 W was selected to be used in the subsequent tests, as it had the greatest virucidal effect and there was no significant difference (P ≥ 0.05) between its effect and the effect of 3 W of power at all exposure times.
The absence of virucidal effect of any feeding gas mixture when the power was off (Fig. 3A to D) confirms that the virucidal effects of CGP are not due to disturbance of the solution by the flow of gases. The increase in virucidal effect with increasing plasma exposure time is probably due to an increase in the production of reactive species in solution, as is illustrated by the reduction of the pH with increasing treatment time. This is in agreement with the results of Alekseev et al. (23), who found that reduction in the infectivity of herpes simplex virus 1 was proportional to the exposure time when the infection medium was treated with nonthermal DBD air-based plasma. A similar effect was found with this plasma source in treating Pseudomonas aeruginosa (24).
The FCV virucidal activities of all tested plasma types were inversely proportional to the exposure distance. This is consistent with a smaller reduction in pH at the high level of plasma than at the low level (Table 2). Also, experiments with plasma plumes showed that the densities of reactive species decreased with increasing distance from the nozzle (26, 29). In contrast, Niemira (42) found that E. coli O157:H7 on the surfaces of almonds was reduced more effectively with greater distance from the dry-air-based cold-plasma emitter. These results clearly illustrate the difference between plasma treatments of pathogens in solution versus those that are directly treated on surfaces.
One of the major factors that determine the reactive species produced by plasma is the gas composition, which leads to significant differences in their biological impacts (42). All four gas mixtures investigated in this study had significant virucidal effects against FCV (Fig. 3A to D). The addition of 1% air or O2 led to an increase in the virucidal effect compared to Ar alone, indicating that the addition of O2 and air enhances the FCV virucidal effect of Ar plasma. This is also consistent with the increase of O3 and NO densities found for these plasma conditions (29). Obviously, the Ar plus 1% O2 plasma was the best among all the tested feed gas mixtures, since FCV was completely inactivated at the low exposure level with only 15 s of exposure followed by 2 h posttreatment incubation at room temperature. We believe that this phenomenon may be attributed to an increase in the reactive oxygen species (ROS), particularly ozone, released from plasma discharge (29) to the treated solution, which may have the main virucidal effect against FCV.
The beneficial effect of adding O2 in the inactivation of bacteria has been reported by many researchers, although these studies were performed to study the effects against bacteria on surfaces rather than in solution. In a study conducted on S. aureus, the authors found that the antibacterial activity of plasma was more significant when 97%:3% He-O2 was used than using 97%:3% He-N2 (43). Sureshkumar et al. (44) reported that addition of 2% O2 to the gas mixture (N2) enhanced the anti-S. aureus effect of RF plasma, leading to a 6.0-log-unit reduction. Noriega et al. (19) observed that the presence of O2 in helium-based atmospheric cold plasma enhanced reductions of Listeria innocua on chicken samples. Chen et al. (45) studied the antibacterial effect of an atmospheric low-temperature helium-based plasma and found that the He-O2 plasma killed Enterococcus faecalis more effectively than the pure He plasma.
Our results show that the addition of 0.27% water to the Ar flow decreased the virucidal activity of the Ar plasma. This is not surprising, because it is known that the augmentation of feed gas humidity increases H2O2 production (46) but decreases certain ROS species, such as ozone (47). Indeed, we found that the H2O2 concentration in distilled water increased from ≤120 μM for Ar, Ar plus 1% O2, and Ar plus 1% air to 2,785 μM for Ar plus 0.27% water after a 2-min treatment time.
The results for virus suspension solutions (Fig. 4A to D) indicate that the FCV virucidal activity of plasma is greatly affected by the type of virus suspension medium. Generally, the greatest reduction in FCV was observed when the virus was suspended in distilled water. In MEM, all types of plasma showed minimum virucidal activity. When FCV was suspended in NTE buffer, FCV virucidal activity was found to be variable; virus suspension in NTE buffer did not affect the virucidal activity of Ar plus 1% O2 plasma but did decrease the virucidal activities of all other plasma types. It is clear that pH has an important effect on the plasma-induced liquid-phase chemistry, as has been shown for the same plasma source using Ar for bacterial inactivation (48). However, the effect of Ar plus 1% O2 seems to be pH independent. The reduced virucidal effect in MEM may be due to the presence of bovine serum and proteins, which may act as scavengers of the ROS produced by the plasma through an oxidation process with the molecules. We hypothesize, based on this observation, that the virucidal efficiency of this new technology might be positively or negatively affected by the chemical composition of the treated foods, and it indicates that plasma might not be applicable with high-protein foods. However, further investigations of various food models are needed to study the effect of food composition on the virucidal efficacy of plasma.
We believe that the virucidal effect of plasma is not due to the production of H2O2 in solution, as the maximum virucidal effect was not more than 2.7 log10 TCID50/0.1 ml of FCV when treated with 100 mM liquid H2O2 for 2 h (Fig. 5). However, there was more than a 4.5 log10 TCID50/0.1 ml decrease with Ar plus 0.27% water plasma, which did not yield more than 2.8 mM H2O2 (Table 3). The key factor for the FCV virucidal activity is the chemical interaction of ROS and reactive nitrogen species (RNS), such as singlet oxygen (O2*), ozone (O3), and superoxide (O2−) or peroxynitrous acid. These species potentially react with the capsid protein of FCV, leading to protein peroxidation and destruction of the capsid. In addition, they can damage the viral RNA, leading to reduced gene expression and elimination of viral RNA, or both. This hypothetical mode of action has been suggested in several reports. Yasuda et al. (49) suggested that CGP mainly affects the bacteriophage lambda coat protein and, to a lesser extent, causes damage to the bacteriophage DNA. They also showed experimental evidence that degradation of proteins and DNA was detectable only at long plasma exposures. However, lambda coat protein can be inactivated quickly, suggesting chemical modification, such as oxidation or reduction. A similar mode of action was suggested by Zimmermann et al. (22), explaining the inactivation of a recombinant strain of human adenovirus using CGP. Since FCV consists of RNA, packaged in the capsid protein coat, the mechanism of inactivation by our CGP system may be similar.
In the plasma exposure protocol used in this study, the plasma-exposed samples are kept at room temperature for 2 h before titration of the surviving virus is performed. This was done to provide ample time for the chemical reactions to occur between the plasma-produced species in the liquid and the virus. Hence, an experiment was conducted to measure the effective virucidal time of the virus exposed to all tested types of plasma (Fig. 6). The titers of surviving virus were measured immediately after the exposure time and addition of a general scavenger of oxidative species present in the liquid at the end of the exposure time. This effectively eliminated all potential post-plasma exposure chemical reactivity that might influence the virus inactivation. The results show that the plasma system has fast and efficient virucidal activity up to 6.0 log10 reduction after 3 min with a very low D value (0.38 min) in the case of Ar plus 1% O2 plasma, as seen in Fig. 6 and 7 and Table 4. The results show that our plasma system has fast and effective virucidal activity in comparison with the other systems reported in the published literature. Terrier et al. (21) reported 6.5, 3.8, and 4 log10 TCID50/ml reductions in the titers of influenza virus type A (H5N2), human parainfluenza virus type 3, and RSV suspensions, respectively, as a result of treating them for 3 min in a newly designed system based on cold oxygen plasma treatment. Within 4 min of CGP treatment, 6.0 log10 units of human adenovirus was inactivated (22). Furthermore, the virucidal efficacy of our CGP seems to be faster and more effective against FCV than a number of novel nonthermal food-processing techniques and is comparable with some others (Table 5). Compared to the strong virucidal activity of short exposure followed by 2 h of incubation, longer exposure times were needed for virus inactivation when the scavenger (sodium thiosulfate) was added immediately after the plasma treatment. This indicates that virus inactivation is partially caused by long-lived ROS or RNS. When plasma-treated solution was added to the virus after different intervals, we found that in most cases, the virucidal effect was no longer present 5 min after the plasma treatment (data not shown).
FIG 7.
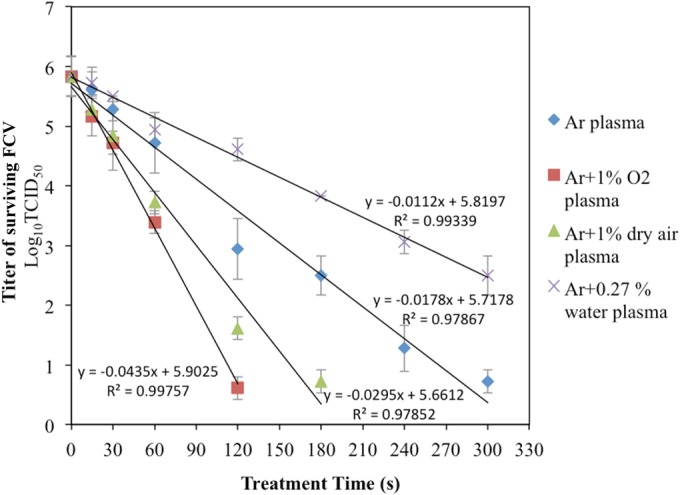
Survival kinetic curves of FCV exposed to Ar, Ar plus 1% O2, Ar plus 1% air, and Ar plus 0.27% water plasmas showing the slopes of regression lines using the linear portions of the survival curves. The D values are the negative reciprocals of the slopes of the regression lines. The plasma dissipated power was kept constant at 2.5 ± 0.1 W. The exposure distance from the nozzle to the surface of the virus suspension was 11.25 mm.
TABLE 4.
D values and estimated times for 4 log10 reduction and complete reduction (5.83 log10) of FCV under different plasma conditionsa
| Treatment | D value | t4b | tTc |
|---|---|---|---|
| Ar plasma | 0.94 | 3.75 | 5.46 |
| Ar + 1% O2 plasma | 0.38 | 1.53 | 2.23 |
| Ar + 1% dry air plasma | 0.56 | 2.26 | 3.29 |
| Ar + 0.27 % water plasma | 1.49 | 5.95 | 8.68 |
The exposure times needed for 4 log10 TCID50/0.1 ml reduction and complete reduction of FCV were determined from the following equation: t = D × (log No − log Nf) = D × n, where D is the D value (in minutes) under the specified conditions; No is the initial titer of the virus; Nf is the surviving titer after an exposure time, t (in minutes), to the selected plasma type; and n is (log No − log Nf), which is equal to the log10 reduction of the initial titer of the virus.
t4, estimated plasma exposure time (in minutes) needed for 4 log10 TCID50 reduction. t4 = D × n; n = 4 log10.
tT, estimated exposure time (in minutes) needed for complete reduction of the initial FCV titer (5.83 log10 TCID50). tT = D × n; n = 5.83 log10.
TABLE 5.
Comparison of known virucidal effects of several nonthermal food-processing techniques
| Technique | Virusa | Log10 reduction in virus titer | Treatment time | Sample typeb | Reference |
|---|---|---|---|---|---|
| 5,000 mg/liter sodium hypochlorite in water (4 times the FDA-allowed concn) | FCV | 3.0 | Water | 50 | |
| Chlorine (3,000 mg/liter) | NV | No effect | 10 min | Water | 51 |
| Ozone treatment (ozone/h at a flow rate of 2.4 liters/min [6.25 ppm]) | FCV | >6.0 | 5 min | Water | 9 |
| High-hydrostatic-pressure processing (HHP) (275 MPa) | FCV | 7.0 | 5 min | DMEM | 52 |
| UV irradiation (130 mW/cm2) | FCV | 3.0 | Phosphate buffer | 53 | |
| Ionized gamma irradiation | |||||
| 500 kGy | FCV | 3.0 | Tap water | 54 | |
| 5.6 kGy | MNV1 | 1.7–2.4 | Fresh produce | 10 | |
| Pulsed electric field treatment (20–29 kV/cm) | RV | No effect | 145.6 μs | MEM | 55 |
| Ar + 1% O2 plasma | FCV | >6.0 | 2 min | Water | Present study |
MNV1, murine norovirus type 1; RV, rotavirus (nonenveloped enteric RNA virus, like human norovirus).
DMEM, Dulbecco's modified Eagle medium.
In conclusion, oxygen-based CGP is a novel, versatile nonthermal tool that has great virucidal potential against FCV, a surrogate for human norovirus, which is the most important foodborne virus. This novel technology has strong potential in food-processing and food safety applications for controlling viral contamination of food-processing surfaces and fresh and minimally processed foods. Further studies are recommended to determine the mechanism of action of CGP used in this study. Experiments should also be conducted to determine if this CGP system can inactivate FCV, HAV, and NV on ready-to-eat foods and foods eaten fresh and on food contact surfaces.
ACKNOWLEDGMENTS
Partial funding provided by the Cultural Affairs and Mission Sector, Ministry of Higher Education and Scientific Research, Egypt, is gratefully acknowledged. We also acknowledge funding from the Department of Energy Plasma Science Center through the U.S. Department of Energy, Office of Fusion Energy Sciences, contract DE-SC0001939, and the University of Minnesota.
We thank Nhungoc Ti Luong for technical help.
REFERENCES
- 1.Scharff RL. 3 March 2010. Health-related costs from foodborne illness in the United States. http://www.publichealth.lacounty.gov/eh/docs/ReportPublication/HlthRelatedCostsFromFoodborneIllinessUS.pdf.
- 2.Anonymous. 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in the European Union in 2010. EFSA J 10:2597. doi: 10.2903/j.efsa.2012.2597. [DOI] [PubMed] [Google Scholar]
- 3.European Center for Disease Prevention and Control. 2013. Factsheet for health professionals. http://www.ecdc.europa.eu/en/healthtopics/norovirus_infection/factsheet-health-professionals/Pages/factsheet_health_professionals.aspx.
- 4.CDC. 30 December 2014. U.S. trends and outbreaks. http://www.cdc.gov/norovirus/trends-outbreaks.html.
- 5.European Food Safety Authority. 30 April 2009. Community summary report. Food-borne outbreaks in the European Union in 2007. European Food Safety Authority, Parma, Italy: http://www.efsa.europa.eu/de/efsajournal/doc/271r.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra NN, Tiwari BK, Raghavarao KSMS, Cullen PJ. 2011. Nonthermal plasma inactivation of food-borne pathogens. Food Eng Rev 3:159–170. doi: 10.1007/s12393-011-9041-9. [DOI] [Google Scholar]
- 7.Hirneisen KA, Black EP, Cascarino JL, Fino VR, Hoover DG, Kniel KE. 2010. Viral inactivation in foods: a review of traditional and novel food-processing technologies. Compr Rev Food Sci Food Saf 9:3–20. doi: 10.1111/j.1541-4337.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- 8.Mormann S, Dabisch-Ruthe M, Becker B. 2010. Inactivation of norovirus in foods: inoculation study using human norovirus. Fleischwirtschaft 90:116–121. [Google Scholar]
- 9.Hirneisen KA, Markland SM, Kniel KE. 2011. Ozone inactivation of norovirus surrogates on fresh produce. J Food Prot 74:836–839. doi: 10.4315/0362-028X.JFP-10-438. [DOI] [PubMed] [Google Scholar]
- 10.Feng K, Divers E, Ma Y, Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboubakr HA, El-Banna AA, Youssef MM, Al-Sohaimy SAA, Goyal SM. 2014. Antiviral effects of Lactococcus lactis on feline calicivirus, a human norovirus surrogate. Food EnvironVirol 6:282–289. doi: 10.1007/s12560-014-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves DB. 2012. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45:263001. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 13.Fridman A. 2008. Plasma chemistry. Cambridge University Press, New York, NY. [Google Scholar]
- 14.Bruggeman P, Locke BR. 2013. Assessment of potential applications of plasma with liquid water, p 13–40. In Chu PK, Lu XP (ed), Low temperature plasma technology: methods and applications. CRC Press, Boca Raton, FL. [Google Scholar]
- 15.Cecchi JL. 1990. Introduction to plasma concept and discharge, p 14–69. In Rossnagel SM, Cuomo JJ, Westwood WD (ed), Handbook of plasma processing technology, fundamentals, etching, deposition and surface interactions. Noyes Publications, Saddle River, NJ. [Google Scholar]
- 16.Azharonok V, Krat'ko L, Nekrashevich YI, Filatova I, Mel'nikova L, Dudchik N, Yanetskaya S, Bologa M. 2009. Bactericidal action of the plasma of high-frequency capacitive and barrier discharges on microorganisms. J Eng Phys Thermophys 82:419–426. doi: 10.1007/s10891-009-0210-0. [DOI] [Google Scholar]
- 17.Fernández A, Noriega E, Thompson A. 2013. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol 33:24–29. doi: 10.1016/j.fm.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Sun P, Bai N, Tian Y, Zhou H, Wei S, Zhou Y, Zhang J, Zhu W, Becker K. 2010. Inactivation of bacteria in an aqueous environment by a direct current, cold atmospheric pressure air plasma micro jet. Plasma Process Polym 7:231–236. doi: 10.1002/ppap.200900070. [DOI] [Google Scholar]
- 19.Noriega E, Sharma G, Laca A, Diaz M, Kong MG. 2011. Cold atmospheric gas plasma disinfection of chicken muscle and chicken skin contaminated with Listeria monocytogenes. Food Microbiol 28:1293–1300. doi: 10.1016/j.fm.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Alkawareek MY, Algwari QT, Gorman SP, Graham WG, O'Connell D, Gilmore BF. 2012. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol Med Microbiol 65:381–384. doi: 10.1111/j.1574-695X.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 21.Terrier O, Essere B, Yver M, Barthélémy M, Bouscambert-Duchamp M, Kurtz P, van Mechelen D, Morfin F, Billaud G, Ferraris O, Lina B, Rosa-Calatrava M, Moules V. 2009. Cold oxygen plasma technology efficiency against different airborne respiratory viruses. J Clin Virol 45:119–124. doi: 10.1016/j.jcv.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer Schlegel VJ, Gansbacher B, Anton M. 2011. Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys 44:505201. doi: 10.1088/0022-3727/44/50/505201. [DOI] [Google Scholar]
- 23.Alekseev O, Donovan K, Limonnik V, Azizkhan-Clifford J. 2014. Nonthermal dielectric barrier discharge (DBD) plasma suppresses herpes simplex virus type 1 (HSV-1) replication in corneal epithelium. Trans Vis Sci Technol 3:2. doi: 10.1167/tvst.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gils CAJ, Hofmann S, Boekema BKHL, Brandenburg R, Bruggeman PJ. 2013. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J Phys D Appl Phys 46:175203. doi: 10.1088/0022-3727/46/17/175203. [DOI] [Google Scholar]
- 25.Hofmann S, Gessel A, Bruggeman P. 2011. Power dissipation, gas temperatures and electron densities of cold atmospheric pressure helium and argon RF plasma jets. Plasma Sources Sci Technol 20:065010. doi: 10.1088/0963-0252/20/6/065010. [DOI] [Google Scholar]
- 26.van Gessel AFH, Alards KMJ, Bruggeman PJ. 2013. NO production in an RF plasma jet at atmospheric pressure. J Phys D Appl Phys 46:265202. doi: 10.1088/0022-3727/46/26/265202. [DOI] [Google Scholar]
- 27.Malik YS, Maherchandani S, Allwood PB, Goyal SM. 2005. Evaluation of animal origin cell cultures for in vitro cultivation of noroviruses. J Appl Res Clin Exp Ther 5:312–317. [Google Scholar]
- 28.van Gaens W, Bogaerts A. 2014. Reaction pathways of biomedically active species in an Ar plasma jet. Plasma Sources Sci Technol 23:035015. doi: 10.1088/0963-0252/23/3/035015. [DOI] [Google Scholar]
- 29.van Ham BTJ, Hofmann S, Brandenburg R, Bruggeman PJ. 2014. In situ absolute air, O3 and NO densities in the effluent of a cold RF argon atmospheric pressure plasma jet obtained by molecular beam mass spectrometry. J Phys D Appl Phys 47:224013. doi: 10.1088/0022-3727/47/22/224013. [DOI] [Google Scholar]
- 30.Bruggeman P, Leys C. 2009. Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42:053001. doi: 10.1088/0022-3727/42/5/053001. [DOI] [Google Scholar]
- 31.Kärber G. 1931. 50% end point calculation. Arch Exp Pathol Pharmacol 162:480–483. doi: 10.1007/BF01863914. [DOI] [Google Scholar]
- 32.Satterfield CH, Bonnell AH. 1995. Interferences in the titanium sulfate method for hydrogen peroxide. Anal Chem 27:1174–1175. doi: 10.1021/ac60103a042. [DOI] [Google Scholar]
- 33.Eisenberg GM. 1943. Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal 15:327–328. doi: 10.1021/i560117a011. [DOI] [Google Scholar]
- 34.Straub TM, Honerzu BK, Orosz-Coghlan P, Dohnalkova A, Mayer BK, Bartholomew RA, Valdez CO, Bruckner-Lea CJ, Gerba CP, Abbaszadegan MA, Nickerson CA. 2007. In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis 13:396–403. doi: 10.3201/eid1303.060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Souza DH, Sair A, Williams K, Papafragkou E, Jean J, Moore C, Jaykus L. 2006. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol 108:84–91. doi: 10.1016/j.ijfoodmicro.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus L, Vinjé J. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot 69:2761–2765. [DOI] [PubMed] [Google Scholar]
- 37.Sattar SA, Ali M, Tetro JA. 2011. In vivo comparison of two norovirus surrogates for testing ethanol-based handrubs: the mouse chasing the cat! PLoS One 6:e17340. doi: 10.1371/journal.pone.0017340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinjé J. 2010. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot 73:2232–2238. [DOI] [PubMed] [Google Scholar]
- 39.Butot S, Putallaz T, Sanchez G. 2008. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int J Food Microbiol 126:30–35. doi: 10.1016/j.ijfoodmicro.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Hury S, Vidal DR, Desor F, Pelletier J, Lagarde T. 1998. A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-based plasmas. Lett Appl Microbiol 26:417–421. doi: 10.1046/j.1472-765X.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 41.Allwood PB, Malik YS, Maherchandani S, Hedberg CW, Goyal SM. 2005. Effect of temperature on the survival of F-specific RNA coliphage, feline calicivirus, and Escherichia coli in chlorinated water. Int J Environ Res Public Health 2:442–446. doi: 10.3390/ijerph2005030008. [DOI] [PubMed] [Google Scholar]
- 42.Niemira BA. 2012. Cold plasma reduction of Salmonella and Escherichia coli O157:H7 on almonds using ambient pressure gases. J Food Sci 77:M171. doi: 10.1111/j.1750-3841.2011.02594.x. [DOI] [PubMed] [Google Scholar]
- 43.Lu XP, Ye T, Cao YG, Sun ZY, Xiong Q, Tang Z, Xiong Z, Hu J, Jiang Z, Pan Y. 2008. The roles of the various plasma agents in the inactivation of bacteria. J Appl Phys 104:053309. doi: 10.1063/1.2977674. [DOI] [Google Scholar]
- 44.Sureshkumar S, Sankar R, Mandal M, Neogi S. 2010. Effective bacterial inactivation using low temperature radio frequency plasma. Int J Pharm 396:17–22. doi: 10.1016/j.ijpharm.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Huang J, Du N, Liu X, Wang X, Lv G-H, Zhang G, Guo L, Yang S. 2012. Treatment of Enterococcus faecalis bacteria by a helium atmospheric cold plasma brush with oxygen addition. J Appl Phys 112:013304. doi: 10.1063/1.4732135. [DOI] [Google Scholar]
- 46.Winter J, Wende K, Masur K, Iseni S, Dünnbier M, Hammer MU, Tresp H, Weltmann K-D, Reuter S. 2013. Feed gas humidity: a vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J Phys D Appl Phys 46:295401. doi: 10.1088/0022-3727/46/29/295401. [DOI] [Google Scholar]
- 47.Liu DX, Iza F, Wang XH, Kong MG, Rong MZ. 2011. He+O2+H2O plasmas as a source of reactive oxygen species. Appl Phys Lett 98:221501. doi: 10.1063/1.3592775. [DOI] [Google Scholar]
- 48.Boekema BKHL, Hofmann S, van Ham BJT, Bruggeman PJ, Middelkoop E. 2013. Antibacterial plasma at safe levels for skin cells. J Phys D Appl Phys 46:422001. doi: 10.1088/0022-3727/46/42/422001. [DOI] [Google Scholar]
- 49.Yasuda H, Miura T, Kurita H, Takashima K, Mizuno A. 2010. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym 7:301–308. doi: 10.1002/ppap.200900088. [DOI] [Google Scholar]
- 50.Gulati BR, Allwood PB, Hedberg CW, Goyal SM. 2001. Efficacy of commonly used disinfectants for the inactivation of calicivirus on strawberry, lettuce and a food-contact surface. J Food Prot 64:1430–1434. [DOI] [PubMed] [Google Scholar]
- 51.Duizer E, Bijkerk P, Rockx B, de Groot A, Twisk F, Koopmans M. 2004. Inactivation of caliciviruses. Appl Environ Microbiol 70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kingsley DH, Hoover DG, Papafragkou E, Richards GP. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J Food Prot 65:1605–1609. [DOI] [PubMed] [Google Scholar]
- 53.Nuanualsuwan S, Mariam T, Himathongkham S, Cliver D. 2002. Ultraviolet Inactivation of feline calicivirus, human enteric viruses and coliphages. Photochem Photobiol 76:406–410. doi: 10.1562/0031-8655(2002)0760406UIOFCH2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 54.De Roda Husman AM, Bijkerk P, Lodder W, Van Den Berg H, Pribil W, Cabaj A, Gehringer P, Sommer R, Duizer E. 2004. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl Environ Microbiol 70:5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khadre MA, Yousef AE. 2002. Susceptibility of human rotavirus to ozone, high pressure and pulsed electric field. J Food Prot 65:1441–1446. [DOI] [PubMed] [Google Scholar]