Abstract
Akkermansia muciniphila is a Gram-negative mucin-degrading bacterium that resides in the gastrointestinal tracts of humans and animals. A. muciniphila has been linked with intestinal health and improved metabolic status in obese and type 2 diabetic subjects. Specifically, A. muciniphila has been shown to reduce high-fat-diet-induced endotoxemia, which develops as a result of an impaired gut barrier. Despite the accumulating evidence of the health-promoting effects of A. muciniphila, the mechanisms of interaction of the bacterium with the host have received little attention. In this study, we used several in vitro models to investigate the adhesion of A. muciniphila to the intestinal epithelium and its interaction with the host mucosa. We found that A. muciniphila adheres strongly to the Caco-2 and HT-29 human colonic cell lines but not to human colonic mucus. In addition, A. muciniphila showed binding to the extracellular matrix protein laminin but not to collagen I or IV, fibronectin, or fetuin. Importantly, A. muciniphila improved enterocyte monolayer integrity, as shown by a significant increase in the transepithelial electrical resistance (TER) of cocultures of Caco-2 cells with the bacterium. Further, A. muciniphila induced interleukin 8 (IL-8) production by enterocytes at cell concentrations 100-fold higher than those for Escherichia coli, suggesting a very low level of proinflammatory activity in the epithelium. In conclusion, our results demonstrate that A. muciniphila adheres to the intestinal epithelium and strengthens enterocyte monolayer integrity in vitro, suggesting an ability to fortify an impaired gut barrier. These results support earlier associative in vivo studies and provide insights into the interaction of A. muciniphila with the host.
INTRODUCTION
Akkermansia muciniphila is a Gram-negative anaerobe belonging to the Planctomycetes-Verrucomicrobia-Chlamydiae superphylum (1). A. muciniphila has been found to inhabit the gastrointestinal (GI) tracts of more than 90% of adult subjects analyzed, and it constitutes 1 to 4% of the fecal microbiota (2). A. muciniphila is capable of using intestinal mucins, the highly glycosylated proteins of the epithelial mucus layer, as its sole source of carbon and nitrogen (1). Therefore, it is not surprising that this organism has also been detected in high numbers in mucosal biopsy specimens of the human colon (3). The genome of A. muciniphila contains a large proportion of genes encoding secreted proteins (567 of the 2,176 open reading frames), 61 of which have been assigned protease, sugar hydrolase, sialidase, or sulfatase activities, suggesting specialization in mucus utilization and adaptation to the gut environment (4). There is growing evidence that A. muciniphila is associated with gut health; e.g., fewer A. muciniphila cells have been detected in ulcerative colitis (UC) and Crohn's disease (CD) patients, both in clinically active disease and during remission, than in healthy individuals (5, 6). An inverse correlation between A. muciniphila levels and the severity of acute appendicitis has also been shown (7). Moreover, fecal counts of A. muciniphila cells correlate with the richness of bacterial species and correlate inversely with type 1 diabetes, body weight, and markers of inflammation (8–10). The effects of A. muciniphila and its metabolites on mucosal gene expression patterns have been studied using gnotobiotic mice and mouse gut organoids, respectively (11, 12). These studies showed that A. muciniphila elicits distinctive changes in the expression of pathways involved in metabolic homeostasis and immune tolerance (11, 12). In addition, A. muciniphila has been demonstrated to improve the metabolic profiles of type 2 diabetic mice, to restore mucus layer thickness, and to counteract high-fat-diet-induced lipopolysaccharide (LPS) endotoxemia in obese mice (13).
The ability of intestinal bacteria to adhere to the host epithelium is considered important for efficient colonization and interaction with the host, although experimental data are scarce. In principle, colonizing bacteria can adhere either to the protective mucus gel covering the epithelial cell layer or directly to the enterocytes. In a healthy colon, the epithelial cell layer is fully covered by a thick mucus gel layer, whereas in the small intestine, the mucus layer is thinner and discontinuous (14), allowing for direct contacts between bacteria and host enterocytes. Despite the accumulating evidence for the involvement of A. muciniphila in intestinal and metabolic health, the basic mechanisms of interaction with the host have received little attention. In this study, we investigated the adhesion of A. muciniphila to human colonic mucus, the Caco-2 and HT-29 intestinal epithelial cell lines, and several extracellular matrix (ECM) proteins. In addition, we used in vitro models to study the effects of A. muciniphila on epithelial integrity and interleukin-8 (IL-8) release by enterocytes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. muciniphila BAA-835T (American Type Culture Collection) cells were grown in mucin medium (1), and Bacteroides fragilis E-022248T (= DSM 2151 = ATCC 25285) obtained from the VTT Culture Collection (VTT Technical Research Center of Finland, Espoo, Finland) was cultivated on Brucella agar with hemin and vitamin K (Fluka) supplemented with 5% sheep blood. Both strains were grown at 37°C for 2 days in an anaerobic incubator under an atmosphere of 5% H2, 10% CO2, and 85% N2 (Concept Plus anaerobic workstation; Ruskinn Technology Ltd.). Escherichia coli K-12-derived TOP10 cells (Invitrogen) were grown with agitation (220 rpm) overnight at 37°C in Luria-Bertani (LB) medium. Lactobacillus rhamnosus GG (ATCC 53103) was cultivated overnight in static de Man-Rogosa-Sharpe (MRS) broth (Difco) at 37°C. For the adhesion experiments, bacterial cells were metabolically labeled by supplementing the growth medium with 10 μl ml−1 of [6′-3H]thymidine (14.4 Ci/mmol; PerkinElmer).
Epithelial cell lines.
The Caco-2 (ACC 169) and HT-29 (ACC 299) human colonic epithelial cell lines were purchased from the Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Cultures). The cell lines were grown at 37°C under an oxic atmosphere in an incubator supplemented with 5% CO2. Caco-2 cells were cultivated in RPMI 1640 (Sigma) containing 20% heat-inactivated (30 min at 56°C) fetal calf serum (FCS; Integro B.V.), 1% nonessential amino acids (Lonza), 15 mM HEPES (Lonza), 2 mM l-glutamine (Lonza), and 100 U ml−1 penicillin and streptomycin (PEST; Lonza). HT-29 cells were grown in McCoy 5A medium (Lonza) supplemented with 10% heat-inactivated FCS and with PEST.
Assessment of viability of Akkermansia muciniphila under an oxic atmosphere.
Anaerobically grown A. muciniphila cells were washed once with phosphate-buffered saline (PBS; pH 7.4), which was kept overnight in the anaerobic incubator to remove any oxygen from the buffer. The bacterial cell suspension was diluted to an optical density at 600 nm (OD600) of 0.25. The cell suspension was divided into three 96-well microplates, with 100 μl well−1, and the microplates were incubated for 1 h at 37°C either in the anaerobic incubator, in an incubator with an oxic atmosphere supplemented with 5% CO2, or under a normal oxic atmosphere. Next, the cells were live-dead stained by adding 4′,6-diamidino-2-phenylindole (DAPI) and propidium iodide (PI) at final concentrations of 1 μg ml−1 and 0.75 μg ml−1, respectively, and incubating for 15 min at room temperature, followed by one wash with PBS. The fluorescence of the stained cell suspensions was measured with a Wallac 1420-012 multilabel counter, using 340-nm and 460-nm (DAPI) and 545-nm and 616-nm (PI) excitation and emission filters, respectively. The stained cell suspensions were then streaked onto microscopic slides and were analyzed by a Leica DM4000 B fluorescence microscope using filter cube A for DAPI and filter cube N2.1 for PI.
Isolation of human intestinal mucus.
Human colonic mucus was isolated as described previously (15, 16) from healthy parts of colons from colorectal cancer patients undergoing surgery. The use of human intestinal mucus in the adhesion studies was approved by the ethical committee of the Hospital District of Southwest Finland. All patients who donated intestinal tissue provided written informed consent.
Preparation of a whole-cell antiserum against Akkermansia muciniphila.
Live Akkermansia muciniphila cells were used to produce a polyclonal rabbit antiserum at the Laboratory Animal Centre of the University of Helsinki. Immunization was carried out as described previously (15). Briefly, overnight-grown cells were washed once with PBS and were resuspended in PBS to a final concentration of 109 ml−1. This preparation was diluted 1:1 in Freund's complete adjuvant (first injection) or Freund's incomplete adjuvant (booster injections). A 200-μl volume of the cell-adjuvant suspension was injected into the rabbit once every 3 weeks, and the animal was sacrificed and blood collected 10 days after the third booster injection. The blood was allowed to clot for 1 h at +37°C, followed by an overnight incubation at +4°C, after which the blood clot was separated from the serum by centrifugation at 5,000 × g for 10 min. The serum was divided into aliquots and was stored at −80°C prior to usage.
Immunofluorescence labeling of A. muciniphila cells adhering to the Caco-2 and HT-29 cell lines.
A. muciniphila cells were washed once with PBS, adjusted to an OD600 of 0.25, and applied to Caco-2 or HT29 cell monolayers grown for 3 days on 8-well microscope slides. The microscope slides were incubated for 1 h at 37°C either under a normal atmosphere, in an incubator with 5% CO2, or in the anaerobic incubator. After incubation, the slides were first washed 3 times with PBS and then fixed for 10 min with 4% paraformaldehyde (PFA) in PBS, followed by additional washes with PBS. A 1:100 dilution of the rabbit antiserum raised against whole cells of A. muciniphila was applied to the microscope slides (with plain PBS for conjugate control), and the slides were incubated for 1 h at room temperature. Next, the slides were washed 3 times with PBS and were mounted for 1 h at room temperature with Alexa Fluor 594-conjugated goat anti-rabbit IgG (Invitrogen) and DAPI in PBS (each at 1 μg ml−1). Unbound stains were removed by 3 washes with PBS, and the slides were analyzed by a Leica DM4000 B fluorescence microscope with filter cube A for DAPI and filter cube TX2 for Alexa Fluor 594.
Adhesion to extracellular matrix proteins.
MaxiSorp 96-well microtiter plates were prepared for adhesion assays by coating the wells overnight at 4°C with 2.5 pmol well−1 bovine serum albumin (BSA; Sigma-Aldrich), collagen I (Sigma-Aldrich), collagen IV (Sigma-Aldrich), fetuin (Sigma-Aldrich), fibronectin (Calbiochem), or laminin (Sigma-Aldrich). Coating was carried out in PBS. Next, the wells were washed twice with PBS and were blocked for 1 h at room temperature with 0.5% (wt/vol) BSA in PBS, followed by three additional washes with PBS. The [3H]thymidine-labeled bacteria were washed once with PBS, and the OD600 of the bacterial suspension was adjusted to 0.25 before the cells were added to the microtiter wells. A. muciniphila cells were allowed to bind to immobilized ECM proteins for 1 h at 37°C, after which the wells were washed three times with PBS. Bound bacteria were lysed by adding 1% SDS–0.1 M NaOH to the wells and incubating the plates for 1 h at 60°C. The radioactivity was determined with a liquid scintillator, and the fraction of bound cells was expressed as the percentage of the radioactivity of the cell suspension initially added to the wells that was retained in the wells after washing.
Binding to human intestinal epithelial cell lines and mucus.
Thymidine-labeled A. muciniphila and L. rhamnosus GG cells were collected by centrifugation and were washed once with RPMI 1640 or McCoy 5A medium without supplements, or with PBS, for the assay of adhesion to Caco-2 cells, HT-29 cells, or mucus, respectively. After washing, the OD600 of the bacterial suspension was adjusted to 0.25 in the respective medium or PBS. Caco-2 and HT-29 cells were grown for 3, 8, and 21 days and were washed twice with culture medium before the addition of bacteria. Mucus-coated wells were prepared as described for ECM protein-coated wells above, by incubating the wells with 50 μg well−1 human mucus in PBS, followed by blocking with 0.5% BSA in PBS. The OD-adjusted bacterial suspensions were added to the wells, which were then incubated for 1 h at 37°C. The epithelial cells with bacteria were incubated in the CO2 incubator. The wells were then washed three times with PBS, and the bound bacteria were lysed and quantified as described above.
TER assay.
Caco-2 cells (5 × 104/insert) were seeded in Millicell cell culture inserts (pore size, 3 μm; Millipore) and were grown for 8 days. Bacterial cells were washed once with RPMI 1640 and were applied to the inserts at an OD600 of 0.25 in RPMI 1640. Transepithelial electrical resistance (TER) was determined with a Millicell ERS-2 TER meter (Millipore) from cell cultures at 0 h, 24 h, and 48 h after the addition of bacterial cells.
Induction of IL-8 production in HT-29 cells.
Ten thousand HT-29 cells per well were seeded onto 96-well microplates and were grown for 8 days in McCoy 5A medium with supplements. A. muciniphila, B. fragilis, and E. coli cells were washed, and the OD600 was adjusted as described above in McCoy 5A medium. Bacteria were serially diluted in McCoy 5A medium to 1:100, 1:1,000, and 1:10,000, which corresponded to 106, 105, and 104 cells/ml, respectively. Two hundred microliters of each dilution was added to HT-29 wells, and the cocultures were kept for 3 h at 37°C in the CO2 incubator. For positive-control wells, 1 ng/ml of E. coli lipopolysaccharide (LPS; Sigma) was added to the culture medium, whereas plain culture medium served as a negative control. After the 3 h of incubation, the concentration of interleukin-8 (IL-8) in the culture supernatant was measured with an OptEIA Human IL-8 ELISA set (BD Biosciences) according to the manufacturer's instructions.
Western blotting.
A. muciniphila and E. coli cells were suspended in loading buffer and were boiled for 5 min, followed by SDS-PAGE under denaturing conditions on 4-to-15% gradient gels (Bio-Rad) and electroblotting onto polyvinylidene difluoride (PVDF) Immobilon P membranes (Millipore). The membrane was first probed with a rabbit polyclonal antiserum against E. coli LPS (Bioss Inc.) and then incubated with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Bio-Rad) diluted 1:100,000. The blot was visualized using the Amersham ECL Advance Western blotting detection kit (GE Healthcare Bio-Sciences) according to the manufacturer's instructions.
Preparation of immunogold-labeled thin sections and TEM.
Lowicryl HM20-embedded thin sections of A. muciniphila and E. coli cells were prepared as described previously (17). Briefly, the cells were washed once with phosphate buffer (0.1 M Na phosphate, pH 7.4) and were fixed for 4 h at room temperature in 4% PFA and 0.1% glutaraldehyde in phosphate buffer. After fixation, the cells were collected and were resuspended in 2% PFA in phosphate buffer, followed by embedding in Lowicryl HM20 resin by freeze substitution. Ultrathin sections cut from polymerized Lowicryl were placed on nickel grids and were blocked for 20 min in 1% BSA, 0.5% fish skin gelatinase (FSG), and 1% FCS in phosphate buffer, after which they were incubated for 1 h with antisera against E. coli LPS (Bioss Inc.) or lipid A (Glycobiotech) in 2% BSA, 0.1% Tween 20, and 0.1% FSG in phosphate buffer. The grids were then washed several times with pAg (colloidal gold particles conjugated to protein A) buffer (0.2% BSA, 0.01% Tween 20, and 0.01% FSG in phosphate buffer) and were incubated for 20 min with 10-nm pAg diluted 1:55 in pAg buffer. The grids were washed several times with phosphate buffer and were washed extensively with water. Prior to analysis by transmission electron microscopy (TEM), the grids were poststained with uranyl acetate and lead citrate. The grids were analyzed with a JEOL 1400 transmission electron microscope.
Statistical analysis.
Three to five parallel wells (i.e., technical replicates) were used in each experiment, and all experiments were repeated two to six times. A pairwise Student t test was used to determine significant differences (P < 0.05) between the control and samples or between two different experimental conditions. In the figures, mean values ± standard deviations for technical replicates (parallel wells) of representative experiments are shown.
RESULTS
Oxygen sensitivity of A. muciniphila.
In order to assess the viability of A. muciniphila under the experimental conditions used in our in vitro assays, we first analyzed the effects of different incubation atmospheres on A. muciniphila cells by using live-dead fluorescence staining. Importantly, A. muciniphila cells that were kept for 1 h under oxic, 5% CO2, or anoxic conditions showed similar staining patterns, and more than 90% of the cells stained live, i.e., stained only with DAPI (Fig. 1; see also Fig. S1 in the supplemental material), indicating that A. muciniphila can tolerate oxygen. Since A. muciniphila cells were not severely compromised by the use of oxic incubation conditions, the different incubation conditions were also compared in an adhesion experiment. We incubated the bacterium with Caco-2 or HT29 cell monolayers under an anaerobic or 5% CO2 atmosphere, followed by immunofluorescence microscopy with antibodies raised against whole A. muciniphila cells. We could not see any difference between the levels of binding under the two different atmospheres (Fig. 2). Similarly, binding to ECM proteins was not affected by the presence of oxygen (data not shown). On the basis of these results, and for practical reasons, we performed all subsequent experiments with enterocytes under a 5% CO2 atmosphere and other experiments under a normal atmosphere.
FIG 1.
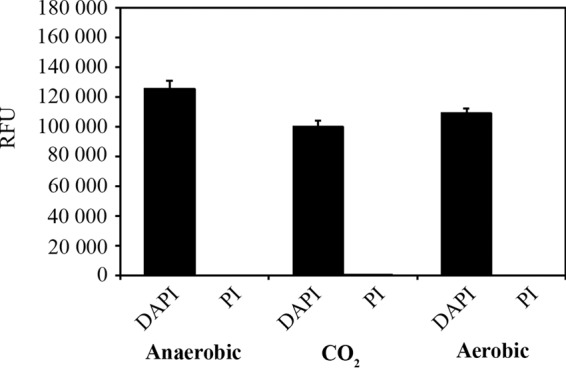
Live-dead fluorescence staining of A. muciniphila cells exposed to different atmospheres. A. muciniphila cells were incubated for 1 h under an aerobic, 5% CO2, or anaerobic atmosphere. Relative fluorescence units (RFU) were measured after staining of the cells with DAPI or propidium iodide (PI), which stains only dead or seriously impaired cells. Background fluorescence from nonstained cells has been subtracted from the RFU values obtained. The results shown are means and standard deviations for four parallel samples.
FIG 2.
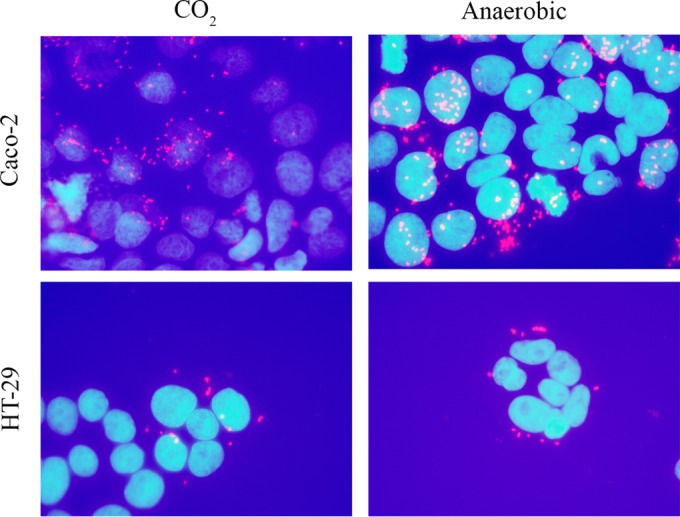
Adhesion of A. muciniphila cells to the Caco-2 and HT29 human epithelial cell lines. Adhesion was carried out under an anaerobic or 5% CO2 atmosphere and was assessed by immunofluorescence microscopy. Enterocyte nuclei were strained with DAPI (blue), and A. muciniphila cells were stained with a polyclonal rabbit antiserum raised against whole A. muciniphila cells and a secondary antibody conjugated with Alexa Fluor 594 (red).
Adhesion of A. muciniphila to extracellular matrix proteins.
The adhesion of A. muciniphila to human ECM proteins (collagens I and IV, fibronectin, and laminin) was studied by determining the binding of radiolabeled A. muciniphila cells to immobilized ligands. Bovine serum albumin was used as a negative control, and fetuin was used as a representative highly glycosylated protein. In comparison with background-level binding to BSA, A. muciniphila bound significantly only to laminin (Fig. 3). Binding to collagens I and IV, fibronectin, and fetuin was at the background level.
FIG 3.
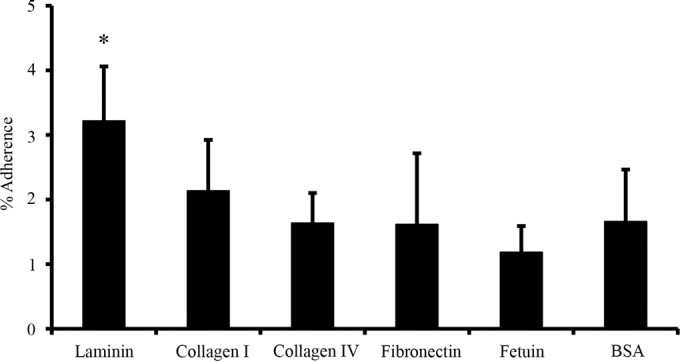
Binding of A. muciniphila to ECM proteins. Metabolically labeled A. muciniphila cells were allowed to bind to different human extracellular matrix proteins. The results shown are means and standard deviations for five parallel wells. The asterisk indicates a level of binding significantly different (P < 0.05) from background binding (to BSA).
Binding to human intestinal mucus and epithelial cells.
Next, we studied the adhesion of A. muciniphila to the Caco-2 and HT-29 cell lines and to mucus. L. rhamnosus GG was included in the experiments as a positive-control strain, since its ability to bind to human mucus and enterocytes has been well established (18, 19). Surprisingly, A. muciniphila did not bind human colonic mucus—the level of adhesion was less than 1%, which can be considered nonspecific, background-level binding—while approximately 20% of the added L. rhamnosus GG cells were found to be mucus bound (Fig. 4). In contrast, the level of adhesion of A. muciniphila to human enterocytes was comparable to that of L. rhamnosus GG (Fig. 4). Next, the binding of A. muciniphila to enterocytes grown for different periods (3 days, 8 days, and 21 days) was studied. The differentiation of Caco-2 cells starts within 3 to 4 days after confluence (20, 21), and we used undifferentiated cells with confluent growth (3 days) and cells at two differentiation stages, grown for 8 and 21 days (i.e., 5 and 18 days after confluence, respectively). The HT-29 cell line does not differentiate, but we used the same growth times for comparison. In general, A. muciniphila adhered equally well to both enterocyte lines at all growth states, except for 3-day-old HT-29 cells, to which it adhered at a lower level (Fig. 5).
FIG 4.
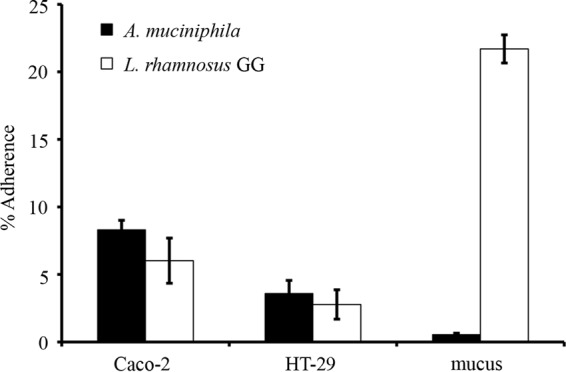
Adherence of A. muciniphila to the Caco-2 and HT-29 cell lines and to human intestinal mucus. A. muciniphila and L. rhamnosus GG (positive-control strain) cells were allowed to bind to human enterocytes or immobilized intestinal mucus. Means and standard deviations for five parallel wells are shown.
FIG 5.
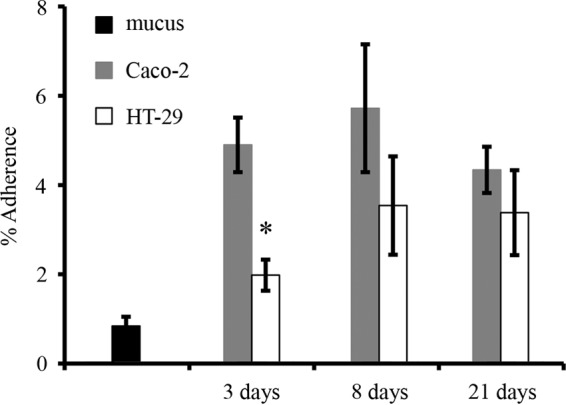
Adherence of A. muciniphila to Caco-2 and HT-29 cells at different growth stages. Levels of binding to mucus are shown for comparison. Data are means and standard deviations for five parallel wells. The asterisk indicates a significant difference (P < 0.05) in adhesion to the different cell lines at the same growth stage.
Effect of A. muciniphila on Caco-2 monolayer integrity.
The impact of A. muciniphila on the integrity of the epithelial cell layer was assessed by determining the development of the transepithelial electrical resistance (TER) of a Caco-2 monolayer, which was cocultured with A. muciniphila. TER is a measure of ion passage across tissue or a cultured enterocyte monolayer (22), and therefore, the epithelial barrier function is commonly assessed by determining the TER. E. coli was chosen as a representative bacterium that has adverse effects on epithelial cell monolayer integrity (23), and B. fragilis was included in the assay for comparison. The bacteria were administered to 8-day-old Caco-2 cells, and TER was measured at 24-h intervals. After 24 h of cocultivation, both A. muciniphila and B. fragilis had significantly increased the TER, whereas the TER of Caco-2 cocultures with E. coli had decreased significantly, relative to that of Caco-2 cultures without added bacteria (Fig. 6). At this time point, the OD600 values for A. muciniphila and B. fragilis cell suspensions showed essentially no growth, whereas the number of E. coli cells had increased slightly (from an OD600 of 0.25 to an OD600 of 0.38) (see Fig. S2 in the supplemental material). At 24 h, the positive impact on cell monolayer integrity was more profound with B. fragilis than with A. muciniphila, whereas E. coli with an essentially similar cellular density clearly affected TER development negatively. At 48 h after the establishment of cocultures, the TER of a Caco-2 cell layer cultured with E. coli had decreased even more, while the TER of Caco-2 cocultures with A. muciniphila had risen to the same level as that of B. fragilis cocultures (Fig. 6). The cell densities of B. fragilis and A. muciniphila did not change during the 48 h of incubation in the Caco-2 medium, and the bacterial cells did not seem to be severely compromised either, as evidenced by the live-dead staining results (see Fig. S2). The E. coli cell suspension, on the other hand, showed an increase in the OD600 from 0.25 to 0.5, i.e., one cell division occurred during 48 h, which is likely to have affected the further decrease of TER in E. coli cocultures.
FIG 6.
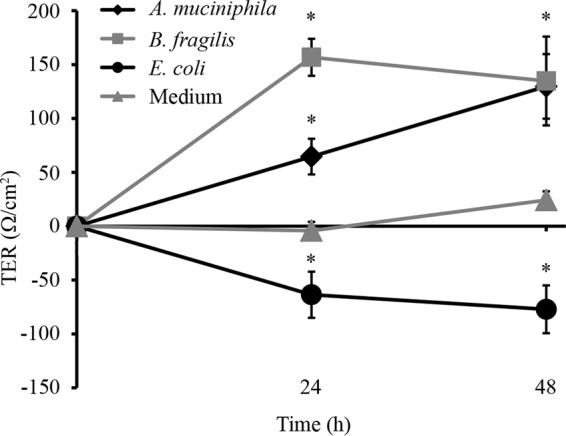
Impact of A. muciniphila, B. fragilis, or E. coli on the development of the TER of a Caco-2 monolayer. Means and standard deviations for three parallel wells are shown. Asterisks indicate TER values significantly different (P < 0.05) from that of the control (growth medium without bacteria).
Effect of A. muciniphila on interleukin-8 production in HT-29 cells.
The proinflammatory capacity of A. muciniphila was assayed by measuring its effect on IL-8 production by HT-29 cells. The HT-29 cells were incubated with different numbers of A. muciniphila, B. fragilis, or E. coli cells, while pure culture medium served as a background control and pure LPS as a positive control. E. coli was used in this assay because it has been shown to trigger IL-8 secretion in vitro, whereas B. fragilis was included because its LPS has been demonstrated to differ markedly from that of E. coli (24, 25). As expected, E. coli elicited a strong, dose-dependent IL-8 response in HT-29 epithelial cells, whereas no IL-8 production was induced when the cells were exposed to B. fragilis (Fig. 7). While lower doses of A. muciniphila failed to show any effect on IL-8 production, an increase in IL-8 release was observed with the largest number of A. muciniphila cells (1:100 dilution; 106 bacteria/ml−1) (Fig. 7). However, a similar level of IL-8 release was achieved with only 104 E. coli cells ml−1, suggesting that the proinflammatory effect of A. muciniphila on enterocytes is minor compared to that of E. coli (Fig. 7).
FIG 7.
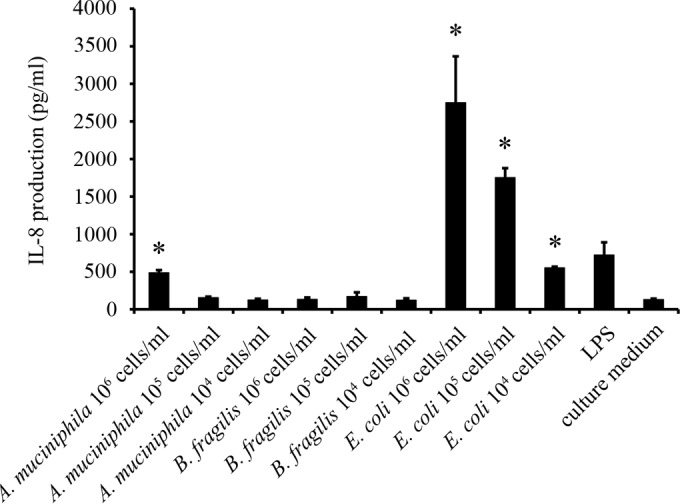
Induction of IL-8 production in HT-29 cells by A. muciniphila, B. fragilis, and E. coli. Means and standard deviations for three parallel wells are shown. Asterisks indicate IL-8 production levels significantly (P < 0.05) above the background level (growth medium without bacteria or LPS). LPS (1 ng ml−1) from E. coli was included as a positive control.
Presence of LPS in A. muciniphila cells.
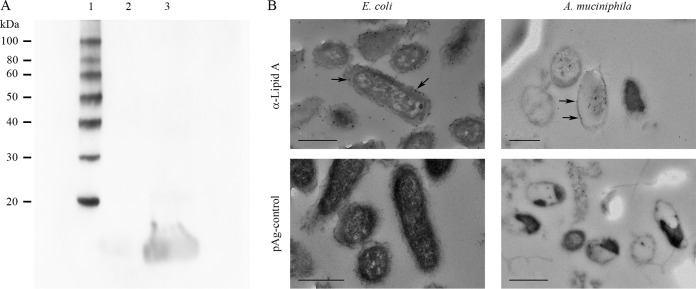
Since A. muciniphila showed only minor proinflammatory activity on HT-29 cells, we investigated whether A. muciniphila produces LPS and, if so, whether A. muciniphila LPS is distinct from that of E. coli. For that purpose, we first analyzed whole-cell preparations of A. muciniphila and E. coli by Western blotting. The antiserum against E. coli LPS reacted with E. coli, whereas only a weak signal, if any, could be detected with an A. muciniphila lysate (Fig. 8A). Next, we analyzed thin sections of A. muciniphila cells immunolabeled with antibodies against lipid A from E. coli by TEM. As shown in Fig. 8B, anti-lipid A antibodies reacted with the envelopes of both A. muciniphila and E. coli cells.
FIG 8.
Analysis of LPS and lipid A contents of A. muciniphila. (A) Western blotting of whole-cell lysates of A. muciniphila (lane 2) and E. coli (positive control) (lane 3) using an antiserum against E. coli LPS. Lane 1, molecular mass standard. (B) Electron micrographs of thin-sectioned and immunostained A. muciniphila and E. coli bacteria. The bacteria were immunostained using an antiserum against E. coli lipid A and 10-nm colloidal gold particles conjugated to protein A (pAg). Arrows indicate 10-nm gold particles. Bars, 500 nm.
DISCUSSION
Akkermansia muciniphila is an anaerobic bacterium isolated from human feces. Our results show that A. muciniphila is an aerotolerant anaerobic bacterium rather than a strict anaerobe, in contrast to many other members of the human intestinal microbiota. Our preliminary plate count data indicate that 80% of A. muciniphila cells exposed to atmospheric oxygen levels for 1 h survive (data not shown). Currently, we cultivate A. muciniphila successfully by inoculating the cultures in a laminar hood under a normal oxic atmosphere and incubating them in an anaerobic jar with a chemically created oxygen-free CO2 atmosphere (Anaerocult; Merck) instead of the anaerobic cabinet. Thus, there is no need to treat the organism as a highly oxygen sensitive anaerobe.
The ability to bind to the epithelial surface is thought to enhance the colonization of the digestive tract by a bacterial strain. The bacterium might, in principle, adhere to intact epithelium through binding to enterocytes, different components of the mucus gel, or other bacteria inhabiting the epithelial surface. In the event of disruption of the mucosal surface, epithelial binding could also be achieved by binding to various components of the extracellular matrix. In this study, we analyzed the adhesion of the human commensal A. muciniphila to various components of the human GI tract epithelium. Remarkably, A. muciniphila showed no binding to human colonic mucin, even though it lives in an intimate relationship with the intestinal mucosa and also utilizes it as a nutrient. We have shown previously, by using the same mucus binding assay, that the human intestinal isolates L. rhamnosus GG and Bifidobacterium bifidum DSM20456 and MIMBb75 bind strongly to human colonic mucus (15, 18). However, L. rhamnosus and B. bifidum are nonmucolytic bacteria, whereas A. muciniphila is a mucin-degrading bacterium. Therefore, the result may reflect the mucinolytic nature of A. muciniphila; i.e., we cannot presently rule out the possibility that A. muciniphila cells might be detached from the immobilized mucus as a result of their mucin-degrading enzymatic activity. The binding assay incubations were carried out under aerobic conditions, which did not severely damage A. muciniphila cells. Presumably, the main mucolytic enzymes of A. muciniphila may stay active under these conditions. On the other hand, since A. muciniphila adhered to cultured enterocytes, it is possible that epithelial enterocytes could serve as bona fide intestinal docking sites for adherent A. muciniphila cells. Therefore, in future work, we shall investigate whether this counterintuitive observation, i.e., the lack of mucus-binding ability of A. muciniphila, is due to the experimental conditions used in the mucus binding assay. Nevertheless, since A. muciniphila is found to reside on the colonic mucus, the question of how this organism manages to stably occupy this constantly renewing ecological niche remains to be answered.
In contrast to its lack of binding to mucus, A. muciniphila showed firm binding to the cultured colonic epithelial cell lines Caco-2 and HT-29. Although the highest A. muciniphila cell counts are found in the colon, the small intestine is also colonized by substantial numbers of A. muciniphila cells (5). In the small intestine, the mucus layer is permeable to bacteria, allowing direct contacts between bacteria and enterocyte surfaces (14). Therefore, A. muciniphila may use direct binding to enterocytes as a colonization strategy in the small intestine. The epithelial cell layer in the GI tract is renewed approximately once every 4 to 5 days in a process in which undifferentiated enterocyte progenitors derived from stem cells move from the intestinal crypts toward the tips of villi (26). The immature enterocytes differentiate en route to the villus tip, where they replace the old enterocytes, which are shed into the intestinal lumen (27). Interestingly, the enterocyte binding strength of A. muciniphila was independent of the growth state of Caco-2 cells, indicating that the host-binding site utilized by the bacterium is expressed on the cell surface irrespective of the state of cell differentiation. It is well known that the expression of surface molecules is influenced by the differentiation stage in epithelial cells (28, 29) and that bacterial adhesion to enterocytes is affected by the profile of surface-associated molecules in the enterocytes (30). Based on our findings, it seems possible that A. muciniphila is able to bind enterocytes at various differentiation stages in vivo.
The intestinal epithelium is subject to mechanical stress during the digestion of food, and as a result, the epithelium frequently suffers minor breaks in its integrity (26, 31). These wounds expose the subepithelium, along with its ECM network, to the intestinal lumen, allowing intestinal bacteria temporary access to the ECM components. ECM components, being natural constituents of the network of macromolecules, are also found in the mucus (32). Since we found that A. muciniphila binds laminin and undifferentiated Caco-2 cells, it is tempting to speculate that A. muciniphila might participate in the competitive exclusion of pathobionts from the sites of injury and fortify the de novo-established enterocyte monolayer after an epithelial insult. In favor of this view is our observation that A. muciniphila significantly increased the TER in coculture with Caco-2 cells, whereas E. coli decreased the TER under the same culture conditions. The strengthening of epithelial barrier function could also explain several in vivo observations linking A. muciniphila not only to gut health but also to systemic health. The impaired integrity of intestinal epithelium leads to the accumulation of LPS from Gram-negative gut inhabitants in the serum, resulting in metabolic endotoxemia with concomitant inflammation (33, 34). Since diabetes and obesity have been linked with increased gut permeability and low-grade inflammation (35, 36), LPS-induced endotoxemia has been suggested as one of the causative agents of obesity and its related metabolic disorders (33, 37, 38). Our in vitro observation that A. muciniphila fortifies epithelial barrier function could provide a working hypothesis for attempts to rationalize the in vivo findings connecting decreased fecal A. muciniphila levels with diabetes and obesity (8, 9) and could reveal one possible mechanism behind the protective effect of the bacterium against high-fat-diet-induced LPS endotoxemia in obese mice (13).
IL-8 is a mediator of inflammation, causing immune cells to migrate to the site of infection and inducing phagocytosis, and it plays an important role in host defense against pathogens (39). However, the stimulation of massive IL-8 production in a healthy, intact epithelium would lead to unnecessary inflammation and disturbance of mucosal homeostasis. We found that A. muciniphila induced IL-8 production in enterocytes at cell concentrations 100-fold higher than those for E. coli. Thus, A. muciniphila does not seem to be able to provoke a strong inflammatory cascade in the epithelium. The finding is in line with the reported in vivo investigations linking A. muciniphila with noninflamed rather than inflamed mucosa (5–7). On the other hand, the low-level proinflammatory stimulation of enterocytes by A. muciniphila may keep the mucosa-associated immune system alerted at an appropriate level. The A. muciniphila genome sequence contains the genetic elements necessary for the production of LPS (4). In contrast to E. coli or its LPS, A. muciniphila did not induce strong IL-8 release from HT-29 cells. Thus, A. muciniphila LPS does not seem to be a powerful activator of host Toll-like receptor 4 (TLR4) and likely differs structurally from E. coli LPS. Recently, B. fragilis LPS has been shown to signal through TLR2, not through the well-recognized LPS receptor TLR4 (24). B. fragilis LPS is structurally atypical, differing from classical LPS by the length of the O-antigen polysaccharide and the phosphorylation of lipid A diglucosamine (24, 25). We attempted to detect LPS in A. muciniphila by Western blotting using polyclonal antisera raised against E. coli LPS. Since this approach was unsuccessful with A. muciniphila, we performed immunoelectron microscopic analysis of thin-sectioned A. muciniphila and E. coli cells. By this method, we were able to immunostain thin sections of A. muciniphila cells with anti-lipid A antiserum, indicating that the bacterium produces lipid A and therefore, most likely, also LPS. We propose that there are structural and antigenic disparities between the LPS polysaccharide structures of A. muciniphila and E. coli, as evidenced by the reactivity of anti-lipid A antibodies but the nonreactivity of antibodies raised against E. coli LPS. These findings warrant future exploration of the precise immunosignaling properties of A. muciniphila, including the identification of the host side receptors involved therein.
In this study, by using in vitro methods, we have revealed several interactions of A. muciniphila with intestinal epithelium. The somewhat unexpected binding preference of A. muciniphila for epithelial cells and laminin over colonic mucus raises questions about the possible physical niches utilized by this organism to stably colonize the human GI tract. Our findings that A. muciniphila is capable of adhering to both nondifferentiated and mature enterocytes and that upon this interaction, the bacterium does not provoke a proinflammatory reaction but instead elicits the strengthening of epithelial integrity open multiple exciting paths for further study of the putative beneficial interactions of A. muciniphila with the host.
Supplementary Material
ACKNOWLEDGMENTS
The financial support of the Academy of Finland is gratefully acknowledged (grant 252803 to J.R., grants 138902 and 258439 to R.S., and grants 137389, 141140, and 1272870 to W.M.D.V.), as is the European Research Council (ERC Advanced Grant 250172, to W.M.D.V.). We thank the Electron Microscopy Unit of the Institute of Biotechnology, University of Helsinki, for providing laboratory facilities.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04050-14.
REFERENCES
- 1.Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 2.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. 2007. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol 73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyra A, Forssten S, Rolny P, Wettergren Y, Lahtinen SJ, Salli K, Cedgård L, Odin E, Gustavsson B, Ouwehand AC. 2012. Comparison of bacterial quantities in left and right colon biopsies and faeces. World J Gastroenterol 18:4404–4411. doi: 10.3748/wjg.v18.i32.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Passel MWJ, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, Chain PSG, Woyke T, Palva A, de Vos WM, Smidt H. 2011. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS One 6(3):e16876. doi: 10.1371/journal.pone.0016876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. 2010. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 6.Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. 2013. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis 2013 19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 7.Swidsinski A, Dörffel Y, Loening-Baucke V, Theissig F, Rückert JC, Ismail M, Rau WA, Gaschler D, Weizenegger M, Kühn S, Schilling J, Dörffel WV. 2011. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60:34–40. doi: 10.1136/gut.2009.191320. [DOI] [PubMed] [Google Scholar]
- 8.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Buschard K, Hansen AK. 2012. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. 2012. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 20:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 10.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 11.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. 2011. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front Microbiol 2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. 2014. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 5(4):e01438-14. doi: 10.1128/mBio.01438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson MEV, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, Subramani DB, Holmén-Larsson JM, Thomsson KA, Bergström JH, van der Post S, Rodriguez-Pinẽiro AM, Sjövall H, Bäckström M, Hansson GC. 2011. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kainulainen V, Reunanen J, Hiippala K, Guglielmetti S, Vesterlund S, Palva A, Satokari R. 2013. BopA does not have a major role in the adhesion of Bifidobacterium bifidum to intestinal epithelial cells, extracellular matrix proteins, and mucus. Appl Environ Microbiol 79:6989–6997. doi: 10.1128/AEM.01993-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vesterlund S, Paltta J, Karp M, Ouwehand AC. 2005. Measurement of bacterial adhesion—in vitro evaluation of different methods. J Microbiol Methods 60:225–233. doi: 10.1016/j.mimet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Reunanen J, von Ossowski I, Hendrickx APA, Palva A, de Vos WM. 2012. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl Environ Microbiol 78:2337–2344. doi: 10.1128/AEM.07047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx AP, Lebeer S, De Keersmaecker SC, Vanderleyden J, Hämäläinen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjärvi T, Auvinen P, de Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci U S A 106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebeer S, Claes I, Tytgat HL, Verhoeven TL, Marien E, von Ossowski I, Reunanen J, Palva A, de Vos WM, Keersmaecker SC, Vanderleyden J. 2012. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78:185–193. doi: 10.1128/AEM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto M, Robine-Leon S, Appay M-D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. 1983. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47:323–330. [Google Scholar]
- 21.Evers BM, Zhou Z, Celano P, Li J. 1995. The neurotensin gene is a downstream target for Ras activation. J Clin Invest 95:2822–2830. doi: 10.1172/JCI117987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blikslager AT, Adam J, Moeser AJ, Jody L, Gookin JL, Jones SL, Odle J. 2007. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 23.Geens MM, Niewold TA. 2010. Preliminary characterization of the transcriptional response of the porcine intestinal cell line IPEC-J2 to enterotoxigenic Escherichia coli, Escherichia coli, and E. coli lipopolysaccharide. Comp Funct Genomics 2010:469583. doi: 10.1155/2010/469583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhawi M, Stewart J, Erridge C, Patrick S, Poxton IR. 2009. Bacteroides fragilis signals through Toll-like receptor (TLR) 2 and not through TLR4. J Med Microbiol 58:1015–1022. doi: 10.1099/jmm.0.009936-0. [DOI] [PubMed] [Google Scholar]
- 25.Patrick S, Houston S, Thacker Z, Blakely GW. 2009. Mutational analysis of genes implicated in LPS and capsular polysaccharide biosynthesis in the opportunistic pathogen Bacteroides fragilis. Microbiology 155:1039–1049. doi: 10.1099/mic.0.025361-0. [DOI] [PubMed] [Google Scholar]
- 26.Mammen JM, Matthews JB. 2003. Mucosal repair in the gastrointestinal tract. Crit Care Med 31(Suppl):S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 27.Vereecke L, Beyaert R, van Loo G. 2011. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med 17:584–593. doi: 10.1016/j.molmed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. 2005. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol 21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 29.García-Lorenzo A, Rodríguez-Piñeiro AM, Rodríguez-Berrocal FJ, Cadena MP, Martínez-Zorzano VS. 2012. Changes on the Caco-2 secretome through differentiation analyzed by 2-D differential in-gel electrophoresis (DIGE). Int J Mol Sci 13:14401–14420. doi: 10.3390/ijms131114401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coconnier MH, Bernet MF, Kerneis S, Chauviere G, Fourniat J, Servin AL. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett 110:299–305. doi: 10.1111/j.1574-6968.1993.tb06339.x. [DOI] [PubMed] [Google Scholar]
- 31.Iizuka M, Konno S. 2011. Wound healing of intestinal epithelial cells. World J Gastroenterol 17:2161–2171. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson MEV, Thomsson KA, Hansson GC. 2009. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 8:3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- 33.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 34.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. 2008. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 37.Stenman LK, Holma R, Korpela R. 2012. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol 18:923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clément K, Hu FB, Lin X. 2010. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33:1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baggiolini M, Clark-Lewis I. 1992. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 307:97–101. doi: 10.1016/0014-5793(92)80909-Z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.