Abstract
Secondary hyperparathyroidism is characterised by excessive secretion of parathyroid hormone and parathyroid hyperplasia, resulting in both skeletal and extraskeletal consequences. Recent basic and clinical studies have brought considerable advances in our understanding of the pathophysiology of parathyroid hyperplasia and have also provided practical therapeutic approaches, especially with regard to indications for parathyroid intervention. In this context, it is quite important to recognize the development of nodular hyperplasia, because the cells in nodular hyperplasia are usually resistant to calcitriol treatment. Patients with nodular hyperplasia should undergo parathyroid intervention including percutaneous ethanol injection therapy (PEIT). Selective PEIT of the parathyroid gland is an effective approach in which the enlarged parathyroid gland with nodular hyperplasia is ‘selectively’ destroyed by ethanol injection, and other glands with diffuse hyperplasia are then managed by medical therapy. With a more focused attention to applying parathyroid intervention, we can expect significant improvement in the management of secondary hyperparathyroidism in dialysis patients.
Keywords: chronic kidney disease, fibroblast growth factor 23, parathyroid hyperplasia, parathyroid intervention, secondary hyperparathyroidism
Introduction
Secondary hyperparathyroidism develops universally in patients with chronic kidney disease (CKD), especially those on long-term dialysis therapy [1,2]. It is characterised by excessive secretion of parathyroid hormone (PTH) and parathyroid hyperplasia, resulting in bone disorder, soft tissue calcification and significantly increased risk of morbidity and mortality [3,4]. Despite recent progress in therapeutic modalities, severe hyperparathyroidism with marked hyperplasia usually becomes refractory to medical treatment [5]. Recent insights into the pathophysiological mechanisms underlying the development of parathyroid hyperplasia provide a rationale for selecting therapeutic strategies, including parathyroid intervention [5–7]. This article surveys the pathophysiological aspects of parathyroid hyperplasia in CKD and describes the potential of parathyroid intervention to attenuate this disorder, based on emerging data from preclinical and clinical studies.
Secondary hyperparathyroidism in CKD
Ever since the observations of Albright et al. in 1937 [8], it has been known that chronic renal insufficiency is frequently accompanied by enlargement of the parathyroid glands and osteitis fibrosa cystica. Following the development of the first-generation PTH assay [9], elevated levels of PTH in patients with mild to moderate CKD were reported by Reiss et al. in 1969 [10]. Subsequent experimental studies have shown that a restriction of dietary phosphorus in proportion to the decrease in renal function can prevent the development of secondary hyperparathyroidism [11]. Consequently, Bricker proposed the ‘trade-off hypothesis’, which stated that phosphate retention, as a result of decreased renal function, would cause transient reduction of ionized calcium, which would in turn stimulate PTH secretion [12]. In other words, phosphorus retention and subsequent hypocalcaemia were considered to be major factors in the development of hyperparathyroidism. Several clinical studies produced substantial support for this proposal [13,14]; however, it has been shown that hypocalcaemia and hyperphosphataemia are not always present in patients with CKD, in whom serum PTH levels are already elevated [15,16]. Furthermore, experimental studies in which hypocalcaemia was prevented by feeding a high-calcium diet demonstrated a slight increase in PTH levels [17]. Thus, hypocalcaemia was not considered to be an essential factor for the development of hyperparathyroidism in CKD.
Decreased production of calcitriol also contributes to the development of secondary hyperparathyroidism [17]. Calcitriol has been shown to decrease PTH gene expression both in vivo and in vitro studies [18,19]. In the setting of CKD, decreases in nephron number lead to a decrease in the ability of the kidneys to produce calcitriol, thereby resulting in hyperparathyroidism. Several clinical studies demonstrated the mechanism and efficacy of calcitriol treatment [20,21], and calcitriol and its analogues are the mainstays for the prevention and treatment of secondary hyperparathyroidism in dialysis patients [22,23]. The production of calcitriol is also regulated by phosphorus retention [15,24], because this can inhibit 1-α-hydroxylase. Thus, restriction of phosphate load might play a role, at least in part, in mediating the effects of calcitriol on hyperparathyroidism.
Phosphorus has also been considered to mediate parathyroid function directly, because several studies showed that dietary phosphorus restriction suppressed PTH hypersecretion independent of calcium or calcitriol [25,26]. This possibility was confirmed by in vitro studies that demonstrated that changes in extracellular phosphorus concentrations resulted in an increased secretion of PTH in the absence of changes in ionized calcium [27,28]. However, the manner in which phosphorus affects parathyroid function has not been fully elucidated. It has been shown that a type III phosphate transporter exists in parathyroid glands [29], but it is not known whether this transporter mediates the effects of phosphorus on PTH secretion. Recent detailed studies have suggested that phosphorus regulates the stability of PTH mRNA, and this effect seems to be mediated by Au-rich RNA-binding factor 1, proteins that bind to the PTH mRNA 3′ untranslated region [30,31]. It has also been demonstrated that phosphorus affects the production of arachidonic acid, a potent inhibitor of PTH release, thereby contributing to PTH hypersecretion [32].
Recent studies have helped to clarify the potential relationship between phosphorus retention and calcitriol deficiency by uncovering the role of fibroblast growth factor 23 (FGF23). FGF23 is a newly discovered peptide hormone involved in the pathogenesis of several hypophosphataemic diseases, such as X-linked hypophosphataemia [33], autosomal-dominant hypophosphataemic rickets [34] and tumour-induced osteomalacia [35]. Factors stimulating FGF23 secretion are dietary phosphate load [36] and the administration of calcitriol [37–39]. It has been shown that FGF23 is expressed primarily in osteocytes [40] and osteoblasts [41]. In the normal kidney, FGF23 acts to excrete phosphorus in the urine by decreasing mRNA and protein levels of the type IIa sodium phosphate cotransporter. FGF23 also suppresses calcitriol production by decreasing mRNA for 25-hydroxyvitamin D-1-α-hydroxylase [42]. In patients with CKD, serum FGF23 levels progressively increase as kidney function declines, even before the development of hyperphosphataemia [43–45]. Given that FGF23 physiologically promotes phosphaturia and suppresses the synthesis of calcitriol in response to phosphorus retention [36,42], it has been proposed that increasing levels of FGF23 in the setting of CKD prevent hyperphosphataemia, at the expense of low calcitriol and secondary hyperparathyroidism [46].
Development of parathyroid hyperplasia in CKD
Parathyroid cells are generally quiescent and rarely divide under normal physiological conditions [47], but the rate of cell proliferation can increase in response to mitogenic stimuli such as hypocalcaemia, calcitriol deficiency and phosphorus retention, as seen in the setting of CKD [1,48]. Thus, as kidney disease progresses, persistent hyperparathyroidism leads to the development of parathyroid hyperplasia. A similar phenomenon may also occur in many other endocrine organs, in which overactive secretion is generally associated with hypertrophy and/or hyperplasia; however, parathyroid hyperplasia in CKD is unique, in that the size and the nature of the glands may vary markedly in the same patient [2,7]. It is well accepted that development of parathyroid hyperplasia is associated with down-regulation of the vitamin D receptor (VDR) [49,50] and the calcium-sensing receptor (CaSR) [51,52]. As kidney disease progresses, parathyroid VDR and CaSR levels decrease in parallel with the severity of parathyroid hyperplasia. Besides the down-regulation of VDR and CaSR, changes in expression of various molecules have been observed in parathyroid hyperplasia [1]. Recently, enhanced parathyroid expression of the potent growth promoter transforming growth factor alpha (TGF-α) and its receptor, the epidermal growth factor receptor (EGFR), has been identified as one of the main causes of parathyroid hyperplasia and the reduction of VDR in CKD [53–55].
In the initial stage of CKD, the parathyroid glands secrete and synthesize PTH in response to increased demand, and parathyroid cells subsequently begin to proliferate, leading to diffuse hyperplasia [1]. Some cells in the parathyroid with diffuse hyperplasia escape from cell cycle control mechanisms and proliferate vigorously, forming small nodules, each of which is monoclonal in origin [7,56]. Such nodules are composed of more tightly packed cells featuring larger nuclei and a greater prevalence of cell cycle markers, oxyphil cells and acinar cell arrangements compared with those seen in diffuse hyperplasia [1]. When these nodules grossly enlarge and become encapsulated, the glands are termed as nodular hyperplasia. In the most severe cases, one of these nodules occupies the entire gland (single nodule) [2,7].
FGF23 and advanced hyperparathyroidism
As mentioned above, FGF23 is a new player in the classic ‘trade-off’ theory that has been proposed to explain the pathogenesis of secondary hyperparathyroidism [46]. Along with the decline of kidney function in CKD patients, serum intact FGF23 levels increase progressively [43–45]. Once patients are placed on dialysis therapy, serum FGF23 levels increase markedly, showing a positive correlation with serum phosphate levels and intact PTH levels. We have recently shown that measurement of the initial serum FGF23 level is a good screening test for predicting patients in whom secondary hyperparathyroidism will develop within 2 years [57]. We also demonstrated that serum FGF23 levels could be used as an additional marker for the resistance to intravenous calcitriol therapy in patients with established secondary hyperparathyroidism [58]. In another clinical study, we showed that intravenous calcitriol therapy not only suppressed PTH levels but also further increased serum FGF23 levels [37]. In accordance with this observation, it has recently been reported that calcitriol administration increased FGF23 levels in vivo and in vitro [38,39]. After surgical parathyroidectomy, serum FGF23 levels decrease gradually, indicating a possible association between abnormal PTH secretion and FGF23 regulation [59]. With regard to the skeletal effect of FGF23, a recent clinical study revealed that serum FGF23 level is not associated with decreased bone mineral density, nor with several circulating biomarkers of bone remodelling [60], despite the presence of the FGF receptor (FGFR) 1 in osteoblast and osteoclast cells [61].
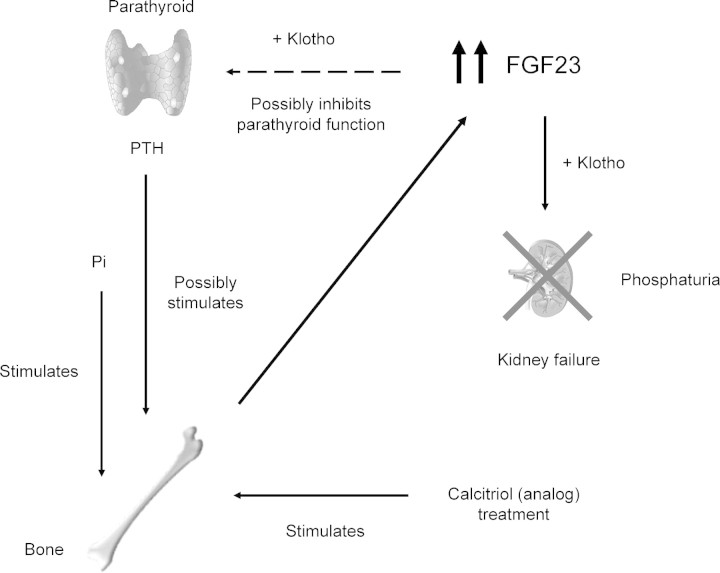
Accordingly, it has been recognized that FGF23 production in dialysis patients is continuously stimulated by phosphate load, calcitriol (analogue) treatment and possibly by high PTH [2,46]; however, it remains unclear whether FGF23 directly modulates PTH expression, or whether the correlation is secondary to abnormalities in phosphorus and calcitriol metabolism. In this regard, the FGF23-klotho axis in regulating mineral homeostasis is a very attractive issue. It has been reported that FGF23 acts on its target tissues by binding to and activating its cognate FGFRs in the presence of its obligatory coreceptor, klotho [62,63]. Klotho binds to FGF23 and converts the canonical FGFR 1c to a receptor specific for FGF23 [63]. Administration of FGF23 to rats increases early growth response 1 (Egr-1) mRNA levels in the kidney and also in the parathyroid and pituitary [63]. Moreover, using both rats and in vitro rat parathyroid cultures, it has recently been shown that FGF23 directly suppresses both PTH secretion and its gene expression [64]. These findings may imply that FGF23, at least in part, has an inhibitory role in secondary hyperparathyroidism. Furthermore, it is also possible that the beneficial effects of calcitriol (analogue) treatment in secondary hyperparathyroidism may partly be attributed to an increase in FGF23. The resistance of the parathyroid to elevated levels of FGF23 in uraemia remains to be studied. Current understanding of the role of very high levels of serum FGF23 in severe hyperparathyroidism is summarized in Figure 1.
Fig. 1.
The regulation and action of FGF23 without the functioning kidney. FGF23 production in the bone is continuously stimulated by phosphate load, calcitriol (analogue) treatment and possibly by high PTH. It remains to be elucidated whether very high FGF23 in dialysis patients would inhibit PTH expression by activating its cognate FGFRs in a Klotho-dependent fashion.
Refractory hyperparathyroidism with marked parathyroid hyperplasia
Development of hyperplasia not only leads to the increased volume of parathyroid mass but also to altered qualities, such as the down-regulation of VDR and CaSR. Nodular hyperplasia in patients with CKD is associated with a lower density of both CaSR and VDR than that noted in diffuse hyperplasia [49,50,52]. Density of VDR was reported to be negatively correlated with both the weight and proliferative activity of the glands [50]. These altered qualities are currently considered to be the central feature responsible for refractory hyperparathyroidism in patients with nodular hyperplasia [1,2]. Although calcitriol therapy may induce regression in glands with diffuse hyperplasia [65], regression of nodular hyperplasia may not occur except in those rare cases associated with spontaneous remission due to autoinfarction of the gland [66,67].
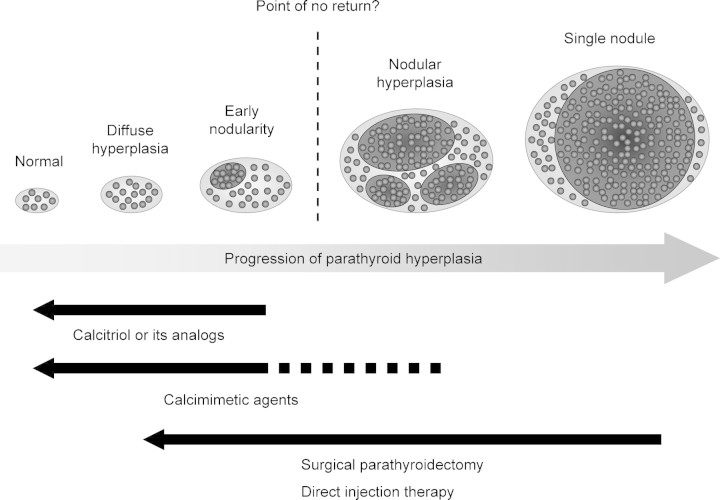
Several clinical and histological observations have shown that the size of the parathyroid gland, evaluated by ultrasonography, can be an indicator for the controllability of hyperparathyroidism. According to our clinical experience, patients with one or more enlarged glands larger than 0.5 cm3 or 1 cm in diameter are usually refractory to calcitriol therapy in the long term [68]. Other researchers also reported that patients with enlarged parathyroid glands larger than 11 mm in diameter [69] or 300 mm3 [70] were less responsive to maxacalcitol therapy than those with smaller glands. Histological studies reported that glands heavier than 0.5 g were composed of nodular hyperplasia in most cases [7]. Taken together, these findings suggest that the critical size is ∼0.5 cm3 or 1 cm in diameter [5]. Patients with hyperplastic glands that meet this criterion should undergo parathyroid intervention, i.e. surgical parathyroidectomy and direct injection therapy, particularly if they do not respond to a short course of calcitriol (analogue) therapy [5] (Figure 2).
Fig. 2.
Progression of parathyroid hyperplasia and current therapeutic strategy. Parathyroid intervention, such as surgical parathyroidectomy and direct injection therapy, is recommended for nodular hyperplasia refractory to calcitriol (analogue) treatment. Calcimimetic agents are promising tools, but further research is required to examine whether they can effectively control hyperparathyroidism associated with nodular hyperplasia.
The selection of parathyroid intervention depends on the pattern of parathyroid hyperplasia. In patients with three or more enlarged parathyroid glands, there is consensus that surgical parathyroidectomy is indicated. Total parathyroidectomy with forearm autograft is preferred for secondary hyperparathyroidism, especially in patients who require long-term haemodialysis, because a recurrent, enlarged autograft can easily be removed from the forearm [71]. In contrast, direct injection therapy such as percutaneous ethanol injection therapy (PEIT) should be indicated for patients with only one or two enlarged glands. In a recent clinical study, PEIT was effective in patients with no more than one hyperplastic gland larger than 0.5 cm3 [72]. The basis of PEIT is that enlarged parathyroid glands with nodular hyperplasia are destroyed ‘selectively’ by ethanol injection, and other glands with diffuse hyperplasia are then managed by adjuvant calcitriol (analogues) therapy. Another recent technique is direct calcitriol (analogue) injection therapy, which has been shown to induce the regression of nodular hyperplasia [73–75]. This therapy suppresses PTH levels and also restores the responsiveness of parathyroid cells to medical therapy. Recent studies have clearly shown that direct calcitriol injection not only induces apoptosis in parathyroid cells, but also up-regulates VDR and CaSR, resulting in normalization of the shifted sigmoidal curve between calcium and PTH [76].
Calcimimetic agents suppress parathyroid function by enhancing the sensitivity of the parathyroid CaSR to extracellular calcium ion levels [77]. Activation of the CaSR by calcimimetics allows long-term control of PTH in dialysis patients without increasing plasma levels of calcium, phosphorus and/or calcitriol [78,79]. We have also confirmed the efficacy of calcimimetics in reducing serum PTH levels in long-term dialysis patients [80]. However, it has not been fully elucidated whether patients with established nodular hyperplasia can be controlled by calcimimetics. With respect to this issue, recent clinical studies with calcimimetics in patients with hypercalcaemia due to persistent hyperparathyroidism after kidney transplantation seem to be promising [81–93]. Given that calcimimetics effectively suppressed PTH levels in such recipients with hypercalcaemia, in whom the numbers of VDRs and CaSRs remained reduced [84], calcimimetics might control severe hyperparathyroidism associated with nodular hyperplasia. Further clinical studies should be performed in the near future to examine whether calcimimetics alone or in combination with calcitriol (analogue) can control hyperparathyroidism in CKD patients with nodular hyperplasia.
Conclusions
Detailed research in the past three decades has brought considerable advances in the understanding of basic and clinical aspects of parathyroid hyperplasia. Recent data from preclinical and clinical studies have provided practical therapeutic approaches, especially with regard to the indications for parathyroid intervention. With a more focused attention to applying parathyroid intervention to patients with nodular hyperplasia, we can expect a significant improvement in morbidity and mortality among CKD patients. Further research and progress in this area are required to establish a more rational approach with a view towards improving patient outcomes.
Conflict of interest statement. None declared.
References
- 1.Drueke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11:1141–1152. doi: 10.1681/ASN.V1161141. [DOI] [PubMed] [Google Scholar]
- 2.Fukagawa M, Nakanishi S, Kazama JJ. Basic and clinical aspects of parathyroid hyperplasia in chronic kidney disease. Kidney Int. 2006;70(Suppl 102):S3–S7. doi: 10.1038/sj.ki.5001594. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 4.Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875–885. doi: 10.1681/ASN.2006070771. [DOI] [PubMed] [Google Scholar]
- 5.Fukagawa M, Kazama JJ, Shigematsu T. Management of the patients with advanced secondary hyperparathyroidism: the Japanese approach. Nephrol Dial Transplant. 2002;17:1553–1557. doi: 10.1093/ndt/17.9.1553. [DOI] [PubMed] [Google Scholar]
- 6.Fukagawa M, Kitaoka M, Tominaga Y, et al. Guideline for percutaneous ethanol injection therapy of the parathyroid glands in chronic dialysis patients. Nephrol Dial Transplant. 2003;18(Suppl 3):iii31–iii33. doi: 10.1093/ndt/gfg1008. [DOI] [PubMed] [Google Scholar]
- 7.Tominaga Y, Tanaka Y, Sato K, et al. Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997;13:78–86. doi: 10.1002/(sici)1098-2388(199703/04)13:2<78::aid-ssu3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Albright F, Drake TG, Sulkowitch HW. Renal osteitis fibrosa cystica. Report of a case with discussion of metabolic aspects. Johns Hopkins Med J. 1937;60:377–385. [Google Scholar]
- 9.Berson SA, Yalow RS. Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab. 1968;28:1037–1047. doi: 10.1210/jcem-28-7-1037. [DOI] [PubMed] [Google Scholar]
- 10.Reiss E, Canterbury JM, Kanter A. Circulating parathyroid hormone concentration in chronic renal insufficiency. Arch Intern Med. 1969;124:417–422. [PubMed] [Google Scholar]
- 11.Slatopolsky E, Caglar S, Pennell JP, et al. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971;50:492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bricker NS. On the pathogenesis of the uremic state. An exposition of the ‘trade-off hypothesis’. N Engl J Med. 1972;286:1093–1099. doi: 10.1056/NEJM197205182862009. [DOI] [PubMed] [Google Scholar]
- 13.Laflamme GH, Jowsey J. Bone and soft tissue changes with oral phosphate supplements. J Clin Invest. 1972;51:2834–2840. doi: 10.1172/JCI107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford WE, Bordier P, Marie P, et al. Phosphate control and 25-hydroxycholecalciferol administration in preventing experimental renal osteodystrophy in the dog. J Clin Invest. 1977;60:332–341. doi: 10.1172/JCI108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portale AA, Booth BE, Halloran BP, et al. Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest. 1984;73:1580–1589. doi: 10.1172/JCI111365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson L, Felsenfeld A, Drezner MK, et al. Altered divalent ion metabolism in early renal failure: role of 1,25(OH)2D. Kidney Int. 1985;27:565–573. doi: 10.1038/ki.1985.48. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Hilker S, Galceran T, Chan YL, et al. Hypocalcemia may not be essential for the development of secondary hyperparathyroidism in chronic renal failure. J Clin Invest. 1986;78:1097–1102. doi: 10.1172/JCI112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantley LK, Russell J, Lettieri D, et al. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology. 1985;117:2114–2119. doi: 10.1210/endo-117-5-2114. [DOI] [PubMed] [Google Scholar]
- 19.Silver J, Naveh-Many T, Mayer H, et al. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78:1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slatopolsky E, Weerts C, Thielan J, et al. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andress DL, Norris KC, Coburn JW, et al. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989;321:274–279. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 22.Martin KJ, Gonzalez EA, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9:1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 23.Tan AU, Jr, Levine BS, Mazess RB, et al. Effective suppression of parathyroid hormone by 1 alpha-hydroxy-vitamin D2 in hemodialysis patients with moderate to severe secondary hyperparathyroidism. Kidney Int. 1997;51:317–323. doi: 10.1038/ki.1997.39. [DOI] [PubMed] [Google Scholar]
- 24.Portale AA, Halloran BP, Murphy MM, et al. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in human. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi H, Fukagawa M, Yamato H, et al. Prevention of enhanced parathyroid hormone secretion, synthesis and hyperplasia by mild dietary phosphorus restriction in early chronic renal failure rats: possible direct role of phosphorus. Nephron. 1995;70:242–248. doi: 10.1159/000188591. [DOI] [PubMed] [Google Scholar]
- 26.Combe C, Aparicio M. Phosphorus and protein restriction and parathyroid function in chronic renal failure. Kidney Int. 1994;46:970–976. doi: 10.1038/ki.1994.408. [DOI] [PubMed] [Google Scholar]
- 27.Slatopolsky E, Finch J, Denda M, et al. Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest. 1996;97:2534–2540. doi: 10.1172/JCI118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almaden Y, Canalejo A, Hernandez A, et al. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res. 1996;11:970–976. doi: 10.1002/jbmr.5650110714. [DOI] [PubMed] [Google Scholar]
- 29.Tatsumi S, Segawa H, Morita K, et al. Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology. 1998;139:1692–1699. doi: 10.1210/endo.139.4.5925. [DOI] [PubMed] [Google Scholar]
- 30.Yalcindag C, Silver J, Naveh-Many T. Mechanism of increased parathyroid hormone mRNA in experimental uremia: roles of protein RNA binding and RNA degradation. J Am Soc Nephrol. 1999;10:2562–2568. doi: 10.1681/ASN.V10122562. [DOI] [PubMed] [Google Scholar]
- 31.Sela-Brown A, Silver J, Brewer G, et al. Identification of AUF1 as a parathyroid hormone mRNA 3′ untranslated region-binding protein that determines parathyroid hormone mRNA stability. J Biol Chem. 2000;275:7424–7429. doi: 10.1074/jbc.275.10.7424. [DOI] [PubMed] [Google Scholar]
- 32.Almaden Y, Canalejo A, Ballesteros E, et al. Effect of high extracellular phosphate concentration on arachidonic acid production by parathyroid tissue in vitro. J Am Soc Nephrol. 2000;11:1712–1718. doi: 10.1681/ASN.V1191712. [DOI] [PubMed] [Google Scholar]
- 33.The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 34.The ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutation in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 35.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 37.Nishi H, Nii-Kono T, Nakanishi S, et al. Intravenous calcitriol therapy increases serum concentration of fibroblast growth factor 23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract. 2005;101:c94–c99. doi: 10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 38.Saito H, Meda A, Ohtomo S, et al. Circulating FGF-23 is regulated by 1 alpha, 25-dihydroxyvitamine D3 and phosphate in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 39.Collins MT, Lindsay JR, Jain A, et al. Fibroblast growth factor-23 is regulated by 1 alpha, 25-dihydroxyvitamin D. J Bone Miner Res. 2005;20:1944–1950. doi: 10.1359/JBMR.050718. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 41.Masuyama R, Stockmans I, Torrekens S, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116:3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 43.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 44.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor-23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 46.Fukagawa M, Kazama JJ. With or without the kidney: the role of FGF23 in CKD. Nephrol Dial Transplant. 2005;20:1295–1298. doi: 10.1093/ndt/gfh827. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Palnitkar S, Parfitt AM. The basal rate of cell proliferation in normal human parathyroid tissue: implications for the pathogenesis of hyperparathyroidism. Clin Endocrinol. 1997;46:343–349. doi: 10.1046/j.1365-2265.1997.1420959.x. [DOI] [PubMed] [Google Scholar]
- 48.Naveh-Many T, Rahaminov R, Livni N, et al. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest. 1995;96:1786–1793. doi: 10.1172/JCI118224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuda N, Tanaka H, Tominaga Y, et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokumoto M, Tsuruya K, Fukuda K, et al. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 51.Kifor O, Moore FD, Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 52.Gogusev J, Duchambon P, Hory B, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 53.Dusso AS, Pavlopoulos T, Naumovich L, et al. p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int. 2001;59:855–865. doi: 10.1046/j.1523-1755.2001.059003855.x. [DOI] [PubMed] [Google Scholar]
- 54.Dusso AS, Sato T, Arcidiacono MV, et al. Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int. 2006;70(Suppl 102):S8–S11. doi: 10.1038/sj.ki.5001595. [DOI] [PubMed] [Google Scholar]
- 55.Arcidiacono MV, Sato T, Alvarez-Hernandez D, et al. EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol. 2008;19:310–320. doi: 10.1681/ASN.2007040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnold A, Brown MF, Urena P, et al. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest. 1995;95:2047–2053. doi: 10.1172/JCI117890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanishi S, Kazama JJ, Nii-Kono T, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 58.Kazama JJ, Sato F, Omori K, et al. Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int. 2005;67:1120–1125. doi: 10.1111/j.1523-1755.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 59.Sato T, Tominaga Y, Ueki T, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487. [PubMed] [Google Scholar]
- 60.Urena-Torres P, Friedlander G, de Vernejoul MC, et al. Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int. 2008;73:102–107. doi: 10.1038/sj.ki.5002622. [DOI] [PubMed] [Google Scholar]
- 61.Chikazu D, Hakeda Y, Ogata N, et al. Fibroblast growth factor (FGF)-2 directly stimulates mature osteoclast function through activation of FGF receptor 1 and p42/p44 MAP kinase. J Biol Chem. 2000;275:31444–31450. doi: 10.1074/jbc.M910132199. [DOI] [PubMed] [Google Scholar]
- 62.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukagawa M, Okazaki R, Takano K, et al. Regression of parathyroid hyperplasia by calcitriol-pulse therapy in patients on long-term dialysis. N Engl J Med. 1990;323:421–422. doi: 10.1056/NEJM199008093230617. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka M, Tominaga Y, Sawatari E, et al. Infarction of mediastinal parathyroid gland causing spontaneous remission of secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:762–767. [PubMed] [Google Scholar]
- 67.Komaba H, Takeda Y, Abe T, et al. Spontaneous remission of severe hyperparathyroidism with normalization of the reversed whole PTH/intact PTH ratio in a haemodialysis patient. Nephrol Dial Transplant. 2008;23:1760–1762. doi: 10.1093/ndt/gfm891. [DOI] [PubMed] [Google Scholar]
- 68.Fukagawa M, Kitaoka M, Yi H, et al. Serial evaluation of parathyroid size by ultrasonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patient. Nephron. 1994;68:221–228. doi: 10.1159/000188261. [DOI] [PubMed] [Google Scholar]
- 69.Okuno S, Ishimura E, Kitatani K, et al. Relationship between parathyroid gland size and responsiveness to maxacalcitol therapy in patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2003;18:2613–2621. doi: 10.1093/ndt/gfg451. [DOI] [PubMed] [Google Scholar]
- 70.Tominaga Y, Inaguma D, Matsuoka S, et al. Is the volume of the parathyroid gland a predictor of Maxacalcitol response in advanced secondary hyperparathyroidism? Ther Apher Dial. 2006;10:198–204. doi: 10.1111/j.1744-9987.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 71.Tominaga Y, Uchida K, Haba T, et al. More than 1,000 cases of total parathyroidectomy with forearm autograft for renal hyperparathyroidism. Am J Kidney Dis. 2001;38(Suppl l):S166–S171. doi: 10.1053/ajkd.2001.27432. [DOI] [PubMed] [Google Scholar]
- 72.Koiwa F, Kakuta T, Tanaka R, et al. Efficacy of percutaneous ethanol injection therapy (PEIT) is related to the number of parathyroid glands in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2007;22:522–528. doi: 10.1093/ndt/gfl620. [DOI] [PubMed] [Google Scholar]
- 73.Kitaoka M, Fukagawa M, Fukuda N, et al. Direct injections of calciriol into parathyroid hyperplasia in chronic dialysis patients with severe parathyroid hyperfunction. Nephrology. 1995;1:563–568. [Google Scholar]
- 74.Kitaoka M, Onoda NM, Kitamura H, et al. Percutaneous calcitriol injection therapy (PCIT) for secondary hyperparathyroidism: multicenter trial. Nephrol Dial Transplant. 2003;18(Suppl 3):iii38–iii41. doi: 10.1093/ndt/gfg1010. [DOI] [PubMed] [Google Scholar]
- 75.Shiizaki K, Hatamura I, Negi S, et al. Percutaneous maxacalcitol injection therapy regresses hyperplasia of parathyroid and induces apoptosis in uremia. Kidney Int. 2003;64:992–1003. doi: 10.1046/j.1523-1755.2003.00154.x. [DOI] [PubMed] [Google Scholar]
- 76.Shiizaki K, Negi S, Hatamura I, et al. Biochemical and cellular effect of direct maxacalcitol injection into parathyroid gland in uremic rat. J Am Soc Nephrol. 2005;16:97–108. doi: 10.1681/ASN.2004030236. [DOI] [PubMed] [Google Scholar]
- 77.Nagano N, Nemeth EF. Functional proteins involved in regulation of intracellular Ca2+ for drug development: the extracellular calcium receptor and an innovative medical approach to control secondary hyperparathyroidism by calcimimetics. J Pharmacol Sci. 2005;97:355–360. doi: 10.1254/jphs.fmj04007x6. [DOI] [PubMed] [Google Scholar]
- 78.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 79.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 80.Fukagawa M, Yumita S, Akizawa T, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328–335. doi: 10.1093/ndt/gfm534. [DOI] [PubMed] [Google Scholar]
- 81.Srinivas TR, Schold JD, Womer KL, et al. Improvement in hypercalcemia with cinacalcet after kidney transplantation. Clin J Am Soc Nephrol. 2006;1:323–326. doi: 10.2215/CJN.00500705. [DOI] [PubMed] [Google Scholar]
- 82.Serra AL, Savoca R, Huber AR, et al. Effective control of persistent hyperparathyroidism with cinacalcet in renal allograft recipients. Nephrol Dial Transplant. 2007;22:577–583. doi: 10.1093/ndt/gfl560. [DOI] [PubMed] [Google Scholar]
- 83.Kruse AE, Eisenberger U, Frey FJ, et al. Effect of cinacalcet cessation in renal transplant recipients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2007;22:2362–2365. doi: 10.1093/ndt/gfm270. [DOI] [PubMed] [Google Scholar]
- 84.Taniguchi M, Tokumoto M, Matuo D, et al. Persistent hyperparathyroidism in renal allograft recipients: vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int. 2006;70:363–370. doi: 10.1038/sj.ki.5001549. [DOI] [PubMed] [Google Scholar]