Abstract
Background. This review describes the peritoneal dialysis (PD) catheter implantation techniques for the treatment of PD. The PD catheter-related complications still cause significant morbidity and mortality, resulting in the necessity to switch to haemodialysis (HD) treatment.
Methods. Several catheter insertion techniques, using an open surgical approach, laparoscopic and percutaneous techniques have been employed, with their specific early and late complications and failure rates.
Results. Despite the similar outcomes of open surgical versus laparoscopic techniques from randomized studies, the laparoscopic insertion has the major advantage of correct catheter positioning in the lower abdomen, with the possibility of adhesiolysis. The minimal invasive percutaneous insertion bears the risk of bowel perforation and catheter malpositioning, and the outcome of this technique is strongly related to the experience of the surgeon. The major complications of these implantation techniques, like bleeding, dialysate leakage and catheter malpositioning, and their management are discussed in our study. Late peritonitis remains the major drawback of PD treatment, with the need of temporary or permanent changeover to the HD treatment in 10% of the patients.
Conclusions. Enrichment of the physician's interest and experience, along with a multidisciplinary approach to outline the optimal strategy of PD-catheter insertion and complication of the treatment, may improve the patients’ survival and decrease the morbidity.
Keywords: peritoneal dialysis, catheter, complications, peritonitis, insertion techniques
Introduction
In 1959, Richard Ruben was the first to use peritoneal dialysis (PD) successfully in a patient with end-stage renal disease (ESRD) for 6 months. Three years later, Fred Boen from the Netherlands described the first automatic cycling PD machine. In 1964, he reported about two patients who were treated with this machine, with long-term survival of 2 years [1]. In 1968, Henry Tenckhoff developed the indwelling peritoneal catheter, which was inserted following an open surgical technique. In 1970, he reported about 16 patients being treated with the self-PD for up to 4 years [2]. The PD was popularized by Popovich and Moncrief who developed continuous ambulatory peritoneal dialysis (CAPD) [3]. The introduction of the percutaneous [4] and later the laparoscopic technique [5] was a major step towards the implantation of PD catheters. In 2004, the National End-Stage Renal Disease program in the USA reported that ∼25 765 patients used CAPD, accounting for 8% of the prevalent dialysis population [6]. In Europe, the PD rates in the prevalent patients were higher, whereas in the UK, ∼35% of the ESRD population was on PD [7]. In the Netherlands, during the last few years, the PD rate varied from 26% to 32% among all the dialysis patients [8].
Several advantages of PD over haemodialysis (HD) have been described, including the quality of life due to superior patient mobility and independence, its simplicity in use, along with the clinical advantages like the maintenance of residual renal function and lower mortality in the first years after the beginning of PD. A significant disadvantage is the poor blood pressure control due to fluid overload [9].
Several techniques and modifications have been described for the insertion of the catheter into the abdominal cavity. We describe the currently available catheter designs and insertion techniques with its early and late complications. The strategy for an optimal catheter implantation together with the preventive and therapeutic means for complicated treatment will be discussed.
Catheter type and design
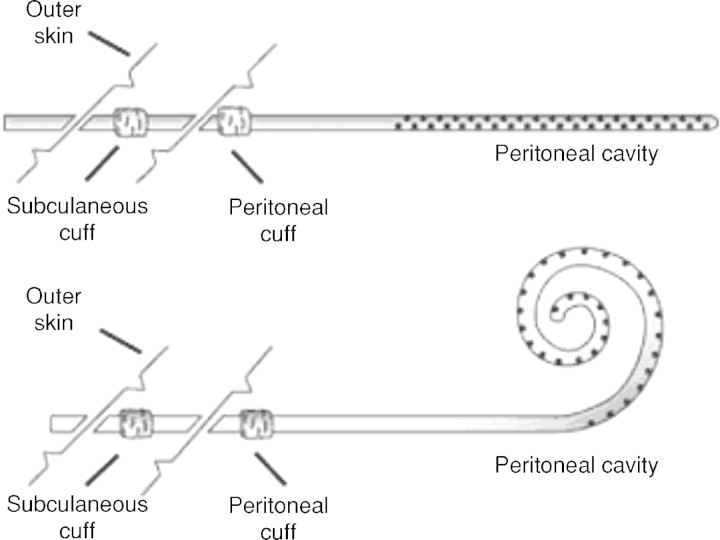
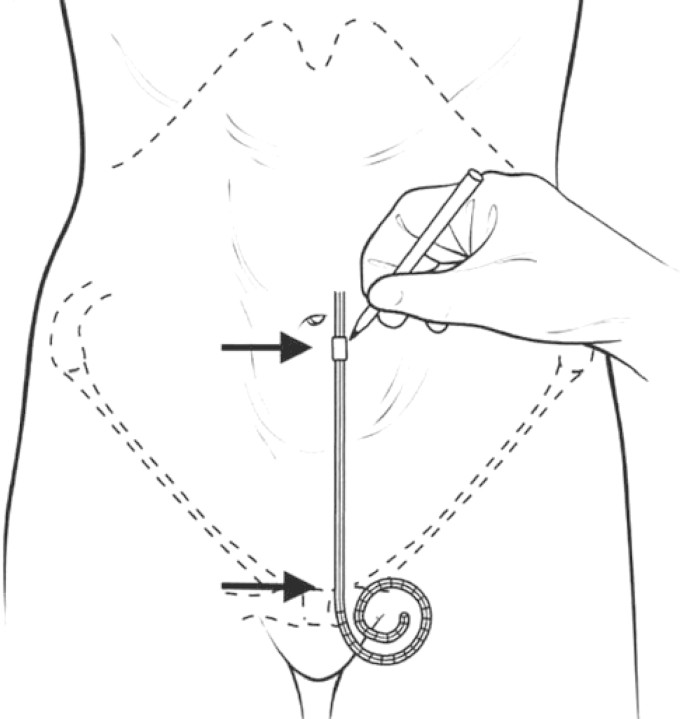
The PD catheters come in a variety of shapes (straight, pigtail-curled, swan-neck), length and number of Dacron cuffs for optimal ingrowth and fixation. It consists of a flexible silicone tube with an open-end port and several side holes for optimal drainage and absorption of the dialysate. The extraperitoneal segment of the PD catheter has either one or two Dacron cuffs. Most catheters used in the adult population have a double cuff: the proximal one implanted in the preperitoneal space and the distal one in the subcutaneous tissue. The proximal cuff holds the catheter in place and the distal cuff acts as a barrier to infection (Figure 1). Selection of the appropriate catheter type and exit location is done prior to the catheter implantation procedure. During the preoperative evaluation, patients are examined fully dressed to mark the belt-line location. Catheter selection then begins with the determination of the catheter-insertion site. With the patient in the supine position, the insertion site, which coincides with the deep cuff location, is established by aligning the upper border of the catheter coil with the upper border of the pubic symphysis and marking the upper border of the deep cuff in the paramedian plane, 3 cm lateral of midline (Figure 2). The pubic symphysis has been recommended as a reliable marker for the ideal location of the catheter tip in the true pelvis [10].
Fig. 1.
Two-cuffed straight (top) and curled PD catheter types.
Fig. 2.
Schematic illustration indicating the manner in which the catheter-insertion site and deep cuff location are selected, to achieve the proper pelvic position of the curled catheter tip (with permission from Crabtree, 2006 [10]).
Straight and curled catheters have similar performance and the implantation technique for both the catheter types minimally varies and is simple. Less pain due to fluid inflow into the abdomen and a little catheter tip dislocation may be more favourable with curled catheters, although recent research showed a preference for the straight catheter in terms of dialysis efficacy. In this randomized trial, the effects of straight versus curled PD catheters with respect to catheter malposition, catheter-associated infection, technique failure and all-cause mortality were investigated in 132 PD patients. No difference in laparoscopic insertion time was observed; however, the median technique survival was significantly worse than the curled and straight catheters (1.5 versus 2.1 years), primarily, owing to the increased risk of inadequate dialytic clearance. No difference was observed between both the groups with respect to the catheter-associated infections or overall patient survival [11]. Catheter-type selection is usually surgeon dependent and is based on the method of insertion, ease of insertion and local expertise.
Technique of implantation
There are several techniques used for the introduction of the PD catheter into the abdominal cavity. Open surgical and laparoscopic techniques are preferred because of their safety and good initial results. The laparoscopic technique is becoming more popular because of its advantage in performing partial omentectomy or adhesiolysis during the initial catheter placement. Percutaneous (radiological) catheter insertion may be less invasive, but bears the risk of unsatisfactory catheter placement and danger of bowel perforation.
Open surgical technique
In this technique, the patient is placed in the supine position. General anaesthesia is inducted and the intravenous antibiotics are administered. A vertical incision of ∼5 cm is made in the midline, 2–3 cm below the umbilicus. The subcutaneous layer is dissected, till the sheath of the rectal abdominal muscle is reached. The anterior rectus sheath is opened and the muscle fibres are bluntly dissected. Subsequently, the posterior sheath is cut to 3–4 cm and the abdominal cavity is opened after dissecting the peritoneum. The abdominal wall is inspected for adhesions. After this, a retractor is used to lift the anterior abdominal wall. If the adhesions are present close to the abdominal wall, they are dissected. The patient is placed in a Trendelenburg position and the catheter is placed over a stylet and advanced into the peritoneal cavity. The intraperitoneal segment is slid off the stylet and the cuff is advanced to the preperitoneal space. The peritoneum and rectus sheaths (posterior and anterior) are closed carefully with resorbable sutures, ensuring not to obstruct the catheter and to prevent dialysate leakage. A tunnel is created to the preferred exit site using a needle and care should be maintained to ensure that the exit site is facing downward. The distal cuff is placed subcutaneously, 2 cm from the exit site. The exit site is usually lateral and caudal to the entrance site. After haemostasis, the incision is closed and the catheter itself is not fixated with a suture. The functioning of the catheter is tested by filling the abdomen with 100 cc saline and the entrance site is checked for leakage. The saline is allowed to drain and isinspected for evidence of haemoperitoneum and faecal contamination.
Laparoscopic technique
In this technique, the patient is placed in the supine position. General anaesthesia is inducted and intravenous antibiotics are administered. It is preferable to create a pneumoperitoneum with an open procedure. A small subumbilical incision is made (2–3 cm) and the umbilical cord is grasped with forceps and lifted. Subsequently, the subcutaneous layer is transected. The anterior rectus sheath is opened and a suture is placed to lift the anterior sheath. The posterior sheath and subsequently the peritoneum are digitally opened. If adhesions are present close to the abdominal wall, they are transected. A 5 mm trocar or a screw trocar is inserted into the abdomen and insufflated with CO2 gas to create a pneumoperitoneum of 12–14 mmHg. A Veres needle technique can also be adopted. Several methods have been described. One is to place the needle in the upper-left quadrant of the abdomen. Another way is to open the anterior sheath as explained in the open procedure, but the Veres needle is used for the last one or two steps (the posterior sheath or the peritoneum). After the needle is in place, its correct position is tested by the water drop test, which should disappear into the abdomen through the needle, and by insufflating and aspirating 10 ml saline. After creating a pneumoperitoneum, a 5 mm trocar is inserted in the subumbilical position. After the 5 mm trocar is in place, the patient is placed in a Trendelenburg position and a diagnostic laparoscopy is performed with a 5 mm 0° scoop. In case the Veres needle is placed in the left-upper quadrant of the abdomen, its position is checked and the needle removed. An extra 5 mm trocar is inserted under direct vision at the site of the planned exit-site position of the PD catheter (paraumbilical left or right 2– 3 cm below the umbilicus). This trocar is introduced through the anterior and posterior rectus sheaths, but not through the peritoneum. Under direct vision, the trocar is directed in the preperitoneal space, 2–4 cm downwards and to the midline of the abdomen. If adhesions are present, the trocar is introduced into the peritoneal cavity. Adhesions close to the abdominal wall are ligated with electrocoagulation or with the ligature device (US Surgical). A double-cuffed curled-tip PD catheter is then introduced through the paraumbilical port, ensuring no torsion has occurred and is placed with the curled tip into the Cavum Douglasi. If no adhesions are present, then the second trocar is not introduced into the peritoneal cavity, but is left in the preperitoneal space. Now, the stiff stylet is used to introduce the catheter into the peritoneal cavity. If the placement is troublesome, an extra 5 mm trocar is used, which can be inserted under the direct vision to grasp the catheter for proper positioning. The distal cuff of the PD catheter should be outside the peritoneum (in the preperitoneal space or between both the rectus sheaths). The paraumbilical trocar is removed and the catheter is now directed to its exit-site position. A needle is used to create the subcutaneous tunnel to the left or the right abdomen. The proximal cuff should be in this tunnel. The catheter is tested and then the abdomen is desufflated, with the camera still in position to check on the location of the catheter. The trocar is removed and the rectus sheaths are closed carefully with resorbable sutures. The wounds are closed with a resorbable monofilament suture, intracutaneously.
Percutaneous technique
Placement of PD catheters with a guide wire and peel-away sheath is performed using a Seldinger technique. The procedure can be performed under local or general anaesthesia with prophylactic antibiotics. A small incision is created above the entrance site, usually in the midline with blunt dissection of the abdominal rectus sheath. The peritoneal cavity is cannulated with an 18-gauge needle and filled with either air or 500 cc of saline. With proper needle placement, the patient should not experience pain or resistance to filling the cavity with fluid. A 0.035-inch guide wire is advanced into the abdomen and the introduction needle is removed. A dilator and the peel-away sheath are advanced over the wire into the abdominal cavity. The wire and the dilator are removed and the catheter is placed on the stylet, advanced through the sheath. The intraperitoneal segment is advanced until the proximal cuff is located in the preperitoneal space. The peel-away sheath and stylet are removed and the catheter position is checked. A tunnel is created to the selected exit site with the placement of the distal cuff subcutaneously, 2 cm from the exit site. The entrance site is closed. The abdomen is filled with 500 cc saline and drained.
Comparison of implantation techniques
Randomized prospective studies show similar outcomes of open surgically and laparoscopically placed PD catheters. The conventional procedure is faster than the laparoscopic one (14.3 versus 21.9 min, P < 0.0001), but there is no difference in the early complication rate. In another study, 50 patients were enrolled and randomly allocated to an open surgical technique or laparoscopic placement, with fixation into the pelvis and suture closure of the port wounds. Fluid leakage was observed in eight patients in the surgical group, but in none in the laparoscopic group. Peritonitis occurred equally in both the groups. Tip migration occurred in five patients in the open surgical group and in none of the patients in the laparoscopy group. Thus, we can conclude that the laparoscopic placement of a PD catheter leads to better functioning than the open procedure. It allows immediate start of dialysis without fluid leakage and permits simultaneous performance of other laparoscopic procedures [12]. Another advantage is the ability to perform the other procedures, simultaneously [13–15]. These advantages may favour the laparoscopic technique over the open surgical approach. Percutaneous PD-catheter placement by experienced hands is a well-tolerated procedure that allows a rapid initiation of CAPD and avoids the necessity for operating room time, and the requirement for a peritoneal incision. It has a high technical success rate and can be performed on an outpatient basis [16]. Catheter survival is comparable with that achieved with the surgical methods of catheter placement. In a retrospective study, the clinical outcome of 230 PD catheters was reviewed. About 50 catheters were placed percutaneously and 180 were placed using conventional surgical techniques. Percutaneous insertion was non-elective, and was reserved for patients unfit for general anaesthesia or HD. These patients were older and had increased susceptibility to early mortality because of underlying pathology. Death and early mechanical failure contributed to a shorter mean duration of catheter use in the percutaneous group. The peritonitis rate was similar in both the groups [17].
A recent meta-analysis could not demonstrate any advantage of one technique over the others, with respect to the risk of peritonitis, catheter removal or replacement, technical failure and all-cause mortality [18].
In our opinion, both the open surgical and laparoscopic techniques can be used in patients who receive a primary PD catheter and have no history of previous abdominal surgery, which could lead to PD catheter malfunction. When abdominal surgery has been performed, a laparoscopic technique is to be preferred, with the advantage of additional adhesiolysis. Also, the cause of persistent PD catheter malfunctioning can be elucidated with a diagnostic laparoscopy, and if possible, solved under the same conditions. For instance, adhesions can be dissected and omental wrapping or fibrin clotting can be removed from the catheter. Percutaneous placement is particularly well suited for ailing patients, who cannot tolerate general anaesthesia [19,20].
Complications of PD catheter placement
Complications after PD catheter placement are defined as those occurring early (<30 days) or late (>30 days), after surgery.
Early complications
Bowel perforation occurs rarely in ∼1% of the patients and is usually initiated during the entry into the abdominal cavity or when advancing the catheter with the stylet into the lower abdomen. Significant perforation is suspected with the onset of pain, nausea or a rigid abdomen. Surgical exploration is mandatory with the repair of the perforation and removal of the catheter. Intravenous antibiotics should be administrated after the surgery. Bleeding is rarely a significant problem after catheter implantation and usually occurs at the exit site. Blood may be present initially in the effluent drained, owing to the trauma of insertion, but the drainage should return to normal within a few days. Manual pressure or additional suturing can stop persistent bleeding. Wound infection is uncommon and usually antibiotics are sufficient to treat superficial wound infections. Rarely, the entrance site may have to be drained.
The outflow failure may be due to multiple causes, including clots or fibrin in the catheter, kink in the subcutaneous tunnel, placement of the catheter in the omentum, development of omental wrap or adhesions in the abdomen. Obstructed catheters may be forcefully irrigated by saline or urokinase (Medicinase: 100 000 IU; 5 cc during 1 h). As an alternative, a stiff guide wire may be advanced into the catheter under direct fluoroscopic control. If the subcutaneous tunnel is kinked, incision over the kink and repositioning of the catheter is worthwhile. Laparoscopic diagnosis and treatment of omental wrap or adhesions are advised with the additional advantage of instantaneous omentectomy or adhesiolysis [21].
Malpositioning of the catheter into the upper abdomen usually causes pain and sometimes outflow failure. A plain abdominal radiological examination, eventually with a fluoroscopic contrast study, can reveal this problem (Table 1). Conservative measures with laxatives to activate bowel movements, which will carry the catheter into the right position, are usually disappointing. Catheter repositioning with a stiff guide wire or forceps can be successful and causes little morbidity [22]. In this technique, a device such as a malleable rod, guide wire, cannula or tip-deflecting wire, is inserted into the catheter and is used to redirect or reposition the catheter tip in a more favourable position for PD. Laparoscopic repositioning with catheter fixation into the lower abdomen may be the ultimate therapy to solve this problem. Prevention of catheter malposition remains the major goal and can be adjusted by a laparoscopic insertion technique and correct measurement of catheter length.
Table 1.
Causes and management of PD catheter obstruction
| Causes | Management |
|---|---|
| Constipation | Relief of constipation |
| Clot | Syringe flushing with heparin/urokinase |
| Omental wrap | Omentectomy |
| Adhesions | Adhesiolysis |
| Catheter tip migration | Fluoroscopic repositioning with fogarty catheter or trocar, laparoscopic repositioning |
Leakage of dialysate can be deducted with fluid drainage from the exit site or with the appearance of a bulge underneath the entrance site. Causes of leaks may be due to hernia at the entrance site as a result of very large incision, positioning of the proximal cuff on the rectus muscle, and trauma. Catheter rest without dialysate instillation for some weeks is most likely to solve this problem. Temporary HD treatment is usually required.
Early peritonitis with catheter placement may be a sign of a poor surgical technique. If the peritoneal fluid becomes cloudy, associated with pain, then the dialysate should be cultured and appropriate antibiotics must be administered. Eradication of nasal staphylococcus carriers by mupirocin and antibiotic prophylaxis with vancomycin may substantially decrease the rate of early peritonitis [23].
Late complications
Late complications (>30 days) include exit-site infection, tunnel infection, cuff protrusion, outflow failure, and dialysate leaks or hernias. The incidence of infections attributable to the exit-site positioning can be reduced by proper placement of the exit site. Irritation and even cuff protrusion can occur when the exit is placed directly beneath the belt line. Superficial cuffs placed close to the skin may be prone to extrusion and infection. An upward-directed site may collect fluid, leading to an increased incidence of infection. Catheter exchange is indicated in most instances and can be performed in one session with the emphasis on the fact to choose a different exit site through the skin for the new catheter.
Outflow failure beyond 30 days is quite likely due to constipation and is relieved by laxatives. Leaks and hernias may become symptomatic because of the increased intra-abdominal pressure, secondary to the dialysate. In patients with residual renal function, temporary nocturnal automatic PD can be applied to decrease leakage. Leaks can also result from umbilical hernias or the presence of a patent processus vaginalis, resulting in scrotal oedema. Surgical repair of hernias or processus vaginalis may be indicated with a temporary shift to HD for adequate wound healing.
Peritonitis is a major problem for patients on PD and the main reason for patients to switch to HD. Peritonitis often results from contamination with skin bacteria. In addition, gram-negative bacteria, associated with diarrhoea or diverticulitis may be the causative organisms. The diagnosis and treatment of PD-associated peritonitis are straightforward. Patients suffer from abdominal pain, sometimes accompanied with fever. The PD fluid white blood cell counts and cultures may reveal leucocytes and bacteria. Systemic or intraperitoneal antibiotics, according to the cultured organisms, are administered and the exchange volumes are reduced. Usually, a related PD peritonitis resolves after proper treatment. With persistent infection, catheter removal and shift to HD for 4–6 weeks is sufficient to solve the peritonitis [24].
Summary
A successful PD program is dependent on the proper placement of the permanent PD catheters. The knowledge of implantation techniques and complications is also very essential. Usually, the catheter type does not influence the outcome. The advantage of the open surgical technique is based on its simplicity. Surgical residents who are familiar with opening the abdomen can perform the procedure. Intra-abdominal adhesions near the incision site can be easily dissected close to the abdominal wall and require fewer skills than those in the laparoscopic setting. The closure of the peritoneum and the rectus sheets is easily performed, which might lead to less dialysate leakage postoperatively. Advantages of the laparoscopic technique are the ability to inspect the abdominal cavity more thoroughly and to accomplish better placement with eventual suture fixation of the catheter. Adhesiolysis can be performed, reducing the catheter placement failure. Several complications of PD implantation have been described like dialysate leakage, exit-site infections, hernias, genital oedema and other discomforts. Catheter malfunction, which has been estimated to occur in 60% of the patients with PD, can be caused by kinking, catheter displacement, omental wrapping, catheter-fibrin coating and adhesions caused by abdominal infections. Besides exit-site and subcutaneous tract infections, peritonitis is a feared complication responsible for 30% of the catheter failures. Peritonitis can be recurrent, with a rate of relapse of ±0.5 episodes/patient/year.
A successful PD program depends on the knowledge of the placement techniques and complications. A multidisciplinary approach with great enthusiasm from the healthcare team will improve the catheter outcome and long-term results.
Conflict of interest statement. None declared.
References
- 1.Blagg CR. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am J Kidney Dis. 2007;3:482–496. doi: 10.1053/j.ajkd.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Tenckhoff H, Curtis FK. Experience with maintenance peritoneal dialysis in the home. Trans Am Soc Artif Intern Organs. 1970;16:90–95. [PubMed] [Google Scholar]
- 3.Popovich RP, Moncrief JW, Nolph KD, et al. Continuous ambulatory peritoneal dialysis. Ann Intern Med. 1978;88:449–456. doi: 10.7326/0003-4819-88-4-449. [DOI] [PubMed] [Google Scholar]
- 4.Allon M, Soucie JM, Macon EJ. Complications with permanent peritoneal dialysis catheters: experience with 154 percutaneously placed catheters. Nephron. 1988;48:8–11. doi: 10.1159/000184860. [DOI] [PubMed] [Google Scholar]
- 5.Amerling R, Cruz C. A new laparoscopic method for implantation of peritoneal catheters. ASAIO J. 1993;39:M787–M789. [PubMed] [Google Scholar]
- 6.U.S. Renal Data System . USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. Available at http://www.usrds.org/adr.htm . [Google Scholar]
- 7.Moeller S, Gioberge S, Brown G. ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant. 2002;17:2071–2076. doi: 10.1093/ndt/17.12.2071. [DOI] [PubMed] [Google Scholar]
- 8. http://www.renine.nl/index.php?page=stt_2005 .
- 9.Konings CJ, Kooman JP, Schonck M, et al. Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int. 2002;22:477–487. [PubMed] [Google Scholar]
- 10.Crabtree JH. Selected best demonstrated practices in peritoneal dialysis access. Kidney Int Suppl. 2006;103:S27–S37. doi: 10.1038/sj.ki.5001913. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Wong J, Wiggins KJ, et al. A randomized controlled trial of coiled vs. straight swan-neck Tenckhoff catheters in peritoneal dialysis patients. Am J Kidney Dis. 2006;48:812–821. doi: 10.1053/j.ajkd.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Wright MJ, Bel’eed K, Johnson BF, et al. Randomized prospective comparison of laparoscopic and open peritoneal dialysis catheter insertion. Perit Dial Int. 1999;19:372–375. [PubMed] [Google Scholar]
- 13.Tsimoyiannis EC, Siakas P, Glantzounis G, et al. Laparoscopic placement of the Tenckhoff catheter for peritoneal dialysis. Surg Laparosc Endosc Percutan Tech. 2000;10:218–221. [PubMed] [Google Scholar]
- 14.Öğűnc G, Tuncer M, Öğűnc D, et al. Laparoscopic omental fixation technique vs open surgical placement of peritoneal dialysis catheters. Surg Endosc. 2003;17:1749–1755. doi: 10.1007/s00464-002-8586-3. [DOI] [PubMed] [Google Scholar]
- 15.Lund L, Jonler M. Peritoneal dialysis catheter placement—is laparoscopy an option? Int Urol Nephrol. 2007;39:625–628. doi: 10.1007/s11255-007-9193-y. [DOI] [PubMed] [Google Scholar]
- 16.Savader SJ, Geschwind JF, Lund GB, et al. Percutaneous radiologic placement of peritoneal dialysis catheters: long-term results. J Vasc Interv Radiol. 2000;11:965–970. doi: 10.1016/s1051-0443(07)61323-2. [DOI] [PubMed] [Google Scholar]
- 17.Georgiades CS, Geschwind JF. Percutaneous peritoneal dialysis catheter placement for the management of end-stage renal disease: technique and comparison with the surgical approach. Tech Vasc Interv Radiol. 2002;5:103–107. doi: 10.1053/tvir.2002.36054. [DOI] [PubMed] [Google Scholar]
- 18.Strippoli GFM, Tong A, Johnson D, et al. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized, controlled trials. J Am Soc Nephrol. 2004;15:2735–2746. doi: 10.1097/01.ASN.0000141463.95561.79. [DOI] [PubMed] [Google Scholar]
- 19.Mellotte GJ, Ho CA, Morgan SH, et al. Peritoneal dialysis catheters: a comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant. 1993;8:626–630. [PubMed] [Google Scholar]
- 20.Ozener C, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant. 2001;16:1893–1899. doi: 10.1093/ndt/16.9.1893. [DOI] [PubMed] [Google Scholar]
- 21.Skipper K, Dickerman R, Dunn E. Laparoscopic placement and revision of peritoneal dialysis catheters. J Surg Laparosc Surg. 1999;3:63–65. [PMC free article] [PubMed] [Google Scholar]
- 22.Savader SJ, Lund G, Scheel PJ, et al. Guide wire directed manipulation of malfunctioning peritoneal dialysis catheters: a critical analysis. J Vasc Interv Radiol. 1997;8:957–963. doi: 10.1016/s1051-0443(97)70693-6. [DOI] [PubMed] [Google Scholar]
- 23.Gadallah MF, Ramdeen G, Mignone J, et al. Role of preoperative antibiotic prophylaxis in preventing postoperative peritonitis in newly placed peritoneal dialysis catheters. Am J Kidney Dis. 2000;36:1014–1019. doi: 10.1053/ajkd.2000.19104. [DOI] [PubMed] [Google Scholar]
- 24.Piraino B, Bailie GR, Bernardini J, et al. ISPD Ad Hoc Advisory Committee. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–131. [PubMed] [Google Scholar]