Abstract
Vitamin D is an important physiologic regulator of bone and mineral metabolism. In chronic kidney disease, reduced renal production of calcitriol contributes to secondary hyperparathyroidism (SHPT). Consequently, supplementation with vitamin D sterols is an important treatment for SHPT and its associated mineral and bone disorders. However, doses of vitamin D sterols required to suppress parathyroid hormone (PTH) secretion often promote hypercalcaemia and hyperphosphataemia. Therefore, there is a trade-off between reduced serum PTH and increased levels of serum calcium, phosphorus and calcium–phosphorus product. It has been suggested that treatment of SHPT with cinacalcet, a type II calcimimetic, with reduced doses of vitamin D sterols could enhance achievement of calcium and phosphorus treatment targets while maintaining goals for PTH. Recent clinical trials have evaluated this hypothesis and demonstrated that treatment with cinacalcet in combination with reduced doses of vitamin D sterols is an effective treatment for the management of SHPT.
Keywords: calcimimetics, calcium, parathyroid hormone, phosphorus, secondary hyperparathyroidism, vitamin D
Biologic role of calcitriol
Physiologic effects
The chief physiologic role of calcitriol (the active form of vitamin D) is the maintenance of bone mineralization and turnover through increased absorption of calcium and phosphorus and suppression of parathyroid hormone (PTH) synthesis. Calcitriol increases calcium and phosphorus absorption from the gut; in excess, it mobilizes calcium from bone; and, in the parathyroid gland, suppresses PTH synthesis [1]. These effects of calcitriol are mediated through the vitamin D receptor (VDR), a ligand-activated nuclear receptor that modulates gene transcription in vitamin D-sensitive tissues through interaction with vitamin D-response elements (VDREs) [2–4]. The VDR is localized not only in target organs involved in mineral homeostasis but also in many other tissues. Nonclassic vitamin D systems and tissues include the immune system [5,6], myeloid tissue [7–9], the heart [10], skeletal muscle [11], the brain, and nerve tissue [12–14]. This wide distribution of the VDR suggests that calcitriol has physiologic roles other than mineral homeostasis. These physiologic roles have been extensively reviewed and, consistent with the nonclassic vitamin D tissues described above, include effects on the haematopoietic system, immune system, skin, muscle and nervous system [1,2,15,16]. Evidence also suggests that calcitriol can mediate nongenomic actions through a cell surface receptor distinct from the nuclear VDR [1].
The synthesis of calcitriol (the most potent endogenously produced vitamin D metabolite) occurs primarily in the kidney but also in a variety of other tissues [17,18]. The renal production of calcitriol is tightly regulated and is thought to account primarily for the level of calcitriol in serum under normal physiologic circumstances [1]. Control of calcitriol is achieved through the coordinated action of the kidneys, intestine, bone and parathyroid glands in response to physiologic calcium and phosphorus requirements [1]. Serum calcium, phosphorus and PTH regulate vitamin D through activation or suppression of the enzymes involved in its synthesis, bioactivation and catabolism [17].
Role of calcitriol in the development of SHPT in chronic kidney disease
A common complication of chronic kidney disease (CKD) is the development of secondary hyperparathyroidism (SHPT) [19]. SHPT begins to develop when reduced renal function results in dysregulation of the complex interactions involved in calcium and phosphorus homeostasis. Reduced renal excretion of phosphorus and synthesis of calcitriol both contribute to the development of hypocalcaemia that, in turn, leads to increased serum PTH [19]. Both the parathyroid gland calcium-sensing receptor (CaR) and VDR are down-regulated in the hyperplastic parathyroid gland, resulting in reduced sensitivity to calcium and vitamin D, respectively [19–22]. This abnormal calcium sensing leads to elevated serum PTH and abnormalities in divalent ion homeostasis, which have been associated with increased morbidity and mortality [23]. In particular, increased calcium–phosphorus product (Ca × P) is associated with vascular calcification [24,25].
In addition to the effects of reduced calcitriol on serum calcium that contribute to SHPT, a decrease in calcitriol has a number of direct effects that influence the development of SHPT. Calcitriol reduces parathyroid gland synthesis of PTH through the actions of the VDR on two VDREs located in the 5′ promoter region of the PTH gene [1,4]. In animal studies, calcitriol was shown to regulate the CaR in the parathyroid gland. Compared with controls, rats fed a vitamin D-deficient diet had reduced parathyroid gland CaR mRNA content [26]. Administration of calcitriol increased CaR mRNA. Interestingly, rat CaR mRNA was unaffected by diet-induced changes in serum ionized calcium [26].
Vitamin D sterol therapy in patients with CKD
Treatment with vitamin D sterols in CKD patients on dialysis has two principle goals. First, it corrects the vitamin D deficiency that commonly develops as renal function declines [27], and second, treatment with higher doses of vitamin D sterols has been demonstrated to decrease serum PTH [28,29]. Therefore, therapy with vitamin D sterols has become a primary treatment for SHPT [30]. Because vitamin D sterols also act to promote intestinal absorption of calcium and phosphorus and, in excess, mobilize calcium from bone [1], this effect on PTH comes at the expense of increased serum calcium, phosphorus and Ca × P. Indeed, clinical studies have shown that treatment with vitamin D sterols can increase serum calcium [31–34]. In some instances, increases in serum phosphorus [34] and Ca × P have been reported [33,34]. Thus, as with other traditional therapies for SHPT in CKD (such as phosphate binders), there is a trade-off between reduced serum PTH and increased Ca × P [35,36]. Potentially, hypercalcaemia and hyperphosphataemia associated with vitamin D sterol use could result in elevations of calcium and phosphorus and their product, leading to an increased risk of cardiovascular calcification and subsequent mortality [24,37]. Moreover, oversuppression of serum PTH from excessive vitamin D sterol treatment can result in reduced bone turnover (adynamic bone) [38].
The recognition of the hyperphosphataemic and hyercalcaemic effects of vitamin D sterols has led to the development of novel vitamin D sterols, such as paricalcitol, that retain the ability to suppress PTH secretion with reduced calcaemic effects [39]. This benefit was demonstrated in a study comparing paricalcitol with calcitriol in rats [40], yet only a small benefit was observed in a clinical trial [41]. These findings may be attributed to the fact that, although the calcaemic effects of paricalcitol may be reduced in comparison with calcitriol, they are not absent [36]. In patients on haemodialysis, paricalcitol treatment was shown to significantly increase serum calcium and serum phosphorus compared with placebo [28].
Observational studies have investigated the effects of vitamin D sterols, such as paricalcitol, calcitriol and alfacalcidol (1α-hydroxyvitamin D3) on survival in haemodialysis patients. In a retrospective epidemiologic analysis of a large database of haemodialysis patients [42], those treated with injectable vitamin D sterols (>95% receiving paricalcitol or calcitriol) were shown to have a 2-year survival advantage of 20% compared with those not receiving vitamin D sterol treatment (hazard ratio, 0.80; 95% confidence interval, 0.76–0.83). Cardiovascular mortality was also lower in the vitamin D sterol-treated group (7.6/100 person-years) compared with the control group (14.6/100 person-years; P < 0.001). Similarly, in an observational study of 242 haemodialysis patients with stage 5 kidney disease, patients receiving alfacalcidol had a significantly lower risk of cardiovascular death compared with nonusers (hazard ratio, 0.287; P = 0.003) [43]. However, not all studies have demonstrated a survival benefit associated with vitamin D sterol treatment. In the observational Dialysis Outcomes and Practice Patterns Study, no association between vitamin D sterol use and mortality was observed [44]. The results of a retrospective study investigating the relative effects of paricalcitol and calcitriol on survival are also controversial. Patients treated with paricalcitol had a 16% lower mortality rate than patients treated with calcitriol [45]. It should be noted that all of these studies are observational, not randomized, and thus there was a fundamental difference in patients selected/not selected to receive vitamin D. In addition, the two groups were not matched with respect to a number of baseline characteristics, including comorbidities and demographics and, pre-study treatment data were not collected. Thus, that a difference in outcome was found is not unexpected. Prospective, randomized trials will be necessary to conclusively demonstrate if there is a survival benefit associated with vitamin D sterol treatment and whether the potential reduced calcaemic effects of paricalcitol compared with calcitriol translate into lower mortality rates.
Combining cinacalcet and vitamin D sterols to maximize control of SHPT
Because the therapeutic window for treatment with vitamin D sterols is narrow, it is difficult to determine an optimal dose that will sufficiently suppress PTH secretion without also increasing calcium and phosphorus absorption. Cinacalcet has a novel mechanism of action, acting on the CaR to simultaneously reduce serum PTH, calcium, phosphorus and Ca × P [46]. A new treatment paradigm has been proposed in which cinacalcet is used in combination with conventional therapies, such as vitamin D sterols and phosphate binders, for the treatment of SHPT. Phase 2 and phase 3 registrational studies were designed to compare cinacalcet in combination with traditional therapies with placebo and traditional therapies. In these studies, strict protocol-defined rules were followed to maintain the dose of vitamin D sterols at a constant level throughout the study period. These trials demonstrated that cinacalcet enabled significantly more haemodialysis patients with SHPT to achieve individual and combined National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQITM) targets and that cinacalcet induced simultaneous reductions in PTH and Ca × P [47–50].
It has been postulated that treatment with cinacalcet in combination with doses of vitamin D sterols sufficient for replenishment might limit the hypercalcaemia and hyperphosphataemia associated with vitamin D sterol therapy while maintaining control of SHPT. The following section of this review describes the results of two trials that investigated the effects of reduced-dose vitamin D combined with cinacalcet therapy on the achievement of KDOQITM goals: Cinacalcet Open-Label Study to Reach KDOQITM Levels (CONTROL) [51] and An Open-Label, Randomized Study Using Cinacalcet to Improve Achievement of KDOQITM Targets in Patients With End-Stage Renal Disease (OPTIMA) [52–55].
The CONTROL study
The CONTROL study was a 16-week open-label trial in 72 adult haemodialysis patients in the United States with controlled biointact PTH (biPTH) levels (80–160 pg/mL) and uncontrolled Ca × P (>55 mg2/dL2) (Table 1) [51]. At study entry, all patients were receiving moderate to high doses of intravenous vitamin D sterols (paricalcitol, doxercalciferol or calcitriol; mean ± standard deviation paricalcitol equivalent dose, 14.1 ± 7.8 μg/week) and phosphate binders. Doses of vitamin D sterols were reduced on day 1 of the dose-titration phase (to 2 μg paricalcitol equivalent dose per dialysis session) but could be increased during the 8-week titration phase if serum calcium was <8.4 mg/dL or if biPTH was >270 pg/mL and Ca × P was <70 mg2/dL2 and cinacalcet could not be titrated further. The vitamin D sterol dose could be decreased after reaching two consecutive biPTH values of <80 pg/mL. During the 8-week titration phase, cinacalcet doses were titrated in stepwise increments from 30 to 180 mg/d (when biPTH was >160 pg/mL or when biPTH was 80–160 pg/mL and Ca × P was >55 mg2/dL2) to reach treatment targets. Cinacalcet dose adjustments were allowed during the assessment phase, and dose reductions were allowed at any time during the study. The primary efficacy measures were the achievement of biPTH ≤160 pg/mL and Ca × P ≤55 mg2/dL2.
Table 1.
Early-use cinacalcet/vitamin D sterol combination trials: study design
| CONTROL | OPTIMA | |
|---|---|---|
| Study design | Titration phase: Weeks 1–8; Assessment phase: Weeks 8–16 | Dose optimization phase: Weeks 1–16; Assessment phase: Weeks 16–23 |
| Patients | (n = 53); PTH controlled (biPTH: 80–160 pg/mL), elevatedCa × P at baseline | (n = 552); Baseline PTH elevated (iPTH: 300–800 pg/mL) |
| Objective | Evaluate KDOQITM target achievement | Compare treatment strategy with conventional therapy for KDOQITMtarget achievement |
| Treatment | Cinacalcet: titrate to optimum at 8 weeks; vitamin D steroldose reduced at day 1 to levels equivalent to ≤2 μgparicalcitol | Algorithm to optimize combination of cinacalcet, vitamin D sterols andphosphate binder or use best conventional methods; vitamin D steroldose adjusted according to algorithm to a minimum 2 μg paricalcitolequivalent |
Although the dose of vitamin D sterols was reduced, the introduction of cinacalcet enabled PTH control to be maintained (85% achieved their biPTH target versus 91% of patients at baseline, P = not significant), and Ca × P control was markedly improved (72% versus 21% at baseline; P < 0.0001). Serum calcium and phosphorus levels were simultaneously reduced by 9.7% and 11.1% from baseline, respectively. More patients treated with cinacalcet achieved both biPTH and Ca × P goals at assessment compared with baseline (47% versus 17%; P < 0.01) (Figure 1A). At the assessment phase, vitamin D sterol use was discontinued in 21% of patients, and in the other patients, the dose was reduced by 49% (to 6.9 μg/week) compared with baseline (Figure 1B). Taken together, these results demonstrate that cinacalcet and reduced-dose vitamin D sterols can simultaneously address all four KDOQITM targets.
Fig. 1.
CONTROL trial: comparison of standard care with cinacalcet; (A) percentage of patients achieving KDOQITM targets and (B) dose of paricalcitol at baseline and endpoint. Note that the biPTH target in this study was ≤160 pg/mL. The KDOQITM target range is approximately 80–160 pg/mL. *P < 0.01, **P < 0.001. Adapted with permission from Chertow et al. [51].
The OPTIMA study
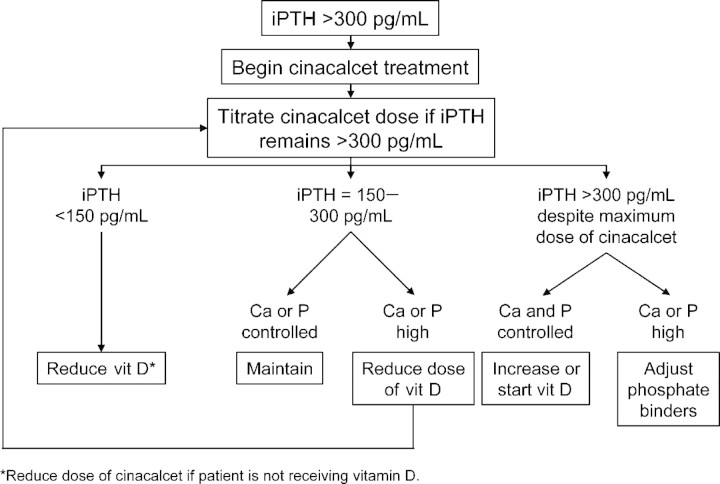
The OPTIMA trial was a 23-week, randomized, standard care-controlled, multicentre, open-label study in 552 dialysis patients in Europe (Table 1) [52–55]. This study used a predefined algorithm designed to optimize the combination of cinacalcet and existing treatments of vitamin D sterols and phosphate binders to control intact PTH (iPTH) and reduce serum calcium and phosphorus (Figure 2). Patients were assigned to cinacalcet or conventional therapy in a 2:1 ratio. Conventional therapy gave investigators full freedom to administer vitamin D sterols and phosphate binders as appropriate to maximize target achievement. Cinacalcet was titrated from a starting dose of 30 mg/d until an iPTH of 150–300 pg/mL was achieved. Dose reductions related to patient safety were allowed at any time during the 23-week study. Vitamin D sterol dose was reduced in patients with calcium ≥9.5 mg2/dL2 or phosphorus ≥5.5 mg2/dL2. The dose of vitamin D sterols was reduced by 50% in sequential steps until a minimum dose (calcitriol, 0.5 μg three times per week [TIW] intravenous or 0.25 μg TIW oral; alfacalcidol, 1 μg TIW intravenous or 0.25 μg daily oral; paricalcitol, 2 μg TIW intravenous) was prescribed. Efficacy as measured by achievement of KDOQITM targets was evaluated during Weeks 17–23.
Fig. 2.
OPTIMA treatment algorithm. Ca, calcium; P, phosphorus; vit D, vitamin D. Adapted with permission from Messa et al. [53].
More patients achieved the primary endpoint of iPTH control (≤300 pg/mL) with cinacalcet compared with standard care (71% versus 22%; P < 0.001). Cinacalcet reduced iPTH levels irrespective of the baseline PTH level (Table 2). When patients were stratified into a less severe SHPT group (baseline iPTH, 300–500 pg/mL) and more severe SHPT group (baseline iPTH, 500–800 pg/mL), the greatest reduction in serum iPTH was achieved in patients with more severe SHPT (Table 2). However, the proportion of patients in the cinacalcet group reaching the primary endpoint of iPTH ≤300 pg/mL was greater in the less severe SHPT group (Figure 3). Additionally, the median dose of cinacalcet was lower in the less severe SHPT group (30 mg/d) than in the more severe SHPT group (60 mg/d).
Table 2.
OPTIMA study efficacy outcomes
| Best conventional treatment | Cinacalcet | |||
|---|---|---|---|---|
| iPTH 300–500 pg/mL(n = 92) | iPTH >500–800 pg/mL(n = 89) | iPTH 300–500 pg/mL(n = 193) | iPTH >500–800 pg/mL(n = 167) | |
| iPTH, % change, mean ± SD | 2.0 ± 46.2 | 1.8 ± 42.5 | −39.9 ± 33.7 | −53.4 ± 27.7 |
| Ca × P, % change, mean ± SD | 3.5 ± 27.3 | 5.9 ± 31.2 | −11.1 ± 27.4 | −13.0 ± 26.0 |
SD, standard deviation.
Adapted with permission from Messa et al. [53].
Fig. 3.
OPTIMA trial: percentage of patients achieving endpoint targets by baseline iPTH level. BCT, best conventional treatment. Adapted with permission from Messa et al. [53].
Compared with the best standard care, the cinacalcet-based algorithm used in the OPTIMA study provided superior control of SHPT. Attempts by physicians to maximize therapy with standard care to attain the recommended targets were generally unsuccessful, with just 30% of patients in the less severe SHPT group achieving the primary endpoint (iPTH ≤300 pg/mL) and 20% of patients reaching the composite endpoint (iPTH ≤300 pg/mL and Ca × P <55 mg2/dL2). In contrast, 80% of cinacalcet-treated patients in the less severe SHPT group reached the primary endpoint, and 64% reached the composite endpoint (Figure 3).
As previously discussed, treatment with vitamin D sterols is often associated with hypercalcaemia and hyperphosphataemia, with consequent risk of increased mortality [24,37,45]. Importantly, in this study, treatment with cinacalcet in combination with vitamin D sterols enabled approximately a 24% reduction in the dose of vitamin D sterols compared with baseline.
Summary and conclusions
Overall, evidence from the CONTROL and OPTIMA trials demonstrates the clinical utility of treatment with cinacalcet in combination with reduced doses of vitamin D sterols. These treatment regimens allowed for both replenishment of vitamin D and improved control of SHPT. Compared with conventional therapy, an increased proportion of CKD patients were able to achieve their individual KDOQITM targets, as well as combined targets of reduced iPTH, calcium, phosphorus and Ca × P. Furthermore, in the OPTIMA trial, this treatment regimen improved control of both PTH and Ca × P in patients with moderate (300–500 pg/mL PTH) and severe (500–800 pg/mL PTH) SHPT. Given the potential risks involved in therapy with vitamin D sterols, including vascular calcification and adynamic bone, the ability to lower vitamin D sterol doses while maintaining, and indeed improving, KDOQITM target achievement is an exciting development. Prospective, long-term clinical trials will be necessary to definitively evaluate the effects of this treatment on mortality, cardiovascular calcification and other outcomes.
Acknowledgments
The authors wish to thank Carol Berry and Ali Hassan for providing medical writing assistance in the preparation of this manuscript. This supplement and online open access are sponsored by Amgen Inc.
Conflict of interest statement. David Bushinsky has given lectures supported by Amgen, Genzyme and Shire. He has also participated in advisory boards for Amgen, Ilypsa, Shire and Genzyme. Piergiorgio Messa has given lectures supported by Amgen, Janssen-Cilag, Abbott, Novartis and Roche. He has also participated in advisory boards for Abbott and Novartis.
References
- 1.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277:F157–F175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 2.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 3.Nishishita T, Okazaki T, Ishikawa T, et al. A negative vitamin D response DNA element in the human parathyroid hormone-related peptide gene binds to vitamin D receptor along with Ku antigen to mediate negative gene regulation by vitamin D. J Biol Chem. 1998;273:10901–10907. doi: 10.1074/jbc.273.18.10901. [DOI] [PubMed] [Google Scholar]
- 4.Demay MB, Kiernan MS, DeLuca HF, et al. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1992;89:8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manolagas SC, Hustmyer FG, Yu XP. 1,25-Dihydroxyvitamin D3 and the immune system. Proc Soc Exp Biol Med. 1989;191:238–245. doi: 10.3181/00379727-191-42915. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Smith C, Prahl JM, et al. Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys. 1993;303:98–106. doi: 10.1006/abbi.1993.1260. [DOI] [PubMed] [Google Scholar]
- 7.Yetgin S, Yalcin SS. The effect of vitamin D3 on CD34 progenitor cells in vitamin D deficiency rickets. Turk J Pediatr. 2004;46:164–166. [PubMed] [Google Scholar]
- 8.Yetgin S, Ozsoylu S. Myeloid metaplasia in vitamin D deficiency rickets. Scand J Haematol. 1982;28:180–185. doi: 10.1111/j.1600-0609.1982.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Bastie JN, Balitrand N, Guidez F, et al. 1alpha,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol Endocrinol. 2004;18:2685–2699. doi: 10.1210/me.2003-0412. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Garami M, Cheng T, et al. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434–448. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 12.Cai Q, Tapper DN, Gilmour RF, Jr, et al. Modulation of the excitability of avian peripheral nerves by vitamin D: relation to calbindin-D28k, calcium status and lipid composition. Cell Calcium. 1994;15:401–410. doi: 10.1016/0143-4160(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 15.Staud R, Vitamin D. More than just affecting calcium and bone. Curr Rheumatol Rep. 2005;7:356–364. doi: 10.1007/s11926-005-0020-0. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca HF, Zierold C. Mechanisms and functions of vitamin D. Nutr Rev. 1998;56:S4–S10. doi: 10.1111/j.1753-4887.1998.tb01686.x. discussion S54–S75. [DOI] [PubMed] [Google Scholar]
- 17.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 18.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol. 2005;288:F253–F264. doi: 10.1152/ajprenal.00302.2004. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda N, Tanaka H, Tominaga Y, et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kifor O, Moore FD, Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 22.Gogusev J, Duchambon P, Hory B, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 23.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 24.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 25.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 26.Brown AJ, Zhong M, Finch J, et al. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270:F454–F460. doi: 10.1152/ajprenal.1996.270.3.F454. [DOI] [PubMed] [Google Scholar]
- 27.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55:1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin KJ, Gonzalez EA, Gellens M, et al. 19-nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9:1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 29.Berl T, Berns AS, Hufer WE, et al. 1,25-Dihydroxycholecalciferol effects in chronic dialysis. A double-blind controlled study. Ann Intern Med. 1978;88:774–780. doi: 10.7326/0003-4819-88-6-774. [DOI] [PubMed] [Google Scholar]
- 30.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 31.Khajehdehi P, Taheri S. Effect of oral calcitriol pulse therapy on the lipid, calcium, and glucose homeostasis of hemodialysis-patients: its safety in a combination with oral calcium carbonate. J Ren Nutr. 2003;13:78–83. doi: 10.1053/jren.2003.50026. [DOI] [PubMed] [Google Scholar]
- 32.Koshikawa S, Akizawa T, Kurokawa K, et al. Clinical effect of intravenous calcitriol administration on secondary hyperparathyroidism. A double-blind study among 4 doses. Nephron. 2002;90:413–423. doi: 10.1159/000054729. [DOI] [PubMed] [Google Scholar]
- 33.Moe SM, Zekonis M, Harezlak J, et al. A placebo-controlled trial to evaluate immunomodulatory effects of paricalcitol. Am J Kidney Dis. 2001;38:792–802. doi: 10.1053/ajkd.2001.27697. [DOI] [PubMed] [Google Scholar]
- 34.Akizawa T, Ohashi Y, Akiba T, et al. Dose-response study of 22-oxacalcitriol in patients with secondary hyperparathyroidism. Ther Apher Dial. 2004;8:480–491. doi: 10.1111/j.1774-9987.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 35.Goodman WG. Recent developments in the management of secondary hyperparathyroidism. Kidney Int. 2001;59:1187–1201. doi: 10.1046/j.1523-1755.2001.0590031187.x. [DOI] [PubMed] [Google Scholar]
- 36.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 37.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 38.Malluche HH, Monier-Faugere MC, Koszewski NJ. Use and indication of vitamin D and vitamin D analogues in patients with renal bone disease. Nephrol Dial Transplant. 2002;17(Suppl 10):6–9. doi: 10.1093/ndt/17.suppl_10.6. [DOI] [PubMed] [Google Scholar]
- 39.Martin KJ, Gonzalez EA. Vitamin D analogues for the management of secondary hyperparathyroidism. Am J Kidney Dis. 2001;38:S34–S40. doi: 10.1053/ajkd.2001.28109. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi F, Finch JL, Denda M, et al. A new analog of 1,25-(OH)2D3, 19-nor-1,25-(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. Am J Kidney Dis. 1997;30:105–112. doi: 10.1016/s0272-6386(97)90571-0. [DOI] [PubMed] [Google Scholar]
- 41.Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 42.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 43.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 44.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the dialysis outcomes and practice patterns study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 45.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 46.Steddon SJ, Fan SL, Cunningham J. New prospects for the management of renal bone disease. Nephron Clin Pract. 2005;99:c1–c7. doi: 10.1159/000081787. [DOI] [PubMed] [Google Scholar]
- 47.Moe SM, Chertow GM, Coburn JW, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67:760–771. doi: 10.1111/j.1523-1755.2005.67139.x. [DOI] [PubMed] [Google Scholar]
- 48.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 49.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 50.Quarles LD, Sherrard DJ, Adler S, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14:575–583. doi: 10.1097/01.asn.0000050224.03126.ad. [DOI] [PubMed] [Google Scholar]
- 51.Chertow G, Blumenthal S, Turner S, et al. Cinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin D derivatives with controlled PTH and elevated calcium × phosphate. Clin J Am Soc Nephrol. 2006;1:305–312. doi: 10.2215/CJN.00870805. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez M, Hutchison A, Girndt M, et al. Secondary hyperparathyroidism (HPT) in patients receiving peritoneal dialysis (PD) can be effectively managed with cinacalcet (Mimpara/Sensipar) [Abstract SP359] Presented at: ERA-EDTA Congress, 15–18 July 2006, Glasgow, UK.
- 53.Messa P, Villa G, Braun J, et al. The OPTIMA study: lower doses of cinacalcet (Mimpara/Sensipar) are required to achieve KDOQI secondary hyperparathyroidism (HPT) targets in patients with less severe disease [Abstract and Presentation MP324] Presented at: ERA-EDTA Congress, 15–18 July 2006, Glasgow, UK.
- 54.Wilkie M, Salvadori M, De Meester J, et al. The OPTIMA study: optimising the dose of vitamin D (vit D) in the presence of cinacalcet (Mimpara/Sensipar) to obtain maximum clinical benefit [Abstract SP358] Presented at: ERA-EDTA Congress, 15–18 July 2006, Glasgow, UK.
- 55.Locatelli F, Macario F, Brink H, et al. The OPTIMA study; efficacy of cinacalcet (Mimpara/Sensipar) treatment algorithm to treat dialysis patients with elevated PTH and calcium–phosphorus product (Ca × P) [Abstract SP357] Presented at: ERA-EDTA Congress, 15–18 July 2006, Glasgow, UK.