Abstract
A series of 5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-1-substituted-4,5-dihydropyrazole derivatives 4a–e and 6a–g have been synthesized and spectrally characterized. The antibacterial activity of the novel candidates has been screened using the agar diffusion test. These compounds were endowed with high antibacterial activity against different Gram +ve and Gram −ve bacteria when compared with standard antibacterial drugs. In the light of zone of inhibition and MIC results, Sarcina and Staphylococcus aureus are the most sensitive bacteria where pyrrolidinomethanone derivative 4e showed MICs at 80 and 110 nM, respectively. While hydroxypiperidinoethanone derivative 6c showed MIC at 90 nM for Sarcina.
Keywords: 1,3-Benzodioxole; 2-Pyrazoline; Antibacterial
1. Introduction
Recently, after years of misuse and overuse of antibiotics, potential global health crisis has become very apparent due to remarkable increase in bacterial resistance (Kathiravan et al., 2012) which has been firmly correlated to higher rates of morbidity and mortality (Ozdemir et al., 2007). In order to overcome such resistance new antibacterial candidates have to be introduced that consist of chemical features which differ from those of the present drugs. Thus, these leads have to be novel nevertheless resemble known biologically active molecules by the presence of critical pharmacophoric structural moieties. This impetus led to innovative heterocyclic permutations with unique action or diverse function. Also, it could be achievable via using small heterocyclic molecules which are highly functionalized scaffolds and are well known pharmacophores incorporated in a great number of molecules characterized by antibacterial activity.
During discovery of novel antibacterial agents, powerful activity has been displayed by natural molecules containing the 1,3-benzodioxole system for example protopine (Carradori et al., 2012) and egonol (Emirdag-Ozturk et al., 2011) and also by several chemically synthesized antimicrobial candidates such as compounds I and II (Chimenti et al., 2011, Wani et al., 2011, Secci et al., 2012) (Fig. 1).
Figure 1.
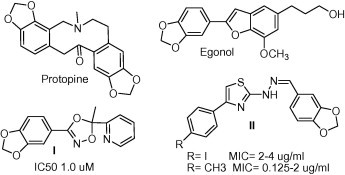
Antimicrobial candidates containing 1,3-benzodioxol system.
2-Pyrazoline is a ubiquitous small heterocyclic pharmacophore in medicinal chemistry. Many pharmacological activities have been reported for various 2-pyrazolines such as hypoglycemic (Cottineau et al., 2002), analgesic (Carradori et al., 2012), anti-inflammatory (Chakrabarthi et al., 1987), antitumor (Rostom et al., 2003), antibacterial (Akbas and Berber, 2005), and antifungal activities (Akbas and Berber, 2005). In particular pyrazoline-methanones (e.g. compound III) and pyrazoline-ethanones (e.g. compound IV) have shown an intense antimicrobial potency against TB and several candida species (Mamolo et al., 2001, Mamolo et al., 2003) (Fig. 2).
Figure 2.
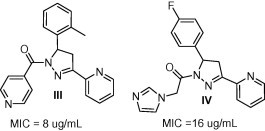
Antimicrobial candidates containing pyrazoline-methanone and ethanone scaffolds.
On the other hand, alicyclic amines occur widely in drugs (Gorrod and Aislaitner, 1994). Many of these amines such as piperidine and morpholine fostered the antimicrobial activity of chemically synthesized candidates (Qing et al., 2010, Sangshetti et al., 2011). These amines are susceptible to metabolism giving rise to diverse end products. Noteworthy, one of the most common metabolic pathways of alicyclic amines is α-carbonyl formation leading to lactam structures (Gorrod and Aislaitner, 1994) which are well known pharmacophores in antibacterial agents (Fig. 3).
Figure 3.
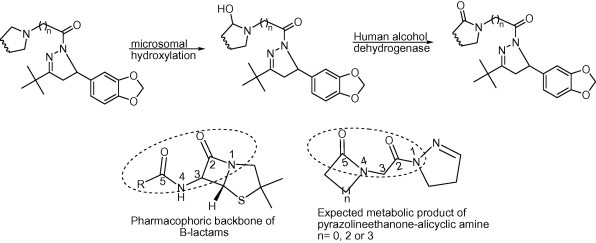
Pharmacophoric similarity between β-lactams and the expected metabolic products.
Thus it was of interest to implement the symbiotic approach in drug design (Baldwin et al., 1979, Christiaans and Timmerman, 1996) to synthesize novel candidates by joining the benzodioxolpyrazoline moiety with different alicyclic amines via methanone or ethanone linker in order to screen their antibacterial potentials.
2. Materials and methods
2.1. Chemistry
All melting points were determined using an Electrothermal Capillary melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded as thin film (for oils) in NaCl discs or as KBr pellets (for solids) with a JASCO FT/IR-6100 Spectrometer (Japan) and values are represented in cm−1. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Jeol ECA 500 MHz spectrometer (Japan) using TMS as internal standard and chemical shift values were recorded in ppm on the δ scale. Silica gel TLC (thin layer chromatography) cards from Merck (silica gel precoated aluminium cards with fluorescent indicator at 254 nm) were used for thin layer chromatography. Visualization was performed by illumination with UV light source (254 nm). Column chromatography was carried out on silica gel 60 (0.063–0.200 mm) obtained from Merck. The mobile phase consisted of chloroform or chloroform/ethyl acetate 1/1 v/v. Tetracycline, gentamicin and ofloxacin standard antibiotic discs were purchased from Bioanalyse®, Turkey.
2.1.1. Synthesis of 1,3-benzodioxole-5-carbaldehyde (1, Piperonal) (Aboul-Enein et al., 2012)
The Aboul-Enein method has been followed to afford light brown solid mp 37 °C.
2.1.2. Synthesis of (1E)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-one (2) (Aboul-Enein et al., 2012, Vallet, 1975)
The Aboul-Enein method has been followed to afford 13 g (78%) of 2 as pale yellow crystals mp 92 °C.
2.1.3. Synthesis of 5-(1,3-benzodioxol-5-yl)-3-tert-butyl-4,5-dihydro-1H-pyrazole (3) (Aboul-Enein et al., 2012)
Hydrazine hydrate (0.62 mL, 0.64 g, 0.012 mol) was added to a solution of 2 (1.0 g, 0.0043 mol) in absolute ethanol (30 mL). The reaction mixture was stirred under reflux for 2 h, and evaporated under vacuum to afford 1.0 g (90%) of 3 as yellowish oil.
1H-NMR (CDCl3): 1.0 (s, 9H, t-butyl), 2.3 (dd, J = 10.7, 16.1 Hz, 1H, CHCH2), 2.9 (dd, J = 9.95, 16.1 Hz, 1H, CHCH2), 4.5 (t, J = 9.9, 10.7 Hz, 1H, CHCH2), 5.9 (s, 2H, O–CH2–O), 6.7 (s, 1H, NH), 6.75 (dd, J = 1.5, 7.6 Hz, 1H, H-6), 6.7 (d, J = 8.4 Hz, 1H, H-7), 6.8 (s, 1H, H-4) ppm. 13C NMR (CDCl3) of 3 (base): 27.9 (C(CH3)3), 35.5 (C(CH3)3), 40.0 (CHCH2), 60.0 (CHCH2), 101 (OCH2O), 107, 108, 120 (CHar), 137 (Car), 146, 147 (Car), 160 (C N) ppm.
2.1.4. General procedures for synthesis of (5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(heteroalicyclic)methanones (4a-e)
To a stirred solution of 3 (0.5 gm, 2 mmol) in 20 ml CHCl3, phosgene (12.5% W/V solution in toluene, 1.6 ml, 2 mmol) was added. The mixture was stirred at room temperature for 10 min followed by the addition of the appropriate amine (6 mmol, 3 mol equivalents). The reaction mixture was kept under stirring at room temperature overnight then washed with NaHCO3 (10%, 20 ml), water and the organic layer was separated, dried (Na2SO4), and evaporated under vacuum to afford the corresponding products 4a–e. Solid products were further purified by recrystallization from ethanol while oils by column chromatography.
2.1.4.1. (5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(morpholino) methanone (4a)
Brown solid, mp 138–140 °C; yield 77%. IR (KBr, cm−1): 1658 (C O), 1493 (C N). 1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 2.55 (dd, J = 9.2, 17.6 Hz, 1H, CHCH2), 3.15 (dd, J = 6.1, 17.55 Hz, 1H, CHCH2), 3.65 (m, 8H, H for morpholine), 5.2 (t, J = 11.45, 2.4 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.67–6.7 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 34 (C(CH3)3), 41 (CHCH2), 46 (N-(CH2)2 for morpholine), 62 (CHCH2), 67 (O-(CH2)2 for morpholine), 101 (O–CH2–O), 106, 108, 119, 136, 146, 148 (CHar, Car), 157 (C O), 162 (C N) ppm. MS: for C19H25N3O4, calcd. 359.42 (M++1), found 360.24.
2.1.4.2. (5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(piperidin-1-yl)methanone (4b)
Yellow oil, yield 80%, IR (thin film, cm−1): 1647 (C O), 1443 (C N). 1H NMR (CDCl3): δ 1.0 (s, 9H, t-butyl), 1.5 (m, 6H, H for piperidine), 2.52 (dd, J = 9.95, 17.55 Hz, 1H, CHCH2), 3.13 (dd, J = 10.7, 16.8 Hz, 1H, CHCH2), 3.45 (m, 4H, N-(CH2)2 for piperidine), 5.2 (t, J = 9.95, 10.7 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.67–6.7 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 24.8, 26, 28 (C(CH3)3), 33 (C(CH3)3), 41 (CHCH2), 47 (N-(CH2)2 for piperidine), 62 (CHCH2), 101 (O–CH2–O), 106, 108, 119, 137, 146, 148 (CHar, Car), 157 (C O), 161 (C N) ppm. MS: for C20H27N3O3, calcd. 357.45 (M++1), found 358.20.
2.1.4.3. 5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(4-hydroxy piperidin-1-yl)methanone (4c)
Pale yellow solid, mp 156–158 °C; yield 95%. IR (KBr, cm−1): 3399 (OH), 1637 (C O), 1447 (C N). 1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 1.5 (m, 2H, H for piperidine), 1.8 (m, 2H, H for piperidine), 2.55 (dd, J = 9.2, 17.6 Hz, 1H, CHCH2), 2.9 (s, 1H, OH), 3.0 (m, 1H, H for piperidine), 3.15 (m, 1H, CHCH2), 3.6 (m, 1H, N-(CH2)2 for piperidine), 3.75 (m, 1H, N-(CH2)2 for piperidine), 3.95 (m, 1H, N-(CH2)2 for piperidine), 4.0 (m, 1H, N-(CH2)2 for piperidine), 5.2 (t, J = 9.2, 1.5 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.67–6.72 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 33, 34, 41 (C(CH3)3), 47 (N-(CH2)2 for piperidine), 62, 67 (CHOH), 101 (O–CH2–O), 106, 108, 119, 136, 146, 148 (CHar, Car), 157 (C O), 162 (C N) ppm. MS: for C20H27N3O4, calcd. 373.45 (M++1), found 374.26.
2.1.4.4. (5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(4-ethyl piperazin-1-yl)methanone (4d)
Yellow oil, yield 90%; IR (thin film, cm−1): 1648 (C O), 1455 (C N). 1H NMR (CDCl3): δ 1.0 (t, J = 6.9, 6.85 Hz, 3H, CH3CH2) 1.1 (s, 9H, t-butyl), 2.32–2.45 (m, 6H, CH3CH2 & H for piperazine), 2.55 (dd, J = 5.4, 17.6 Hz, 1H, CHCH2), 3.15 (dd, J = 5.35, 16.8 Hz, 1H, CHCH2), 3.7 (t, J = 6.9, 5.53 Hz, 4H, H for piperazine), 5.2 (t, J = 11.45, 9.2 Hz, 1H, CHCH2), 5.9 (s, 2H, O–CH2–O), 6.7–6.8 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 12 (CH3), 28 (C(CH3)3), 34 (C(CH3)3), 41 (CHCH2), 45 (CH2), 52 (C for piperazine), 53 (C for piperazine), 62 (CHCH2), 101 (O–CH2–O), 106, 108, 120, 137, 147, 148 (CHar, Car), 157 (C O), 162 (C N) ppm. MS: for C21H30N4O3, calcd. 386.49 (M++1), found 387.24.
2.1.4.5. (5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(pyrrolidin-1-yl)methanone (4e)
Yellowish white solid, mp 160–162 °C; yield 95%. IR (KBr, cm−1): 1641 (amide, C O), 1503 (C N). 1H NMR (CDCl3): δ 1.0 (s, 9H, t-butyl), 1.7 (m, 4H, H for pyrrolidine), 2.4 (m, 1H, CHCH2), 3.1 (m, 1H, CHCH2), 3.4 (m, 4H, H for pyrrolidine), 5.1 (m, 1H, CHCH2), 5.7 (s, 2H, O–CH2–O), 6.6 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 24, 28 (C(CH3)3), 33 (CHCH2), 40 (C(CH3)3), 48 (N-(CH2)2) for pyrrolidine, 61 (CHCH2), 101 (O–CH2–O), 106, 108, 119, 137, 146, 147 (CHar, Car), 156 (C O, amide), 161 (C N) ppm. MS: for C19H25N3O3, calcd. 343.42 (M++1), found 344.29.
2.1.5. Synthesis of 1-(5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-chloroethanone (5)
To a stirred solution of 3 (0.5 g, 2 mmol) in 20 ml CHCl3, chloroacetyl chloride (0.16 ml, 0.23gm, 2 mmol) was added. The mixture was stirred at room temperature for 30 min then the reaction mixture was washed with NaHCO3 (10%, 20 ml) and the organic layer was separated, dried (Na2SO4), and evaporated under vacuum to afford crude 5. Crystallization from ethanol afforded 0.6 g (100%) of yellowish white solid mp 172 °C.
1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 2.7 (dd, J = 4, 17.75 Hz, 1H, CHCH2), 3.3 (dd, J = 11.45, 18.35 Hz, 1H, CHCH2),4.4 (s, 2H, CH2Cl), 5.3 (dd, J = 4.6, 16.05 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.5–6.8 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 34 (CHCH2), 41 (C(CH3)3), 42 (CH2Cl), 60 (CHCH2), 101 (O–CH2–O), 105, 108, 119, 135, 147, 148 (CHar, Car), 163 (C O, amide), 167 (C N) ppm.
2.1.6. General procedures for synthesis of (5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(heteroalicyclic)ethanones (6a–c and 6g)
To a stirred solution of 5 (0.5 gm, 1.5 mmol) in 30 ml ethanol, the appropriate alicyclic amine (4.5 mmol) was added and the mixture was stirred under reflux overnight. The solvent was evaporated under reduced pressure and the residue was dissolved in ethyl acetate, washed with NaHCO3 (10%, 20 ml) then water, dried (Na2SO4), and evaporated under vacuum to afford the corresponding products 6a–g. Solid products were further purified by recrystallization from ethanol while oils by column chromatography (silica gel 60 (0.063–0.200 mm) mobile phase: chloroform 100% or chloroform/ethyl acetate 1/1 v/v).
2.1.6.1. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-morpholinoethanone (6a)
Yellowish white solid, mp 136 °C; yield 87%; IR (KBr, cm−1): 1658 (C O), 1492 (C N). 1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 2.5 (m, 4H, N-(CH2)2 for morpholine), 2.7 (m, 1H, CHCH2), 3.27 (m, 1H, CHCH2), 3.5 (m, 2H, COCH2N), 3.7 (m, 4H, O-(CH2)2 for morpholine), 5.2 (dd, J = 3.85, 11.5 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.5–6.7 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 34 (CHCH2), 41 (C(CH3)3), 43 (COCH2N), 54 (N-(CH2)2 for morpholine), 59 (CHCH2), 66 (O-(CH2)2 for morpholine), 101 (O–CH2–O), 105, 108, 119, 136, 146, 148 (CHar, Car), 166 (C O), 167 (C N) ppm. MS: for C20H27N3O4, calcd. 373.2 (M+1), found 374.3.
2.1.6.2. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(piperidin-1-yl)ethanone (6b)
Yellowish white solid, mp 108–110 °C; yield 79%; IR (KBr, cm−1): 1668 (C O), 1502 (C N). 1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 1.3–1.5 (m, 6H, H for piperidine), 2.5 (m, 4H, N-(CH2)2 for piperidine), 2.66 (m, 1H, CHCH2), 3.25 (m, 1H, CHCH2), 3.5 (m, 2H, COCH2N), 5.2 (m, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.4–6.7 (m, 3H, H aromatic) ppm.13C NMR (CDCl3): δ 24, 26, 28 (C(CH3)3), 33 (CHCH2), 41 (C(CH3)3), 54 (N-(CH2)2 for piperidine), 59 (COCH2N), 60 (CHCH2), 101 (O–CH2–O), 105, 108, 118, 136, 146, 147 (CHar, Car), 165 (C O), 167 (C N) ppm. MS: for C20H28N4O3, calcd. 372.46 (M++1), found 373.24.
2.1.6.3. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(4-hydroxypiperidin-1-yl)ethanone (6c)
Yellowish liquid; yield 80%; IR (thin film, cm−1): 3406 (OH), 1651 (C O), 1453 (C N). 1H NMR (CDCl3): δ 1.0 (s, 9H, t-butyl), 1.45 (d, J = 9.2 Hz, 2H, H for piperidine), 1.6 (s, 2H, H for piperidine), 2.1 (d, J = 9.9 Hz, 2H, N-(CH2)2 for piperidine), 2.55 (d, J = 18.35 Hz, 1H, CHCH2), 2.7 (d, J = 12.25 Hz, 2H, N-(CH2)2 for piperidine), 3.1 (m, 1H, CHCH2), 3.4–3.6 (m, 4H,CHOH, COCH2N), 5.1 (d, J = 11.45 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.4–6.6 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 34 ((CH2)2CHOH for piperidine), 34.1 (CHCH2), 41 (C(CH3)3), 51 (N-(CH2)2 for piperidine), 58 (COCH2N),59 (CHCH2), 67 (CHOH), 101 (O–CH2–O), 105, 108, 118, 136, 146, 147 (CHar, Car), 166 (C O), 167 (C N) ppm. MS: for C21H29N3O4, calcd. 387.22, (M+1), found 388.32.
2.1.6.4. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(piperazin-1-yl)ethanone (6g)
Yellowish oil; yield 87%; IR (thin film, cm−1): 3310 (NH), 1661 (C O), 1484 (C N). 1H NMR (CDCl3): δ 1.0 (s, 9H, t-butyl), 2.3–2.9 (m, 10H, 1H for CHCH2 and 8H for piperazine, NH), 3.1 (m, 1H, CHCH2), 3.4 (m, 2H, COCH2N), 5.2 (m, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.4–6.6 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 28 (C(CH3)3), 34 (CHCH2), 41 (C(CH3)3), 45 (C for piperazine), 54 (C for piperazine), 59.5 (COCH2N), 59.6 (CHCH2), 101 (O–CH2–O), 105, 108, 118, 136, 146, 148 (CHar, Car), 165 (C O), 167 (C N) ppm. MS: for C20H28N4O3, calcd. 372.22 (M+1), found 373.25.
2.1.7. General procedures for synthesis of (5-(benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)(heteroalicyclic)ethanones (6d–f)
To a stirred solution of 5 (0.5 g, 1.5 mmol) in 30 ml ethanol, the appropriate alicyclic amine (4.5 mmol) was added followed by NaOH (0.06 g, 1.5 mmol). The mixture was stirred under reflux overnight then the solvent was evaporated under reduced pressure and the residue was dissolved in ethyl acetate, washed with NaHCO3 (10%, 20 ml) then water, dried (Na2SO4), and evaporated under vacuum to afford the corresponding products 6d–f which were further purified by recrystallization from ethanol.
2.1.7.1. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(4-ethyl piperazin-1-yl)ethanone (6d)
Yellow solid, mp 118–120 °C; yield 65%, IR (KBr, cm−1): 1675 (C O), 1485 (C N). 1H NMR (CDCl3): δ 1.0 (m, 12H, CH3CH2, t-butyl), 2.3–3.6 (m, 11H, CHCH2, CH3CH2 & 8H for piperazine), 4.3 (m, 2H, COCH2N), 5.3 (dd, J = 3.8, 11.45 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.5–6.7 (m, 3H, H aromatic) ppm.13C NMR (CDCl3): δ 15 (CH3), 28 (C(CH3)3), 34 (CHCH2), 41 (C(CH3)3), 52 (CH2), 53 (C for piperazine), 59 (C for piperazine), 67 (COCH2N), 68 (CHCH2), 101 (O–CH2–O), 105, 108, 119, 135, 147, 148 (CHar, Car), 166 (C O), 167 (C N) ppm. MS: for C22H32N4O3, calcd. 400.25 (M+), found 400.25.
2.1.7.2. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(pyrrolidin-1-yl)ethanone (6e)
Yellowish white solid, mp 134–136 °C; yield 81%. IR (KBr, cm−1): 1670 (amide, C O), 1485 (C N). 1H NMR (CDCl3): δ 1.0 (s, 9H, t-butyl), 1.7 (m, 4H, H for pyrrolidine), 2.65 (m, 5H, CHCH2, (N-(CH2)2) for pyrrolidine), 3.2 (dd, J = 11.45, 17.55 Hz, 1H, CHCH2), 3.6 (dd, J = 16.8, 16.8 Hz, 2H, COCH2N), 5.3 (dd, J = 3.85, 11.5 Hz, 1H, CHCH2), 5.9 (s, 2H, O–CH2–O), 6.5–6.7 (m, 3H, H aromatic) ppm.13C NMR (CDCl3): δ 23, 28 (C(CH3)3), 34 (CHCH2), 41 (C(CH3)3), 54 (N-(CH2)2) for pyrrolidine), 57 (COCH2N), 59 (CHCH2), 101 (O–CH2–O), 105, 108, 119, 136, 146, 148 (CHar, Car), 165 (C O), 168 (C N) ppm. MS: for C20H27N3O3, calcd. 357.21 (M+), found 357.24.
2.1.7.3. 1-(5-(Benzo[d][1,3]dioxol-5-yl)-3-tert-butyl-4,5-dihydropyrazol-1-yl)-2-(pyrrolidin-2-one-1-yl)ethanone (6f)
Yellowish white solid, mp 114 °C; yield 65%; IR (KBr, cm−1): 1681 (ketone C O), 1617 (amide, C O), 1501 (C N). 1H NMR (CDCl3): δ 1.1 (s, 9H, t-butyl), 2.1–2.3 (m, 2H, CHCH2), 2.7 (m, 2H, H for pyrrolidin-2-one), 3.25–3.37 (m, 2H, H for pyrrolidin-2-one), 3.5 (m, 2H, H for pyrrolidin-2-one), 4.4 (dd, J = 16.05, 16.05 Hz, 2H, COCH2N), 5.3 (dd, J = 3.8, 11.45 Hz, 1H, CHCH2), 5.8 (s, 2H, O–CH2–O), 6.5–6.7 (m, 3H, H aromatic) ppm. 13C NMR (CDCl3): δ 15, 28 (C(CH3)3), 30 (CH2CO for pyrrolidin-2-one), 34 (CHCH2), 41 (C(CH3)3), 59 (CH2N for pyrrolidin-2-one), 67 (COCH2N), 68 (CHCH2), 101 (O–CH2–O), 105, 108, 119, 135, 147, 148 (CHar, Car), 166 (C O, amide), 167 (C N), 179 (C O, ketone) ppm. MS: for C20H25N3O4, calcd. 371.18 (M+), found 371.25.
2.2. Antibacterial assay
Antibacterial activities of compounds 4a–e and 6a–g were tested using the agar well diffusion method. The concentration of the microbial suspensions was adjusted to 0.5 McFarland standards. The bacterial suspensions were seeded on nutrient agar plates. In each of these plates many wells were cut using a sterilize cork borer. Using a micropipette, 200 μL of sample was added into different wells. A positive control antibiotic disc was placed in the plate. Bacterial plates were incubated for 24 h at 37 °C. Antimicrobial activity was evaluated by measuring the zone of inhibition.
3. Results and discussion
3.1. Chemistry
The synthesis of the target compounds 4a–e and 6a–g, and their intermediates 1–3 and 5 is depicted in Scheme 1, Scheme 2. Piperonal (1) (Aboul-Enein et al., 2012, Nakatani et al., 1977) was obtained by reacting 3,4-dihydroxybenzaldehyde with CH2Cl2 in the presence of K2CO3 under reflux conditions. Subsequent Claisen–Schmidt condensation with pinacolone afforded α,β-unsaturated ketone 2 in good yield. Chalcone 2 was reacted with hydrazine hydrate in ethanol under reflux conditions to yield pyrazoline derivative 3 (Aboul-Enein et al., 2012) (Scheme 1).
Scheme 1.
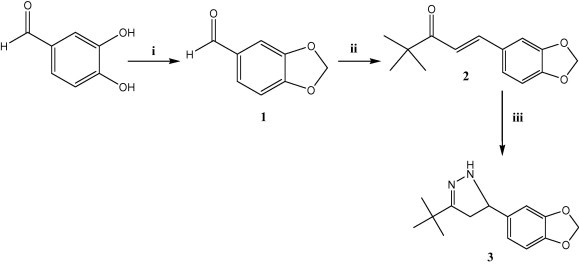
Reagents and conditions: (i) CH2Cl2, K2CO3, DMF, reflux, 4 h; (ii) pinacolone 50% KOH, CH3OH, 70 °C, 5 h; (iii) H2N–NH2·H2O, ethanol, reflux, 2 h;
Scheme 2.
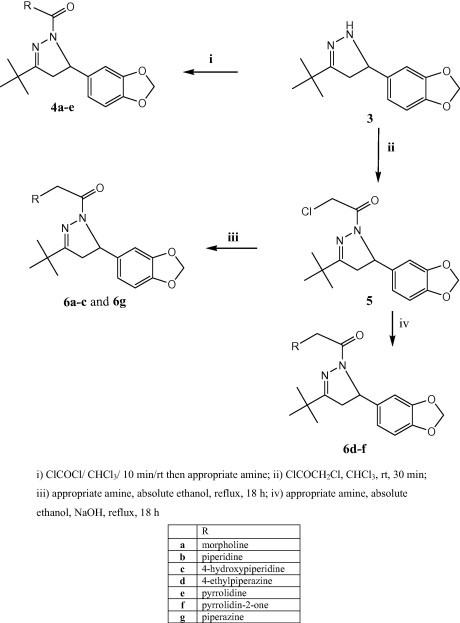
Key pyrazoline 3 was further reacted with phosgene at room temperature to afford the carbamoyl chloride intermediate which was – in the same pot – reacted with the appropriate amine to afford pyrazolinemethanones 4a–e (Scheme 2). On the contrary, the intermediate pyrazoline chloroethanone 5 was stable enough to be isolated after the reaction of pyrazoline 3 with chloroacetyl chloride in CHCl3 at room temperature. Further reaction of 5 with different alicyclic amines in ethanol under reflux gave the target compounds 6a–c and 6g. For compounds 6d–f the reaction was performed in the presence of 1 mol equivalent of NaOH (Scheme 2).
The spectral data of the synthesized compounds were investigated. The 1H NMR spectra of α,β-unsaturated ketone 2 showed the olefinic protons as doublet of doublet at δ 6.1 and doublet at δ 6.5 with coupling constant J = 15.3 which indicates trans geometric isomerism and it was in accordance with the assigned structure and the reported data (Aboul-Enein et al., 2012). For pyrazoline 3, 1H NMR spectra showed two doublet of doublet signals at 2.3 and 2.9 ppm corresponding to the prochiral CH2 protons while CH proton responded as triplet signal at 4.5 ppm. One singlet signal at 6.7 ppm was found corresponding to the NH proton at position 1 of the pyrazoline ring. In addition, singlet signals were found at 1.0 and 5.9 ppm for t-butyl and the ethereal methylene bridge protons, respectively. The aromatic protons were elucidated at 6.4–6.8 ppm. 1H NMR spectra of pyrazolinechloroethanone 5 revealed the characteristic signals of 3 in addition to the singlet signal corresponding to the methylene protons at δ 4.4. However in the final products 6f and 6e, methylene protons were magnetically un-equivalent and displayed as doublet of doublet signals at δ 4.4 for 6f and δ 3.6 for 6e with identical coupling constant. For 13C NMR, the urea carbonyl of 4a–e was elucidated at 156–157 ppm while the amidic carbonyl of 6a–g was elucidated at 165–167 ppm.
3.2. Antibacterial activity
Antibacterial activities of compounds 4a–e and 6a–g were tested using the agar well diffusion method (Tagg et al., 1976). Tested microorganism strains are: Bacillus subtilis (ATCC 8037), Staphylococcus aureus (ATCC 29213), Klebsiella pneumonia (ATCC 13883), Pseudomonas aeruginosa (ATCC 27953), Enterobacter cloacae (ATCC), Enterococcus faecalis (ATCC 29212), and Sarcina sp. (NRC isolate). Tetracycline (0.067 μM) (Aghatabay et al., 2009), gentamicin (0.021 μM) (Al-Abbas, 2012), and ofloxacin (0.013 μM) (Aghatabay et al., 2009) were used as positive control and DMSO as negative control. The observed data on the antibacterial activity of the compounds and control drugs are given in Table 1, Table 2.
Table 1.
Antibacterial activity of compounds 4a–e and 6a–g.
| Microorganism | Zone of inhibition (mm) |
||||||
|---|---|---|---|---|---|---|---|
| Gram +ve |
Gram −ve |
||||||
| Bacillus subtilis | Staphylococcus aureus | Enterococcus faecalis | Sarcina | Pseudomonas aeruginosa | Klebsiella pneumonia | Enterobacter cloacae | |
| Test compounds (μM) | |||||||
| 4a 2.7 | 24 | 25 | 18 | 14 | 20 | 18 | 20 |
| 4b 2.8 | 25 | 17 | 20 | 16 | 19 | 21 | 20 |
| 4c 2.6 | 16 | 19 | 17 | +/− | 17 | 14 | 13 |
| 4d 2.5 | 15 | 24 | 17 | 15 | 12 | 16 | 13 |
| 4e 2.9 | 29 | 25 | 28 | 20 | 15 | 25 | 28 |
| 6a 2.6 | 15 | 19 | 14 | 16 | 18 | 20 | 15 |
| 6b 2.6 | 15 | 23 | 21 | 23 | 14 | +/− | 20 |
| 6c 2.5 | 17 | 12 | 11 | 17 | 20 | +/− | 17 |
| 6d 2.5 | 18 | 18 | 17 | 14 | 15 | 18 | 21 |
| 6e 2.8 | 15 | 19 | 19 | 22 | 20 | 15 | 19 |
| 6f 2.6 | 20 | 17 | 19 | 15 | 17 | 12 | 21 |
| 6g 2.6 | 16 | 19 | 17 | 17 | 17 | 15 | 20 |
| Gentamicin 0.021 | 20 | 25 | 20 | 22 | 11 | 18 | 28 |
| Tetracycline 0.067 | 30 | 25 | 25 | 28 | 9 | 35 | 18 |
| Ofloxacin 0.013 | 16 | 30 | 16 | 19 | 10 | 25 | 30 |
| DMSO | – | – | – | – | – | – | – |
(+/−) = unclear zone of inhibition.
Table 2.
MICs⁎ for the most potent compounds (4a, 4b, 4e, 6c and 6e) against certain pathogenic bacteria.
| Compound | Staphylococcus aureus (μM) | Klebsiella pneumonia (μM) | Pseudomonas aeruginosa (μM) | Sarcina (μM) |
|---|---|---|---|---|
| 4a | 0.7 | 0.55 | 0.9 | 0.11 |
| 4b | 0.14 | 0.35 | 0.35 | 0.14 |
| 4e | 0.11 | 0.48 | 0.96 | 0.09 |
| 6c | 0.12 | 0.51 | 0.64 | 0.08 |
| 6e | 0.18 | 0.46 | 0.92 | 0.11 |
The minimum concentration of a compound that inhibits the growth of tested microorganisms.
Broad spectrum activities of the newly synthesized candidates (4a–e and 6a–g) have been revealed being active against all tested human bacteria (B. subtilis, S. aureus, K. pneumonia, P. aeruginosa, E. cloacae, E. faecalis, and Sarcina sp.) in micromolar concentrations. In the light of zone of inhibition (Table 1) particular candidates have been selected for the estimation of MICs against pathogenic bacteria. In general, MICs were in the micromolar range while the most active candidates showed MICs in nanomolar range (80 and 90 nM by compounds 6c and 4e against Sarcina, respectively) which reflect the ingenuity of the novel candidates (Table 2).
It was found that Sarcina sp. is the most susceptible bacteria to our candidates which showed MIC range 0.08–0.14 μM. The ethanone derivative 6c with R as the hydroxypiperidine is the most potent inhibitor for Sarcina at 0.08 μM followed by 4e (pyrrolidinomethanone) at 0.09 μM and 4a (morpholinomethanone), 6e (pyrrolidinoethanone) at 0.11 μM. As well, very good activity against S. aureus has been discovered. The maximum activity against S. aureus was displayed by 4e (pyrrolidinomethanone) at 0.11 μM and 6c (hydroxypiperidinoethanone) at 0.12 μM. For both K. pneumonia and P. aeruginosa, compound 4b (piperidinomethanone) was the most active candidate with MIC at 0.35 μM.
3.3. Conclusion
Broad spectrum antibacterial derivatives of 5-(1,3-benzodioxol)-4,5-dihydropyrazol-1-yl methanones 4a–e and ethanones 6a–g have been revealed. The novel candidates have shown activity against 7 Gram +ve and Gram −ve bacteria. Particular potency has been discovered against Sarcina sp. and S. aureus by compounds 4e and 6c at nanomolar concentrations.
Conflict of Interest
None.
Acknowledgements
The authors would like to thank the National Research Centre, Dokki, Cairo, Egypt for the support and financing the research through project No. 10010302.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammed F. El-Behairy, Email: mohabeha@gmail.com.
Tarek E. Mazeed, Email: tarek_mazeed@yahoo.co.uk.
Aida A. El-Azzouny, Email: elazzounyaida@yahoo.com.
Mohamed N. Aboul-Enein, Email: mnaboulenein@yahoo.com.
References
- Aboul-Enein M.N., El-Azzouny A.A., Attia M.I., Maklad Y.A., Amin K.M., Abdel-Rehim M., El-Behairy M.F. Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur. J. Med. Chem. 2012;47:360–369. doi: 10.1016/j.ejmech.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Aghatabay N.M., Mahmiani Y., Cevik H., Dulger B. Synthesis, Raman, FT-IR, NMR spectroscopic data and antimicrobial activity of mixed aza-oxo-thia macrocyclic compounds. Eur. J. Med. Chem. 2009;44:365–372. doi: 10.1016/j.ejmech.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Akbas E., Berber I. Antibacterial and antifungal activities of new pyrazolo[3,4-d]pyridazin derivatives. Eur. J. Med. Chem. 2005;40:401–405. doi: 10.1016/j.ejmech.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Al-Abbas M.J.A. Antimicrobial susceptibility of Enterococcus faecalis and a novel Planomicrobium isolate of bacteremia. Int. J. Med. Med. Sci. 2012;4:19–27. [Google Scholar]
- Baldwin J.J., Lumma W.C., Lundell G.F., Ponticello G.S., Raab A.W., Engelhardt E.L., Hirschmann R., Sweet C.S., Scriabine A. Symbiotic approach to drug design: antihypertensive beta-adrenergic blocking agents. J. Med. Chem. 1979;22:1284–1290. doi: 10.1021/jm00197a002. [DOI] [PubMed] [Google Scholar]
- Carradori S., Secci D., Bolasco A., De Monte C., Yáñez M. Synthesis and selective inhibitory activity against human COX-1 of novel 1-(4-substituted-thiazol-2-yl)-3,5-di(hetero)aryl-pyrazoline derivatives. Arch Pharm (Weinheim) 2012;345:973–979. doi: 10.1002/ardp.201200249. [DOI] [PubMed] [Google Scholar]
- Chakrabarthi K.J.E., Richard J.G., Peter T., Janette H., Terrence H.A. 5-Acyl-3-substituted benzofuran-2(3H)-ones as potential antiinflammatory agents. J. Med. Chem. 1987;30:1663–1668. doi: 10.1021/jm00392a024. [DOI] [PubMed] [Google Scholar]
- Chimenti F., Bizzarri B., Bolasco A., Secci D., Chimenti P., Granese A., Carradori S., D’Ascenzio M., Lilli D., Rivanera D. Synthesis and biological evaluation of novel 2,4-disubstituted-1,3-thiazoles as anti-Candida spp. agents. Eur. J. Med. Chem. 2011;46:378–382. doi: 10.1016/j.ejmech.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Christiaans J.A.M., Timmerman H. Cardiovascular hybrid drug: combination of more than one pharmacological property in one single molecule. Eur. J. Pharm. Sci. 1996;4:1–22. [Google Scholar]
- Cottineau B., Toto P., Marot C., Pipaud A., Chenault J. Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorg. Med. Chem. Lett. 2002;12:2105–2108. doi: 10.1016/s0960-894x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- Emirdag-Ozturk S., Karayildirim T., Anil H. Synthesis of egonol derivatives and their antimicrobial activities. Bioorg. Med. Chem. 2011;19:1179–1188. doi: 10.1016/j.bmc.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Gorrod l.W., Aislaitner G. The metabolism of alicyclic amines to reactive iminium ion intermediates. Eur. J. Drug Metab. Pharmacokinet. 1994;19:209–217. doi: 10.1007/BF03188923. [DOI] [PubMed] [Google Scholar]
- Kathiravan M.K., Salake A.B., Chothe A.S., Dudhe P.B., Watode R.P., Mukta M.S., Gadhwe S. The biology and chemistry of antifungal agents. Bioorg. Med. Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Mamolo M.G., Zampieri D., Falagiani V., Vio L., Banfi E. Synthesis and antimycobacterial activity of 5-aryl-1-isonicotinoyl-3-(pyridin-2-yl)-4,5-dihydro-1H-pyrazole derivatives. Il Farmaco. 2001;56:593–599. [PubMed] [Google Scholar]
- Mamolo M.G., Zampieri D., Falagiani V., Vio L., Banfi E. Synthesis and antifungal activity of (9/)-1-(5-aryl-3-pyridin-2-yl-4,5-dihydro-pyrazol-1-yl)-2-imidazol-1-yl-ethanone derivatives. Il Farmaco. 2003;58:315–322. doi: 10.1016/S0014-827X(02)00006-X. [DOI] [PubMed] [Google Scholar]
- Nakatani, K., Inoue, T., Nishizawa, T., Numata, S., Ishii, T., 1977. Process for preparing piperonal. US 4157333.
- Ozdemir A., Turan-Zitouni G., Kaplancıklı Z.A., Revial G., Gu¨ven K. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives. Eur. J. Med. Chem. 2007;42:403–409. doi: 10.1016/j.ejmech.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Qing Z., Kui C., Xiao Z., Kai L., Qing J., Hai Z. Synthesis of some N-alkyl substituted urea derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010;45:3207–3212. doi: 10.1016/j.ejmech.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Rostom S.A.F., Shalaby M.A., El-Demellawy M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003;38:959–974. doi: 10.1016/j.ejmech.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Sangshetti J.N., Chabukswar A.R., Shinde D.B. Microwave assisted one pot synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazoles as antifungal agents. Bioorg. Med. Chem. Lett. 2011;21:444–448. doi: 10.1016/j.bmcl.2010.10.120. [DOI] [PubMed] [Google Scholar]
- Secci D., Bizzarri B., Bolasco A., Carradori S., D’Ascenzio M., Rivanera D., Mari E., Polletta L., Zicari A. Synthesis, anti-Candida activity, and cytotoxicity of new (4-(4-iodophenyl)thiazol-2-yl)hydrazine derivatives. Eur. J. Med. Chem. 2012;53:246–253. doi: 10.1016/j.ejmech.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Tagg T.R., Dajani A.S., Wannamaker L.W. Bacteriocin of Gram positive bacteria. Bacteriol. Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet, F.M.J., 1975. 1-[3,4(methylenedioxy)-phenyl]-4,4-dimethyl-pent-1-en-3-ol. US 3,910,959 A.
- Wani M.Y., Athar F., Salauddin A., Agarwal S.M., Azam A., Choi H., Bhat A. Novel terpene based 1,4,2-dioxazoles: synthesis, characterization, molecular properties and screening against Entamoeba histolytica. Eur. J. Med. Chem. 2011;46:4742–4752. doi: 10.1016/j.ejmech.2011.06.005. [DOI] [PubMed] [Google Scholar]


