Abstract
Objective
Roux-en-Y gastric bypass (RYGB) is an effective method of weight loss and remediation of type-2 diabetes; however, the mechanisms leading to these improvements are unclear. Additionally, adipocytes within white adipose tissue (WAT) depots can manifest characteristics of brown adipocytes. These ‘BRITE/beige’ adipocytes express uncoupling protein 1 (UCP1) and are associated with improvements in glucose homeostasis and protection from obesity. Interestingly, atrial and B-type natriuretic peptides (NPs) promote BRITE/beige adipocyte enrichment of WAT depots, an effect known as “browning.” Here, we investigate the effect of RYGB surgery on NP, NP receptors, and browning in the gonadal adipose tissues of female mice. We propose that such changes may lead to improvements in metabolic homeostasis commonly observed following RYGB.
Methods
Wild type, female, C57/Bl6 mice were fed a 60% fat diet ad libitum for six months. Mice were divided into three groups: Sham operated (SO), Roux-en-Y gastric bypass (RYGB), and Weight matched, sham operated (WM-SO). Mice were sacrificed six weeks following surgery and evaluated for differences in body weight, glucose homeostasis, adipocyte morphology, and adipose tissue gene expression.
Results
RYGB and calorie restriction induced similar weight loss and improved glucose metabolism without decreasing food intake. β3-adrenergic receptor expression increased in gonadal adipose tissue, in addition to Nppb (BNP), and NP receptors, Npr1, and Npr2. The ratio of Npr1:Npr3 and Npr2:Npr3 increased in RYGB, but not WM-SO groups. Ucp1 protein and mRNA, as well as additional markers of BRITE/beige adipose tissue and lipolytic genes increased in RYGB mice to a greater extent than calorie-restricted mice.
Conclusions
Upregulation of Nppb, Npr1, Npr2, and β3-adrenergic receptors in gonadal adipose tissue following RYGB was associated with increased markers of browning. This browning of gonadal adipose tissue may underpin the positive effect of RYGB on metabolic parameters and may in part be mediated through upregulation of natriuretic peptides.
Keywords: High fat diet (HFD), Roux-en-Y gastric bypass (RYGB), Natriuretic peptide receptor, Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), Browning
1. Introduction
Obesity and its associated co-morbidities are international public health concerns. As the prevalence of obesity has increased over the past decade, it has become clear that bariatric surgery can be more effective than diet and lifestyle interventions for weight loss and remediation of obesity-related morbidities [1–4]. Roux-en-Y gastric bypass (RYGB) is the most commonly performed bariatric surgery worldwide and involves physical reconstruction of the gastrointestinal tract [5]. In RYGB, a small stomach pouch is formed by ligation of the proximal from the distal stomach. The jejunum is then separated from the distal duodenum and attached to the newly created stomach pouch. Ingested food moves quickly from the stomach pouch into the new jejunal limb; thus, food bypasses a significant portion of proximal small intestine [5]. RYGB produces rapid initial weight loss, which, unlike conventional diet-induced weight loss, can be maintained for years after surgery, as well as improvements in diabetes and cardiovascular health [6,7]. Interestingly, reductions in caloric intake following RYGB do not fully account for the magnitude of weight loss commonly seen after the surgery [6,8]. This suggests that molecular mechanisms affecting metabolism and nutrient utilization underlie the effect of RYGB. Understanding the nature of these mechanisms is essential to developing novel, non-surgical obesity interventions.
Human and rodent adipose tissues comprise several distinct adipocyte sub-types [9–11]. White adipocytes are the prototypical fat cell, capable of storing a large amount of lipid and secreting a wide variety of cytokines. Brown adipocytes are found in depots discrete from white adipose tissue (the interscapular depot in mice and supraclavicular and paraspinal depots in humans [10]), have more mitochondria, and constitutively express uncoupling protein 1 (UCP1) [12,13]. Expression of Ucp1 conveys a thermogenic function to brown adipocytes; rather than oxidizing substrates to produce ATP, cellular respiration in brown adipocytes results in heat production [14]. A third sub-type, Brown-in-white (BRITE)/beige adipocytes, arises within white adipose tissue depots from the same progenitor population as white adipocytes but functionally resembles brown adipocytes [12]. Both brown and beige adipocytes express Ucp1 [15,16]; in both cell types thermogenic activity can be induced by exposure to cold temperatures, sympathetic nervous system (SNS) signaling (such as β-adrenergic agonists), and the cardiac natriuretic peptides, namely, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) [17]. Interestingly, it has been shown that RYGB surgery elevates cardiac natriuretic peptide expression in humans [18].
Brown and beige adipocytes provide beneficial whole-body and tissue-specific metabolic effects beyond thermal insulation. For example, beige adipocyte enrichment following treatment with β3-adrenergic agonists improves glucose uptake in insulin resistant subcutaneous and visceral white adipose depots in mice [19], and the amount of brown adipose tissue correlates inversely with body mass index (BMI) and fasting glucose in humans [11]. Since known inducers of beige adipocyte activity are elevated following RYGB surgery, it makes sense to investigate whether beige-enrichment of WAT depots follows RYGB and whether these inducible beige enrichments are associated with improved metabolic health in a weight-loss independent manner. The purpose of the present study was to determine whether changes in energy metabolism and glucose homeostasis after RYGB surgery result, in part, from beige enrichment due to upregulation of NPs and altered SNS signaling. Importantly, approximately 80% of all bariatric surgeries in the United States are performed on women [20]. As such, we chose to examine the effects of RYGB on beige enrichment in female murine adipose tissues.
Here, we report for the first time, increased levels of natriuretic peptide receptors 1 and 2 (Npr1, Npr2) and B-type natriuretic peptide (Nppb, aka BNP) mRNA, as well as increased levels of β3-adrenergic receptors and lipolytic genes in the gonadal adipose tissue of RYGB mice. Furthermore, we recognize a general pattern of gene expression and adipocyte morphology in gonadal adipose tissue that strongly suggests beige enrichment. Our data suggest the positive effect of RYGB on metabolic parameters may in part be mediated through upregulation of natriuretic peptides but do not exclude the possibility that other mechanisms may be involved. This evidence supports the hypothesis that improvements in metabolism seen following RYGB surgery are driven by mechanisms beyond weight loss per se.
2. Material and methods
2.1. Animal care
Studies were conducted in accordance with University of Texas Southwestern Medical Center (UTSW) Institutional Animal Care and Use Committee and the Association of Assessment and Accreditation of Laboratory Animal Care policies. Mice were individually housed in a temperature-controlled environment at 22°C–24 °C using 12-h light/12-h dark cycles (Light cycle: 0600–1800).
2.2. Study design
Female C57BL/6 mice were placed on high fat diet (HFD) (D12492, Research Diets) from 6 weeks of age. Upon reaching approximately 40–45 g (12–14 weeks on HFD), mice were randomized to receive Roux-en-Y gastric bypass surgery (RYGB) or sham operations (SO). To control for the effects of weight loss per se an additional subset of female sham-operated diet-induced obese mice were weight-matched to the RYGB group by calorie restriction (WM-SO). After recovery, SO and RYGB mice were provided HFD ad libitum. Body weight was monitored daily and body composition evaluated using a Minispec mq10 NMR (Bruker Optics) prior to sacrifice. Food intake was measured over four consecutive days during post-operative week four. Oral glucose tolerance test and insulin tolerance tests were conducted during post-operative week six. Animals were sacrificed at post-operative week six.
2.3. Surgical intervention
RYGB surgery was performed as described [21]. Briefly, RYGB involved gastrointestinal reconstruction such that ingested nutrients pass from a proximal gastric pouch into a jejunal afferent limb. Distal stomach and proximal intestine were excluded from alimentary flow using a vascular clip (Ethicon) placed just distal to the gastro-jejunostomy. The sham procedure involved gastrotomy, enterotomy, and repair. Mice were anesthetized using a scavenged circuit of isoflurane; anesthesia time was standardized between groups. Mice were maintained on a post-operative feeding protocol during which liquid diet was provided from post-operative days two through seven. On post-operative day six, 0.25 g of HFD was provided on a daily basis until consumed in its entirety. Subsequently, solid diet was re-introduced ad libitum. WM-SO mice were provided food once a day at the onset of the dark phase. The amount of food given to the WM-SO group was adjusted in order to induce weight loss equal to that of the RYGB group.
2.4. Oral glucose tolerance test (OGTT)
SO and RYGB mice were fasted for three hours (beginning two hours into light cycle) prior to administration of glucose (1 g/kg body weight; Sigma–Aldrich) by gavage. Importantly, WM-SO mice were fed at the onset of the dark phase and regularly consumed all food within one hour; therefore, at the time of OGTT this group had been without food for a longer duration than the other groups. Mice did not have access to food throughout the experiment. At the indicated time points, blood samples were collected in heparin-coated capillary tubes from the tail vein and assayed as described in the Hormone and metabolite measurements section.
2.5. Insulin tolerance test (ITT)
Mice were fasted for three hours (beginning two hours into light cycle) prior to administration of insulin (0.75 U/kg body weight; Eli Lilly) via intraperitoneal injection. Importantly, WM-SO mice were fed at the onset of the dark phase and regularly consumed all food within one hour; therefore, at the time of OGTT this group had been without food for a longer duration than the other groups. Mice did not have access to food throughout the experiment. At the indicated time points, blood samples were collected in heparin-coated capillary tubes from the tail vein and assayed as described in the Hormone and metabolite measurements section.
2.6. Hormone and metabolite measurements
Plasma was obtained by centrifugation and assayed using enzyme–linked immunosorbent assay for leptin (Invitrogen, Carlsbad, CA). Glucose concentrations were determined using an oxidase–peroxidase assay (Sigma–Aldrich, St. Louis, MO) for OGTT, fasting glucose, and ITT.
2.7. Fat histological analyses
Fat pads were weighed, fixed overnight in 10% formalin (in phosphate-buffered saline), and stored in 50% ethanol. Fixed fat pads were further processed by the Richardson Molecular Pathology Core at UT Southwestern Medical Center. UCP1 staining was done with Fisher Scientific Rabbit polyclonal anti-UCP1 (Catalog # PA1-24894) at a dilution of 1:500. Adipocyte size was determined using epifluorescent microscopy. Images were captured using an Optronics Microfire Color CCD Camera, and analyzed using ImageJ software (NIH, Bethesda, MD; http://rsb.info.nih.gov/ij/). Approximately two hundred cells for each sample were included in the analysis.
2.8. Tissue mRNA analyses
Adipose tissue samples were homogenized in Trizol (Invitrogen) using a TissueLyser (Qiagen). Total mRNA was extracted using the RNeasy RNA extraction kit and protocol (Qiagen). Quality and quantity of RNA were determined by UV Spectroscopy (absorbance at 260/280 nm). cDNA was prepared from 2.0 μg mRNA using Superscript III reverse transcriptase (Invitrogen) and oligo (dT) (Invitrogen). Real time quantitative polymerase chain reactions (RT-qPCR) assays were performed according to published protocols [22] using ABI 7900HT and ViiA7 RT-qPCR systems. TaqMan gene expression assays (Life Technologies) were used to determine expression levels of the following genes: Ucp1, Dio2, Pgc1, Prdm16, Dio2, Egr1, Adrb3, Atgl, Hsl, Nppa (ANP), Nppb (BNP), Npr1, Npr2, Npr3. B2m used as the reference gene. Relative gene expression was determined by the ΔΔCq quantification method using both manual calculation and DataAssist software (ABI, v.3.01). The identification and catalog numbers of each assay are available upon request.
2.9. Statistics
Data are presented as means ± SEM, unless otherwise specified in figure legend. Experiments comparing two means were analyzed using t-tests (two tailed, unpaired with Welch's correction). Experiments comparing three or more means were analyzed using one-way ANOVA followed by Tukey post hoc for multiple comparisons. The post-operative body weight curve (Figure 1A), oral glucose tolerance test curve (Figure 2A), and insulin tolerance test curve (Figure 2C) were analyzed using repeated measures, two-way ANOVA (with surgery group and time of measurement as the two independent variables) followed by Tukey post hoc for multiple comparisons. P values less than 0.05 were considered statistically significant. * denotes statistical significance between SO vs. RYGB or SO vs. WM-SO groups. ˆ denotes statistical significance between only SO vs. WM-SO. # denotes statistical significance between only RYGB vs. WM-SO groups. Statistical analyses were performed using R v3.1.0 and GraphPad Prism 6 software.
Figure 1.
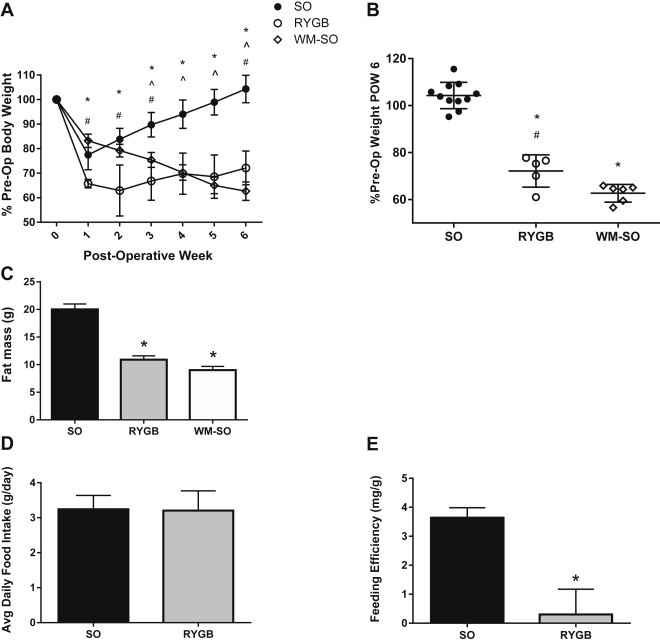
Effects of Roux-en-Y gastric bypass surgery on body weight and feeding efficiency. Three experimental groups received either sham operation (SO), Roux-en-Y gastric bypass (RYGB), or sham operation with a calorie-restricted diet (WM-SO). (A) Body weight changes over six weeks post-surgery as percentage of pre-operative body weight (SO n = 11, RYGB n = 5, WM-SO n = 6). Data presented as mean ± s.d. (B) Body weight as percentage of pre-operative body weight at post-operative week six. Each data point represents a single subject, horizontal lines denote mean and s.d. of the group (C) Fat mass as determined by nuclear magnetic resonance imaging at time of sacrifice (SO n = 6, RYGB n = 5, WM-SO n = 6). (D) Food intake during post-operative week four (SO n = 7, RYGB n = 5). (E) Feeding efficiency (calculated as average daily change in body weight divided by average daily food intake) during post-operative week four (SO n = 7, RYGB n = 5). ∗P < 0.05 RYGB or WM-SO versus SO. ˆ P < 0.05 WM-SO only versus SO. #P < 0.05 RYGB versus WM-SO.
Figure 2.
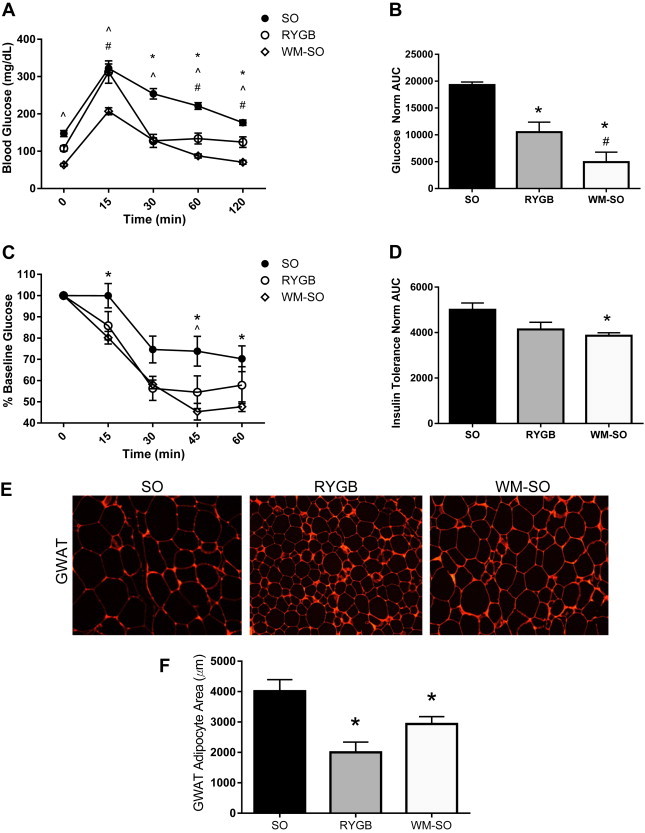
Effects of Roux-en-Y gastric bypass surgery on glucose homeostasis and adipocyte size. At post-operative week six, SO (n = 10), RYGB (n = 4), and WM-SO (n = 6) groups underwent tests of glucose homeostasis. All data presented as mean ± s.e.m. (A) Oral glucose tolerance test, plasma glucose concentrations. (B) Normalized area under curve graph for Oral glucose tolerance test. (C) Insulin tolerance test, percentage of baseline plasma glucose. (D) Normalized area under curve graph for Insulin tolerance test. (E) Representative immunohistochemical images and (F) Quantification of average adipocyte size in gonadal adipose tissues from SO, RYGB, and WM-SO groups. ∗P < 0.05 RYGB or WM-SO versus SO. ˆ P < 0.05 WM-SO only versus SO. #P < 0.05 RYGB versus WM-SO.
3. Results
3.1. RYGB decreases body weight but does not change food intake
3.1.1. Postsurgical weight loss
Over the six week post-operative course, RYGB surgery and calorie restriction induced significant body weight and fat mass loss in female diet-induced obese mice. By post-operative week six, RYGB mice lost 27.86% of their pre-operative weight, while calorie-restricted WM-SO mice lost 37.33% (Figure 1A,B). Additionally, both RYGB and WM-SO mice significantly reduced fat mass in both groups (Figure 1C).
3.1.2. Postsurgical feeding
During post-operative week four, we assessed food intake in addition to body weight changes in all three groups. Food intake did not differ between RYGB and SO groups (Figure 1D); however, SO mice gained an average of 0.31 g of body weight per day, while RYGB gained only an average of 0.03 g body weight per day during this time. Thus, during this period, feeding efficiency (average daily change in body weight divided by average daily food intake) declined by an average of 91.7% in the RYGB group versus SO (Figure 1E).
3.2. RYGB and calorie restriction improve glucose homeostasis
3.2.1. Glucose homeostasis
Both RYGB and weight loss improve glucose tolerance in mice and humans [23,24]. To evaluate the effects of RYGB on glucose and insulin homeostasis irrespective of weight loss, we performed fasting blood glucose tests, oral glucose tolerance tests (OGTT), and insulin tolerance tests (ITT) during post-operative week six. Both oral glucose tolerance and fasting blood glucose improved in RYGB mice relative to SO (Figure 2A,B, Supplementary Figure 1A), though not to the extent seen in the WM-SO group. RYGB and WM-SO showed similar initial responses to the ITT (Figure 2C, Supplementary Figure 1B); however, area under the curve (AUC) measurements were only significantly reduced in the WM-SO group (Figure 2D). These greater improvements in glucose and insulin sensitivity in the WM-SO group may be the result of a greater total fasting time prior to testing. On the basis of comparable or even less pronounced effects in RYGB compared to WM-SO mice, however, the improvements may also be associated with reductions of body weight/fat.
3.2.2. Adipocyte morphology and serum leptin
Average gonadal adipocyte size decreased to a greater extent in RYGB versus the WM-SO or the SO groups (Figure 2E,F). Consistent with the overall decrease in adiposity, serum leptin levels were lower in RYGB and WM-SO groups compared to SO (Supplementary Figure 1C); however, while average leptin levels were lower in RYGB mice versus WM-SO, the difference did not reach statistical significance.
3.3. RYGB increases markers of brown adipocytes in gonadal adipose tissue depots
3.3.1. BRITE/beige adipocytes markers
Ucp1 is expressed in brown/beige adipocytes and not in white adipose tissue; therefore, Ucp1 is used as a selective marker for beige/brown adipose tissue [13]. UCP1 protein increased in GWAT from RYGB mice (Figure 3A). Ucp1 mRNA levels increased as well, but did not reach statistical significance (Figure 3B). There was no trend of increased Ucp1 mRNA in either the inguinal (IWAT) or brown (BAT) adipose depots (Figure 3B).
Figure 3.
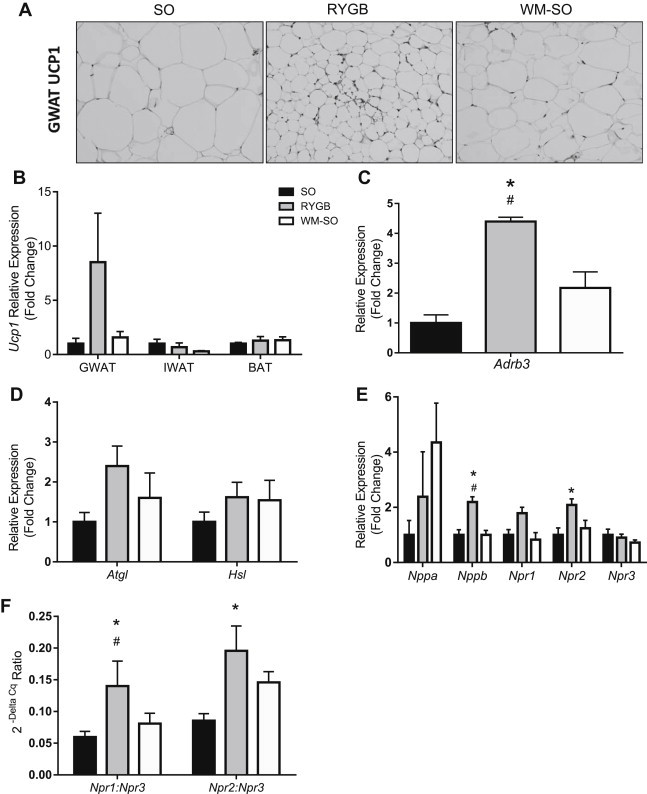
Roux-en-Y gastric bypass surgery increases markers of beige adipocytes, natriuretic peptides and natriuretic peptide receptors in gonadal adipose tissue. (A) Representative immunohistochemical image of UCP1 protein in gonadal adipose tissue taken from SO, RYGB, and WM-SO mice. Original magnification, 20x (B) Relative expression determined by RT-qPCR of Ucp1 mRNA in gonadal, inguinal, and brown adipose tissues taken from SO (n = 6), RYGB (n = 4) and WM-SO (n = 6) mice. (B) Relative expression determined by RT-qPCR of (C) Adrb3 mRNA (D) Atgl and Hsl mRNA, and (E) Nppa (ANP), Nppb (BNP), Npr1, Npr2, Npr3 mRNA in gonadal adipose tissue taken from SO (n = 6), RYGB (n = 4) and WM-SO (n = 6) mice. (F) Ratio of Npr1:Npr3 mRNA expression and Npr2:Npr3 mRNA expression in gonadal adipose tissue taken from SO (n = 6), RYGB (n = 4) and WM-SO (n = 6) mice. All data presented as mean ± SEM. ∗P < 0.05 RYGB or WM-SO versus SO. #P < 0.05 RYGB versus WM-SO.
Brown/beige adipocyte populations are characterized by increased expression of Prdm16, Pgc1α, Dio2, and Egr1 [25]. While there was a trend toward increased expression of each of these genes in the GWAT RYGB relative to SO and WM-SO, only expression of the early growth response protein 1 (Egr1) reached statistical significance (Supplementary Figure 1D).
3.3.2. Sympathetic activity markers in GWAT
Norepinephrine affects BAT thermogenesis via interaction with β3-adrenergic receptors, and it is well known that β3-adrenergic receptors exist on both brown and white adipocytes [26]. WAT depots are more poorly innervated than BAT depots; nevertheless, we observed significantly increased β3-adrenergic receptor (Adbr3) expression in the GWAT of RYGB mice relative to SO and WM-SO groups (Figure 3C), suggesting an association of RYGB with increased sympathetic sensitivity and browning in GWAT.
3.3.3. Lipolytic markers in GWAT
Increased sympathetic activation of adipose tissues is associated with increased Ucp1 transcription and increases in markers of lipolysis [13]. Consistent with a putative relationship between RYGB and browning of adipose tissue, we found elevations of Atgl and Hsl (Figure 3D) in the GWAT of RYGB relative to SO and WM-SO; however, these trends did not reach statistical significance.
3.3.4. Cardiac natriuretic peptides in GWAT
Cardiac natriuretic peptides (NPs) work synergistically with β3-adrenergic receptor agonists to induce lipolysis in human [27] and browning in both human and mouse adipocytes, as evidenced by enhanced expression of Ucp1, Pgc-1α and other brown/beige adipocyte markers [17]. Importantly, in humans, NP concentrations increase following gastric bypass [18]; therefore, we assessed adipose tissue expression of NPs and NP receptors to determine if RYGB surgery was associated with similar increases in mice. Expression of Npr1, Npr2, and Bnp were elevated in GWAT of RYGB mice, suggesting that the surgery per se, and not weight loss, may promote increased Ucp1 expression in GWAT (Figure 3E) through activation of NPs.
Wild-type mice express high levels of the NP clearance receptor (Npr3), abrogating the lipolytic effect of circulating NPs [28]; however, Npr3 knock-out mice have reduced body weight and increased brown adipocyte formation [17]. Additionally, cold exposure itself increases expression of Npr1 and Npr2 and reduces Npr3 in mice [17]. This evidence suggests that the ratio of Nprs in adipose tissues mediates the effect of NPs in mice. With this in mind, we evaluated the relative expression ratios of Npr1 and Npr2 to Npr3 in our treatment groups. Indeed, the ratios of GWAT Npr1:Npr3 and Npr2:Npr3 significantly increased in RYGB versus both SO and WM-SO groups (Figure 3F) indicating greater effectiveness of NPs in facilitating browning.
4. Discussion
Here, for the first time, we suggest a possible mechanism by which gastric bypass surgery improves obesity-related metabolic sequelae. RYGB significantly reduced adiposity without decreasing food intake, suggesting that, in female mice, RYGB influences energy expenditure. When we examined potential mechanisms underlying this effect, we found increases of natriuretic peptides (NPs) and natriuretic peptide receptors (NPRs), along with markers of increased sympathetic nervous system (SNS) activity, as well as markers of beige adipocytes in gonadal adipose tissues. These findings advance the field of adipose tissue physiology by positing a link between RYGB surgery and improvements in glucose and energy metabolism independent of weight loss, mediated through NPs.
Consistent with our previous report [21], we found that RYGB in female mice induces significant and persistent weight loss, and that fat mass was not preferentially spared compared to calorie-restricted (WM-SO) mice (Figure 1A–C). Importantly, RYGB did not affect food intake (Figure 1D). The literature is inconsistent with respect to the contribution of reduced food intake to the efficacy of RYGB surgery. Some studies report significant, though transient, reductions in intake post-surgery [29,30]; nevertheless, metabolic benefits of RYGB have been described in the absence of caloric reduction in both obese human and rodent models [31]. Here, while food intake did not change appreciably following surgery (Figure 1D), RYGB mice lost significant weight in the post-operative period (Figure 1A). Furthermore, RYGB mice did not experience the same magnitude of weight regain as SO mice four to six weeks after surgery; SO mice gained significantly more bodyweight per gram of food consumed than RYGB mice (Figure 1E). In light of studies suggesting that caloric malabsorption is not the operative mechanism underlying RYGB-induced weight loss [32,33], this reduced feeding efficiency is consistent with the notion of a metabolic effect of RYGB surgery that operates independently of weight loss. Increases in whole animal energy expenditure or adipose tissue thermogenesis exist as possible mechanisms underlying this effect [29,31].
It is well established that RYGB improves glucose tolerance and insulin sensitivity in a weight-loss independent manner in both humans and rodents [7,34,35]. In our dataset OGTT and ITT were performed at post-operative week six, when RYGB and WM-SO had lost approximately equivalent amounts of weight. Both fasting blood glucose and oral glucose tolerance AUC improved in RYGB and WM-SO groups (Figure 2A,B, Supplementary Figure 1A). Interestingly, while the SO and RYGB groups had a similar response to the oral glucose challenge by t = 15 min, the RYGB mice returned to a blood glucose concentration equivalent to WM-SO by t = 30 min (Figure 2A). This rate of glucose disposal in the RYGB OGTT is remarkable in comparison to both the SO and WM-SO groups. Taken with the fact that, in the ITT, the initial response of both RYGB and WM-SO groups was similar and diverged only in the later stages of the test (Figure 2C), this is consistent with the notion of an improved ability to regain glucose homeostasis following RYGB.
That said, the WM-SO group did show greater overall improvements in glucose homeostasis than RYGB. This could be explained by the fact that the WM-SO mice were fed a small amount of food only once per day; therefore, they endured a much longer time period of fasting prior to the OGTT and ITT than the other groups. Since weight loss was similar in RYGB and WM-SO, this discrepancy in fasting length could account for the increased insulin sensitization of the WM-SO mice. However, since the magnitude of weight loss was similar between the two groups, we cannot definitively separate these metabolic improvements from weight loss per se. It is also important to note that both OGTT and ITT were carried out six weeks after surgery to allow the groups to fully recover from surgery. Improvements in glucose tolerance and insulin sensitivity due to RYGB could appear well before these time points. Future time course studies will be required to determine precisely when improvements in glucose homeostasis appear in this RYGB mouse model.
Another interesting effect of RYGB relates to adipocyte size and leptin secretion. GWAT adipocytes appear much smaller in RYGB mice compared to SO and WM-SO groups. Such reductions in adipocyte size may be associated with enhanced lipolysis and/or increased SNS at the level of adipose tissue. Consistent with this notion, we found significantly elevated levels of β3-adrenergic receptor and a trend toward increased Atgl and Hsl expression in RYGB mice. Leptin concentrations were also reduced in the RYGB and WM-SO mice (Supplemental Figure 1C), which is consistent with human and rodent studies and the degree of adipose tissue loss [35]. Leptin infusion has been associated with activation of the SNS [36]; in the context of lower leptin concentrations, our present data showing increased SNS-related gene expression (Figure 3C) could seem counterintuitive. However, taken in the context of leptin resistance – a common feature of obesity [37] – reduced absolute levels of leptin may be less important than the degree of leptin sensitivity. Our observations are consistent with a model wherein RYGB promotes re-sensitization to leptin. Moreover, RYGB mice lost weight without decreasing food intake, while the WM-SO group was significantly calorie-restricted; therefore, it is reasonable to hypothesize that the method by which weight loss is achieved could influence leptin sensitivity and SNS activity.
It is well established that SNS stimulation promotes brown adipocyte thermogenesis, as well as BRITE/beige adipocyte enrichment in prototypical white adipose tissue depots. Norepinephrine release from sympathetic nerve terminals to β-adrenergic receptors activates beige adipocytes [38] and we found a significant increase in β3-adrenergic receptor (Adrb3) gene expression in the gonadal depot of RYGB mice (Figure 3C). Moreover, sympathetic activation increases Atgl and Hsl [39], liberating fatty acids which may serve as the principle substrate for brown/beige thermogenesis. Consistent with this, we observed elevated Atgl and Hsl expression in RYGB mice (Figure 3D). These data suggest that RYGB may increase sympathetic signaling to GWAT, thereby inducing beige adipocyte formation and/or activity.
In addition to increased markers of SNS activation, we observed increased expression of both Npr1 and Npr2 in GWAT and, somewhat surprisingly given the traditional notion that NPs are secreted primarily by the heart, increased Nppb (BNP) expression as well (Figure 3E). The three known natriuretic peptides (ANP, BNP, and CNP) interact with three natriuretic peptide receptors (NPR1, NPR2, and NPR3) to modulate a wide variety of physiologic phenomenon, including blood pressure, cardiac hypertrophy and remodeling, and bone growth [40]. However, recent studies have expanded our understanding of these genes to metabolism, characterizing novel effects on substrate utilization and thermogenesis in adipose tissues. For example, ANP infusion in subcutaneous adipose of humans increases plasma free fatty acid concentration, and in vitro studies in human adipocytes show that ANP induces lipolysis through cGMP-dependent protein kinase I (cGKI) phosphorylation of hormone sensitive lipase and perilipin A [27,41]. Infusion of BNP increases Ucp1 and Pgc-1α transcription through a p38 mitogen-activated protein kinase in mouse adipocytes [17]. While not in adipose tissue per se, Miyashuta et al. demonstrated that transgenic overexpression of BNP increases mitochondrial biogenesis in muscle tissue, protecting against diet induced obesity in vivo [42]. Critically, NP expression has been shown to increase following gastric bypass [18], and increases in BNP due to gene polymorphisms have been associated with protection against type-2 diabetes in humans [43]. Thus, in our data, elevated BNP represents a plausible mechanism linking RYGB with browning in GWAT and, possibly, improved glucose disposal.
It is known that rodents exhibit far greater expression of NPR3 in adipose tissues than humans [28]. This difference is important because NPR3 functions mainly as a clearance receptor, binding and internalizing all three forms of NPs for degradation [40]. Thus, the metabolic effect of NPs observed in human adipocytes is largely absent in wild-type mice [28]. However, deletion of Npr3 confers lipolytic and thermogenic characteristics to murine adipose tissues [17]. This is evidence that the extent to which NP and NPR interactions induce lipolysis and browning in mouse adipose likely depends on the ratio of NP receptor types present in the tissue, with lower relative expression of Npr3 enabling more pronounced effects. In our study, we observed increased levels of Npr1 and Npr2 mRNA in RYGB (Figure 3E) and a significant increase in the ratio of Npr1:Npr3 and Npr2:Npr3 in RYGB versus SO and WM-SO groups (Figure 3F). This favorable change in Npr ratio, in combination with increased β-adrenergic signaling, could provide the context for NP-mediated increases in thermogenesis and beige adipocyte gene expression observed in the RYGB group.
Indeed, we observed increased expression of the brown adipocyte specific uncoupling protein 1 (UCP1) at both the protein and mRNA level in the GWAT of RYGB females (Figure 3A,B). Ucp1 drives the unique thermogenic program of brown and beige adipose tissue by uncoupling cellular respiration from ATP synthesis, thereby dissipating energy normally used for cellular work as heat [26,44]. Ucp1 expression is considered specific to brown and beige adipocytes [15,45] and its upregulation in GWAT was surprising given the prevailing notion that visceral adipose is resistant to beige enrichment. Investigations into browning of adipose tissue have typically focused on the inguinal/subcutaneous tissue depot; to our knowledge beige induction in the gonadal adipose tissue depot has not been previously demonstrated. Interestingly, we did not find increased markers of beige adipocytes in the inguinal or brown depots (Figure 3B). This lack of Ucp1 induction in BAT and IWAT suggests that the browning effect brought on by RYGB uniquely influences GWAT.
Even though the RYGB – NP pathway stands as a plausible driver of beige adipocyte activation and weight loss following gastric bypass, other mechanisms may also contribute to white adipose tissue browning. For instance, it is known that the fibroblast growth factor 21 (FGF21) promotes brown adipocyte thermogenesis [46] through interaction with adipose tissue fibroblast growth factor receptor 1 (Fgfr1) [47]. Moreover, FGF21 increases following RYGB in humans [48]. In our study, we did not see increases in Fgf21 or Fgfr1 following RYGB (data not shown), lending support to the notion of a primary role of NPs and Nprs in adipose tissue browning; however, additional studies utilizing knockdown models of known browning mediators, including both Fgf21 and the NPs, are required to precisely define the contributions of each of these pathways to beige adipocyte activation.
In addition, since weight loss and glucose homeostasis were similarly altered in both RYGB and WM-SO groups, the results of this study do not definitively characterize weight-loss independent effects of RYGB surgery on glucose metabolism. Future time course studies should be conducted to describe precisely when improvements in glucose metabolism occur, and to what extent weight-loss per se contributes to overall metabolic improvements in this model. Finally, these studies were carried out in female mice due to the fact that the overwhelming majority of human bariatric surgeries are performed on females. Future investigations should assess the effect of sex on RYGB-induced browning in order to better understand the hormonal and genetic contributions to the phenomenon.
5. Conclusions
Our findings demonstrate that RYGB in female mice induces significant and persistent weight loss without reductions in food intake. RYGB or WM-SO mice lost equal amounts of weight with proportionate reductions in fat mass and had improved glucose tolerance. Critically, expression of beige adipocyte markers increased only in GWAT tissue following RYGB, strongly suggesting an increased beige adipocyte population. Finally, we found significant elevations in adipose tissue expression of Nppb, Npr2, Abdr3, as well as significant increases in the ratio of Npr1:Npr3 in RYGB but not WM-SO mice, indicating that RYGB may affect beige adipocyte enrichment of adipose tissues by altering sympathetic and natriuretic peptide-related circuits. While not all of it reaches statistical significance, the preponderance of this data suggests that RYGB induces browning of GWAT potentially through increased NP and SNS activity. Future lines of investigation should determine to what extent this pattern is recapitulated in human RYGB patients.
Conflict of interest
There is no conflict of interest.
Funding
This research was supported by the National Institutes of Health grant, DK 073689, and the Women's Health Institute.
Author contributions
The RYGB, sham operation and subsequent post-operative care were performed by VA and JLF. Gene expression studies were performed by MN, AF. Histology was performed by LV. Statistics were performed by QL and AF. AF and DJC prepared the manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Three experimental groups received either sham operation (SO), Roux-en-Y gastric bypass (RYGB), or sham operation with caloric restriction (WM-SO). Data presented as mean ± SEM. (A) Fasting blood glucose (SO n = 10, RYGB n = 4, WM-SO n = 6). (B) Insulin tolerance test, plasma glucose concentrations. (C) Plasma leptin concentration at time of sacrifice (SO n = 5, RYGB n = 3, WM-SO n = 5). (D) Relative expression determined by RT-qPCR of Pgc1α, Prdm16, Dio2, and Egr1 mRNA. *=p < 0.05 by 1-way ANOVA with Tukey post hoc test for SO vs. RYGB/WM-SO. ∗P < 0.05 RYGB or WM-SO versus SO. ˆ P < 0.05 WM-SO only versus SO. #P < 0.05 RYGB versus WM-SO.
References
- 1.Sanchis P., Frances C., Nicolau J., Rivera R., Fortuny R., Julian X. Cardiovascular risk profile in Mediterranean patients submitted to bariatric surgery and intensive lifestyle intervention: impact of both interventions after 1 year of follow-up. Obesity Surgery. 2014 doi: 10.1007/s11695-014-1321-z. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K. Bariatric surgery versus intensive medical therapy for diabetes. The New England Journal of Medicine. 2014;371(7):681–682. doi: 10.1056/NEJMc1407393. [DOI] [PubMed] [Google Scholar]
- 3.Adams T.D., Davidson L.E., Litwin S.E., Kolotkin R.L., LaMonte M.J., Pendleton R.C. Health benefits of gastric bypass surgery after 6 years. The Journal of the American Medical Association. 2012;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating C.L., Dixon J.B., Moodie M.L., Peeters A., Playfair J., O'Brien P.E. Cost-efficacy of surgically induced weight loss for the management of type 2 diabetes: a randomized controlled trial. Diabetes Care. 2009;32(4):580–584. doi: 10.2337/dc08-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surgery, A.S.f.M.a.B., Bariatric Surgery Procedures.
- 6.Lips M.A., Van Klinken J.B., van Harmelen V., Dharuri H.K., t Hoen P.A., Laros J.F. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes mellitus. Diabetes Care. 2014 doi: 10.2337/dc14-0195. [DOI] [PubMed] [Google Scholar]
- 7.Wickremesekera K., Miller G., Naotunne T.D., Knowles G., Stubbs R.S. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obesity Surgery. 2005;15(4):474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 8.Laferrere B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 11.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 13.Ricquier D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: a historical perspective. Frontiers in Endocrinology (Lausanne) 2011;2:85. doi: 10.3389/fendo.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q.A., Scherer P.E., Gupta R.K. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. The Journal of Lipid Research. 2014;55(4):605–624. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajimura S., Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual Review of Physiology. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoneshiro T., Aita S., Matsushita M., Kameya T., Nakada K., Kawai Y. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19(1):13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 17.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessi-Fulgheri P., Zhang C. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. Journal of Clinical Investigation. 2012;122(3):1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changchien E.M., Ahmed S., Betti F., Higa J., Kiely K., Hernandez-Boussard T. B-type natriuretic peptide increases after gastric bypass surgery and correlates with weight loss. Surgical Endoscopy. 2011;25(7):2338–2343. doi: 10.1007/s00464-010-1565-1. [DOI] [PubMed] [Google Scholar]
- 19.Mossenbock K., Vegiopoulos A., Rose A.J., Sijmonsma T.P., Herzig S., Schafmeier T. Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One. 2014;9(10):e110428. doi: 10.1371/journal.pone.0110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maggard M.A., Yermilov I., Li Z., Maglione M., Newberry S., Suttorp M. Pregnancy and fertility following bariatric surgery: a systematic review. The Journal of the American Medical Association. 2008;300(19):2286–2296. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 21.Zechner J.F., Mirshahi U.L., Satapati S., Berglund E.D., Rossi J., Scott M.M. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580–590. doi: 10.1053/j.gastro.2012.11.022. e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookout A.L., Mangelsdorf D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nuclear Receptor Signaling. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colman E., Katzel L.I., Rogus E., Coon P., Muller D., Goldberg A.P. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism. 1995;44(11):1502–1508. doi: 10.1016/0026-0495(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 24.Bays H.E., Toth P.P., Kris-Etherton P.M., Abate N., Aronne L.J., Brown W.V. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. The Journal of Clinical Lipidology. 2013;7(4):304–383. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Seale P., Conroe H.M., Estall J., Kajimura S., Frontini A., Ishibashi J. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. Journal of Clinical Investigation. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 27.Sengenes C., Berlan M., De Glisezinski I., Lafontan M., Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB Journal. 2000;14(10):1345–1351. [PubMed] [Google Scholar]
- 28.Sengenes C., Zakaroff-Girard A., Moulin A., Berlan M., Bouloumie A., Lafontan M. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;283(1):R257–R265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 29.Shin A.C., Zheng H., Townsend R.L., Patterson L.M., Holmes G.M., Berthoud H.R. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obesity Surgery. 2013;23(4):531–540. doi: 10.1007/s11695-012-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirksen C., Jorgensen N.B., Bojsen-Moller K.N., Kielgast U., Jacobsen S.H., Clausen T.R. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. International Journal of Obesity (London) 2013;37(11):1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 31.Nestoridi E., Kvas S., Kucharczyk J., Stylopoulos N. Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology. 2012;153(5):2234–2244. doi: 10.1210/en.2011-2041. [DOI] [PubMed] [Google Scholar]
- 32.Lutz T.A., Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2014;307(11):R1275–R1291. doi: 10.1152/ajpregu.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bueter M., Lowenstein C., Olbers T., Wang M., Cluny N.L., Bloom S.R. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Fried M., Ribaric G., Buchwald J.N., Svacina S., Dolezalova K., Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2: an integrative review of early studies. Obesity Surgery. 2010;20(6):776–790. doi: 10.1007/s11695-010-0113-3. [DOI] [PubMed] [Google Scholar]
- 35.Rubino F., Gagner M., Gentileschi P., Kini S., Fukuyama S., Feng J. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. The Annals of Surgery. 2004;240(2):236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Z., Brooks V.L. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of estrogen. The Journal of Physiology. 2014 doi: 10.1113/jphysiol.2014.284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munzberg H., Morrison C.D. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Frontiers in Neuroendocrinology. 2014;35(4):473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter L.R., Yoder A.R., Flora D.R., Antos L.K., Dickey D.M. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. The Handbook of Experimental Pharmacology. 2009;(191):341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengenes C., Bouloumie A., Hauner H., Berlan M., Busse R., Lafontan M. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. The Journal of Biological Chemistry. 2003;278(49):48617–48626. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 42.Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., Sone M. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meirhaeghe A., Sandhu M.S., McCarthy M.I., de Groote P., Cottel D., Arveiler D. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Human Molecular Genetics. 2007;16(11):1343–1350. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 44.Inokuma K., Okamatsu-Ogura Y., Omachi A., Matsushita Y., Kimura K., Yamashita H. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. The American Journal of Physiology – Endocrinology and Metabolism. 2006;290(5):E1014–E1021. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 45.Cannon B., Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) International Journal of Obesity (London) 2010;34(Suppl 1):S7–S16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 46.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu T., Seok S., Choi S., Huang Z., Suino-Powell K., Xu H.E. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Molecular and Cellular Biology. 2014;34(22):4130–4142. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen P.L., van Werven J., Aarts E., Berends F., Janssen I., Stoker J. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Digestive Diseases. 2011;29(1):48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three experimental groups received either sham operation (SO), Roux-en-Y gastric bypass (RYGB), or sham operation with caloric restriction (WM-SO). Data presented as mean ± SEM. (A) Fasting blood glucose (SO n = 10, RYGB n = 4, WM-SO n = 6). (B) Insulin tolerance test, plasma glucose concentrations. (C) Plasma leptin concentration at time of sacrifice (SO n = 5, RYGB n = 3, WM-SO n = 5). (D) Relative expression determined by RT-qPCR of Pgc1α, Prdm16, Dio2, and Egr1 mRNA. *=p < 0.05 by 1-way ANOVA with Tukey post hoc test for SO vs. RYGB/WM-SO. ∗P < 0.05 RYGB or WM-SO versus SO. ˆ P < 0.05 WM-SO only versus SO. #P < 0.05 RYGB versus WM-SO.


