Abstract
Standard therapy for secondary hyperparathyroidism (SHPT) includes dietary calcium supplementation, active vitamin D, and phosphate binders; however, these are often insufficient to allow patients to achieve their serum parathyroid hormone (PTH), calcium and calcium–phosphorus product (Ca × P) targets. Recent preclinical studies have demonstrated that treatment with type II calcimimetics that increase the sensitivity of the calcium-sensing receptor (CaR) to calcium can reverse the alterations in CaR and vitamin D receptor expression and parathyroid cell proliferation that are associated with SHPT. These data suggest that calcimimetic treatment could stabilize disease progression and improve maintenance of treatment goals. In clinical trials involving SHPT patients, the calcimimetic cinacalcet has been shown to decrease PTH, calcium, phosphorus and Ca × P. Significant improvements were seen regardless of initial disease severity, and benefits were maintained over the course of long-term therapy (up to 4 years), indicating effective disease stabilization. In conclusion, preclinical and clinical data provide both theoretical and empirical support for the use of calcimimetics in moderate and advanced SHPT to effectively stabilize disease.
Keywords: calcimimetic, calcium-sensing receptor, cinacalcet, secondary hyperparathyroidism
Stabilization of secondary hyperparathyroidism with calcimimetics: preclinical evidence
Calcimimetics provide an alternative approach to the traditional therapy with active vitamin D and phosphate binders for the management of secondary hyperparathyroidism (SHPT). Type II calcimimetics are positive allosteric modulators of the parathyroid gland calcium-sensing receptor (CaR) that increase its sensitivity to serum calcium, so that lower concentrations of calcium are sufficient to mediate signalling through the CaR [1]. This suggests that, despite the reduced CaR expression observed in hyperplastic parathyroid tissues [2,3], calcimimetics have the potential to control even advanced disease by enhancing the function of the remaining CaR [4–7]. In addition, calcimimetics have the potential to influence both CaR expression and CaR functions that have been implicated in the progression of disease, particularly effects on parathyroid hormone (PTH), cell proliferation and hyperplasia. These characteristics suggest that targeting the CaR presents a therapeutic option for the stabilization of early disease, as well as remaining a viable therapeutic target in the face of advancing disease. In addition to being effective in patients with mild to moderate disease [5], calcimimetics may be the most viable option for chronic kidney disease (CKD) patients with advanced hyperparathyroidism, who are often refractory to standard calcitriol therapy [8–10]. The data below summarize preclinical findings providing a rationale for the clinical use of calcimimetics.
CaR expression
Animal data indicate that calcimimetic therapy can have a direct impact on CaR expression. Administration of cinacalcet or the calcimimetic R-568 to 5/6 nephrectomized rats prevented hyperplasia or increased CaR expression [2,11]. These actions support a possible role for calcimimetics in modifying the course of SHPT. Interestingly, in an in vitro study, the calcimimetic cinacalcet suppressed PTH secretion in human parathyroid cells with advanced hyperparathyroidism, even though CaR expression was significantly diminished [12].
Vitamin D receptor expression
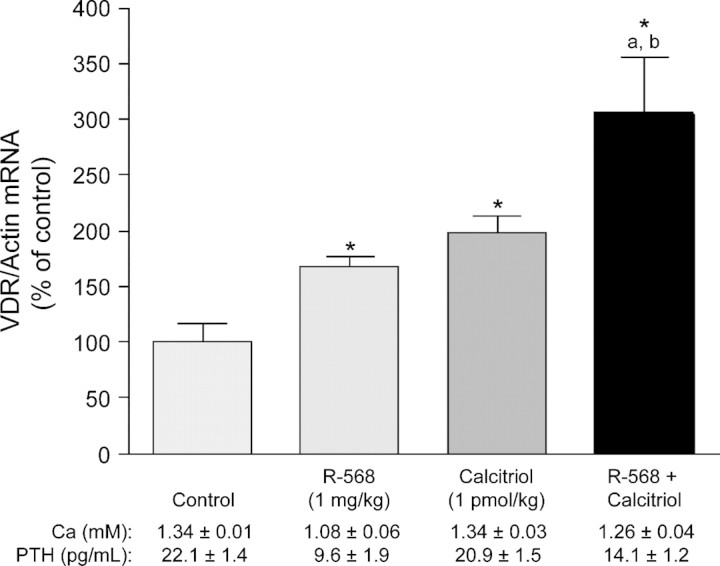
Expression of vitamin D receptor (VDR) mRNA is reduced in the parathyroid gland of hypocalcaemic rats compared with normocalcaemic rats [13]. Because the VDR is a transcriptional repressor of PTH [14], these data suggest that reduced signalling through this receptor might affect PTH secretion. Recent studies indicate that type II calcimimetics, in the presence of low concentrations of calcium, can up-regulate VDR mRNA [15]. Rats injected with a calcimimetic (R-568, 1 mg/kg intravenously 3 and 6 h before euthanasia) exhibited an increase in VDR mRNA expression compared with controls (P < 0.001), with a marked increase observed when R-568 and calcitriol were coadministered (Figure 1).
Fig. 1.
The effect of the calcimimetic R-568 on rat parathyroid gland VDR mRNA in vivo. Rats were treated with R-568 (1 mg/kg intravenously 3 and 6 h before euthanasia; n = 22), calcitriol (10 pmol/kg intraperitoneally every 30 min for 5.5 h before euthanasia; n = 22), or a combination of R-568 and calcitriol (n = 22) before euthanasia and measurement of VDR mRNA by quantitative real-time polymerase chain reaction. A control group (n = 20) received no treatment. Mean serum concentrations of ionized calcium and PTH are also included. *P < 0.01 versus control; aP < 0.05 versus calcimimetic; bP < 0.05 versus control. Data are mean ± standard error. Adapted with permission from Rodriguez et al. [15].
These data indicate that, in addition to regulating PTH levels and restoring CaR density, calcimimetics can up-regulate VDR expression in parathyroid cells. This effect of calcimimetics on VDR expression is of clinical importance because it may result in an additive interaction between calcimimetic and vitamin D sterol therapy.
Parathyroid cell proliferation and parathyroid hyperplasia
Although parathyroid cells are relatively quiescent under normal conditions, the mineral and hormonal imbalances characteristic of SHPT, particularly low serum calcium and high serum phosphorus, drive cell proliferation leading to polyclonal and oligoclonal cell expansion [8]. The importance of maintaining calcium homeostasis is evidenced in weanling rats fed a low-calcium diet for 10 days, in which the number of proliferating cell nuclear antigen (PCNA)-positive parathyroid cells was increased sixfold compared with controls [16]. Calcimimetics, in the presence of low serum calcium, can hold proliferation in check, as evidenced by decreased parathyroid cell proliferation in uraemic rats following treatment [17] and their ability to cause regression of advanced hyperplasia in some experimental animal models [11,17]. Long-term administration of the calcimimetic cinacalcet significantly reduced the number of proliferating (PCNA-positive) cells and glandular hyperplasia, as measured by weight, in 5/6 nephrectomized rats. This effect was parathyroid tissue-specific and was due to a direct effect on cell growth, rather than to induction of apoptosis [11].
Taken together, the mechanistic data presented here, and reviewed in greater detail in another article in this supplement (Riccardi and Martin), suggest that calcimimetics have the potential to retard SHPT disease progression. Preclinical studies in uraemic rats indicate that calcimimetics can modify the defining parameters in SHPT by significantly lowering PTH and blood-ionized calcium in a dose-dependent manner without inducing significant changes in serum phosphate [11]. Clinical investigations supporting this therapeutic modality are evaluated in the remainder of this review.
Unmet medical need in SHPT management
One of the primary treatment goals of SHPT is to restore mineral and hormone balance. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQITM) has established recommendations regarding levels of serum calcium, phosphorus, calcium–phosphorus product (Ca × P), and intact PTH (iPTH) for patients based on their degree of kidney function [18]. As kidney function decreases, mineral homeostasis is increasingly perturbed, resulting in increased PTH, serum phosphorus, and Ca × P. As this dysregulation progresses, the response to standard therapy worsens [8]. Consequently, PTH can become high enough to increase mortality and impede adequate control of phosphate [19–21]. These findings underscore the need for new or adjunct treatments with improved efficacy over current modalities that can stabilize disease progression during a long-term course when initiated early, but can also maintain effectiveness in patients with more severe disease.
Because of the ability of calcimimetics, demonstrated in animal studies, to modulate disease-related processes through multiple mechanisms of action implicated in the development of disease, this therapeutic modality appears to be well suited for use in combination with moderate doses of vitamin D as the primary mode of treatment in SHPT management. As described earlier, calcimimetics up-regulate CaR and VDR expression and inhibit parathyroid cell proliferation and hyperplasia. These multiple mechanisms of action may allow calcimimetics to (1) maintain effectiveness during long-term therapy; (2) maintain enhanced efficacy in patients with severe disease despite decreased CaR and VDR expression and (3) halt development of severe disease if initiated early in patients with mild or moderate SHPT. Each of these goals represents an unmet medical need in the management of SHPT.
Phase 3 clinical trials in SHPT patients with advanced disease have shown that cinacalcet decreased PTH, as well as serum calcium, phosphorus and Ca × P [5,7,22]. Analysis of the data from these trials has been performed to investigate the efficacy of cinacalcet in the treatment of both early- and later-stage SHPT and to investigate the potential of long-term cinacalcet therapy to stabilize disease progression. Results of these analyses, presented below, suggest that calcimimetics have the ability to address these unmet therapeutic needs.
Effect of cinacalcet on disease stabilization in clinical trials
A post-hoc analysis of the combined results of three 26-week, placebo-controlled, phase 3 studies evaluating the efficacy and safety of a once-daily dose of cinacalcet for the treatment of SHPT demonstrated the impact of therapy initiated in early or advanced disease [23]. These studies included adult patients on maintenance dialysis who had iPTH levels ≥300 pg/mL despite standard treatment with active vitamin D and phosphate binders. Patients received standard therapy in combination with cinacalcet (n = 546) or placebo (n = 408). Patients included in the analysis were divided into eight subgroups according to their baseline iPTH level, which was used as a marker of disease severity (300 to >1000 pg/mL). Findings from this analysis indicated that the use of cinacalcet (30 mg titrated up to 180 mg) significantly decreased both iPTH and Ca × P levels regardless of disease severity at baseline, compared with standard therapy (Figure 2). The progressive nature of SHPT, even when patients receive standard therapy, is evidenced by positive changes from baseline in iPTH seen in these patients (Figure 2A) [23]. Furthermore, the effect of cinacalcet on Ca × P (Figure 2B) and serum phosphorus levels was more pronounced in patients with more severe disease, suggesting a more significant impact of cinacalcet on bone turnover in these patients.
Fig. 2.
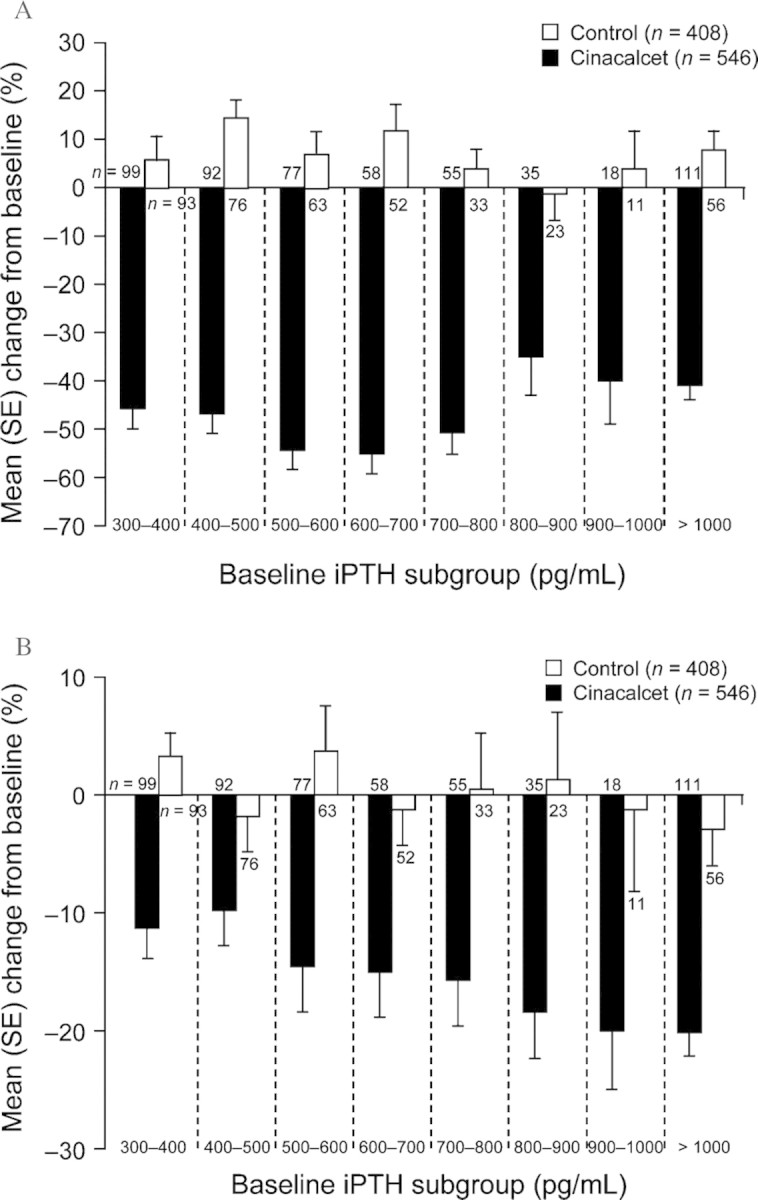
Percentage change in (A) iPTH and (B) Ca × P by disease severity. Patients were treated with cinacalcet (beginning at 30 mg with possible titration up to 180 mg; n = 546) or placebo (n = 408) for 26 weeks. Patients were divided into groups according to baseline iPTH levels (pg/mL). Bars represent the mean (SE) percentage change from baseline for iPTH. SE, standard error. Adapted with permission from Frazão et al. [23].
However, although these data demonstrate that cinacalcet is effective in reducing serum iPTH and Ca × P, even in patients with severe disease, these patients remain less likely to achieve their KDOQITM targets than patients with less severe disease. After 6 months of treatment with cinacalcet, 76% of patients with a baseline iPTH of 300–500 pg/mL achieved a serum iPTH level of <300 pg/mL compared with 55% of patients with a baseline iPTH of 500–800 pg/mL and 16% of patients with a baseline iPTH of >800 pg/mL. Similarly, 54% of patients with a baseline iPTH of 300–500 pg/mL achieved an iPTH of <300 pg/mL and their Ca × P target (<55 mg2/dL2), compared with 36% and 9% of patients in the 500–800 and >800 mg/mL groups, respectively [24]. These data suggest that early treatment of SHPT provides the best opportunity to maintain control of serum PTH, calcium, phosphorus and Ca × P.
Treatment with cinacalcet for up to 1 year resulted in increased attainment of KDOQITM targets compared with standard therapy [25]. After 12 months, 66% of patients on dialysis receiving cinacalcet achieved an iPTH of ≤300 pg/mL compared with 15% in the placebo group. Similarly, 55% of patients receiving cinacalcet achieved their Ca × P target (<55 mg2/dL2) compared with 43% in the placebo group. Furthermore, the proportion of patients able to achieve both an iPTH of ≤300 pg/mL and their Ca × P target was considerably higher in the cinacalcet group than in the placebo group; 42% of patients in the cinacalcet group achieved both targets simultaneously, whereas only 8% of patients in the placebo group reached both targets (Figure 3A).
Fig. 3.
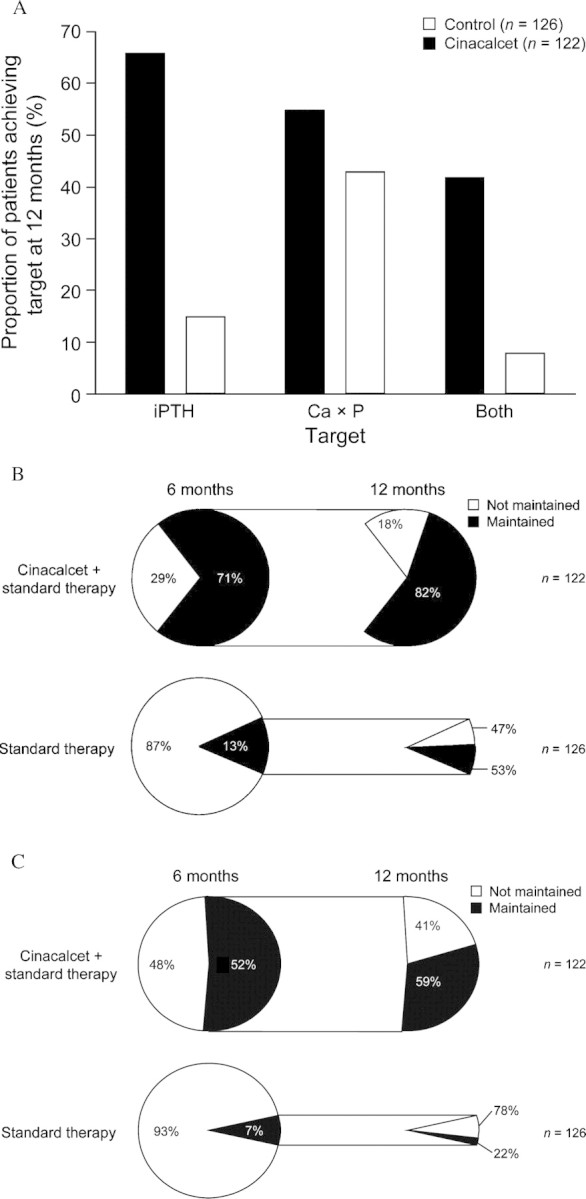
Proportion of patients achieving serum iPTH ≤300 pg/mL and Ca × P <55 mg2/dL2 during treatment with either cinacalcet (n = 122) or placebo (n = 126). (A) Achievement of serum iPTH ≤300 pg/mL and Ca × P <55 mg2/dL2 after 1 year. (B) Maintenance of serum iPTH ≤300 pg/mL after 6 and 12 months of treatment. (C) Maintenance of combined serum iPTH and Ca × P target after 6 and 12 months of treatment. Adapted with permission from Frazão et al. [25].
In this study, treatment with cinacalcet not only improved the ability of patients to achieve their KDOQITM targets, but also their ability to maintain these targets. Seventy-one percent of cinacalcet-treated patients were able to achieve an iPTH of ≤300 pg/mL after 6 months of cinacalcet treatment. Of the group who achieved an iPTH of ≤300 pg/mL at 6 months, 82% also maintained an iPTH of ≤300 pg/mL after 1 year. In contrast, in the standard care group, 13% of patients achieved an iPTH concentration of ≤300 pg/mL, with 53% of this group continuing to maintain an iPTH ≤300 pg/mL after 1 year (Figure 3B). Cinacalcet also improved maintenance of the combined iPTH and Ca × P targets. In the cinacalcet group, 52% of patients reached the combined target after 6 months compared with 7% in the standard care group. Of these patients, 59% in the cinacalcet group maintained the combined target after 1 year compared with just 22% in the standard care group (Figure 3C). These data demonstrate that cinacalcet effectively reduces and maintains the four critical disease biomarkers associated with SHPT in CKD patients. Cinacalcet, therefore, reduces the shifting in and out of targets often observed in patients using standard therapy.
Open-label extension studies have evaluated the ability of cinacalcet to stabilize disease in SHPT patients for up to 4 years [26–28]. These analyses were based on a series of double-blind randomized clinical trials in which patients received standard care treatment in combination with either placebo or cinacalcet for 26 or 52 weeks, after which patients could receive open-label cinacalcet treatment. In one such open-label extension study, serum iPTH was found to progressively increase in patients treated with standard care for 52 weeks [26]. After open-label cinacalcet treatment was started, serum iPTH was rapidly reduced to a level matching that of cinacalcet-treated patients. After 2 years of open-label cinacalcet therapy, approximately 59% of all patients (n = 59) had achieved an iPTH of ≤300 pg/mL at each study visit. Serum Ca × P did not increase during this period.
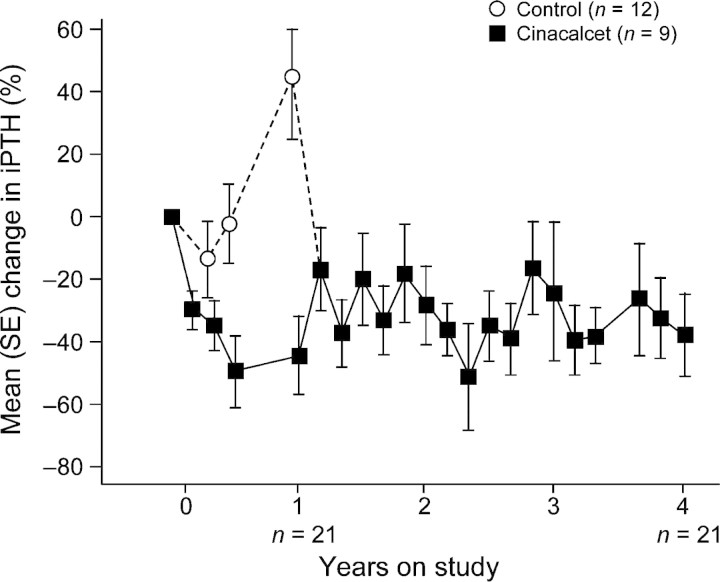
In a separate study, a limited number of patients (n = 21) taking cinacalcet were followed up for as long as 4 years [28]. A substantial upward trend in iPTH was noted in control patients who received standard therapy during the first year of treatment in this study, before switching to cinacalcet therapy, with levels increasing from approximately 600–850 pg/mL. In these patients, cinacalcet reduced and maintained iPTH levels throughout the follow-up period (Figure 4). After 4 years, the mean serum iPTH was 396 pg/mL, 65% of patients achieved a serum iPTH of ≤300 pg/mL, and the mean serum Ca × P was 50.5 mg2/dL2. These findings again highlight the progressive nature of SHPT in patients receiving standard therapy as first-line treatment [28], as well as the inability of traditional therapies to effectively control or stabilize disease in both the short and long term. These and other clinical observations discussed in this section are also consistent with preclinical evidence supporting the CaR as a therapeutic target to optimize the achievement of treatment goals and slow or prevent disease progression.
Fig. 4.
Effect of long-term cinacalcet treatment on serum iPTH in dialysis patients. Patients received double-blind treatment with either cinacalcet (n = 9) or placebo (n = 12) for 1 year followed by a 3-year open-label extension treatment with cinacalcet. SE, standard error. Adapted with permission from Cunningham et al. [28].
Conclusions
Calcimimetics are highly specific therapeutic agents that effectively lower concentrations of plasma PTH and partially correct disturbances in mineral metabolism that are characteristic of SHPT. The mechanism of action involves allosteric modification of the CaR, rendering it more sensitive to calcium signalling, thereby helping counteract the decreased CaR expression and prevent and halt the progression of parathyroid hyperplasia. In addition to effects on CaR signalling, new experimental studies indicate that calcimimetics may act to increase VDR expression. These studies raise the possibility that treatment with calcimimetics might improve the sensitivity of the parathyroid glands to calcitriol and vitamin D analogues, allowing reduced doses to be used and thus minimizing the risk of hypercalcaemia and hyperphosphataemia.
Preclinical mechanistic studies with calcimimetics have suggested that this modality may arrest disease progression, even in patients with advanced disease at treatment onset, and stabilize SHPT, thereby allowing improved maintenance of KDOQITM targets. Thus far, clinical data support this scenario. In clinical use, cinacalcet was found to be effective for the reduction and long-term maintenance of iPTH, calcium and phosphorus levels in patients with SHPT. Cinacalcet has been shown to allow more patients to achieve and maintain KDOQITM targets compared with standard therapy, and extended use (up to 4 years) has been shown to be effective and well tolerated.
Acknowledgments
The authors wish to thank Susan DeRocco for providing medical writing assistance in the preparation of this manuscript. This supplement and online open access are sponsored by Amgen Inc.
Conflict of interest statement. João M. Frazão has been a scientific consultant for Amgen and Genzyme. He has also participated in advisory boards for Amgen, Genzyme and Abbott. Mariano Rodriguez has received honoraria and grants from Amgen and Shire. He has also participated in advisory boards for Amgen and Shire.
References
- 1.Hammerland LG, Garrett JE, Hung BC, et al. Allosteric activation of the Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol. 1998;53:1083–1088. [PubMed] [Google Scholar]
- 2.Mizobuchi M, Hatamura I, Ogata H, et al. Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol. 2004;15:2579–2587. doi: 10.1097/01.ASN.0000141016.20133.33. [DOI] [PubMed] [Google Scholar]
- 3.Kifor O, Moore FD, Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg JS, Moe SM, Goodman WG, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium × phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63:248–254. doi: 10.1046/j.1523-1755.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 6.Quarles LD, Sherrard DJ, Adler S, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14:575–583. doi: 10.1097/01.asn.0000050224.03126.ad. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288:F253–F264. doi: 10.1152/ajprenal.00302.2004. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez M, Canalejo A, Garfia B, et al. Pathogenesis of refractory secondary hyperparathyroidism. Kidney Int Suppl. 2002:155–160. doi: 10.1046/j.1523-1755.61.s80.26.x. [DOI] [PubMed] [Google Scholar]
- 10.Slatopolsky E, Weerts C, Thielan J, et al. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colloton M, Shatzen E, Miller G, et al. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005;67:467–476. doi: 10.1111/j.1523-1755.2005.67103.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawata T, Imanishi Y, Kobayashi K, et al. Direct in vitro evidence of the suppressive effect of cinacalcet HCl on parathyroid hormone secretion in human parathyroid cells with pathologically reduced calcium-sensing receptor levels. J Bone Miner Metab. 2006;24:300–306. doi: 10.1007/s00774-006-0687-y. [DOI] [PubMed] [Google Scholar]
- 13.Garfia B, Canadillas S, Canalejo A, et al. Regulation of parathyroid vitamin D receptor expression by extracellular calcium. J Am Soc Nephrol. 2003;13:2945–2952. doi: 10.1097/01.asn.0000037676.54018.cb. [DOI] [PubMed] [Google Scholar]
- 14.Silver J, Naveh-Many T, Mayer H, et al. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78:1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez ME, Almaden Y, Canadillas S, et al. The calcimimetic R-568 increases vitamin D receptor expression in rat parathyroid glands. Am J Physiol Renal Physiol. 2007;292:F1390–F1395. doi: 10.1152/ajprenal.00262.2006. [DOI] [PubMed] [Google Scholar]
- 16.Naveh-Many T, Rahamimov R, et al. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest. 1995;96:1786–1793. doi: 10.1172/JCI118224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada M, Furuya Y, Sakiyama J, et al. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 1997;100:2977–2983. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 19.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 21.Block GA, Hulbert-Shearon T, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 22.Moe SM, Chertow GM, Coburn JW, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67:760–771. doi: 10.1111/j.1523-1755.2005.67139.x. [DOI] [PubMed] [Google Scholar]
- 23.Frazão J, Nicolini M, Torregrosa V, et al. Cinacalcet HCl effectively reduces intact parathyroid hormone (iPTH) and Ca × P irrespective of the severity of secondary hyperparathyroidism (HPT) [abstract and presentation MO18] Presented at: ERA-EDTA Congress, 15–18 May 2004, Lisbon, Portugal.
- 24.Frazão J, Messa P, Cunningham J, et al. Early use of cinacalcet (MIMPARA/SENSIPAR) in dialysis patients enables greatest achievement of NKF-KDOQI treatment targets for bone metabolism [abstract SO016] Presented at: ERA-EDTA Congress, 15–18 July 2006, Glasgow, UK.
- 25.Frazão J, Holzer H, Stummvoll H, et al. Cinacalcet (Mimpara/Sensipar) maintains achievement of NKF-K/DOQI treatment targets for secondary hyperparathyroidism (HPT) in patients on dialysis [abstract SP209] Presented at: ERA-EDTA Congress, 4–7 June 2005, Istanbul, Turkey.
- 26.Moe SM, Cunningham J, Bommer J, et al. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol Dial Transplant. 2005;20:2186–2193. doi: 10.1093/ndt/gfh966. [DOI] [PubMed] [Google Scholar]
- 27.Sprague SM, Nicolini M, Evenepoel P, et al. Simultaneous control of PTH and Ca × P is sustained over 2 years of treatment with Sensipar (cinacalcet HCL) Presented at: Renal Week, 8–13 November 2005, Philadelphia, PA.
- 28.Cunningham J, Urena P, Reichel H, et al. Long-term efficacy of cinacalcet in secondary hyperparathyroidism (HPT) of end stage renal disease (ESRD) [abstract SP210] Presented at: ERA-EDTA Congress, 4–7 June 2005, Istanbul, Turkey.