Abstract
The needs for safe, therapeutically effective antidiarrheal combination continuously lead to effective treatment. When administered simultaneously, metronidazole–kaolin interactions have been reported by FDA but not studied. This paper is the first to study metronidazole–kaolin interactions. Adsorption isotherms of a metronidazole–kaolin antidiarrheal combination from aqueous solutions at an in vivo simulated pH conditions were obtained at 37 ± 0.5 °C. Langmuir constants for the adsorption are 10.8225, 41.3223 mg g−1 and 11.60, 2.56 l g−1 aimed at the monolayer capacity, and the equilibrium constant at pH 1.2 and 6.8, respectively. pH effect on adsorption of known concentration of metronidazole by kaolin was also studied over the range 1.2–8. A gradual increase in the adsorbed amount was noted with increasing the pH. Elution studies by different eluents showed that drug recovery from adsorbent surface was pH-dependent via competitive mechanism. The elution followed the sequence: 0.1 M HCl > 0.1 M NaCl > H2O. Adsorption–desorption studies revealed physical adsorption. The equilibrium concentration of metronidazole decreased as the adsorbent concentration was increased in the systems.
The dissolution profiles (USP) of commercially available tablets (Riazole® 500 mg) were obtained alone and in the presence of either (ORS®) rehydration salts and 9 or 18 g of kaolin powder. The percentage drug released versus time: 95.01% in 25 min, 101.02% in 30 min, 67.63% in 60 min, 60.59% in 60 min, respectively.
The percentage drug released versus time was increased with ORS® due to common ion effect [Cl−], while, it was decreased with kaolin due to adsorption. The mechanism of reaction of Riazole® (500 mg) tablets in the different dissolution media, confirms with Korsmeyer–Peppas model.
The interaction between metronidazole and kaolin was characterized by melting point determinations, differential scanning calorimetry analysis and infrared spectroscopy. The results obtained were suggestive of physical interaction between metronidazole and kaolin.
Keywords: Metronidazole, Koalin, Adsorbtion–desorbtion, Korsmeyer–Peppas model
1. Introduction
Adsorbents are potentially reactive substances which may interfere with the absorption of concurrently administered drugs (Gugler and Allgayer, 1990) and (Thorpe et al., 1990).
Kaolin is a clay-like material, rocks that are rich in kaolinite are known as kaolin or china clay. In its natural state kaolin is a white, soft powder consisting principally of mineral crystalline flakes, not colloidal material, and its melting point: 1725–1790 °C (Thompson et al., 1992). It is a purified native hydrated silicate of aluminum of a definite composition. Kaolin formula Al2O7Si2, Or Al2Si2O5(OH)4.
1.1. Kaolin Chemical structure
Kaolin is freed from impurities (FeO, CaCO3, MgCO3) by elutriation and dried. Clay impurities will depend on the genesis of the mineral and will affect the degree of disorder and the particle size of the clay (Balan et al., 1999).
Kaolin is insoluble in water, mineral acids and solutions of alkali hydroxides. It is one of the principal intestinal adsorbents, has traditionally been used internally in the treatment of various enteric disorders, colitis, enteritis, dysentery and diarrhea associated with food and alkaloidal poisoning and in traveler’s diarrhea (DuPont, 2007; Ericsson, 2005; Thielman and Guerrant, 2004). It binds to and traps bacteria and their toxins and gases in the gut. It also binds to water in the gut, which helps to make the stools firmer, hence giving symptomatic relief (Carretero and Pozo, 2009). Kaolin is administered in the form of a suspension. The usual daily dose for adults is 26.2 g after each meal; not to exceed more than 26.2 g per 24 h; and not to be used longer than 2 days (Helms and Quan, 2006).
As adjunct to rehydration therapy: Up to 24 g it may be used in divided doses daily. It may also be used in combination with other antidiarrheal agents (Moini, 2008).
There are many reports of adsorbed drugs and chemicals onto kaolin, for example; quinidine (Bucci et al., 1981), propranolol (Al-Gohary et al., 1987), mebeverine hydrochloride (al Gohary, 1997); ampicillin, cloxacillin, amoxicillin, clavulanic acid, chloroquine, chlorpheniramine (Onyekweli et al., 2003), Congo red (Vimonses et al., 2009); ciprofloxacin, chloroquine, piperazine, phyllocontin, digoxin and diazepam (Hassan and Ibrahim, 2011).
Metronidazole C6H9N3O3; 2-(2-methyl-5-nitro-1H-imidazole-1-yl) ethanol; Pka: 2.6; melting point: 159–163; molecular weight: 177.15. It is a synthetic antibacterial agent that is used primarily in the treatment of various anaerobic infections such as intra-abdominal infections, antiprotozoal, and amebicidal (Dukes, 1990; DuPont, 2007; Fekety, 1997; Samuelson, 1999).
The aim of this investigation was to assess metronidazole–kaolin interaction by an adsorption–desorption test in different media and pH values; to determine the changes of the dissolution profiles of metronidazole in the presence of rehydration salts and kaolin powder and to characterize metronidazole–kaolin interaction by melting point determinations, differential scanning calorimetry analysis and infrared spectroscopy.
2. Materials and methods
2.1. Materials and instruments
Metronidazole pure powder and its tablets (Riazole® 500 mg) were obtained from Riyadh Pharma (Saudi Arabia) and Kaolin white fine powder UNI-CHEM® was used as received. ORS® rehydration salts (sodium chloride 3.5 g, potassium chloride 1.5 g, tri sodium citrate dehydrate 209 g, anhydrous dextrose 20 g) were from National Pharmaceutical Industries Co. (SAOG). All other reagents were of pure analytical grade and were used as supplied by BDH (Poole, UK). Freshly deionized distilled water was used throughout the work.
pH meter (P.Selecta, s.a., Barcelona, Spain), Centrifuge (Sigma, Laborzentrifugen, Germany), UV–visible Spectrophotometer (Genesys 5, USA), water bath (Karl Kolb type D-6.72, Bonn, Germany), ERWEKA dissolution rate testing apparatus DT 600 (Heusenstamm, Germany).
2.2. Adsorption studies
Adsorption studies were carried out by shaking 1 g of kaolin adsorbent powder with 20 ml of solution containing different concentrations of metronidazole in 0.1 M HCl (pH 1.2) or phosphate buffer (pH 6.8). Suspensions were shaken in a thermostatically controlled water bath at 80 rpm and 37 ± 0.5 °C until equilibrium was attained after 2 h (Al Gohary, 1995). The suspensions were centrifuged at 13200 rpm for 20 min and then the supernatant layer was filtered using filter paper (Vimonses et al., 2009). The drug concentration remaining in the filtrate was determined by spectrophotometric assay at 277 nm. Kaolin was not found to interfere with the assay. Three runs were obtained and the results averaged (Al Gohary, 1995).
2.3. Effect of pH on the extent of adsorption of metronidazole onto kaolin powder
Adsorption experiments were carried out as previously mentioned at a pH range 1.2–8 and 37 ± 0.5 °C, using a fixed known concentration of metronidazole. The amount of drug adsorbed was calculated and the results were compared.
2.4. Effect of varying the concentration of kaolin powder (g% w/v) on the equilibrium concentration of drug in solution (Ce mg%)
Different weights of kaolin powder (0.5, 1.0, 2.0 and 4.0 g), i.e.; 2.5, 5, 10 and 20 (g% w/v) were suspended in 20 ml of 0.1 M HCl (pH 1.2) or phosphate buffer (pH 6.8) containing a fixed known concentration of the metronidazole and equilibrated for 2 h at 37 ± 0.5 °C, as mentioned under adsorption studies. Then, the amount of metronidazole remained in solution at equilibrium (Ce mg%) was determined spectrophotometrically at 277 nm and the results were compared.
2.5. Desorption (elution) studies
The residue obtained from adsorption process of a certain metronidazole concentration onto kaolin in 0.1 M HCl at 37 ± 0.5 °C was collected and dried at room temperature away from light, shaken at 80 rpm and 37 ± 0.5 °C for 5 min with 20 ml of different elution media (0.1 M HCl,0.1 M NaCl and H2O), then treated as mentioned under adsorption studies. Successive washings for residue were carried out in a similar manner until no drug was detected in the filtrate.
2.6. Dissolution studies
The in vitro drug release from 500 mg metronidazole tablets (Riazole®) was determined according to USP specifications, using 900 ml of 0.1 M HCl at 37 ± 0.5 °C. The basket was rotated at 100 rpm. Five ml of sample was withdrawn at 5 min intervals over 60 min and replaced with a fresh aliquot of the dissolution medium. Then the sample was appropriately diluted and measured spectrophotometrically at 277 nm. The amount of metronidazole in the sample was determined based on the calibration curve generated at a wavelength of 277 nm. Three replicates were run and the mean average was calculated.
In addition, the dissolution profile was constructed as mentioned above, but in the presence of ORS® rehydration salts. Also, the percentage of drug released from (Riazole® 500 mg) tablets was determined with two doses of kaolin; 9 and 18 g powder, was added to the dissolution medium; based on the adult kaolin dose being 26.2 g that may be used daily in divided doses or in combination with other antidiarrheal agents according to severity of disease (Pray, 2006).
2.7. Characterization of the interaction between metronidazole and kaolin
1.0 g of kaolin powder with 20 ml of 0.1 M HCl (pH 1.2) or phosphate buffer (pH 6.8) containing 40 mg of drug was treated as previously mentioned under adsorption studies. The residue obtained from adsorption experiments was collected, dried over calcium chloride in desiccators for 24 h away from light, and then subjected to the following tests:
2.7.1. Melting point (MP) determination
Melting point apparatus (RAC exports, Haryana, India) was used for MP determination of the samples at a heating rate of 2 °C/min, until the desirable temperature was reached, then the rate was lowered to 0.2 °C/min until complete melting occurred. The MP recorded for each sample was the average of five readings.
2.7.2. Differential scanning calorimetry (DSC) analysis
The DSC thermograms were recorded on (TA Instruments, Auto Q20 DSC, United State) calibrated with indium (99.999%). 2 mg of the samples were heated at the heating rate of 30 °C/min over a temperature range of 30–350 °C in closed aluminum pans under an argon purge. At least three determinations were used to calculate mean values and standard deviation.
2.7.3. Infrared spectroscopy (IR) determinations
IR spectra were taken on a Thermo Scientific, Nicolet 6700 FT-IR, USA as KBr pellets. The samples: KBr ratio was 1:300 mg, and a pressure of 15 tons was used. Spectra were recovered over a 4000–250 cm−1 range, with a scan speed of 300 cm−1/min.
Different samples of kaolin dry powder alone, or treated with 0.1 M HCl (pH 1.2) or phosphate buffer (pH 6.8) were shaken at 80 rpm for 2 h. The residue obtained was collected, dried over calcium chloride in desiccators for 24 h away from light. Powdered metronidazole alone or their physical mixtures with kaolin in ratios 1:1 or 1:2 were prepared. All samples were also subjected to MP, DSC and IR determinations and the results were compared.
3. Results and discussion
3.1. Adsorption studies
Kaolin is a two-layered structure (Brindley et al., 1946) which is non-expandable. One layer is an octahedral structure of oxygen and hydroxyl groups surrounding an aluminum atom; the octahedral sheets are joined by neighboring units sharing oxygen atoms. The other layer consists of four oxygen atoms surrounding a silicon atom in a tetrahedral arrangement and the apices of the tetrahedral and one of the layers of the octahedral sheet forms a common layer. There are two surfaces on kaolin on which adsorption is possible. On the exposed hydroxyl layer adsorption is possible by hydrogen bonding and on the edge surface, due to the amphoteric nature of the aluminum ion. The charge will vary with change in pH. The ratio of the surface area edge to face is 1:14 (Armstrong and Clarke, 1971). The structure is electrically neutral but an isomorphous substitution occurs within the lattice and gives rise to a negative charge which is inevitably compensated for by cations being attracted to the surface. Kaolinite is a 1:1 layer mineral and a product of advanced weathering processes. One layer of the mineral consists of an alumina octahedral sheet and a silica tetrahedral sheet that share a common plane of oxygen atoms and repeating layers of the mineral are hydrogen bonded together (Bear, 1965; Miranda-Trevino and Coles, 2003). The forces that hold the kaolinite layers together are hydrogen bond and attractive van der Waals forces.
The adsorption properties of kaolinite are likely determined by its surface structure and the edges (Miranda-Trevino and Coles, 2003; Schoonheydt and Johnston, 2006). The edges possess a variable charge that can be correlated to the reaction between ionizable surface groups along the edges and the clay mineral surface and the ions present in aqueous solution (Vimonses et al., 2009). The comment on adsorption of metronidazole onto kaolin is consistent with the suggestions of Ma and Eggleton, 1999 who proposed that the crystallinity of kaolinite might correlate to its cation exchange capacity and the adsorption process is most likely to take place on the edges and surfaces of the clay mineral.
Adsorption isotherms of metronidazole onto kaolin in 0.1 M HCl (pH 1.2) and phosphate buffer (pH 6.8) at 37 ± 0.5 °C were obtained. The results (Figs. 1 and 2) show that adsorption was in accordance with Langmuir’s equation (Langmuir, 1918) expressed as Ce/(x/m = 1/(ab + Ce/a), where the slope = 1/a, (a) is the monolayer adsorptive capacity of adsorbent for the drug, and the intercept = 1/ab, (b) is the equilibrium constant of the adsorption process or the enthalpy constant, and (Ce) is the equilibrium concentration. (x/m) is the mass of solute (x) adsorbed per g (m) of the adsorbent (mg g−1). The adsorption constants (a) and (b) and correlation coefficients (R2) were estimated by linear regression analysis where (R2) = 0.9984, 0.9926; the value of (a) = reciprocal of the slope (10.8225, 41.3223 mg gm−1); the value of the affinity constant of the adsorption process (b) = 1/a. intercept = 11.60, 2.56 l g−1 in pH 1.2 and 6.8, respectively (Table 1). It is noticed from the previous results that the adsorption was pH dependant (Figs. 3–8).
Figure 1.
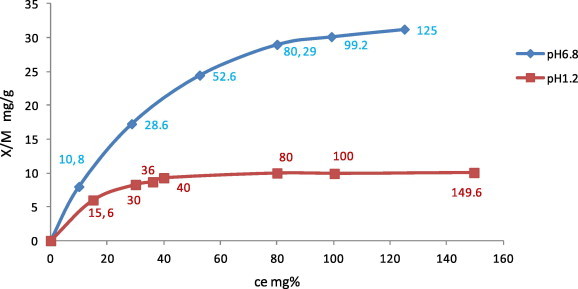
Langmuir adsorption isotherm of metronidazole onto kaolin from 0.1 M HCl, pH 1.2 and phosphate buffer pH 6.8 at 37 °C.
Figure 2.
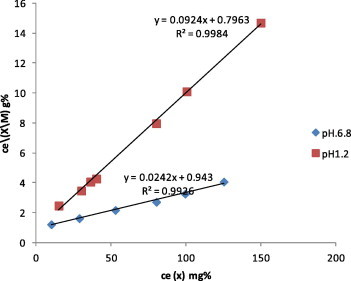
Langmuir adsorption isotherm – linear form- of metronidazole onto kaolin from 0.1 M HCl, pH 1.2 and phosphate buffer pH 6.8 at 37 °C.
Table 1.
Langmuir constants a and b for the adsorption of metronidazole onto kaolin (5.0% w/v) in 0.1 M HCl, pH 1.2 and phosphate buffer, pH 6.8 at 37 °C.
| pH | Regression analysis |
Langmuir constants |
|||
|---|---|---|---|---|---|
| Slope (1/a) | Intercept (1/ab) | R2 | a (mg g−1) 1/slope | b (l g−1) 1/ac | |
| 1.2 | 0.0924 | 0.7963 | 0.9984 | 10.8225 | 11.6 |
| 6.8 | 0.0242 | 0.9430 | 0.9926 | 41.3223 | 2.56 |
Figure 3.
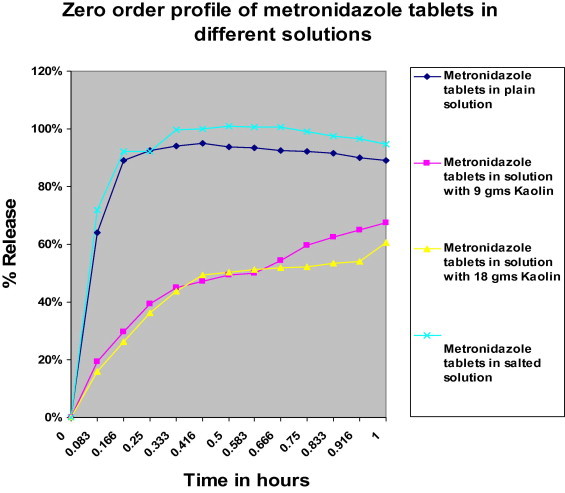
Metronidazole dissolution profile from Riazole (500 mg) tablet in 0.1 M HCl.
Figure 4.
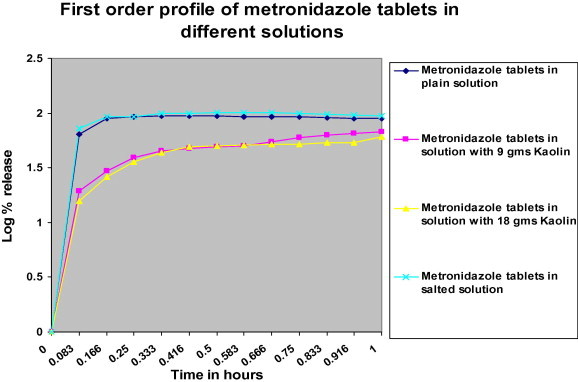
First order profile of log% drug release of metronidazole tablets versus time.
Figure 5.
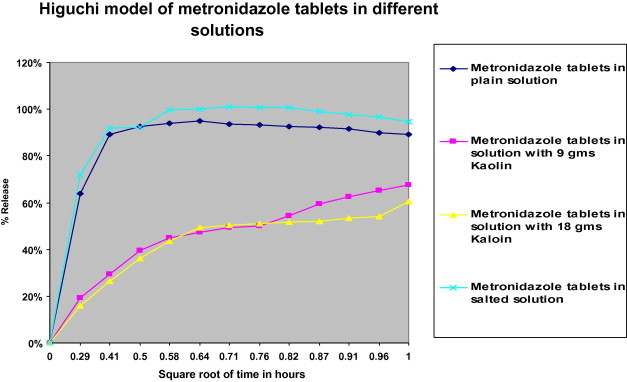
Higuchi model of log% drug release of metronidazole versus log time.
Figure 6.
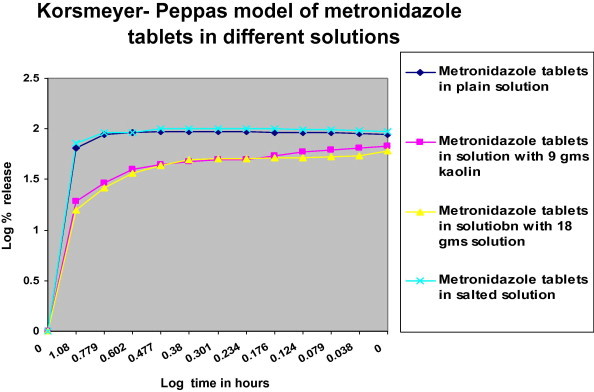
Korsmeyer–Peppas model of log% release of metronidazole tablets versus log time.
Figure 7.
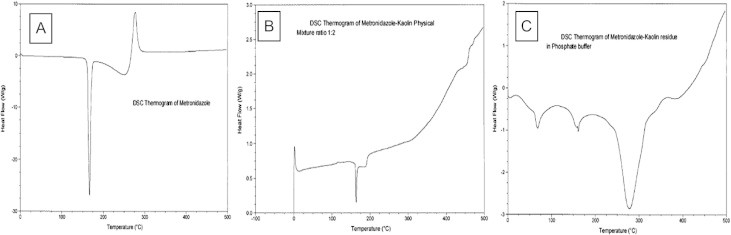
Differential scanning calorimetry (DSC) analysis of metronidazole and kaolin interaction.
Figure 8.
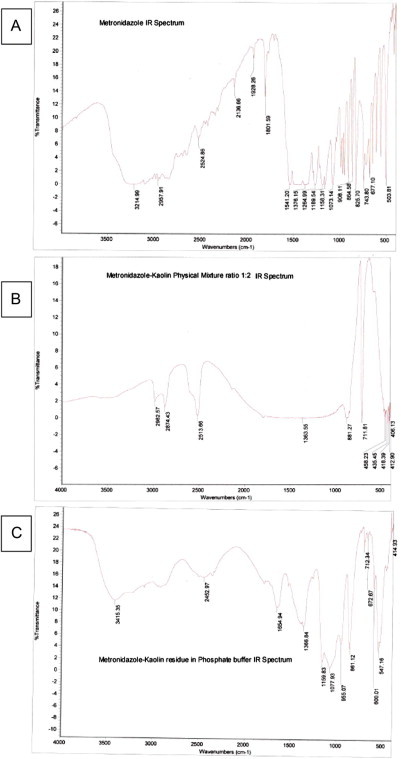
Infrared spectroscopy (IR) determinations of metronidazole and kaolin interaction.
3.2. Effect of pH on the extent of adsorption of metronidazole onto kaolin powder
The effect of pH over a range of 1.2–8 on the extent of adsorption of metronidazole onto kaolin powder was studied. Table 2 shows that the adsorption of drug was increased as the pH increased. This also was noticed in (Table 1). A competitive mechanism may exist involving hydronium ions (H3O+) and the protonated amine. Since metronidazole is a weak base that appears to dissolve maximally around pH ⩽2.0, at high pH values, the drug has low solubility (Wu and Fassihi, 2005), hence, adsorption increases according to Lundelius’ rule; when solubility increases, adsorption decreases: “generally, pH and solubility effects act in concert”. On the other hand, the equilibrium between the protonated and non-protonated forms of the drug is dynamic. At a low pH, hydronium ions increase, thus the system is flocculated; hence, adsorption decreases (Ridout, 1968). This is in accordance with the results obtained for adsorption of divalent cations onto kaolin. Displacement of H+ ions and adsorption of Pb, Zn or Cd create flocculation and an increase in compressibility (Farrah and Pickering, 1987). In addition, the substitution of H+ ions for metal ions could change the van der Waals forces within the kaolinite structure according to Thompson et al. (1992). Metal adsorption of divalent cations of Pb, Zn and Cd, on the broken edges of the kaolinite may cause the DDL thickness to decrease, repulsive forces decrease and flocculation, porosity and the hydraulic conductivity of the kaolinite increase (Chen et al., 2000; Mitchell, 1993; Yong et al., 1992).
Table 2.
Effect of pH on the extent of adsorption of metronidazole (40 mg/20 ml buffer system) onto kaolin powder (5.0% w/v) at 37 °C.
| pH | Amount of drug adsorbed (mg g−1) |
|---|---|
| 1.2 | 10.07 |
| 2.0 | 17.87 |
| 4.2 | 25.67 |
| 6.8 | 25.89 |
| 8.0 | 29.5 |
The adsorption of Cd appears to be more dependent on kaolinite’s edge charge as influenced by pH. The reduced solution pH may cause less Cd to be retained (Ziper et al., 1988). Unlike Cd, the data for Pb and Zn may suggest that some adsorption of these two metals is also taking place along the outer hydroxyl plane described by Frost, R.L., 1998 and, therefore, releasing of the H+ ions located there although the H+ ions at these sites may not be as easily exchanged as those on the broken edges of the kaolinite (Ma and Eggleton, 1999).
3.3. Effect of varying the concentration of kaolin powder (g% w/v) on the equilibrium concentration of drug in solution (Ce mg%)
The effect of kaolin powder concentration (2.5, 5, 10, and 20 g% w/v) on the extent of adsorption is shown in Table 3. The amount of unbound drug (Ce mg%) was decreased as the adsorbent concentration increased up to 10 g% w/v. This is due to the fact that as the adsorbent concentration increased, more active sites are made available for adsorption of a fixed amount of metronidazole, hence, the equilibrium concentration becomes progressively less. This implies that the equilibrium concentration is dependent on the adsorbent concentration in the system. But there was no significant increase in Ce (mg%) between kaolin concentrations (10 and 20 g% w/v), since the concentration of metronidazole in solution was quite enough to form a mono layer on the adsorbent, i.e.; saturation was attained with (10 g% w/v) kaolin.
Table 3.
Effect of kaolin concentration on the equilibrium concentration (Ce) of metronidazole (40 mg/20 ml) of (a) 0.1 M HCl, pH 1.2 and (b) phosphate buffer, pH 6.8, at 37 °C.
| Kaolin concentration (g% w/v) |
Ce (mg%) |
|
|---|---|---|
| a | b | |
| 2.5 | 118.83 | 63.13 |
| 5.0 | 168.7 | 55.70 |
| 10 | 98.67 | 59.94 |
| 20 | 115.12 | 54.64 |
3.4. Desorption (Elution) Studies
The results of desorption (elution) are shown in Table 4. When the eluent (20 ml) was 0.1 M HCl, 61.53% of drug was recovered-desorbed-from kaolin after 4 washes; 34.33% when the eluent was 0.1 M NaCl after 3washes, and only 20.29% after 3washes when the eluent was water. Dry grinding of kaolinite with NaCl, followed by the addition of distilled water and subsequent drying by heating, may result in 95% intercalation with NaCl between the hydrogen-bonded layers of kaolinite. This intercalation changes the structure of the kaolinite and swells the mineral, increasing the space between adjacent layers by as much as 7.8 A°, but the process is reversible in excess water. The elution of metronidazole from kaolin surface by different elution media followed the sequence: 0.1 M HCl > 0.1 M NaCl > H2O (Table 4). NaCl was more efficient than water in displacing metronidazole from the negatively charged kaolin surface, since metronidazole being a weak base is sparingly soluble in water (Wu and Fassihi, 2005), its desorption from kaolin was pH dependant and via competitive mechanism (Tables 5–7).
Table 4.
Desorption of metronidazole from kaolin (5.0% w/v), by different eluents at 37 °C.
| Amount of drug retained (mg g−1) by different eluents |
|||
|---|---|---|---|
| 0.1 M HCl | 0.1 M NaCl | H2O | |
| Amount adsorbed before elution mg g−1 | 11.88 | 19.31 | 23.55 |
| Amount retained mg g−1 after 1st wash | 9.78 | 13.68 | 19.84 |
| Amount retained mg g−1 after 2nd wash | 4.79 | 12.62 | 18.77 |
| Amount retained mg g−1 after 3rd wash | 4.57 | 12.62 | 18.77 |
| Amount retained mg g−1 after 4th wash | 4.57 | – | – |
| pH of eluent before elution | 1.2 | 6.29 | 6.83 |
| pH of filtrate after last wash | 6.49 | 9.36 | 9.27 |
| Total number of washes | 4 | 3 | 3 |
| % Drug desorbed | 61.53 | 34.33 | 20.29 |
Table 5.
Comparative values of R2 and the rate constant of the tested metronidazole tablets for different models of mechanism of drug release.
| Types of solutions | Zero order |
First order |
Higuchi model |
Korsmeyer–Peppas model |
||||
|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | |
| Metronidazole tablet in plain solution | 0.3914 | 0.1084 | 0.4086 | 0.1450 | 0.5130 | 0.1893 | 0.6619 | 63.973 |
| Metronidazole in solution with 9 g kaolin | 0.9661 | 0.4641 | 0.9048 | 1.0860 | 0.9885 | 0.6326 | 0.9878 | 96.310 |
| Metronidazole in solution with 18 g kaolin | 0.8865 | 0.3841 | 0.8191 | 1.0520 | 0.9417 | 0.5434 | 0.9564 | 102.706 |
| Metronidazole in salted solution | 0.5092 | 0.1371 | 0.5110 | 0.1607 | 0.6292 | 0.2256 | 0.7534 | 68.533 |
Table 6.
The estimated (n) values according to Korsmeyer–Peppas equation.
| Types of solutions | Estimated n values |
|---|---|
| Metronidazole tablet in plain solution | 0.09346 |
| Metronidazole in solution with 9 g kaolin | 0.4709 |
| Metronidazole in solution with 18 g kaolin | 0.4877 |
| Metronidazole in salted solution | 0.09423 |
Table 7.
The melting point values of the drugs and their physical mixtures and dried residues after adsorption processes.
| Serial no. | Test | Melting point (MP) |
|---|---|---|
| 1 | Metronidazole (Metro) pure powder | 162 |
| 2 | Kaolin (Kln) powder | >300 |
| 3 | 1:1 (Metro:Kln) powders physical mixture | 290 |
| 4 | 1:2 (Metro:Kln) powders physical mixture | 320 |
| 5 | Dried residue (1 g Kln in 20 ml 0.1 M HCl pH 1.2; shaken 2 h; centrifuged, residue collected and dried at room temperature) | 220 |
| 6 | Dried residue (1 g Kln in 20 ml 0.1 M HCl + 40 mg Metro pH 1.2; shaken 2 h; centrifuged, residue collected and dried at room temperature) | 260 |
| 7 | Dried residue (1 g Kln in 20 ml Phosphate buffer pH 6.8; shaken 2 h; centrifuged, residue collected and dried at room temperature) | 200 |
| 8 | Dried residue (1 g Kln in Phosphate buffer + 40 mg Metro + pH 6.8; shaken 2 h; centrifuged, residue collected and dried at room temperature) | 280 |
In all cases the amount recovered represented the amount that was adsorbed through weak Van der Waals attraction forces or electrostatic hydrogen bond. While the amount retained onto kaolin surface unrecovered could be attributed to forces of ionic type.
Desorption studies of Farrah and Pickering (1987) showed that the percentages of Pb desorbed from kaolinite are around 90% or higher, confirming that the metals are attached by ion exchange mechanisms. The H+ ions that are displaced at about 8 h during adsorption of Pb and Zn appear to be subsequently re-adsorbed by the kaolinite (or the Fe oxides).
3.5. Dissolution studies
According to USP specifications metronidazole tablets contain not less than 90.0% and not more than 110.0% of the labeled amount of drug during 60 min. Generally the initial rate of dissolution is directly proportional to the solubility of drug in the dissolution medium. The dissolution medium is kept at the body temperature of 37 °C, since the dissolution rate of some drug was affected and increased with increasing temperature. The solubility of the drug is affected by the pH of the medium, metronidazole exhibited a greater solubility in pH 1 and 2, since its solubility was reported to be 64.80 mg/ml (Wu and Fassihi, 2005).While its solubility was little affected by the addition of common ion (Cl−) in the presence of ORS® which contains NaCl and KCl. Thus the USP defined the dissolution medium under test; 0.1 M HCl (pH 1.2).
According to n values (Table 2), both Metronidazole tablet in plain solution and Metronidazole in salted solution have n values less than 0.45, this indicates that the drug release follows the Fickian diffusion which describes the diffusion flux according to the concentration (diffusion flux from the region of high concentration to the region of low concentration), and this is indicated in zero order Fig. 1, which indicate that the highest% drug release was with plain solution and with salted solution.
Metronidazole in solution with 9 g and 18 g kaolin, exhibited n value 0.45 < n > 0.89, which indicates anomalous diffusion or non-Fickian diffusion (refers to combination of both diffusion and erosion controlled release) (Gillespie and Seitaridou, 2012).
4. Conclusion
Metronidazole–kaolin interaction was determined by Langmuir adsorption isotherms and desorption tests and characterized to be physical interaction. This antidiarrheal combination exhibited significant suppression of metronidazole release from its tablets in acidic medium in the presence of different kaolin concentrations. Thus, concomitant administration of metronidazole and kaolin is not recommended in general practice. Further in vivo and microbiological investigations should be done.
Acknowledgements
The authors highly acknowledge the Research Center of the Science and Medical studies Departments Deanship of Scientific Research at King Saud University for financial support. The authors also would like to thank Dr. Maha Al-Eid from university of Dammam and Ashwag abahussain for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Fadilah Sfouq Aleanizy, Email: faleanizy@ksu.edu.sa.
Fulwah Alqahtani, Email: fyalqahtani@ksu.edu.sa.
Omaimah Al Gohary, Email: omaimah@ksu.edu.sa.
References
- Al Gohary O. Determination and characterization of the interaction between magaldrate antacid and some drugs: Part II. Pharm. Acta Helv. 1995;70:289–300. [Google Scholar]
- al Gohary O.M. In vitro adsorption of mebeverine hydrochloride onto kaolin and its relationship to pharmacological effects of the drug in vivo. Pharm. Acta Helv. 1997;72:11–21. doi: 10.1016/s0031-6865(96)00042-8. [DOI] [PubMed] [Google Scholar]
- Al-Gohary O., Lyall J., Murray J. Adsorption of antihypertensives by suspensoids. Part I. The adsorption of propranolol hydrochloride by attapulgite, charcoal, kaolin and magnesium trisilicate. Pharm. Acta Helv. 1987;62:66. [PubMed] [Google Scholar]
- Armstrong N., Clarke C. The adsorption of crystal violet by kaolin. J. Pharm. Pharmacol. 1971;23:95S–100S. doi: 10.1111/j.2042-7158.1971.tb08776.x. [DOI] [PubMed] [Google Scholar]
- Balan E., Allard T., Boizot B., Morin G., Muller J.-P. Structural Fe (super 3+) in natural kaolinites; new insights from electron paramagnetic resonance spectra fitting at X and Q-band frequencies. Clay Clay Miner. 1999;47:605–616. [Google Scholar]
- Bear F.E. second ed. Reinhold Publishing; 1965. Introduction in Chemistry of the Soil. [Google Scholar]
- Brindley G., Robinson K., MacEwan D. The clay minerals halloysite and meta-halloysite. Nature. 1946;157:225–226. [Google Scholar]
- Bucci A.J., Myre S.A., Tan H.S., Shenouda L.S. In vitro interaction of quinidine with kaolin and pectin. J. Pharm. Sci. 1981;70:999–1002. doi: 10.1002/jps.2600700907. [DOI] [PubMed] [Google Scholar]
- Carretero M.I., Pozo M. Clay and non-clay minerals in the pharmaceutical industry: Part I. Excipients and medical applications. Appl. Clay Sci. 2009;46:73–80. [Google Scholar]
- Chen J., Anandarajah A., Inyang H. Pore fluid properties and compressibility of kaolinite. J. Geotech. Geoenviron. Eng. 2000;126:798–807. [Google Scholar]
- Dukes G.E. Over-the-counter antidiarrheal medications used for the self-treatment of acute nonspecific diarrhea. Am. J. Med. 1990;88:S24–S26. doi: 10.1016/0002-9343(90)90272-f. [DOI] [PubMed] [Google Scholar]
- DuPont H.L. Therapy for and prevention of traveler’s diarrhea. Clin. Infect. Dis. 2007;45:S78–S84. doi: 10.1086/518155. [DOI] [PubMed] [Google Scholar]
- Ericsson C.D. Nonantimicrobial agents in the prevention and treatment of traveler’ diarrhea. Clin. Infect. Dis. 2005;41:S557–S563. doi: 10.1086/432952. [DOI] [PubMed] [Google Scholar]
- Farrah H., Pickering W.F. Extraction of heavy metal ions adsorbed on clays. Water Air Soil Pollut. 1987;9:491–498. [Google Scholar]
- Fekety R. Diagnosis and management of C. difficile-associated diarrhea and colitis. Am. J. Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- Gillespie D.T., Seitaridou E. Oxford University Press; 2012. Simple Brownian diffusion: an introduction to the standard theoretical model. [Google Scholar]
- Gugler R., Allgayer H. Effects of antacids on the clinical pharmacokinetics of drugs. Clin. Pharmacokinet. 1990;18:210–219. doi: 10.2165/00003088-199018030-00003. [DOI] [PubMed] [Google Scholar]
- Hassan S., Ibrahim J. Adsorption of some drugs onto surface of Iraqi kaolin clay. Pak. J. Chem. 2011;1 [Google Scholar]
- Helms R.A., Quan D.J. Lippincott Williams & Wilkins; 2006. Textbook of therapeutics: drug and disease management. [Google Scholar]
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40:1361–1403. [Google Scholar]
- Ma C., Eggleton R.A. Cation exchange capacity of kaolinite. Clay Clay Miner. 1999;47:174–180. [Google Scholar]
- Miranda-Trevino J.C., Coles C.A. Kaolinite properties, structure and influence of metal retention on pH. Appl. Clay Sci. 2003;23:133–139. [Google Scholar]
- Mitchell J.K. second ed. Wiley; 1993. Fundamentals of Soil Behavior. [Google Scholar]
- Moini J. Cengage Learning; 2008. Fundamental Pharmacology for Pharmacy Technicians: For Pharmacy Technicians. [Google Scholar]
- Onyekweli A.O., Usifoh C.O., Okunrobo L.O., Zuofa J.D. Adsorptive property of kaolin in some drug formulations. Trop. J. Pharm. Res. 2003;2:155–159. [Google Scholar]
- Pray W.S. second edn. Philadelphia; USA, Lippincott Williams & Wilkins: 2006. Nonprescription Product Therapeutics. [Google Scholar]
- Ridout C.W. The effect of pH on the adsorption of atropine from aqueous solution by kaolin. Pharm. Acta Helv. 1968;43:177–181. [PubMed] [Google Scholar]
- Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999;43:1533–1541. doi: 10.1128/aac.43.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheydt R., Johnston C. Surface and interface chemistry of clay minerals. Devel. Clay Sci. 2006;1:87–113. [Google Scholar]
- Thielman N.M., Guerrant R.L. Acute infectious diarrhea. New Engl. J. Med. 2004;350:38–47. doi: 10.1056/NEJMcp031534. [DOI] [PubMed] [Google Scholar]
- Thompson J.G., Uwins P., Whittaker A.K., Mackinnon I. Structural characterization of kaolinite: NaCl intercalate and its derivates. Clay Clay Miner. 1992;40:369–380. [Google Scholar]
- Thorpe J.E., Baker N., Bromet-Petit M. Effect of oral antacid administration on the pharmacokinetics of oral fluconazole. Antimicrob. Agents Chemother. 1990;34:2032. doi: 10.1128/aac.34.10.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimonses V., Lei S., Jin B., Chow C.W., Saint C. Adsorption of congo red by three Australian kaolins. Appl. Clay Sci. 2009;43:465–472. [Google Scholar]
- Wu Y., Fassihi R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int. J. Pharm. 2005;290:1–13. doi: 10.1016/j.ijpharm.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Yong R.N., Mohamed A.M.O., Warketin B.P. Elsevier Science Ltd; 1992. Principles of Contaminants Transport in Soil. [Google Scholar]
- Ziper C., Komarneni S., Baker D.E. Specific cadmium sorption in relation to the crystal chemistry of clay minerals. Soil Sci. Soc. Am. J. 1988;52(52):49–53. [Google Scholar]


