Abstract
Nature, especially the marine environment, provides the most effective drugs used in human therapy. Among the metazoans, the marine sponges (phylum Porifera), which are sessile filter feeders, produce the most potent and highly selective bioactive secondary metabolites. These animals (or their associated symbiotic microorganisms) synthesize secondary metabolites whose activity and selectivity has developed during their long evolutionary history (evochemistry). The exploitation of these resources has become possible due to the progress in molecular and cell biology. BIOTECmarin, the German Center of Excellence follows this rationale. In the past, these animals have been successfully and extensively utilized to isolate bioactive compounds and biomaterials for human benefit. Pharmaceuticals prepared from marine animals, primarily sponges, have been applied since ancient times (Hippocrates, Aristotle and later Plinius). It has been reported that extracts and/or components from sponges can be used for the treatment of specific diseases. For a systematic and applied-oriented exploitation, the successful development of effective compounds largely depends on quality of the institutional infrastructure of marine stations and more so on the biodiversity. The Center for Marine Research in Rovinj (Croatia) fulfils these prerequisites. Founded in 1891, this institute has to its credit major discoveries related to exploitation of secondary metabolites/biomaterials from sponges for therapeutical application and to obtain biomaterials for general wellbeing.
This is the first part of a review focusing on biomedical prospecting. Here, we have mainly described the historic background. The details of techniques, substances, approaches and outlooks will be discussed in the second part.
Keywords: bioactive compounds, bioprospecting, sponges, history, medicine
Introduction
Natural products have been traditionally exploited as sources of bioactive metabolites for human benefit. Prior to the development of synthetic chemicals, plants and animal extracts were the only sources of organochemical and medical compounds. Documents dating back to Hippocrates [460–377 BC; he resided on the Greek island of Cos] (1,2), describe the application of natural products for the treatment of human diseases and injuries. Such naturally occurring bioactive compounds have a defined physiological role in the organism from which they are isolated. Organisms produce such metabolites as a protection mechanism against pathogens (foreign prokaryotes or eukaryotes). Until approximately 100 years ago, nature was the only ‘fermenter’ of compounds with therapeutic potential. One of the major progresses towards the discovery of synthetic bioactive compounds applicable in medicine was brought about by the synthesis of amidophenol-arsenoxide for the treatment of spirillosis (3).
Although religion did not permit establishing evolutionary links between the different taxa, and thereby did not provide the grounds for the discovery of common metabolic pathways, empiric biomedical prospecting for natural compounds was successful. Today, it is well established that, especially in the intermediary metabolism, the basic structural and controlling elements (metabolites and their converting enzymes; transcription factors) and systems (allosteric regulation) are conserved in plants, fungi and animals. They have been optimized during an evolutionary period of more than one billion years. Some metabolic pathways have originated in prokaryotic organisms. In parallel, nature has used the metabolites involved in the intermediary metabolism as a blueprint for the production and subsequent development/specialization of secondary metabolites for the defense against pathogens and control of diseases and injuries. Recently, the establishment of the common ancestry for the entire metazoan phyla (4,5) revealed that secondary metabolites found in the lowest metazoan phylum, namely, Porifera (sponges) act on the same biochemical targets as in the ‘crown’ species—insects (Protostomia) and mammals (Deuterostomia). A striking example is the secondary metabolite macrolide lactone FK506, which acts as an immunosuppressant in sponges as well as in humans by effectively preventing allograft rejection (6,7).
The discovery that secondary metabolites act on the same target within a given kingdom, in this case the Metazoa, led to the establishment of the discipline—evochemistry. During the evolution of multicellular organisms, continuous selection led to formation of secondary metabolites from primary metabolites. Subsequently, the metabolites developed optimal selectivity and activity. Therefore, secondary metabolites produced by plants or animals are superior to compounds synthesized by combinatorial chemistry. While the ‘proof of concept’ for bioactivity of secondary metabolites is clearly evident in nature, this is not the case for chemicals produced through combinatorial chemistry. Natural secondary metabolites are biologically active per se and identifying their mode of action is a challenging task for pharmacologists. In contrast, chemicals obtained by combinatorial chemistry need to be subjected to large-scale high throughput technology in order to detect potential bioactivity. As a result, synthetic organochemical libraries have a lower medicinal value. Out of 10 000 compounds, perhaps only one compound may pass phase III clinical trials.
However, the application of natural secondary metabolites as therapeutic drugs has not been very successful due to limited supply of compounds, which hamper investigation of their mode of action and the potential use/application in human therapy. Nevertheless, secondary metabolites dominate the drug spectrum used for cancer treatment (8).
Marine animals and animals that live as sessile filter feeders are the most promising organisms from which bioactive compounds can be sourced for potential human therapeutic applications. The reasons for this selection are as follows: Firstly, the physical conditions in a marine environment, e.g., greater density of the milieu (seawater against air) and reduced sunlight, allowed the formation of complex organismic interactions, which rapidly established a rich biodiversity. The higher physical density of the aqueous medium also allowed the compartmentation of the biotope, resulting in co-existence and co-evolution of diverse biota. Secondly, the sessile nature of Porifera, Cnidaria, Bryozoa and Tunicata, in combination with an efficient system of filtering water from the surrounding environment, forced the establishment of a primary or secondary reduced simplicity of the body plan with an advantageous access to sufficient nutrients. This evolutionary strategy could be successful only if these animals had effective protection mechanisms against pathogens.
The role of secondary metabolites as defense molecules in such animals was recently elucidated by the application of molecular biology techniques. Most of the studies were performed with marine sponges, primarily with the demosponge Suberites domuncula (reviewed in 9). It became apparent that S. domuncula can recognize bacteria and fungi through specific receptors leading to the activation of signal transduction pathways that discriminate between symbiotic and parasitic microbes. In sponges, the spectrum of self-self and non-self recognition is not restricted to bacteria and fungi but also comprises xenogeneic and allogeneic specimens (10,11). The defense strategies of the sessile marine filter feeders not only relies on an immune system, present from sponges to mammals (10), but also on the production of an array of low molecular weight bioactive compounds that display an unusually high diversity (12). It is, and always will be the task of science to isolate and characterize these compounds for exploitation under the aspect of sustainability.
An efficient bioprospecting program depends on the selection of a suitable biotope, characterized by a high diversity of sessile animals, and an institution with the required technical infrastructure. The marine biology station in Rovinj (Croatia), located at the Northern Adriatic Sea, meets these prerequisites. It facilitates both comprehensive bioprospecting and the subsequent successful procedures for an efficient biotechnological transfer, as performed at the German Center of Excellence BIOTECmarin.
An Example of Biodiversity in the Rovinj Area
The reports on the high biodiversity of marine animals in the Eastern Mediterranean Sea date back to Aristotle [384–322 BC], (cited in 13), who gave an extensive description of three commercially used sponge species near the island of Lesbos, in his 5th book on the ‘History of Animals.’ Focusing on the Adriatic Sea, the first few descriptions of marine animals were published by Donati (14) and Olivi (15). Along with the species, these scientists also depicted their biotope. Sponges were the predominant and prevalent taxon described. Shortly after, Esper (16) gave a detailed description of the marine plants and animals. However, it was only the illustrative descriptions given by Schmidt (17,18) and Drasche (19) that were decisive for a systematic compilation of the marine species in the Adriatic Sea. These authors outlined the vast biodiversity, especially of sponges, sea urchins, bryozoans and mollusks in the vicinity of Rovinj (Rovigno).
Center of Marine Research
Rovinj, a city of 13 000 inhabitants, is located (45°01′ lat./13°38′ long.) at the West coast of Istria in the Northern Adriatic Sea (Fig. 1). Since the city is protected by cliff from the sea and firm walls on the land, it provides a natural harbor for ships. Close to Rovinj there is a unique underwater biotope, the 11 km long ‘Limski Canal’ (a fjord-like bay). Since the 9th century, Istria was under the influence of Central Europe, mainly Germany, Dubrovnik and Venice and later Austria and Italy. In 2002, guidelines were introduced in the city for strategic development of economy in compliance with the rules for environmental protection and demands to protect cultural and historic heritage.
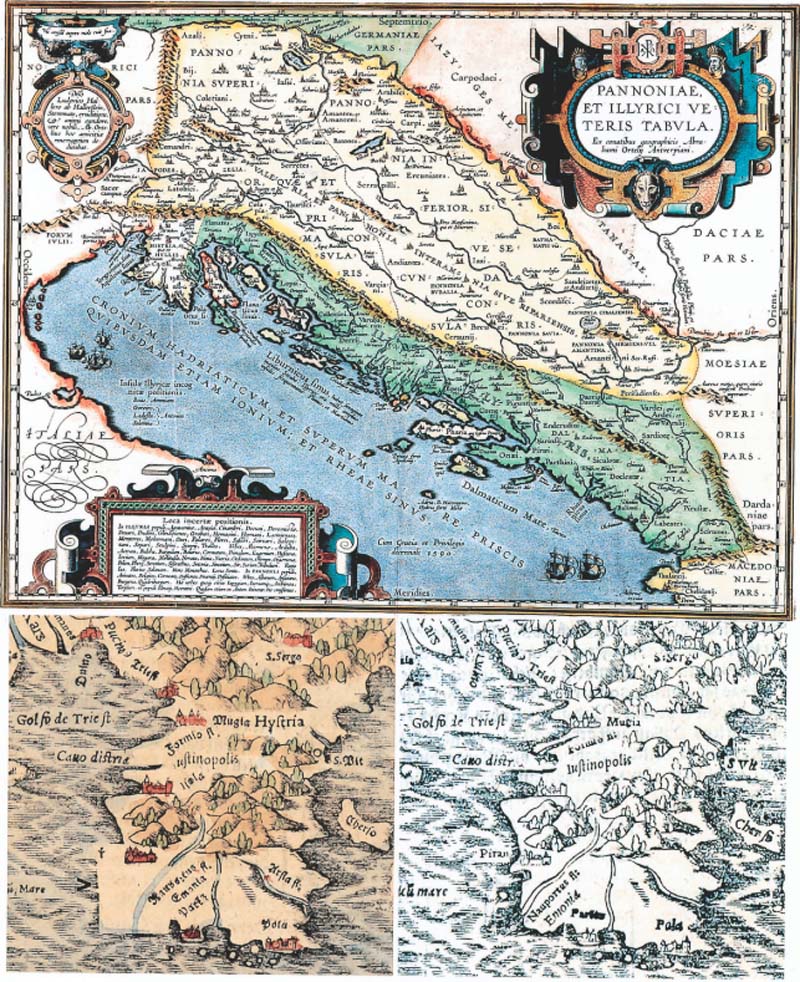
Figure 1.
Map of the Adriatic Sea as given in the ‘Pannoniae et Illyrici ve Teris Tabula’ by A. Ortelius 1590 (upper map). In the first edition of Cosmographia by S. Münster, the area around Rovinj (array) was not described (left) until 1582. In 1612, the name and the region was explicitly mentioned (right).
Close to the center of Rovinj, which is on a peninsula, the marine station was founded in 1891 as a field station of the Berlin Aquarium (Fig. 2). In 1900, the institute was expanded to its current-day size (610 m2). Currently, it is a department of the Institute ‘Ruder Boskovic’—Zagreb. Since the initial aim of the institute was to collect live marine animals for the Berlin Aquarium, it always had ready access to small motor boats, e.g., the Adria (Fig. 2) or the Rudolf Virchow. Presently, the institute possesses standard and sophisticated laboratory facilities, one research vessel Vila Velebita, a motor boat Burin and several small boats with outboard motors (reviewed in 20).
Figure 2.
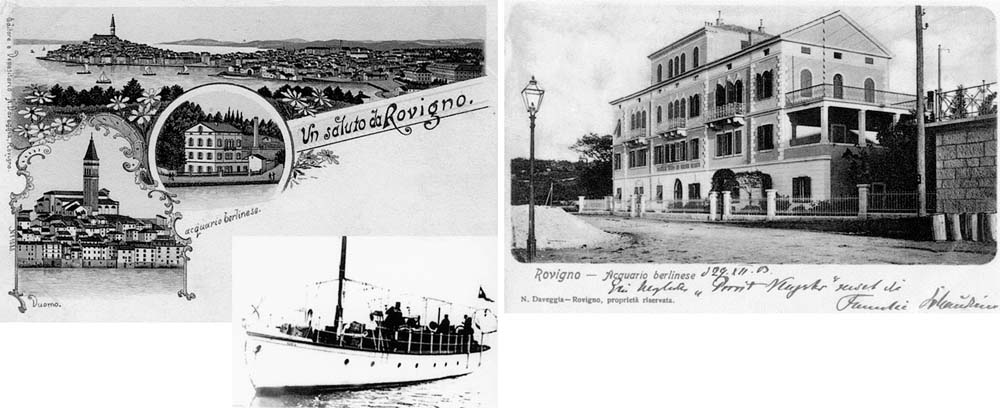
The center of Rovinj is located on a peninsula, 500 m to the north of which is the marine station, ‘Acquario Berlinese.’ It was founded in 1891 (lithography from 1898); left. The institute was expanded in 1900 (photograph taken in 1902; postcard was sent on December 29, 1903 from the famous parasitologist Fritz Schaudinn to Stanislaus von Prowazek); right. The research vessel ‘Adria’ is shown (1898); lower left.
The institute was influenced by a series of eminent scientists, for example, Schaudinn, von Prowazek and Steuer, who worked there in the initial phase of development. The major achievement of Schaudinn is the microscopical demonstration of the spirochaetes in syphilitic smears (21), whereas that of von Prowazek is the discovery of the biological cycle of trypanosomes (22) (Fig. 3). The planktonic migration and systematics of Copepoda have been studied in detail by Steuer (23) (Fig. 3). In the 1920s, M. Sella became the director of the institute and continued the work in the field of sponge biology/biotechnology (24). Prior to the opening of the institute, eminent scientists frequently visited Rovinj. For example, Lazzaro Spallanzani, who is the founder of modern embryology and biotechnology, resided in this region during 1782–1783. This naturalist, who also discovered blood leukocytes, observed that fertilization is essential for the development of new animals (25). He discovered that digestion is a (bio)chemical process and not merely mechanical grinding of food. Moreover, he observed that microorganisms could not be grown in bouillon after it was boiled (26). Spallanzani collected gastric juice by tying a piece of sponge to a string and allowing it to be swallowed. The sponge was then forcefully expelled out and the gastric juice was collected. Using this approach, he discovered that gastric juice can digest meat (26). These experiments were the first demonstrations of (enzymatic) reactions inside the living organism.
Figure 3.
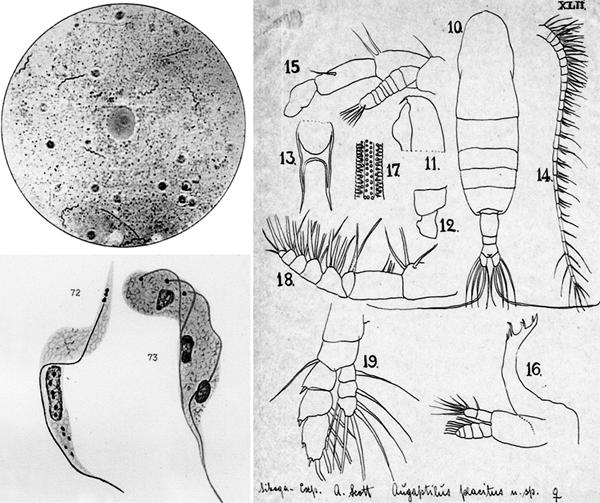
Basic and applied science in the affirmation period of the Marine Station in Rovinj. (Left/above) Photograph of spirochaetes in syphilitic smear, Giemsa staining (21), (left/below) stage of three-division of Trypanosoma brucei, Giemsa staining (22) and (right) Augaptilus placitus, a new copepodan species, described by Steuer (original).
Sponges
Sponges (phylum Porifera) are an outstanding animal taxon that first diverged from the common ancestor of the metazoan phyla—the hypothetical Urmetazoa (27). They possess a unique combination of simplicity and an adaptation ability that qualifies them as the most successful animal phylum. They have been in existence since more than 800 My and the basic body plan of some sponges like the Hexactinellida (28), has remained unchanged. They have developed amazingly rich and diverse arrays of defense mechanisms that give them a unique position among the other metazoan phyla. Therefore, they can be considered as living fossils with an enormous potential to improve the quality of human life, provided that technologies which enable a sustainable exploitation of their biomedical potential are developed (29).
As sessile organisms, they have developed specialized devices to utilize the water current through a more or less complex channel system in a manner that is efficient and energy saving, following the Bernoulli's principle (see 30). This system allows control of water flow and filtration of organic nutrients. The efficiency of the water current systems to maintain the basic requirements for suspension feeding is high. It was calculated that a marine sponge is able to process a water mass corresponding to its own volume every 5 s (30) or > 1 ton of water/day/kg of sponge; bionutrients are extracted from this water mass.
The species diversity of sponges around the Rovinj area is well studied. Research started with Schmidt (17,18) and was continued by Graeffe (31), Zimmermann (32), Vatova (33) and Rützler (34). Till date, approximately 140 to 200 species (reviewed in 35,36) have been identified. The total number of sponge species in the Mediterranean Sea is estimated to be 564 (37) and represents one of the highest diversities in the world. Interestingly, the Limski Canal harbors a series of (most likely) endemic sponge species, such as Tethya limski (38), Geodia rovinjensis (39) and Thoosa istriaca (40).
Other Invertebrates
In the order of evolution, animals that evolved after the Porifera, namely, the Cnidaria, protostomian taxon Ectoprocta (Bryozoa) and the deuterostomian Tunicata, also maintained the sessile organization level and are rich in chemical defense mechanisms as the sponges. The Mollusca with the order Nudibranchia are also well catalogued in the Rovinj area. The synascidians (19) and decapodes (41) have been especially well described.
Sustainable Exploitation
As mentioned above, the potential exploitation of components or bioactive compounds from marine animals and their subsequent therapeutic application in humans is limited by the difficulty of obtaining sufficient supply of the respective sponge species. Solutions to the problem of limited availability can be resolved by (1) chemical synthesis of the compounds, (2) cultivation of sponges in the sea (mariculture) or in a bioreactor and (3) the production of secondary metabolites in bioreactors using cultured sponge cells. These tasks require an understanding of the relationship between the taxon and its environment and also the knowledge of the effect of ecological diversity, e.g., bacterial species, in this particular linkage. Finally, the physiology and molecular biology of a given species must be elucidated to some extent in order to understand the pathway(s) by which these compounds are synthesized. Major progress in the field of biotechnology has been achieved using the sponge species.
Sponges
The commercial use of sponges is an age old concept. It can be dated back to the Stone and Bronze Ages. The use of sponges was considered very fashionable during the Crete/Mycenaean periods (2000 BC) (reviewed in 2). Bath sponges were of primary interest; skin diving or harpooning were the most favorite techniques for their collection (2). In the late 19th century, the first diving equipment, scaphander-diving, was employed for collection of sponges and also for enduring the low water temperatures during the winter season. The most important areas of commercial sponge fishery were in Europe (Spain/France, Adriatic Sea, Aegean Sea) and in America (Florida, Cuba, Bahamas) (see 2,42). It must be stressed here that apparently sponges have also been consumed (43). It has been reported that the sponge Chondrosia reniformis was sold in the market of Trieste (Italy) as fegato di mare (31) and was the poor man's steak on some Greek islands.
In the 19th century, sponge cultivation was attempted for their sustainable use. The sponge species that were predominantly cultivated for commercial use were the bath sponges, Hippospongia communis (of low quality) and Spongia officinalis. The latter species occurs in varieties ranging from medium (var. lacinulosa and var. usitatissima) to high quality (var. agaricina), as shown in Fig. 4. Besides the Caribbean, the Mediterranean Sea, especially the region around the Balkan, from Croatia to Greece (Fig. 4), was a major sponge trading center in the world (2). Further, the area around Rovinj, where the first sponge mariculture experiments were started (18,44), was of great interest for sponge fishing (Fig. 4).
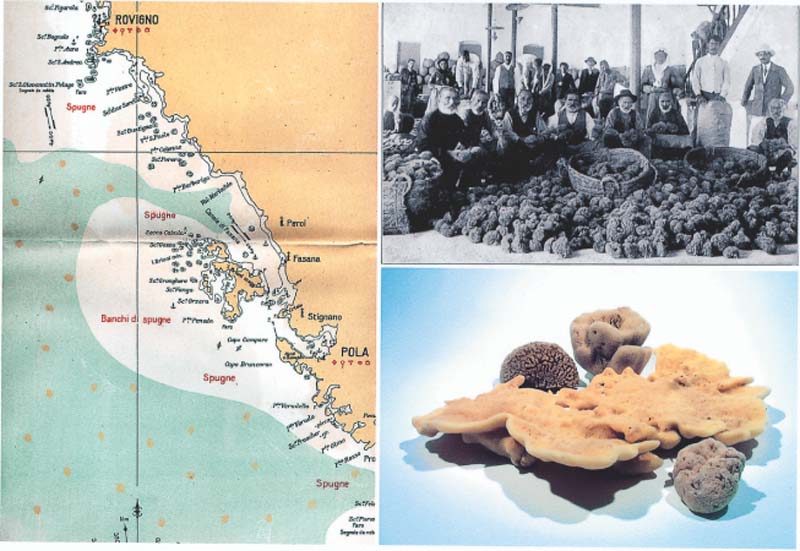
Figure 4.
Mariculture of sponges. (Left) Sponge fishing area around Rovinj (81). (Upper right) In the Eastern Mediterranean Sea, the island of Calymnos was the center of sponge fishing and trade (82). (Lower right) The quality of the bath sponges/spongids ranged from low (upper left; Hippospongia communis) to medium (upper right, Spongia officinalis var. lacinulosa; and below, Spongia officinalis var. usitatissima) to very high (center, Spongia officinalis var. agaricina).
The Romans had knowledge about the high regeneration capacity of sponges and this was highlighted again in the 18th century by Ellis (45 [p. 213]). Based on this property of sponges, Schmidt (18 [p. 25]) started the first rational approach for sponge culture which he named ‘Schwammfischerei’. The term mariculture matches the term ‘pisciculture’ introduced by Phipson (46 [p. 6]). Schmidt (18) proposed two techniques for the propagation of commercial sponges, i.e., either by cultivation of hatched young specimens or by dissection of medium-sized specimens into 1-cm large fragments which were then attached to wooden pegs. He started this approach along with Buccich from Hvar in the Rovinj area (44); they were more than partially successful. This cultivation method by fragmentation/transplantation is still in use in Calymnos, Greece, and has been steadily optimized, as shown in Fig. 5.
Figure 5.

Mariculture of sponges and mussels. Upper row: Technique of mariculture of oysters (Ostrea edulis) in the Canal di Leme, 10 km north of Rovinj, as performed in the 19th century (83). This technique, by which mussels are grown on a rope hanging from a floating scaffold, is conserved even today (lower row; right). (Lower row, left) Cultivation of sponges on a substrate produced by electrochemical precipitation. Here, the cultivation platform with several Tethya lyncurium specimens, growing at the mariculture platform in the Limski Canal is shown (×0.2).
A modern approach has been recently introduced. Fragments from corals or sponges are transplanted to a cathode mesh of a DC electrolytical system (e.g., applying the Arcon technology). The fragments are embedded into the Arcon substrate fed by solar energy during the precipitation phases (Fig. 5). The initial experiments showed that the survival of fragments is almost 100% and their growth rate was encouraging (47).
The second technique for the sustainable use of sponges for applied purposes is cell culture. It appears that sponge cells do not proliferate in suspension. We investigated some metabolic pathways to overcome the growth inhibition. Based on previous findings, which indicated that single cells from S. domuncula, Geodia cydonium or Dysidea avara are telomerase-negative, we concluded that it is more difficult to maintain suspension cultures of single sponge cells than those with tissue-like aggregates. This implies that sponge cells will require stimuli resulting from cell–cell and/or cell–matrix contact in order to proliferate. Our approach was successful (48,49), and it was applied for the production of the bioactive compound avarol (50). The 3D-cell aggregates, termed primmorphs, contain proliferating and differentiating cells (Fig. 6). After the transfer of these circular-shaped aggregates onto a homologous galectin matrix, the primmorphs begin to develop canal-like structures. The successful application of primmorphs for the production of bioactive compounds has been demonstrated: from S. domuncula [(2′-5′)oligoadenylate (51)] and from D. avara [avarol (50)].
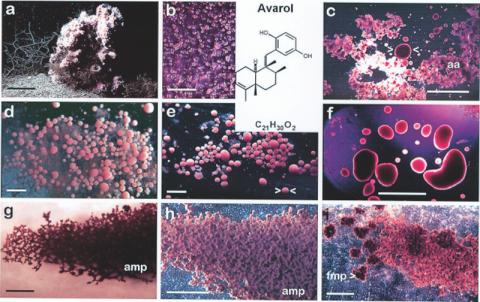
Figure 6.
Primmorph formation with cells from Dysidea avara and bioreactor application. (a) A specimen living on the hard bottom (bar corresponds to 50 mm). Re–aggregation of cells and formation of primmorphs: (b) dissociated single cell suspension (200 µm); (c) circular–shaped primmorphs [> <] surrounded by amorphous cell aggregates [aa] (5 mm); (d) circular–shaped, floating primmorphs associated onto the amorphous cell aggregates (5 mm); (e) circular–shaped primmorphs (5 mm); (f) large circular–shaped primmorphs (5 mm); (g) and (h) adherent mesh–primmorphs [amp] (1 mm), which stick to the plastic dish and (i) adherent mesh–primmorphs with formation of floating mesh–primmorphs [fmp] (1 mm). Modified according to Müller et al. (2000). Inset: structure of avarol.
The third approach is the isolation and expression of gene clusters. Gene clusters may be defined as linearly arranged units of genes. Functional linkage and expression of such clusters forms transcripts that code for intermediary metabolic pathways. These pathways involve (1) the catabolism of nutrients, (2) control of the initial and terminal differentiation and (3) synthesis of secondary metabolites. Gene clusters usually interact through cis-regulatory elements, a system that may be initiated by a trans-regulatory intra- or extracellular stimulus (see 52). Till date, gene clusters isolated from bacteria have been successfully used for the synthesis of macrolide antibiotics (53).
Raw Material—Tools
The use of sponges as food has also been documented (31); however, with the exception of mussels, none of these are currently consumed. On the other hand, sponges have been used as tools in cosmetics and hygiene, as a stabilizer in pottery and as tools for decorating and painting the walls of palaces, e.g., the King's palace in Knossos (1900 BC) and buildings, e.g., the Institute of Philosophy of the University of Genova (reviewed in 2). In 1903, over 2000 persons were employed in sponge fishery in the Florida counties, which was not the largest commercial area for sponge production (42). Therefore, the utilization of natural resources from sponges and other filter feeders for daily use or even religious ceremonies (see: Talmud or Bible) has been an important commercial factor for remote regions.
Other examples date back to the European Middle Ages: the shell design of the pilgrim's scallop (Pecten jacobaeus; a molluscan bivalve) became a religious emblem (the badge of St. James) (Fig. 7), primitive tribes in the Indian Ocean worshiped the Pecten jacobaeus (St. James Scallop) as a symbol of love and fertility. Very often, the Giant European scallop shell is seen on shrines, gravestones or as decoration in churches, like in Santiago (Spain) or in Porec, close to Rovinj (Fig. 7), which have/had been the destination of pilgrimages. The operculum of the molluscan gastropod Astraea rugosa is used locally around Rovinj as decoration and jewelry (Fig. 7).
Figure 7.
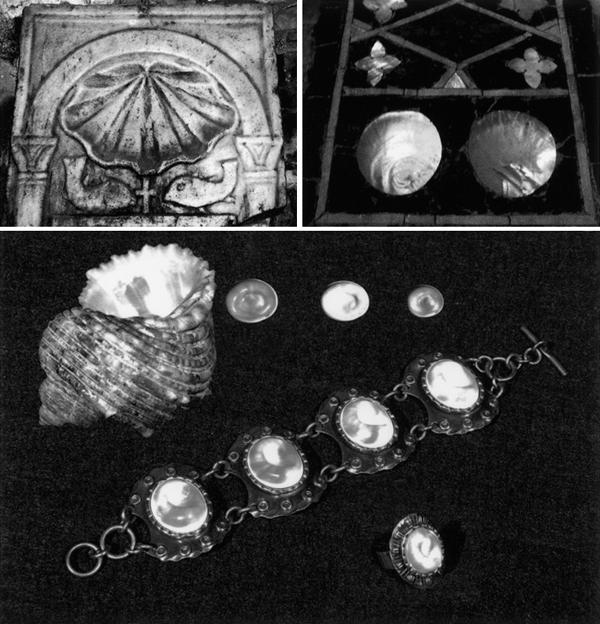
Use of mollusks as religious symbols or jewelry. (Upper panel) In the Euphrasius Basilica in Porec, built in the 6th century, the pilgrim's scallop (Pecten jacobaeus) appears on gravestones as well as on pillars. (Below) The operculum of the gastropod Astraea rugosa is used as jewelry.
Invertebrates
Approaches similar to those for the sponge culture have been undertaken for exploitation of natural resources/bioactive compounds from other invertebrates, with the main focus on mollusks. The Rovinj area, especially the Limski Canal, provides a suitable and natural biotope for mariculture activities. Since over 100 years, raising and cultivation of oysters (Ostrea edulis) and the Mediterranean mussel (Mytilus galloprovincialis) has been well established in the Canal di Leme (old Italian name for Limski Canal; Fig. 5). In the last 20 years, this technology has also been successfully applied for the cultivation of high-quality fish, e.g., the gilt-head bream (Sparus auratus). Besides field cultivation, it has also been applied for in vitro cultivation of mollusk cells from Mytilus galloprovincialis (54) or Pecten maximus (55).
Application for Human Benefit: History
Biomedical Application
Until the end of the 19th century, sponges had been widely used in surgery. An effective disinfection procedure that used sponges for aseptic treatment (57) and for replacement, e.g., during cesarean section (58) has been described (56). Sponges were also impregnated with extracts of opium, nightshade, hemlock, mandragora, ivy and lettuce seed. The ‘wetted’ sponge was inserted into the nostrils until the patient fell asleep and surgery could be performed. The ‘soporific sponge,’ as an anesthetic device, was widely used in the Middle Ages in European and Arabic culture. A comprehensive description has been given by Pfolsprundt (59) who summarized the techniques from the medieval period.
In traditional medicine practiced in Europe, the organic skeleton of the bath sponge was used, for example, Spongia cerata or Spongia gelatina to cover wounds, Spongia compressa to block heavy bleeding or Spongia jodoformiata or Spongia salicylata for disinfection (60,61).
In addition to the use as a matrix for soaking bioactive compounds, sponges were also used as medicine. A target-oriented therapeutic use of sponges in humans started when the successful application of Spongia tosta was established for the treatment of scrofulae, a thyroid gland disease (62 [p. 368]). Further progress was achieved with the discoveries made by Geoffroy (63). Geoffroy described the separation of a sponge into inorganic and organic fractions using a quantitative approach. This work was devoted to an application of the sel volatil for human therapy, for the treatment of struma, tumeurs scrophuleuses and for goiter, goëtres. Sponge extract was prepared and applied as syrup and also advised for topical use. Hufeland (64 [p. 139]) considered the application of Spongia tosta as the most effective treatment against scrofulae. Uhle (65) and Liebig (66 [322]) were of the opinion that roasted, dried resins from sponges used for the treatment of goiter/crop, e.g., Spongiae ustae, are occasionally contaminated with toxins. In 1910, it was demonstrated that iodine was an effective ingredient for sponge preparation, but other unknown parts were also considered active (67). It had been suggested that the use of sponges with their bioactive ingredients can be traced back to Plinius [23-79 AC] (1).
With the exception of corals, other invertebrate taxa are less frequently used for biomedical applications. The most famous description of the medical application of corals has been given by Gans (68 [120]) and it also included an extensive selection of recipes with extracts from corals, mainly focusing on the red coral. The ‘spiritu vitrioli antepileptico’ formulation prepared by him is used even today.
A systematic analysis for bioactive compounds in Porifera started only with the investigations of Richet (69 [598] and 70 [686]) who showed that the aqueous extract from S. domuncula, which had been subsequently fractionated with ethanol, displayed a toxic effect on dogs and rabbits. The active component was subsequently identified as a protein that was termed ‘Suberitin’ (71). Furthermore, lipids, which were associated with the symbiotic relationship between sponges and microorganisms were also identified (71,72). The first nucleoside analogues from sponges had been isolated from G. cydonium (73), among them was methyl-adenine. Since then, the search for bioactive compounds from sponges became a major task for natural chemists and was further advanced by the contributions of Bergmann (74) and Scheuer (75).
Biomaterials
Among the sponges, the Demospongiae and Hexactinellida contain a skeleton that is composed of hydrated, amorphous and noncrystalline silica. This inorganic material is deposited in their spicules, their major skeletal elements (76), as shown in Fig. 8. The morphology of the spicules is highly diverse and ranges from micrometers to centimeters in length. The surface of a freshwater sponge, the Baikalian sponge, Baikalospongia bacillifera, and a sterraster of G. cydonium are shown in Fig. 8 (left panel). The formation of silica in sponges occurs enzymatically. This implies that the spicules are formed at the ambient temperature at which the sponges live, i.e., between 2°C and 35°C (Fig. 8, left panel), while the chemical reaction requires high temperatures, at least >500°C. During the Chernobyl accident in 1986, the fuel rods melted and resulted in the formation of silicate crystals in some cases, the Chernobylites (77). Their formation required at least 4–5 days at 1650°C (Fig. 8, right panel). Sponge biosilicate spicules are formed enzymatically within 1–2 days at low temperature.
Figure 8.
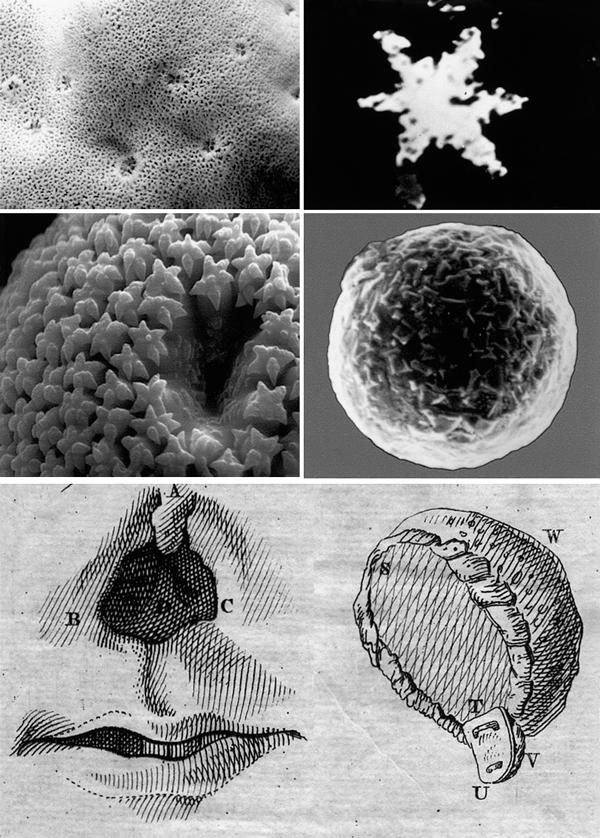
Natural biosilica (sponge spicules) and chemically formed zirconium orthosilicate. Natural biosilica (Upper left) the surface of the freshwater sponge Baikalospongia bacillifera is shown. The size of the larger openings of the oscules (excurrent water canals) is 2 mm, while the small pores (incurrent water canals) have a size of 0.05 mm. (Lower left) A sterraster of G. cydonium of a size of 0.120 mm is shown (courtesy Dr. T. Reiber and Dr. W. Tremel, Mainz). Chemically formed zirconium orthosilicate (right panel) silica crystals formed during the high-temperature process resulting in the formation of Chernobylite (courtesy Dr. E.M. Pazukhin, Chernobyl). Crystal size: above 0.01 mm; below 0.1 mm. (Below) Application of sponges in tissue replacement (Camper 1771). Damaged nose/nostrils (A–D) was modeled by a piece of lime wood covered with a sponge (T, U, V), and fixed in the roof of the mouth (W) via a silk thread that had been surrounded with small sponge slices.
The application of biomaterial obtained from marine animals as plaster and in bone/tissue replacement has been a tradition since Greek times. Camper (78) described that the organic matrix of sponges can be successfully used in plastic surgery of the palate of the skull (Fig. 8, lower panel). He developed a nose using lime wood, covered it with a sponge and fixed it in the roof of the mouth via a silk thread which had been waded by small sponge slices. With this prosthesis, he covered the cavity of the nose with a punch. The application of sponges as a matrix for the preparation of plaster has also been well described (66[142]). It is interesting to note that the application of the siliceous skeleton of sponges as suitable scaffold onto which human stem cells can be seeded has been recently reported (79).
Application for Human Benefit: Modern Developments
Since ancient times, marine filter feeders have been widely used for commercial trade and human benefit. Extracts from sponges and other filter feeders have been analyzed, and the bioactive secondary metabolites have been isolated. Some of them were found to be promising candidates for human therapy (9,80). Furthermore, the elucidation of the genetic blueprint of the skeletal elements of these animals, e.g., the siliceous spicules of sponges led to new horizons in biotechnology, toward exploiting these resources for the synthesis of biomaterials (76). A more comprehensive review on this topic will be described in the second part of this review.
The basis for the rapid and sustainable exploitation of the biodiversity of chemical compounds and structures is evident in the unexpectedly large genomic diversity of the base-stem of metazoan taxa. Especially in the field of chemical ecology of Porifera, a tremendous potential benefit for humans from the application viewpoint can be expected. Chemical ecology is a specialized sub-discipline of ecology. This field of research focuses on the study of secondary metabolites synthesized by the organisms that play a role in the metabolism of the producers and also in their survival strategies in a given environment (Fig. 9A). Taking into account that (1) the chemical diversity of natural bioactive compounds is much higher than that of compounds synthesized by standard combinatorial chemistry approaches and (2) natural compounds display an impressive selectivity, value of the secondary metabolites from natural resources in general and from sponges in particular can only roughly be envisaged. It is also conceivable that especially during first screening programs aimed at discovering new lead molecules that are directed against newly discovered targets, the natural compounds are superior to those generated by combinatorial chemistry. During the long evolutionary period of 800 My, the optimal bioactive compounds against particular organismic disorders were developed. Therefore, the highly diverse chemical compounds obtained from sponges, which possess diverse bioactivities, are extremely valuable.
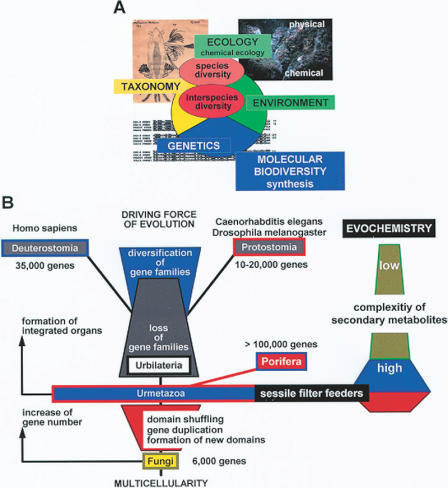
Figure 9.
Evochemistry. (A) Effect of physical and chemical parameters as driving forces of the environment on the organismic world. The interactions between species/interspecies diversity and environment are studied by ecology and chemical ecology. Genetics explains the evolution of the organismic world and molecular biodiversity links taxonomy and genetics with ecology. (B) Proposed evolutionary processes proceed from Yeast to Urmetazoa and subsequently from this hypothetical ancestor of Metazoa to Deuterostomia [Homo sapiens] and Protostomia [Caenorhabditis elegans and in Drosophila melanogaster]. It is proposed that the number of genes calculated to be present in sponges and—very likely also in Urmetazoa—has been created by gene duplication. New domains have also been formed during this process, giving rise to novel mosaic proteins. In the metazoan phyla, derived from Urmetazoa, the number of genes decreased by elimination of gene families and the remaining gene families diversified in parallel. Based on the higher gene complexity in the lower metazoan phyla, their potency to synthesize a more complex spectrum of bioactive compounds also became possible. During their evolutionary history, bioactive secondary metabolites developed, and hence they are more potent and more specific [evochemical history of these compounds].
The reason behind the success of Porifera and other phylogenetically old taxa, which are sessile filter feeders, during the long evolutionary period of 800 My is unclear. Recently, it had been demonstrated that Porifera comprise a large genome of approximately 1670 Mb (52). Since the genome is compact (≈5000 nts/gene), the expected number of genes in the sponge genome might be >100 000, which is comparatively large. The human genome contains ≈35 000 genes, Caenorhabditis elegans (nematode [worm]) ≈19 000, the insect Drosophila melanogaster ≈14 000 and the yeast Saccharomyces cerevisiae 6000 genes. Two major conclusions are drawn: Firstly, during the transition from Yeast to the Urmetazoa, a period of dramatic gene duplication must have occurred; secondly, during the evolutionary period from the Urmetazoa to the Protostomia [C. elegans and D. melanogaster] and the Deuterostomia [Homo sapiens], a reduction in the number of genes must have taken place. In parallel with the metazoan evolution by loss of genes [domains and families] a second evolutionary progress, which occurred after Urmetazoa, must be postulated for the metazoan phyla, an evolution by diversification of gene families (Fig. 9B). The hypothetical Urmetazoa has been proposed as the last common ancestor for all Metazoa (4).
The finding that the number of sponge genes is higher than that in the ‘crown’ phyla also supports the explanation for comparably high diversity of the secondary metabolites in sponges and other filter feeders. In order to survive environmental threats, these animals formed bioactive compounds from the regular metabolites. During their long evolutionary history, the biological/defensive/pharmacological effectiveness of these compounds was streamlined for higher potency and selectivity (evochemistry). As a result, the abundance of bioactive compounds in the sessile filter feeders can be attributed to their growth and nutritional characteristics and their comparably large genetic repertoire.
The identification of bioactive compounds from sponges is the main focus of the German Center of Excellence BIOTECmarin, which is coordinated by the senior author of this review. All activities follow the approach of sustainable exploitation of the biodiverse sponge taxon with respect to its bioactive potential. Therefore, immense efforts have been made to use the starting material in an environmentally tolerable manner, which allows the upscaling of pure bioactive compounds to an extent, allowing animal testing or even human trials.
Acknowledgments
This study was supported by the Bundesministerium für Bildung und Forschung (project: Center of Excellence BIOTECmarin) and the International Human Frontier Science Program [RG-333/96-M].
References
- 1.Arndt W. Die Verwendung der Spongien in der Medizin. Arch Naturgesch. 1925;90:149–174. (Abt A). [Google Scholar]
- 2.Arndt W. Schwämme. In: Pax F, Arndt W, editors. Die Rohstoffe des Tierreichs. Vol. I/2. Berlin: Bornträger; 1937. pp. 1577–2000. [Google Scholar]
- 3.Ehrlich P, Hata S. Die Experimentelle Chemotherapie der Spirillosen. Berlin: Julius Springer; 1910. [Google Scholar]
- 4.Müller WEG. How was metazoan threshold crossed: the hypothetical Urmetazoa. Comp Biochem Physiol. 2001;129:433–460. doi: 10.1016/s1095-6433(00)00360-3. [A]. [DOI] [PubMed] [Google Scholar]
- 5.Müller WEG, Schröder HC, Skorokhod A, Bünz C, Müller IM, Grebenjuk VA. Contribution of sponge genes to unravel the genome of the hypothetical ancestor of Metazoa (Urmetazoa) Gene. 2001;276:161–173. doi: 10.1016/s0378-1119(01)00669-2. [DOI] [PubMed] [Google Scholar]
- 6.Müller WEG, Steffen R, Lorenz B, Batel R, Kruse M, Krasko A, et al. Suppression of allograft rejection in the sponge Suberites domuncula by FK506 and expression of genes encoding FK506-binding proteins in allografts. J Exp Biol. 2001;204:2129–2207. doi: 10.1242/jeb.204.13.2197. [DOI] [PubMed] [Google Scholar]
- 7.Müller WEG, Müller IM. Origin of the metazoan immune system: identification of the molecules and their functions in sponges. Integr Comp Biol. 2003;43:281–292. doi: 10.1093/icb/43.2.281. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RW. Drugs from the sea: harvesting the results of aeons of chemical evolution. Mol Med Today. 1997;3:277–281. doi: 10.1016/S1357-4310(97)01059-9. [DOI] [PubMed] [Google Scholar]
- 9.Müller WEG, editor. Marine Molecular Biotechnology. Berlin: Springer; 2003. Sponge (Porifera) [Google Scholar]
- 10.Müller WEG, Blumbach B, Müller IM. Evolution of the innate and adaptive immune systems: relationships between potential immune molecules in the lowest metazoan phylum [Porifera] and those in vertebrates. Transplantation. 1999;68:1215–1227. doi: 10.1097/00007890-199911150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Müller WEG, Krasko A, Skorokhod A, Bünz C, Grebenjuk VA, Steffen R, et al. Histocompatibility reaction in the sponge Suberites domuncula on tissue and cellular level: central role of the allograft inflammatory factor 1. Immunogenetics. 2002;54:48–58. doi: 10.1007/s00251-002-0441-0. [DOI] [PubMed] [Google Scholar]
- 12.Sarma AS, Daum T, Müller WEG. Akademie gemeinnütziger Wissenschaften zu Erfurt. Berlin: Ullstein-Mosby Verlag; 1993. Secondary Metabolites from Marine Sponges. [Google Scholar]
- 13.Camus M. Histoire des Animaux d'Aristote. Paris: Desaint; 1783. [Google Scholar]
- 14.Donati V. Auszug seiner Natur-Geschichte des Adriatischen Meers. Halle: CP Franckens; 1753. [Google Scholar]
- 15.Olivi G. Zoologia Adriatica ossia catalogo regionato degli animali del golfo e delle lagune di Venezia. Bassano: 1792. pp. 1–32. [Google Scholar]
- 16.Esper EJC. Die Pflanzenthiere. Raspe: Nürnberg; 1794. [Google Scholar]
- 17.Schmidt O. Die Spongien des Adriatischen Meeres. Leipzig: Engelmann; 1862. [Google Scholar]
- 18.Schmidt O. Spongien des Adriatischen Meeres—Supplement. Leipzig: Engelmann; 1864. [Google Scholar]
- 19.Drasche Rv. Synascidien der Bucht von Rovigno (Istrien) Wien: Carl Gerold; 1883. [Google Scholar]
- 20.Zavodnik D. A North Adriatic centenarian: the marine research station at Rovinj. Helgol Meeresunters. 1995;49:441–453. [Google Scholar]
- 21.Schaudinn F, Hoffmann E. Arbeiten aus dem Kaiserlichen Gesundheitsamte. Vol. 22. Berlin: 1905. Vorläufiger Bericht über das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen; pp. 527–534. [PubMed] [Google Scholar]
- 22.Prowazek Sv. Arbeiten aus dem Kaiserlichen Gesundheitsamte. Vol. 22. Berlin: 1905. Studien über Säugetiertrypanosomen; pp. 350–394. [Google Scholar]
- 23.Steuer A. Planktonkunde. Leipzig-Berlin: BG Teubner; 1910. [Google Scholar]
- 24.Sella M. La pesca della spugne nella Libia. R Comitato Talassografico Italiano. 1912;13:1–154. [Google Scholar]
- 25.Spallanzani L, Senebier J. Versuche über die Urzeugung der Thiere und Pflanzen. Leipzig: GJ Göschen; 1786. [Google Scholar]
- 26.Spallanzani L, Senebier J. Experiences sur la Digestion de l'Homme et de Différentes especes d'Animaux. Geneve: B. Chirol; 1784. [Google Scholar]
- 27.Müller WEG. The origin of metazoan complexity: Porifera as integrated animals. Integr Comp Biol. 2003;43:3–10. doi: 10.1093/icb/43.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Mehl D, Müller I, Müller WEG. Molecular biological and palaeontological evidence that Eumetazoa, including Porifera (sponges), are of monophyletic origin. In: Watanabe Y, Fusetani N, editors. Sponge Science—Multidisciplinary Perspectives. Tokyo: Springer-Verlag; 1998. pp. 133–156. [Google Scholar]
- 29.Müller WEG, Brümmer F, Batel R, Müller IM, Schröder HC. Molecular biodiversity. Case study: Porifera (sponges) Naturwissenschaften. 2003;90:103–120. doi: 10.1007/s00114-003-0407-6. [DOI] [PubMed] [Google Scholar]
- 30.Vogel S. Life in Moving Fluids. Princeton: Princeton University Press; 1994. [Google Scholar]
- 31.Graeffe E. Übersicht der Seethierfauna des Golfes von Triest. Arb Zool Inst Wien-Triest. 1882;4:313–321. [Google Scholar]
- 32.Zimmermann H. Tierwelt am Strande der blauen Adria. Z Naturwiss. 1907;1:293–322. [Google Scholar]
- 33.Vatova A. Compendio della Flora e Fauna del Mare Adriatico presso Rovigno. R Comitato Talassografico Italiano. 1928;143:1–613. [Google Scholar]
- 34.Rützler K. Systematik und Ökologie der Poriferen aus Litoral-Schattengebieten der Nordadria. Zeitschr Morphol Ökol Tiere. 1965;55:1–82. [Google Scholar]
- 35.Müller WEG, Zahn RK, Kurelec B, Müller I. Catalogue of the sponges near Rovinj. Thalassia Jugoslavica. 1984;20:13–23. [Google Scholar]
- 36.Brümmer F, Calcinai B, Götz M, Leitermann F, Nickel M, Schillak L, et al. Overview on the sponge fauna of the Limski Kanal, Croatia, Northern Adriatic Sea. Boll Musei Institut Biol. 2004 (Genova), in press. [Google Scholar]
- 37.Pansini M. Considerazione sulla biodiversitá die poriferi die mari italiani e del Mediterraneo. Biol Mar Medit. 1996;3:128–135. [Google Scholar]
- 38.Müller W, Zahn RK. Tethya limski n.sp, eine Tethyide aus der Adria (Porifera: Homosclerophorida: Tethyidae) Senckenbergiana Biol. 1968;49:469–478. [Google Scholar]
- 39.Müller I, Zahn RK, Zahn G, Rijavec M, Batel R, Kurelec B, et al. Description of Geodia rovinjensis n.sp. on the basis of immunological and morphological criteria. Thalassia Jugoslavica. 1983;19:279–283. [Google Scholar]
- 40.Müller WEG, Zahn RK, Rijavec M, Britvic S, Kurelec B, Müller I. Aggregation of sponge cells. The aggregation factor as a tool to establish species. Biochem Syst Ecol. 1979;7:49–55. [Google Scholar]
- 41.Stevcic Z. Decapod fauna of seagrass beds in the Rovinj area. Acta Adriatica. 1991;32:637–653. [Google Scholar]
- 42.Moore HF. The commercial sponges and the sponge fisheries. Bull Bureau Fisheries. 1908;28:399–511. [Google Scholar]
- 43.Darwin E, Brandis JD. Zoonomie oder Gesetze des Organischen Lebens. Hannover: Hahn; 1799. [Google Scholar]
- 44.Buccich G. Alcune spugne dell' Adriatico sonosciute e nuove. Boll Soc Adr Sci Nat Trieste. 1886;9:222–225. [Google Scholar]
- 45.Ellis J. The Natural History of Many Curious and Uncommon Zoophytes, Collected from Various Parts of the Globe. London: Benjamin White; 1786. [Google Scholar]
- 46.Phipson TL. The Utilization of Minute Life. London: Groombridge; 1864. [Google Scholar]
- 47.Schillak L, Ammar MSA, Müller WEG. Transplantation of coral species to electrochemical produced hard substrata: Stylophora pistillata (Esper, 1797 and Acropora humilis Dana, 1846) ACP-EU Fish Res Rep. 2001. pp. 68–84. Mombasa, Kenya, 19–22 June 2000, Brussels.
- 48.Custodio MR, Prokic I, Steffen R, Koziol C, Borojevic R, Brümmer F, et al. Primmorphs generated from dissociated cells of the sponge Suberites domuncula: A model system for studies of cell proliferation and cell death. Mech Ageing Develop. 1998;105:45–59. doi: 10.1016/s0047-6374(98)00078-5. [DOI] [PubMed] [Google Scholar]
- 49.Müller WEG, Wiens M, Batel R, Steffen R, Borojevic R, Custodio MR. Establishment of a primary cell culture from a sponge: primmorphs from Suberites domuncula. Marine Ecol Progr Ser. 1999;178:205–219. [Google Scholar]
- 50.Müller WEG, Böhm M, Batel R, De Rosa S, Tommonaro G, Müller IM, et al. Application of cell culture for the production of bioactive compounds from sponges: synthesis of avarol by primmorphs from Dysidea avara. J Nat Prod. 2000;63:1077–1081. doi: 10.1021/np000003p. [DOI] [PubMed] [Google Scholar]
- 51.Grebenjuk VA, Kuusksalu A, Kelve M, Schütze J, Schröder HC, Müller WEG. Induction of (2′-5′)oligoadenylate synthetase in the marine sponges Suberites domuncula and Geodia cydonium by the bacterial endotoxin lipopolysaccharide, Eur J Biochem. 2002;269:1382–1392. doi: 10.1046/j.1432-1033.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 52.Breter HJ, Grebenjuk VA, Skorokhod A, Müller WEG. Approaches for a sustainable use of the bioactive potential in sponges: analysis of gene clusters, differential display of mRNA and DNA chips. In: Müller WEG, editor. Sponges (Porifera). Marine Molecular Biotechnology. Berlin: Springer; 2003. pp. 199–230. [DOI] [PubMed] [Google Scholar]
- 53.Rawlings BJ. Biosynthesis of polyketides. Nat Prod Rep. 1997:523–556. doi: 10.1039/np9971400523. [DOI] [PubMed] [Google Scholar]
- 54.Micic M, Bihari N, Jakšic Z, Müller WEG, Batel R. DNA Damage and apoptosis in the mussel Mytilus galloprovincialis. Marine Env Res. 2002;53:243–262. doi: 10.1016/s0141-1136(01)00112-x. [DOI] [PubMed] [Google Scholar]
- 55.Le Marrec-Croq F, Glaise D, Guguen-Guillouzo C, Chesné C, Guillouzo A, Boulo V, et al. Primary cultures of heart cells from the scallop Pecten maximus (mollusca-bivalvia) In Vitro Cell Dev Biol. 1999;35:289–295. doi: 10.1007/s11626-999-0073-x. [DOI] [PubMed] [Google Scholar]
- 56.Elsberg CA. Ein neues und einfaches Verfahren zur Sterilisation der Schwämme durch Auskochen. Centralblatt f Chirurgie. 1900;27:1289–1290. [Google Scholar]
- 57.Hacker VR., v . Anleitung zur antiseptischen Wundbehandlung. Wien, Toeplitz & Deuticke: 1883. [Google Scholar]
- 58.Gräfe CF., v. Über Minderung der Gefahr beim Kaiserschnitte, nebst der Geschichte eines Falles, in welchem Mutter und Kind erhalten wurden. J Chirurgie. 1826;9:1–85. [Google Scholar]
- 59.Pfolsprundt H., v . In: Buch der Bündth-Ertznei – 1460. Haeser H, Middeldorpf A, editors. Berlin: Georg Reimer; 1868. [Google Scholar]
- 60.Rust JN. Theoretisch-praktisches Handbuch der Chirurgie, mit Einschluss der syphilitischen und Augen-Krankheiten. Vol. 15. Berlin: Enslin; 1835. [Google Scholar]
- 61.Dieterich E. Neues Pharmaceutisches Manual. Berlin: Julius Springer; 1887. [Google Scholar]
- 62.Muralt J., v . Hippocrates Helveticus oder der getreu-sichere und wohl-bewährte Eydgnössische Stadt-Land-und Hauß Artzt. Basel: König; 1692. [Google Scholar]
- 63.Geoffroy M. Analyse chimique de l'eponge de la moyenne espece. Histoire de l'Acad Roy Scie. 1731:507–508. [Google Scholar]
- 64.Hufeland CW. Ueber die Natur, Erkenntnißmittel und Heilart der Skrofelkrankheit, Wien: Ghelensche Schriften; 1798. [Google Scholar]
- 65.Uhle AF. De Spongia Marina. Lipsiae: 1819. [Google Scholar]
- 66.Liebig J. Handbuch der Chemie. Wien: CF Winter; 1843. [Google Scholar]
- 67.Scott L. Über Spongin. Biochem Z. 1910;27:266–269. [Google Scholar]
- 68.Gans JL. Corallorum Historia. Frankfurt: Lucae Iennis; 1630. [Google Scholar]
- 69.Richet C. De l' action toxique de la Subéritine (extrait aqueux de Suberites domuncula) Comptes Rend Soc Biol. 1906;61:598–600. [Google Scholar]
- 70.Richet C. De la variabilité de la dose toxique de Subéritine. Comptes Rend Soc Biol. 1906;61:686–688. [Google Scholar]
- 71.Lassablière O. Influence des injections intraveneuses de Subéritine sur la resistance globulaire. Comptes Rend Soc Biol. 1906;61:600–601. [Google Scholar]
- 72.Arndt W. Über Lipoide und Lipoidstoffwechsel bei Evertebraten. Verh Deut Zool Ges. 1922;27:76–78. [Google Scholar]
- 73.Ackermann D, Holtz J, Reinwein H. Über das Vorkommen von Methyladenin, Dimethylhistamine, Guanidin, Betain und Eledonin bei Geodia gigas. Z Biol. 1924;82:278–284. [Google Scholar]
- 74.Bergmann W, Feeney RJ. Contribution to the study of marine sponges. 32. The nucleosides of sponges. J Org Chem. 1951;16:981–987. [Google Scholar]
- 75.Scheuer PJ. Chemistry of Marine Natural Products. New York-London: Academic Press; 1973. [Google Scholar]
- 76.Müller WEG, editor. Silicon Biomineralization: Biology-Biochemistry-Molecular Biology-Biotechnology. Progress Molecular Subcellular Biology. Vol. 33. Berlin: Springer; 2003. [Google Scholar]
- 77.Pazukhin EM. Fuel-containing lavas of the Chernobyl NPP fourth block: topography, physicochemical properties, and formation scenario. Radiochemistry. 1994;36:109–154. [Google Scholar]
- 78.Camper Petrus. Naauwkeurige Afbelding en Beschryving van eene geheel en al verloorene, Maar door Kunst herstelde Neus en Verhemelte. Amsterdam: Seep–Boekverkooper; 1771. [Google Scholar]
- 79.Green D, Howard D, Yang X, Kelly M, Oreffo RO. Natural marine sponge fiber skeleton: a biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003;9:1159–1166. doi: 10.1089/10763270360728062. [DOI] [PubMed] [Google Scholar]
- 80.Donia M, Hamann MT. Marine natural products and their applications as anti-infective agents. Lancet Infect Dis. 2003;3:228–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saga A. La carta di pesca del compartimento marittimo di Pola. Bollettino di Pesca, di Piscicoltura e di Idrobiologia, 1927;III/II:15–16. [Google Scholar]
- 82.Volonakis MD. The Island of Roses and her Eleven Sisters. London: MacMillan; 1922. [Google Scholar]
- 83.Krisch A. Die Fischerei im Adriatischen Meere. Pola: Carl Gerold; 1900. [Google Scholar]